Abstract
Background:
Cervical radicular pain is a challenging medical problem in terms of therapeutic management. Recently, pulsed radiofrequency (PRF) stimulation on the dorsal root ganglion (DRG) has been used to control several types of chronic pain. However, its effect on cervical radicular pain is still not well studied. To conduct a meta-analysis of available clinical studies on PRF treatment in patients with cervical radicular pain induced by cervical spine disease that was not responsive to other conservative treatments.
Methods:
A comprehensive database search was conducted on PubMed, Embase, Cochrane Library, and SCOPUS. We included studies published up to August 31, 2017, that fulfilled our inclusion and exclusion criteria. The pain degrees measured using visual analog scale (VAS) at pretreatment and after PRF on the DRG were collected for the meta-analysis. The Cochrane Collaboration's Handbook and Newcastle–Ottawa scale were used for the methodological quality assessments of included studies. The meta-analysis was performed using the Comprehensive Meta-analysis Version 2.
Results:
A total of 67 patients from one RCT study, 2 prospective observational studies, and one retrospective study were included in this meta-analysis. The pooled data of the 4 included studies showed that overall VAS after the PRF treatment was significantly reduced (P ≤ .001). In the subgroup analysis according to follow-up evaluation time points, the pain was significantly reduced at 2 weeks, 1 month, 3 months, and 6 months after the procedure (2 weeks: P = .02; 1, 3, and 6 months: P < .001).
Conclusion:
According to the results of the meta-analysis, the use of PRF on the DRG is effective for alleviating cervical radicular pain, which was unresponsive to oral medications, physical therapy, or epidural steroid injection.
Keywords: cervical radicular pain, disc herniation, meta-analysis, pulsed radiofrequency, spinal stenosis
1. Introduction
Cervical radicular pain is the most frequently occurring neuropathic pain in the upper extremity. Its incidence was reported to be approximately 83 per 100,000 people.[1] The most common causes of cervical radicular pain are herniation of the cervical disc and cervical foraminal stenosis.[2] Mechanical compression of the cervical nerve root and chemical inflammation cause cervical radicular pain.[3,4] For the management of cervical radicular pain, several oral medications, modalities, and procedures have been used.[5] However, despite these treatments, pain persists in some patients.
Recently introduced pulsed radiofrequency (PRF), which works by delivering an electrical field and heat bursts to targeted nerves or tissues without damaging these structures, has been reported to be safe and effective in controlling several types of chronic pain.[6–8] Conventional radiofrequency (RF) thermocoagulation exposes target nerves or tissues to a continuous electrical stimulation and ablates the structures by increasing the temperature around the RF needle tip.[9] In contrast to RF, PRF applies a brief electrical stimulation followed by a long resting phase. Thus, PRF does not produce sufficient heat for structural damage.[10] The mechanism of PRF is not clearly elucidated, but it has been proposed that the electrical field produced by PRF can alter pain signals.[11–13]
Several studies have reported the effectiveness of PRF on the dorsal root ganglion (DRG) in alleviating refractory cervical radicular pain.[14–23] However, the effect of PRF on cervical radicular pain has not been clearly elucidated yet because those previous studies are limited by their small sample size. To further explore this issue, we performed a meta-analysis of all available clinical studies of PRF treatment in patients with cervical radicular pain induced by cervical spine disease.
2. Materials and methods
2.1. Search strategy
This meta-analysis was performed according to the guidelines from Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). We performed systematic searches of the relevant literature contained in PubMed, Embase, Cochrane Library, and SCOPUS for studies published until August 31, 2017. The following keywords were used for the database search: (pulsed radiofrequency AND radicular pain) OR (pulsed radiofrequency AND radiculopathy) OR (pulsed radiofrequency AND spine) OR (pulsed radiofrequency AND spinal stenosis) OR (pulsed radiofrequency AND disc herniation). The filters were used to select studies with human participants. We included all study designs, not limited to randomized control trial (RCT). However, we only included published articles in English.
