Abstract
Background:
The aim of this study was to evaluate the prognostic role of D-dimer level upon admission in patients with traumatic brain injury (TBI) through performing a meta-analysis.
Methods:
PubMed, Web of Science, Cochrane Library, and EMBASE were searched for potential eligible literature. The study characteristics and relevant data were extracted. Poor functional outcome was defined according to the Glasgow Outcome Scale (GOS ≤3). Odds ratios (ORs) with 95% confidence intervals (CIs) were pooled to estimate the predictive value of D-dimer for progressive hemorrhagic injury (PHI) and poor functional outcome at 3 months (3M GOS ≤3) in patients with TBI.
Results:
Eleven studies with 2761 patients were included. Eight studies examined the predictive role of higher D-dimer level for the risk of PHI, and the pooled OR was 1.72 (95% CI, 1.23–2.42). Three studies examined the predictive role of higher D-dimer level for the risk of 3M GOS ≤3, and the pooled OR was 2.00 (95% CI, 0.87–4.59). Significant between-study heterogeneities were observed, and sensitivity analyses and subgroup analyses were performed. No significant publication bias was found.
Conclusions:
In conclusion, in patients with TBI, higher D-dimer level upon admission was associated with higher risk of PHI, yet no significant relationship was found between D-dimer level and the risk of 3M GOS ≤3. In the future, this readily available marker could help identify patients at risk and tailor management of these patients, thus reducing PHI and improving outcome.
Keywords: D-dimer, outcome, prognosis, traumatic brain injury
1. Introduction
The incidence of traumatic brain injury (TBI) is between 180 and 250 per 100,000 population per year in the United States.[1] In China, the incidence of TBI is 200 to 300 per 100,000 population per year.[2] It is a leading cause of morbidity and mortality among young adults, and poses great burden on global public health.[3,4] It is estimated that around 1.5 million TBI victims die each year.[5] The leading causes of TBI were mainly motor vehicle crashes and falls.[5] The Glasgow Coma Score (GCS) is the most commonly used scoring instrument for grading TBI severity.[6] Repeated computed tomography (CT) scans play an important role in the management of TBI.[7] Progressive hemorrhagic injury (PHI) was defined as the appearance of new lesion(s) or an increase in the volume of hemorrhagic lesion(s), that is, a ≥25% increase compared with the first post-injury CT scan.[8–10] The treatment of TBI is complex,[5] and the goal is to promote neuroprotection and cerebral perfusion.[6] Rehabilitation is also essential for functional recovery.[6] Short-term and long-term prognostic markers are still needed, even several prognostic factors have been tested.[5]
Acquired coagulation disorders were reported to occur in TBI, due to the imbalance between anti and procoagulant factors, platelets, endothelium function, and fibrinolysis. Tissue damage, hypoperfusion, hypothermia, and acidaemia are the main contributors to coagulation abnormalities.[11] In recent years, researchers have investigated the relationship between coagulation and fibrinolytic parameters and the outcome of TBI.[12–14] Some investigators demonstrated that abnormal coagulation parameters, such as elevated prothrombin time and decreased platelet count, could predict TBI outcome.[15] Other researchers suggested that fibrinolytic parameters could be reliable markers.[16] Among the fibrinolytic parameters, the role of D-dimer in TBI was a hot spot of research. D-dimer is a degradation product of fibrinogen. Abnormal D-dimer level could reflect the imbalance of coagulation and fibrinolytic systems, thus altering the outcomes of patients with TBI.[17] Many researchers have investigated the prognostic role of D-dimer in patients with TBI; however, the results were not conclusive. For example, with regards to the prediction of PHI, some researchers concluded that higher D-dimer level was associated with higher risk of PHI.[8,10] But other researchers found the association was not statistically significant,[18,19] even some researchers found higher D-dimer level was associated with lower risk of PHI.[20]
Due to the controversy, we aim to systematically evaluate the prognostic role of D-dimer level in patients with TBI through performing a meta-analysis.
