Abstract
Cerebral vasospasm is the most important cause of morbidity after an aneurysm clipping in the early postoperative period. The aim of this retrospective study was to evaluate whether the incidence of vasospasms differs when using propofol or desflurane for an emergent aneurysm clipping.
The data from 102 patients (50 in the propofol group, 52 in the desflurane group) were analyzed. The occurrence of vasospasm based on daily transcranial Doppler, angiography, and cerebral infarction during 14 days after surgery were compared by anesthetic agents. Postoperative data including Glasgow Coma Scale (GCS) score on day 14 after surgery, and the Glasgow Outcome Scale (GOS) score at 3 months were documented.
Patients that intraoperatively received propofol for anesthesia maintenance, had higher incidence of transcranial Doppler (TCD)-evident vasospasm than those that received desflurane (54% vs 30.8%, P = .027). The occurrence of TCD-evident vasospasm was still higher (odds ratio: 2.84; 95% confidence interval: 1.12–7.20) in the propofol group than in the desflurane group after adjusting for confounding factors. However, the incidence of angiographic vasospasm, cerebral infarction, and interventions to treat cerebral vasospasms were similar between both groups. GCS score on day 14 after surgery and the GOS score at 3 months were similar between groups.
No effect of anesthetic agents on angiographic vasospasm, cerebral infarction, or clinical outcome was observed, whereas desflurane anesthesia was associated with a lower incidence of TCD-evident vasospasms compared to propofol anesthesia. Our study provides a basis for further randomized controlled studies in a larger patient population to clarify the effects of anesthetic agents on the occurrence of cerebral vasospasms.
Keywords: desflurane, propofol, subarachnoid hemorrhage, vasospasm
1. Introduction
A subarachnoid hemorrhage (SAH) is a devastating event that often leads to residual neurocognitive deficits or death. Cerebral vasospasm is the most important cause of mortality and morbidity in patients who survive and occurs during the 2 weeks after initial hemorrhage.[1] Many modalities such as calcium channel antagonists, prophylactic hypervolemia, and lumbar drainage of cerebrospinal fluid have been used to reduce the incidence of vasospasms.[2] However, the exact pathophysiology of cerebral vasospasms and the rationale behind therapeutic strategies are not completely understood.[3]
Anesthetic agents produce a considerable effect on cerebral factors such as intracranial pressure (ICP), cerebral blood flow (CBF), and cortical depression. Propofol and desflurane are widely used intravenous and volatile anesthetic agents, respectively, in neurosurgical patients because they facilitate a rapid recovery after surgery compared to other anesthetic agents.[4] Furthermore, several studies have suggested that anesthetic agents may affect the occurrence of vasospasms. Luo et al[5,6] reported that propofol and desflurane anesthesia produce different plasma endothelin (ET) and calcitonin gene–related peptide (CGRP) concentrations, which have been suggested to play key roles in maintaining vascular tone as an intrinsic potent cerebral constrictor and dilator, respectively. Both have roles in the pathogenesis of cerebral vasospasms,[7–10] and ET receptor antagonists reduce the frequency and severity of vasospasms after an SAH.[11]
Until now, there have been no published clinical data that addressed the influence of anesthetic agents on cerebral vasospasms. Therefore, the aim of this retrospective study was to evaluate whether the incidence of vasospasms differs when using propofol or desflurane anesthesia.
2. Materials and methods
We retrospectively investigated the medical records of all patients >19 years old treated with aneurysm clipping within 24 hours after an aneurysmal SAH under general anesthesia using propofol or desflurane anesthesia between July 2008 and September 2015 at Ewha Womans University Hospital. This study was approved by the institutional review board of our hospital (EUMC 2015-12-008-001) on April 27, 2016. Exclusion criteria were patients who had other inhaled anesthesia besides desflurane, had only an unruptured aneurysm without an SAH, those who underwent coil embolization, had received second operation such as decompressive craniectomy or removal of intracerebral hematoma, or had incomplete data.