2.2. Eligibility criteria
Following inclusion criteria were applied for the selection of articles: patients’ radicular pain were secondary to cervical disc herniation or cervical spinal stenosis; pain was not controlled even after conservative management, which included oral medications, physical therapy, or epidural steroid injection; PRF stimulation was applied on the cervical DRG; visual analog scale (VAS) or numeric rating scale (NRS) was used for the evaluation of pain degree; 5) patients were followed-up for at least 2 weeks. We excluded studies from the final analysis if: the study was a review article, abstract, letter or case report; the study reported no data/results.
2.3. Study selection and data extraction
After duplicate publications were deleted, 2 reviewers (DGL and MCCC) independently evaluated potentially eligible studies that were identified by our search. Articles were screened for eligibility based on a review of the title and abstract, and disagreements were resolved by consensus. The full text of eligible articles were accessed and read independently by the 2 reviewers (DGL and MCCC). Then, the following data were independently extracted from each eligible study: first author, publication data, number of patients, demographic information, intervention characteristics, and outcome data, including VAS and NRS. In case of studies reporting pain in NRS, values were converted into VAS.
2.4. Quality assessment
The methodological quality of the included studies was assessed using 2 different tools. For RCTs, the Cochrane Collaboration's Handbook was used to determine adequate sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential sources of bias.[24] The judgments of bias were expressed as “low risk,” “high risk,” or “unclear risk.” For prospective observational and retrospective studies, the Newcastle–Ottawa scale (NOS) was used for the quality assessment, with 3 aspects of selection: selection of subjects, comparability of groups, and assessment of outcome. The quality of each study was graded as low (0–3), moderate (4–6), and high (7–9).[25] All divergences were resolved by consensus.
2.5. Statistical analysis
Comprehensive Meta-analysis Version 2 (Biostat Inc.) was used for statistical analysis of the pooled data. For each analysis, a heterogeneity test was performed using I2 statistics, which measures the extent of inconsistency among results. I2 = 25% was considered low, 50% moderate, and 75% high heterogeneity.[24]I2 values higher than 50% were considered as having substantial heterogeneity, and the random-effects model was used for analysis of the data.[24] VAS or NRS were continuous variables; thus, we analyzed the standardized mean difference (SMD) in change from baseline and the 95% confidence interval (CI) in the analysis. Additionally, we performed subgroup analyses according to the follow-up evaluation time point. P < .05 was considered statistically significant.
3. Results
3.1. Study selection
The primary literature search provided a total of 545 potentially relevant studies (Fig. 1). After discarding the duplicate studies and reading the tiles and abstracts of the articles, 536 publications were excluded. The remaining studies were further assessed for eligibility based on the full text articles. After reviewing, 4 articles were included in the final analysis. One RCT study, 2 prospective observational studies, and 1 retrospective study were included in this meta-analysis.[16,17,19,23] In the RCT study by Lee et al, patients in the control group received transforaminal steroid injection.[19] We extracted the data of patients in the PRF group only.
Figure 1.