2. Methods
2.1. Search strategy
Since this is a meta-analysis, ethical approval was not necessary. We followed the developed guidelines for systematic reviews and meta-analyses in performing our study.[21] PubMed, Web of Science, Cochrane Library, and EMBASE were searched for potential useful literature after consulting a librarian in our university. The following keywords were used: (“D-dimer” OR “fibrin fragment D” OR “fibrin fragment D1 dimer” OR “fibrin fragment DD”) AND (“traumatic brain injury” OR “brain trauma” OR “traumatic encephalopathy” OR “brain concussion” OR “brain contusion”) AND (“prognosis” OR “prognostic” OR “predictive” OR “progression” OR “outcome” OR “mortality”). The last search update was on July 28th, 2017, and no start date was applied. Reference lists of relevant articles were also checked for additional literature. Authors were contacted where additional studies or data were needed. Languages were restricted to English and Chinese.
2.2. Study selection
The study selection process was independently performed by 2 investigators (JZ and MH), with any disagreements resolved by consensus. Titles and abstracts of studies identified in the databases were screened first. Then potentially relevant studies were assessed in full text. Studies were considered eligible if they met all of the following inclusion criteria: the patients were diagnosed with traumatic brain injury and received proper therapy; blood samples were collected upon admission to assess the level of D-dimer; patients were watched closely for disease progression, or followed up for survival outcomes or functional outcomes; enough data were reported to estimate the prognostic role of D-dimer in patients with TBI. Unrelated articles, letters, conference abstracts, case reports, reviews, and studies without enough data were excluded. If multiple studies were performed in the same center and the samples overlapped, the study with the largest sample size was included.
2.3. Data extraction
Two authors (JZ and YS) extracted data from the eligible studies independently, and disagreements were discussed. The primary data was odds ratio (OR) with 95% confidence interval (CI) for disease progression or poorer outcomes, or the data that could be used to calculate the OR and 95% CI. Estimates calculated form multivariate analyses were extracted over those calculated from univariate analyses. The characteristics of the studies and patients were also extracted, including first author, publication year, country, the number of patients, sex of patients, mean or median age of patients, blood sampling time, and so on.
2.4. Study quality assessment
Two investigators (JZ and MH) independently assessed the quality of each study using the Newcastle–Ottawa Scale (NOS) criteria.[22] The NOS assessed 3 aspects of the study: subject selection, 0 to 4; comparability of subject, 0 to 2; and exposure (for case-control studies)/outcome (for cohort studies), 0 to 3. The NOS scores ranged from 0 to 9, and those studies with ≥7 scores were graded as high-quality ones.
2.5. Statistical analysis
Poor functional outcome was defined as severe disability, vegetative state, or dead according to the Glasgow Outcome Scale (GOS ≤3).[23] The logOR and variance were calculated from the OR and 95% CI, and used for aggregation of data. Forest plots were constructed to estimate the pooled prognostic role of D-dimer in patients with TBI. The pooled OR was regarded significant if the P value was <.05 and the 95% CI did not overlap 1. The between-study heterogeneity was assessed, with I2 >30% indicating significant heterogeneity.[24] Random effect models were used throughout this meta-analysis, since some heterogeneity among studies was expected due to differences in study and patient characteristics.[25] Subgroup analyses were also performed according to different patient sources, study designs, treatment strategies, sex composition, and cut-off values of D-dimer. Besides, sensitivity analysis was performed to evaluate the contribution of each study to heterogeneity by excluding individual studies one at a time. Publication bias was assessed by Begg test, with P >.05 implying no significant publication bias. All the above mentioned statistical analyses were performed by STATA 11.0 (STATA Corporation, College Station, TX).
3. Results
3.1. Literature research
The initial literature search retrieved 225 citations. Among them, 62 were found duplicated citations. After removing them, the rest 163 studies were screened by titles and abstracts, and 128 studies were excluded according to the inclusion and exclusion criteria. The rest 35 studies were assessed in full text. Twenty-one studies were further excluded due to unrelated, lacking enough data, or other reasons. Three[10,26,27] of the rest 14 studies were performed in the same center by the same authors, and the study with the longest study period and most patients was included.[10] Two more studies[8,28] were also overlapped research, and the study with more patients was included.[8] Finally, 11 articles were included in our study.[8–10,18–20,23,29–32] The study selection process was shown in Fig. 1.