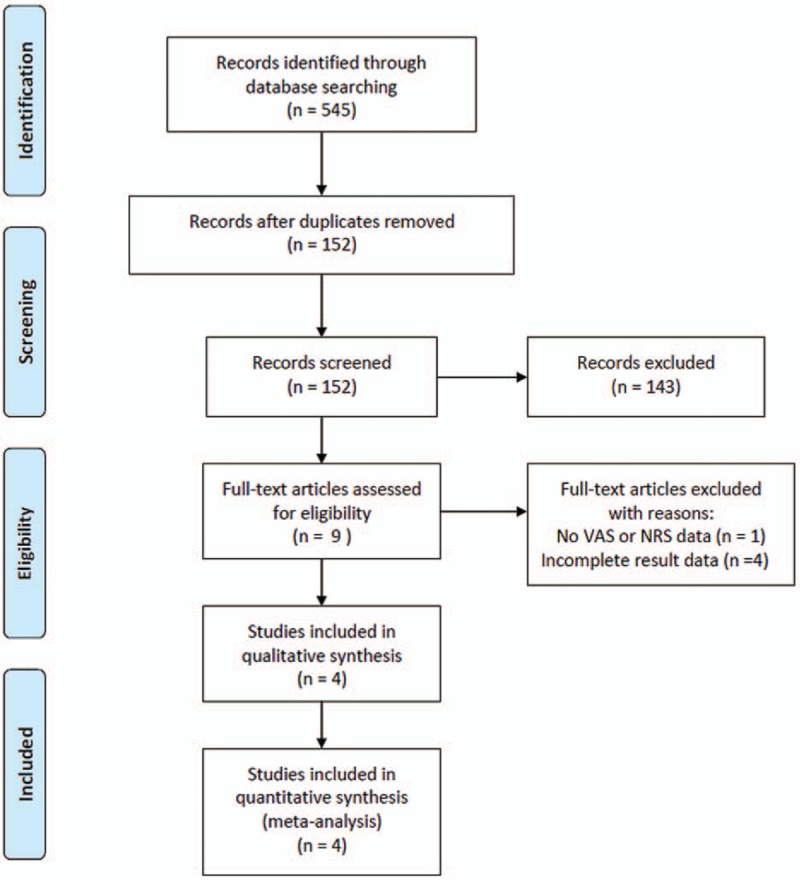
Flow chart showing the search results of the meta-analysis.
3.2. Study characteristics
The selected studies included 67 cases. The study duration ranged from 3 months to 1 year. The basic characteristics of the included studies are shown in Table 1.
Table 1.
Characteristics of the included studies.

3.3. Risk of bias
The study by Lee et al[19] was an RCT. Thus, based on the Cochrane Handbook 5.1 Assessment Tool, the risk of bias was assessed. The study had unclear risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment. Low risk of bias was observed on incomplete outcome data, selective reporting, and other bias. The other studies (2 observational studies and one retrospective study)[16,17,23] were assessed using NOS. All of them were rated 8 stars, which is considered as relatively high-quality (selection of subjects: 4 stars; comparability of groups: 2 stars; assessment of outcome: 2 stars).
3.4. Meta-analysis results
The overall outcome for the pooled analysis of all the outcomes from 2 weeks to 1 year after the PRF treatment in all included studies showed that VAS was significantly reduced after PRF treatment on the DRG (SMD = −1.75, 95% CI = −2.06 to −1.44, P = <.001; Fig. 2). The random-effect model was used because the I2 value was 52.4%. In addition, we conducted the subgroup analysis according to follow-up evaluation time points. On the analysis of pain reduction at 2 weeks after the PRF treatment, I2 was over 50% (2 weeks: I2 = 82.3%; Fig. 3). Accordingly, the random-effect model was used for pain reduction effect at 2 weeks after the procedure. I2 was below 50% at 1, 3, and 6 months after the procedure (1 month: I2 = 0.0%; 3 months: I2 = 36.1%, 6 months: I2 = 13.1%); thus, the fixed effect model was used for subgroup analysis. At each evaluation time points, VAS was significantly reduced after the PRF treatment (2 weeks: SMD = −1.51, 95% CI = −2.77 to −0.26, P = .02; 1 month: SMD = −1.70, 95% CI = −2.07 to −1.32, P = <.001; 3 months: SMD = −1.89, 95% CI = −2.29 to −1.48, P = <.001; 6 months: SMD = −1.84, 95% CI = −2.33 to −1.34, P = <.001; Fig. 3).
Figure 2.
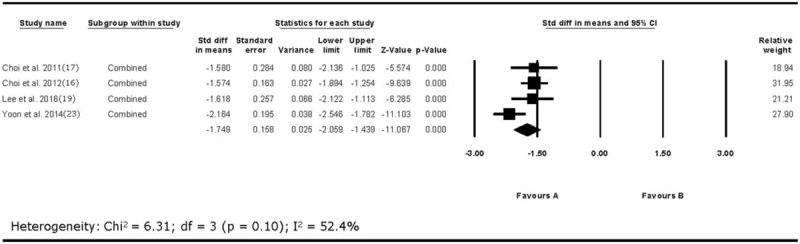
Results of analysis of overall visual analog scale (VAS) changes after pulsed radiofrequency treatment. VAS = visual analog scale.
Figure 3.
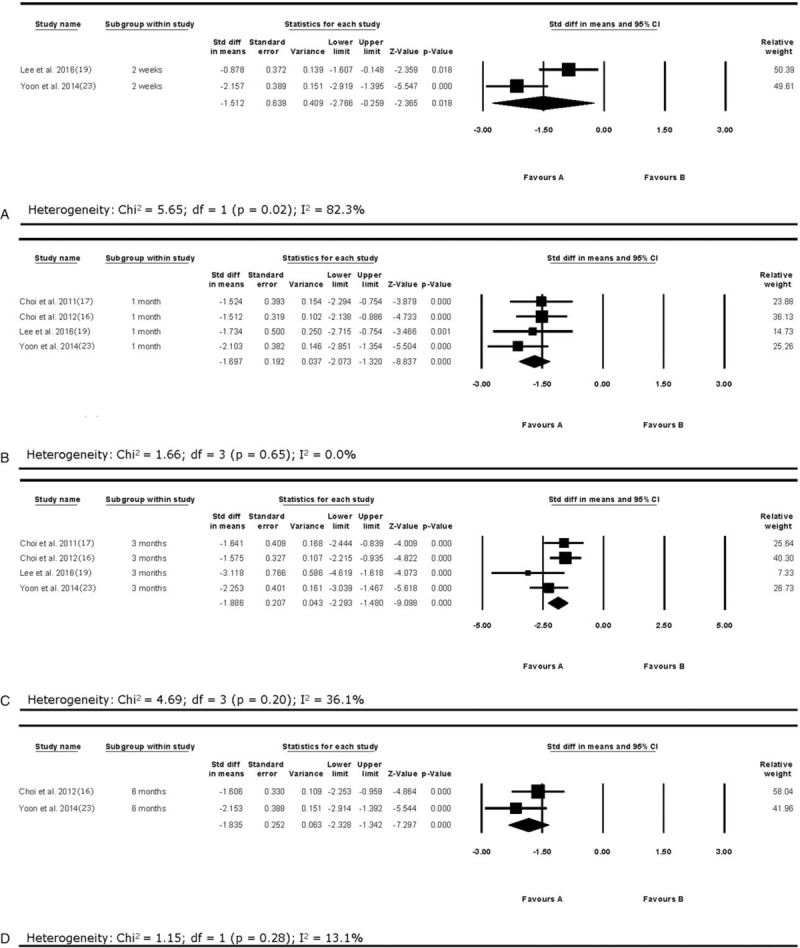
Results of subgroup analysis of visual analog scale (VAS) changes at 2 weeks (A), 1 month (B), 3 months (C), and 6 months (D) after pulsed radiofrequency treatment. VAS = visual analog scale.
Regarding adverse effects, in the study by Choi et al,[16] 2 patients complained of temporary post-procedural radicular pain, which disappeared within 2 weeks. Yoon et al[23] reported no complications after PRF. However, in the studies by Lee et al[19] and Choi et al,[17] there was no exact information on the adverse effects after PRF.
3.5. Publication bias
Funnel plot analysis was conducted for the overall results of VAS changes in the included studies (i.e., the analysis was performed including all the evaluated follow-up outcomes in each study). The graphical funnel pot of the included studies for changes in VAS score seemed to be symmetrical (Fig. 4). In addition, the publication bias was quantified using Egger's test. The intercept was found at 0.558 (P = .904). Therefore, statistically significant publication bias was unlikely to occur.