Figure 1.
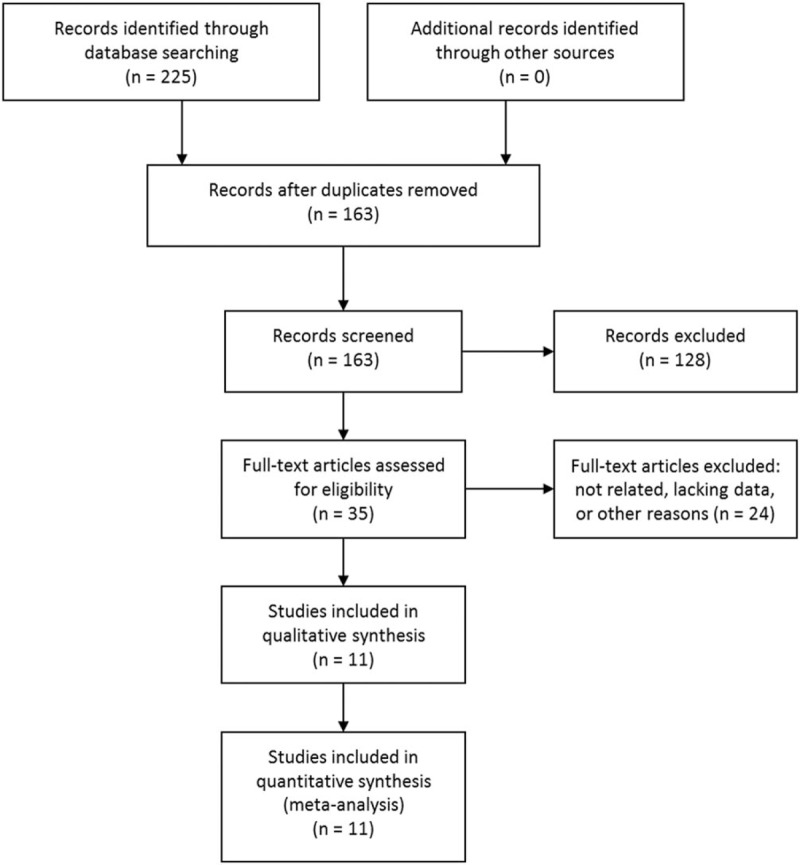
Selection process of studies.
3.2. Study characteristics
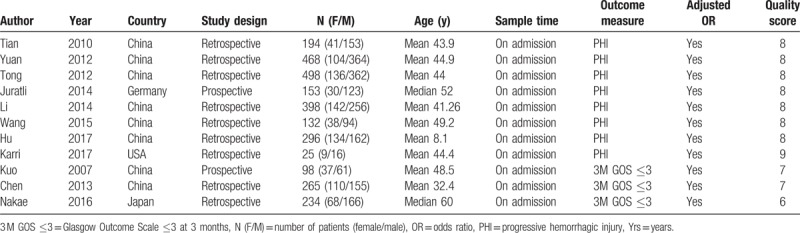
The basic characteristics of the 11 included studies were shown in Table 1. They were published from 2007 to 2017. They were conducted in 4 different countries, mostly in China. Nine studies were retrospective studies and 2 were prospective studies. A total of 2761 patients were included (mean 251, women 849, men 1912). All of the blood samples for D-dimer level tests were collected upon admission. The outcome measures included progressive hemorrhagic injury (PHI) and poor functional outcome at 3 months (3M GOS ≤3). All the ORs were calculated form multivariate analyses. Ten of the 11 included studies got ≥7 scores in methodological assessment. As to the cut-off value of D-dimer level, 3 studies used 5 mg/L,[8,10,30] some studies used other different values and the rest studies did not report the cut-off value they used.
Table 1.
Characteristics of the included studies.
3.3. The risk of PHI
Eight studies examined the predictive role of D-dimer level for PHI.[8–10,18–20,29,30] The pooled OR of the 8 studies was 1.72 (95% CI, 1.23–2.42) (Fig. 2), suggesting that higher D-dimer level was associated with higher risk of PHI. Significant heterogeneity was found between the studies (I2 = 93.7%, P < .001) and sensitivity analysis was then performed. However, after removing individual studies one at a time, the heterogeneities were always >90%.
Figure 2.
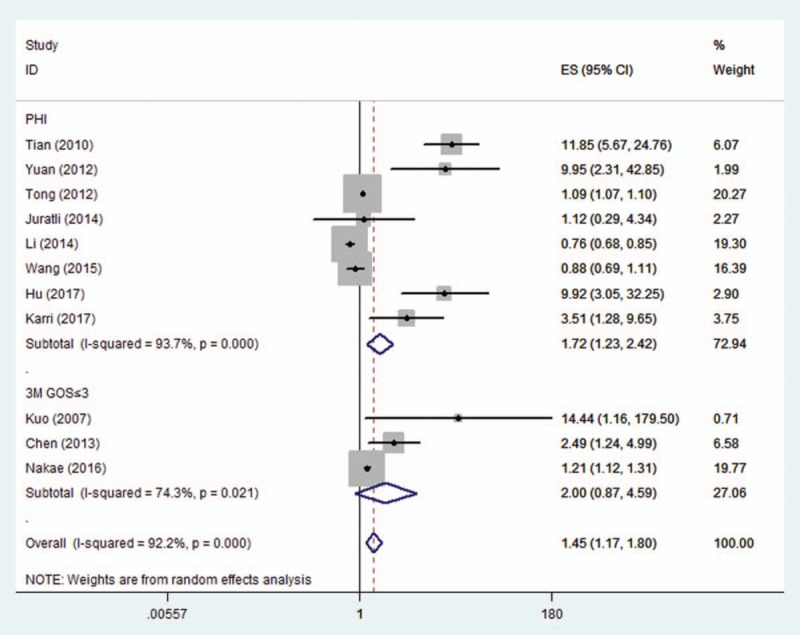
Pooled odds ratios (ORs) of higher D-dimer levels for the risks of progressive hemorrhagic injury (PHI) and poor functional outcome at 3 months (3M GOS ≤3) in patients with traumatic brain injury.
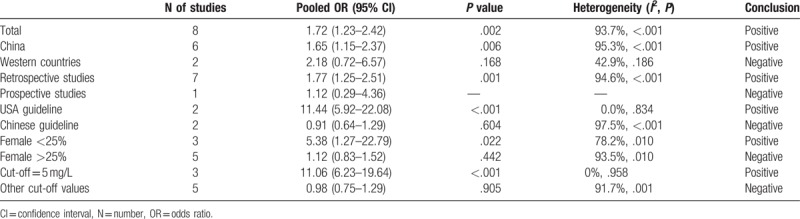
Subgroup analyses were also performed and the results were summarized in Table 2. The pooled OR of the 6 studies from China[8–10,19,20,30] was 1.65 (95% CI, 1.15–2.37), and the heterogeneity was present (I2 = 95.3%, P < .001). After pooling the 2 studies from western countries,[18,29] the heterogeneity decreased to 42.9%, but the pooled OR was not significant 2.18 (95% CI, 0.72–6.57). The pooled OR of the 7 retrospective studies[8–10,19,20,29,30] was 1.77 (95% CI, 1.25–2.51). Among the 8 studies, 4 reported that they followed the currently accepted guidelines to treat patients. The pooled OR of the 2 studies following the guidelines from the USA was 11.44 (95% CI, 5.92–22.08),[8,10] and no heterogeneity was observed (I2 = 0.0%, P = .834). The pooled OR of the 2 studies following the guidelines from China was 0.91 (95% CI, 0.64–1.29).[9,20] The pooled OR of the 3 studies with women <25% was 5.38 (95% CI, 1.27–22.79),[8,10,18] and the pooled OR of the 5 studies with women >25% was 1.12 (95% CI, 0.83–1.52). Three studies used the cut-off value of 5 mg/L for D-dimer level,[8,10,30] and the pooled OR was 11.06 (95% CI, 6.23–19.64), indicating strong association. Moreover, there was no heterogeneity between the 3 studies (I2 = 0%, P = .958). The pooled OR of the rest 5 studies was not statistically significant, and the heterogeneity was present (I2 = 91.7%, P < .001).