Figure 4.
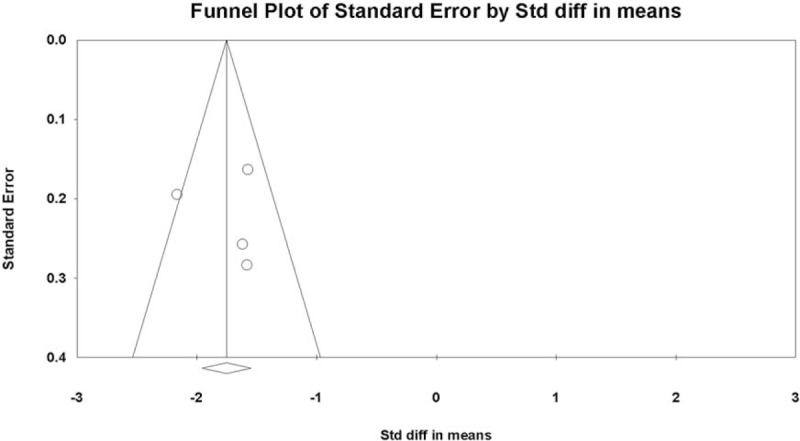
Graphic funnel plot of the included studies depicting overall changes in visual analog scale (VAS) scores. VAS = visual analog scale.
4. Discussion
In the current study, we evaluated the effectiveness of PRF on the DRG in alleviating refractory cervical radiculopathy due to disc herniation or spinal stenosis by meta-analysis. For the meta-analysis, 4 studies were included, and pain scores, which were measured using VAS, were analyzed.
We analyzed all the data in each included study, and the overall outcome showed that cervical radicular pain was significantly reduced after PRF on DRG. In addition, at each evaluation time point (i.e., 2 weeks, 1 month, 3 months, and 6 months after PRF), the pain was significantly alleviated. The effect size was found to range from −1.51 to −1.89. Based on Cohen's study[26], these effect size values can be interpreted as the PRF procedure has a large positive pain reducing effect on cervical radicular pain that did not respond to other conservative treatments, including oral medications, physical therapy, or epidural steroid injection.
As for the mechanism of PRF on pain reduction, some possible proposals have been raised. In 2009, Hagiwara et al[13] proved that PRF activates the noradrenergic and serotonergic descending pain inhibitory pathways and inhibits excitatory nociceptive C-fibers. In 2013, Cho et al[12] found decreased microglial activity in the spinal dorsal horn after applying PRF on the DRG. Because microglia are responsible for the occurrence of chronic neuropathic pain by releasing various cytokines and chemokines that are related to pain signaling, they proposed that downregulation of microglia would prevent the development of chronic neuropathic pain. In addition, Vallejo et al[27] found that pro-inflammatory cytokines, such as tumor necrosis factor-α and interleukin-6, were reduced after a PRF procedure.
Regarding the adverse effects after PRF, in 2 studies[16,23] included in this analysis, a total of 2 out of 43 patients (4.7%) complained of aggravated radicular pain after the PRF. Two patients’ pain aggravation was temporal, which disappeared within 2 weeks after the procedure. In addition, there were no motor or sensory changes. Therefore, PRF on the cervical DRG appears to be safely applied to patients with cervical radicular pain without devastating adverse effects.
In this study, we could not perform the analysis with data extracted from placebo or control group patients. Of the 4 studies included our analysis, only Lee et al[19] performed a study with a control group. They compared the effect of PRF with that of transforaminal steroid injection. The other 3 studies[16,17,23] did not recruit placebo or control subjects. The recruitment of a placebo group is complicated with ethical issues. In addition, clinicians have limited options to manage the pain conservatively other than medications, physical therapy, or epidural steroid injection. Therefore, it seems difficult to find an appropriate procedure for the control group. However, despite these difficulties, the lack of placebo or control group is one of the limitations of the previous studies.