Table 2.
Summary of meta-analysis results.
3.4. The risk of 3M GOS ≤3
Three studies examined the predictive role of D-dimer level for 3M GOS ≤3.[23,31,32] The pooled OR of the 3 studies was 2.00 (95% CI, 0.87–4.59) (Fig. 2), suggesting no significant relationship between higher D-dimer level and the risk of 3M GOS ≤3. Significant heterogeneity was also observed between the studies (I2 = 74.3%, P = .021), and random effect model was used. Sensitivity analysis revealed that the study by Nakae et al[31] contributed greatly to the heterogeneity. After removing this study, the heterogeneity shrinked to 42.4% and the pooled OR was still not statistically significant (OR 3.88; 95% CI, 0.87–17.36). Subgroup analysis was not performed due to the limited number of studies.
3.5. Publication bias
No significant publication bias was found as to the 8 studies examining PHI (P = .902) and the 3 studies examining 3M GOS ≤3 (P = 1.000) (Fig. 3).
Figure 3.
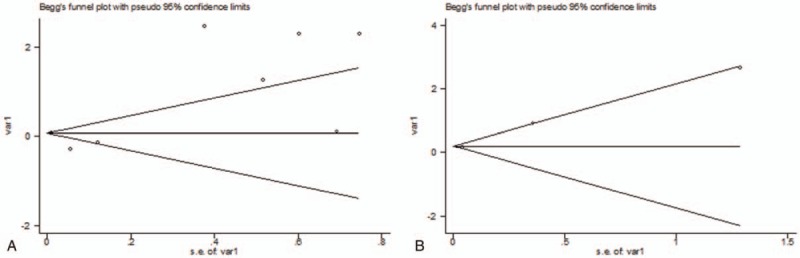
The Begg publication bias plots for the 8 studies examining progressive hemorrhagic injury (PHI) (P = .902) and the 3 studies examining poor functional outcome at 3 months (3M GOS ≤3) (P = 1.000).
4. Discussion
This study aimed to evaluate the prognostic role of D-dimer level upon admission in patients with TBI. We performed a meta-analysis to summarize the present evidence, and 11 studies were included. To our best knowledge, this is the first meta-analysis on this topic. Our results suggested that higher D-dimer level was associated with higher risk of PHI, yet no association was found between D-dimer level and the risk of 3M GOS ≤3. However, significant between-study heterogeneities were observed in the meta-analysis. Random effects models were used and sensitivity analyses were performed. For the 8 studies examining the risk of PHI, no study was found to be a major contributor to the heterogeneity. For the 3 studies examining the risk of 3M GOS ≤3, 1 study was found to contribute to the heterogeneity greatly.[31] After excluding that study, the heterogeneity was still statistically significant and the OR was not significant. However, due to the limited number of studies in this group, more studies are needed to further elucidate our findings.
Subgroup analyses were also performed to examine the role of D-dimer level in predicting PHI in different settings and to further find the source of heterogeneity. Higher D-dimer level was found to be associated with higher risk of PHI in patients from China, patients treated according to the USA guideline and patient population with less women. Subgroup analysis was also performed according to the cut-off value of D-dimer level. Three studies used the cut-off value of 5 mg/L. After pooling the ORs of the 3 studies, the association proved to be very strong (OR = 11.06). Moreover, there was no heterogeneity between the 3 studies (I2 = 0%). Therefore, 5 mg/L might be a good cut-off value of D-dimer for predicting PHI. However, the study number was small, and caution should be applied. Among the subgroup analyses, we could find that the heterogeneities dropped and even disappeared in some subgroups. Thus, confounding factors, like patient sources, treatment strategies, sex composition, and cut-off values of D-dimer, may be contributors to heterogeneity.