In addition, although 2 prospective observational studies[16,17] and 1 retrospective study[23] were of high quality, the RCT[19] included in our meta-analysis was conducted without clear random sequence generation, allocation concealment, and blinding. For a more qualified meta-analysis outcome, more strictly controlled RCTs with a placebo group would be needed.
In conclusion, PRF treatment on the DRG was effective in pain relief of cervical radicular pain that was not responsive to other conservative managements. In addition, the pain reducing effect was significantly manifested at 2 weeks, 1 month, 3 months, and 6 months after the procedure. Our study is first meta-analysis that analyzed the effect of PRF in the reduction of cervical radicular pain. However, our meta-analysis was limited due to the limited number of included trials and sample size. Thus, we could not analyze the influence of other clinically relevant factors, such as functional improvement and patients’ satisfaction after PRF. In the future, meta-analysis with a larger number of trials would be warranted.
Author contributions
Conceptualization: Min Cheol Chang.
Data curation: Dong Gyu Lee, Min Cheol Chang.
Formal analysis: Min Cheol Chang.
Methodology: Sang Gyu Kwak.
Supervision: Min Cheol Chang.
Writing – original draft: Sang Gyu Kwak, Min Cheol Chang.
Writing – review & editing: Min Cheol Chang.
Footnotes
Abbreviations: CI = confidence interval, DRG = dorsal root ganglion, NOS = Newcastle–Ottawa scale, NRS = numeric rating scale, PRF = pulsed radiofrequency, RCT = randomized control trial, RF = radiofrequency, SMD = standardized mean difference, VAS = visual analog scale.
Funding/support: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2017R1C1B5017714).
The authors have no conflicts of interest to disclose.
References
- [1].Radhakrishnan K, Litchy WJ, O’Fallon WM, et al. Epidemiology of cervical radiculopathy. A population-based study from Rochester, Minnesota, 1976 through 1990. Brain 1994;117:325–35. [DOI] [PubMed] [Google Scholar]
- [2].Kim KT, Kim YB. Cervical radiculopathy due to cervical degenerative disease: anatomoy, diagnosis and treatment. J Korean Neurosurg Soc 2010;48:473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ahn SH, Cho YW, Ahn MW, et al. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine 2002;27:911–7. [DOI] [PubMed] [Google Scholar]
- [4].Olmarker K, Rydevik B, Holm S, et al. Effects of experimental graded compression on blood flow in spinal nerve roots. A vital microscopic study on the porcine cauda equina. J Orthop Res 1989;7:817–23. [DOI] [PubMed] [Google Scholar]
- [5].Manchikani L, Hirsch JA. Clinical management of radicular pain. Expert Rev Neurother 2015;15:681–93. [DOI] [PubMed] [Google Scholar]
- [6].Podhajski RJ, Sekiguchi Y, Kikuchi S, et al. The histologic effects of pulsed and continuous radiofrequency lesions at 42 degrees °C to rat dorsal root ganglion and sciatic nerve. Spine 2005;30:1008–13. [DOI] [PubMed] [Google Scholar]
- [7].Vallejo R, Benyamin RM, Kramer J, et al. Pulsed radiofrequency for the treatment of sacroiliac joint syndrome. Pain Med 2006;7:429–34. [DOI] [PubMed] [Google Scholar]
- [8].West M, Wu H. Pulsed radiofrequency ablation for residual and phantom limb pain: a case series. Pain Practice 2010;10:485–91. [DOI] [PubMed] [Google Scholar]
- [9].Vatansever D, Tekin I, Tuglu I, et al. A comparison of the neuroablative effects of conventional and pulsed radiofrequency techniques. Clin J Pain 2008;24:717–24. [DOI] [PubMed] [Google Scholar]
- [10].Sluijter ME, Cosman ER, Rittmann WB, et al. The effects of pulsed radiofrequency fields applied to the dorsal root ganglion—a preliminary report. Pain Clin 1998;11:109–17. [Google Scholar]