Numerous studies have addressed the dysregulation of normal hemostatic processes caused by severe injury and shock.[33–35] The TBI-associated coagulopathies may be driven by platelet dysfunction, thrombocytopenia and factor deficiency.[18,29] The abnormal coagulation and fibrinolytic function may lead to intracranial hemorrhage and result in PHI.[18,33] Besides, the clinical randomization of an antifibrinolytic treatment in significant hemorrhage (CRASH 2) trial demonstrated that early antifibrinolytic treatment could reduce PHI and improve outcomes.[36,37] Since D-dimer is a fibrinolytic parameter, higher D-dimer level may be associated with higher risk of developing PHI, which is in accordance with our study results. Still, the underlining mechanism needs more investigation.
Patients with PHI are reported to be at higher risk for complications, morbidity, and mortality than those with stable hemorrhage.[14,18,29] Thus, as a predictor of PHI, D-dimer may also predict worse outcome. The GOS score is a commonly used instrument to evaluate neurologic outcomes after TBI.[6] In our study, the association between D-dimer level and 3M GOS ≤3 was not significant. However, after excluding 1 study, which was the major contributor to heterogeneity, the result became significant, implying a potential relation between D-dimer level and 3M GOS ≤3. Yuan et al[13] concluded that higher D-dimer level was associated with higher risk of 6M GOS ≤3. Apart from functional outcome, some researchers attempted to find out the relationship between D-dimer level and TBI-associated mortality. Higher level of D-dimer was found to be associated with higher risks of in-hospital or 28-day mortality,[14] 30-day mortality,[13] and 90-day mortality.[38] More studies are still needed to verify the predictive value of D-dimer for functional outcome and mortality.
Considering the promising role of D-dimer for predicting PHI, functional outcome, and mortality in TBI, D-dimer may be used as a valuable marker in the management of TBI. The D-dimer levels on admission are readily available and relatively cheap. It could be used to monitor progressive hemorrhage and initiate proper therapy. Also, the prognostic value of D-dimer may provide future molecular targets for new therapeutic intervention.[29]
There are some limitations in our meta-analysis. First and foremost, the number of included studies was small, especially in the group of 3M GOS ≤3 and some subgroups. The results should be interpreted with caution. Secondly, the characteristics of the studies and patients varied. For example, patients were admitted within different hours from the injury events, and the cut-off values of the D-dimer level were not all the same. Besides, significant heterogeneities were present among the studies and random effects models were used. Although sensitivity analyses and subgroup analyses were performed, the heterogeneities could not be eliminated. Furthermore, although no significant publication bias was found in our meta-analysis, publication bias should not be completely excluded since it was a major concern for all meta-analyses.
In conclusion, our results suggested that, in patients with TBI, higher D-dimer level upon admission was associated with higher risk of PHI, yet no significant relationship was found between D-dimer level and the risk of pool functional outcome at 3 months (3M GOS ≤3). In the future, this readily available marker could help identify patients at risk and tailor management of these patients, thus reducing PHI and improving outcome. Our findings may also help explore new therapeutic targets in the future. However, more well-designed studies are warranted to further verify our results and elucidate the underlining mechanisms.
Acknowledgments
The authors would like to thank the reviewers for their constructive comments.
Author contributions
Conceptualization: Jing Zhang, Yanlin Song, Jianguo Xu.
Data curation: Jing Zhang, Min He, Yanlin Song.
Formal analysis: Jing Zhang, Min He, Yanlin Song, Jianguo Xu.
Investigation: Jing Zhang, Min He, Yanlin Song.
Methodology: Jing Zhang, Min He, Yanlin Song.
Project administration: Jing Zhang, Min He, Yanlin Song.
Resources: Jing Zhang, Min He, Yanlin Song.
Software: Jing Zhang, Min He, Yanlin Song, Jianguo Xu.
Supervision: Jianguo Xu.
Validation: Jing Zhang, Min He, Yanlin Song.
Visualization: Jing Zhang, Min He, Yanlin Song.
Writing – original draft: Jing Zhang, Min He, Yanlin Song, Jianguo Xu.
Writing – review and editing: Jing Zhang, Min He, Yanlin Song, Jianguo Xu.
Footnotes
Abbreviations: 3 M GOS ≤3 = poor functional outcome at 3 months, CI = confidence interval, GCS = Glasgow Coma Score, GOS = Glasgow Outcome Scale, NOS = Newcastle–Ottawa Scale, OR = odds ratio, PHI = progressive hemorrhagic injury, TBI = traumatic brain injury.
The authors received no funding.
The authors declare no conflict of interests.
References
- [1].Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia 2003;44(suppl):2–10. [DOI] [PubMed] [Google Scholar]
- [2].Ma D, Liu B, Hao S, et al. Establishment of database platform in traumatic brain injury field and its preliminary application. Chin J Neurosurg 2014;30:159–61. [Google Scholar]
- [3].Gerber LM, Ni Q, Hartl R, et al. Impact of falls on early mortality from severe traumatic brain injury. J Trauma Manag Outcomes 2009;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol 2008;7:728–41. [DOI] [PubMed] [Google Scholar]
- [5].El-Menyar A, Mekkodathil A, Al-Thani H, et al. Incidence, demographics and outcome of traumatic brain injury in the Middle East: a systematic review. World Neurosurg 2017;107:6–21. [DOI] [PubMed] [Google Scholar]
- [6].Popernack ML, Gray N, Reuter-Rice K. Moderate-to-severe traumatic brain injury in children: complications and rehabilitation strategies. J Pediatr Health Care 2015;29:e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chao A, Pearl J, Perdue P, et al. Utility of routine serial computed tomography for blunt intracranial injury. J Trauma 2001;51:870–5. discussion 875-6. [DOI] [PubMed] [Google Scholar]
- [8].Tian HL, Chen H, Wu BS, et al. D-dimer as a predictor of progressive hemorrhagic injury in patients with traumatic brain injury: analysis of 194 cases. Neurosurg Rev 2010;33:359–65. [DOI] [PubMed] [Google Scholar]
- [9].Tong WS, Zheng P, Zeng JS, et al. Prognosis analysis and risk factors related to progressive intracranial haemorrhage in patients with acute traumatic brain injury. Brain Inj 2012;26:1136–42. [DOI] [PubMed] [Google Scholar]
- [10].Yuan F, Ding J, Chen H, et al. Predicting progressive hemorrhagic injury after traumatic brain injury: derivation and validation of a risk score based on admission characteristics. J Neurotrauma 2012;29:2137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Epstein DS, Mitra B, O’Reilly G, et al. Acute traumatic coagulopathy in the setting of isolated traumatic brain injury: a systematic review and meta-analysis. Injury 2014;45:819–24. [DOI] [PubMed] [Google Scholar]
- [12].DeFazio MV, Rammo RA, Robles JR, et al. The potential utility of blood-derived biochemical markers as indicators of early clinical trends following severe traumatic brain injury. World Neurosurg 2014;81:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yuan F, Ding J, Chen H, et al. Predicting outcomes after traumatic brain injury: the development and validation of prognostic models based on admission characteristics. J Trauma Acute Care Surg 2012;73:137–45. [DOI] [PubMed] [Google Scholar]
- [14].Allard CB, Scarpelini S, Rhind SG, et al. Abnormal coagulation tests are associated with progression of traumatic intracranial hemorrhage. J Trauma 2009;67:959–67. [DOI] [PubMed] [Google Scholar]
- [15].Van Beek JG, Mushkudiani NA, Steyerberg EW, et al. Prognostic value of admission laboratory parameters in traumatic brain injury: results from the IMPACT study. J Neurotrauma 2007;24:315–28. [DOI] [PubMed] [Google Scholar]
- [16].Takahashi H, Urano T, Takada Y, et al. Fibrinolytic parameters as an admission prognostic marker of head injury in patients who talk and deteriorate. J Neurosurg 1997;86:768–72. [DOI] [PubMed] [Google Scholar]