- [11].Higuchi Y, Nashold BS, Jr, Sluijter M, et al. Exposure of the dorsal root ganglion in rats to pulsed radiofrequency currents activates dorsal horn lamina I and II neurons. Neurosurgery 2002;50:850–5. [DOI] [PubMed] [Google Scholar]
- [12].Cho HK, Cho YW, Kim EH, et al. Changes in pain behavior and glial activation in the spinal dorsal horn after pulsed radiofrequency current administration to the dorsal root ganglion in a rat model of lumbar disc herniation: laboratory investigation. J Neurosurg Spine 2013;19:256–63. [DOI] [PubMed] [Google Scholar]
- [13].Hagiwara S, Iwasaka H, Takeshima N, et al. Mechanisms of analgesic action of pulsed radiofrequency on adjuvant-induced pain in the rat: roles of descending adrenergic and serotonergic systems. Eur J Pain 2009;13:249–52. [DOI] [PubMed] [Google Scholar]
- [14].Chang MC. Effect of bipolar pulsed radiofrequency on refractory chronic radicular pain: A report of two cases. Medicine (Baltimore) 2017;96:e6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chao SC, Lee HT, Kao TH, et al. Percutaneous pulsed radiofrequency in the treatment of cervical and lumbar radicular pain. Surg Neurol 2008;70:59–65. discussion 65. [DOI] [PubMed] [Google Scholar]
- [16].Choi GS, Ahn SH, Cho YW, et al. Long-term effect of pulsed radiofrequency on chronic cervical radicular pain refractory to repeated transforaminal epidural steroid injections. Pain Med 2012;13:368–75. [DOI] [PubMed] [Google Scholar]
- [17].Choi GS, Ahn SH, Cho YW, et al. Short-term effects of pulsed radiofrequency on chronic refractory cervical radicular pain. Ann Rehabil Med 2011;35:826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Halim W, van der Weegen W, Lim T, et al. Percutaneous cervical nucleoplasty vs. pulsed radio frequency of the dorsal root ganglion in patients with contained cervical disk herniation; a prospective, randomized controlled trial. Pain Pract 2017;17:729–37. [DOI] [PubMed] [Google Scholar]
- [19].Lee DG, Ahn SH, Lee J. Comparative effectivenesses of pulsed radiofrequency and transforaminal steroid injection for radicular pain due to disc herniation: a prospective randomized trial. J Korean Med Sci 2016;31:1324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Van Zundert J, Lamé IE, de Louw A, et al. Percutaneous pulsed radiofrequency treatment of the cervical dorsal root ganglion in the treatment of chronic cervical pain syndromes: a clinical audit. Neuromodulation 2003;6:6–14. [DOI] [PubMed] [Google Scholar]
- [21].Van Zundert J, Patijn J, Kessels A, et al. Pulsed radiofrequency adjacent to the cervical dorsal root ganglion in chronic cervical radicular pain: a double blind sham controlled randomized clinical trial. Pain 2007;127:173–82. [DOI] [PubMed] [Google Scholar]
- [22].Wang F, Zhou Q, Xiao L, et al. A randomized comparative study of pulsed radiofrequency treatment with or without selective nerve root block for chronic cervical radicular pain. Pain Pract 2017;17:589–95. [DOI] [PubMed] [Google Scholar]
- [23].Yoon YM, Han SR, Lee SJ, et al. The efficacy of pulsed radiofrequency treatment of cervical radicular pain patients. Korean J Spine 2014;11:109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. The Cochrane Collaboration. Available: www.handbook-5-1.cochrane.org 2011. [Google Scholar]
- [25].Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp 2011. [Google Scholar]
- [26].Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed.Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- [27].Vallejo R, Tilley DM, Williams J, et al. Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician 2013;16:E601–13. [PubMed] [Google Scholar]