- [17].Goldenberg NA, Jenkins S, Jack J, et al. Arteriopathy, D-dimer, and risk of poor neurologic outcome in childhood-onset arterial ischemic stroke. J Pediatr 2013;162:1041.e1–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Juratli TA, Zang B, Litz RJ, et al. Early hemorrhagic progression of traumatic brain contusions: frequency, correlation with coagulation disorders, and patient outcome: a prospective study. J Neurotrauma 2014;31:1521–7. [DOI] [PubMed] [Google Scholar]
- [19].Wang K, Zhao Dq, Zhang Jj, et al. Risk factors of progressive brain contusion and relationship with outcome. Zhejiang Da Xue Xue Bao Yi Xue Ban 2015;44:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li X, Ma L, Wang X, et al. Evaluation on the related high-risk factors of progressive hemorrhagic injury after acute traumatic brain injury. Chongqing Med 2014;43:915–7. [Google Scholar]
- [21].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [23].Chen H, Xue LX, Guo Y, et al. The influence of hemocoagulation disorders on the development of posttraumatic cerebral infarction and outcome in patients with moderate or severe head trauma. BioMed Res Int 2013;2013:685174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Specogna AV, Turin TC, Patten SB, et al. Factors associated with early deterioration after spontaneous intracerebral hemorrhage: a systematic review and meta-analysis. PLoS One 2014;9:e96743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ding J, Yuan F, Guo Y, et al. A prospective clinical study of routine repeat computed tomography (CT) after traumatic brain injury (TBI). Brain Injury 2012;26:1211–6. [DOI] [PubMed] [Google Scholar]
- [27].Ding J, Chen SW, Guo Y, et al. Analysis of risk factors of intracranial progressive hemorrhage after traumatic brain injury. J Shanghai Jiaotong Univ Med Sci 2010;30:829–31. [Google Scholar]
- [28].Bingshan WU, Hengli T, Zaikai LIN, et al. Changes and clinical significance of D-dimer in progressive hemorrhagic injury after traumatic brain injury. J Shanghai Jiaotong Univ Med Sci 2008;28:1554–6. [Google Scholar]
- [29].Karri J, Cardenas JC, Matijevic N, et al. Early fibrinolysis associated with hemorrhagic progression following traumatic brain injury. Shock 2017;48:644–50. [DOI] [PubMed] [Google Scholar]
- [30].Hu Gw, Lang Hl, Guo H, et al. A risk score based on admission characteristics to predict progressive hemorrhagic injury from traumatic brain injury in children. Eur J Pediatr 2017;176:689–96. [DOI] [PubMed] [Google Scholar]
- [31].Nakae R, Takayama Y, Kuwamoto K, et al. Time course of coagulation and fibrinolytic parameters in patients with traumatic brain injury. J Neurotrauma 2016;33:688–95. [DOI] [PubMed] [Google Scholar]
- [32].Kuo JR, Lin KC, Lu CL, et al. Correlation of a high D-dimer level with poor outcome in traumatic intracranial hemorrhage. Eur J Neurol 2007;14:1073–8. [DOI] [PubMed] [Google Scholar]
- [33].Stein SC, Smith DH. Coagulopathy in traumatic brain injury. Neurocrit Care 2004;1:479–88. [DOI] [PubMed] [Google Scholar]
- [34].MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma 2003;55:39–44. [DOI] [PubMed] [Google Scholar]
- [35].Talving P, Benfield R, Hadjizacharia P, et al. Coagulopathy in severe traumatic brain injury: a prospective study. J Trauma 2009;66:55–61. discussion 61-2. [DOI] [PubMed] [Google Scholar]
- [36].Collaborators C-, Roberts I, Shakur H, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011;377:1096–101. 1101.e1-1101 e2. [DOI] [PubMed] [Google Scholar]
- [37].Crash-2 Collaborators IBS. Effect of tranexamic acid in traumatic brain injury: a nested randomised, placebo controlled trial (CRASH-2 Intracranial Bleeding Study). BMJ 2011;343:d3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Takayama Y, Yokota H, Sato H, et al. Pathophysiology, mortality, treatment of acute phase of haemostatic disorders of traumatic brain injury. Jpn J Neurosurg 2013;22:837–41. [Google Scholar]