In the propofol anesthesia group, patients received an effect-site target-controlled infusion (TCI) of propofol and remifentanil using a TCI pump (Orchestraw Base Primea, Fresenius Vial, Brezins, France) based on Schnider pharmacokinetic model for propofol and Minto model for remifentanil. Anesthesia was maintained using a 50% oxygen/air or N2O mixture and TCI propofol 3 to 4 μg/mL and remifentanil 2 to 6 ng/mL with an adjuvant bolus of fentanyl if needed. In the desflurane anesthesia group, anesthesia was induced with a bolus of propofol or pentothal sodium and fentanyl, and was maintained using a 50% oxygen/air or N2O mixture and desflurane (1.0–1.5-fold the minimum alveolar concentration) with an adjuvant of fentanyl or remifentanil. In all of the patients, neuromuscular blockade was provided using a bolus of rocuronium or cisatracurium, and an additional bolus or infusion of vecuronium or cisatracurium was used as necessary. Anesthesia was maintained to keep systolic blood pressure within 20% of baseline values, mean arterial pressure greater than 80 mm Hg during aneurysm clipping and a bispectral index value of 40 to 60, if applicable. Ventilation was adjusted to maintain end-tidal CO2 pressure at 35 to 45 mm Hg and maximum airway pressure <30 mm Hg.
All of the patients were managed according to our SAH management protocol.[12] The protocol included surgical clipping within 24 hours after the initial SAH, administration of nimodipine, daily transcranial Doppler (TCD) ultrasound for 14 days after surgery, normovolemia, and control of ICP. Newly developed neurological deterioration such as confusion, disorientation, focal motor or speech deficits, and a change in the pupillary reflex for at least 2 hours were considered signs of vasospasm after excluding an electrolyte imbalance, drug toxicity, or abnormal computed tomography (CT) findings.[12–14] CT scans were performed on day 7 after surgery to evaluate ventricular size, cerebral edema, and early signs of ischemic lesions and when necessary to rule out secondary hemorrhage, cerebral edema, and hydrocephalus. If a vasospasm was suspected based on clinical signs and/or TCD, the patient underwent additional CT or diffusion magnetic resonance imaging and digital subtraction angiography (DSA) and induced hypertension was initiated by maintaining mean arterial blood pressure between 100 and 120 mm Hg with the maintenance of normovolemia.
TCD recordings were conducted daily for 14 days after surgery by the same team of experienced neurosurgical surgeons through the craniotomy site window in the case of the pterional approach, and through the temporal window in the case of a supraorbital approach using a 2-MHz transducer. Mean velocities (MVs) of the proximal middle cerebral artery (MCA) in both hemispheres and the extracranial internal carotid artery (ICA) at the submandibular level were measured. TCD-evident vasospasm was defined when the MCA MV/external ICA MV ratio was >3 or MCA MV was >150 m/s or increased by >50 cm/s a day.[12,15] An angiographic vasospasm was defined as a narrowing of the lumen of the major cerebral arteries based on a global and quantitative assessment by interventional specialists using DSA. If a cerebral vasospasm was confirmed using DSA, either intra-arterial vasodilator infusion or balloon angioplasty was performed by an interventional radiologist. A cerebral infarction was diagnosed as new hypodensity on a CT scan that was not attributable to intracerebral hemorrhage, ventricular drain placement, or other nonvascular etiology by radiologists.[16] The occurrence of vasospasm based on daily TCD, angiography, and cerebral infarction was documented. Preoperative details including Fisher grade and Hunt-Hess grade, intraoperative data including total fluid input, and blood loss during surgery were documented.[17] Postoperative data including Glasgow Coma Scale (GCS) on day 14 after surgery, and the Glasgow Outcome Scale (GOS) at 3 months were documented. The GOS was classified as favorable (good recovery or moderate disability) or unfavorable (severe disability, vegetative, or dead) for analysis.
3. Statistical analysis
SPSS software (ver. 18.0, SPSS Inc, Chicago, IL) was used for the statistical analysis. The primary endpoint was the incidence of cerebral vasospasm during 14 days after surgery. According to previous studies, angiographic vasospasm in patient undergoing neurosurgical clipping develops in 50% to 70% patients during the first 14 days. We considered a difference of 50% in the incidence of vasospasm was considered clinically significant. Assuming an incidence of angiographic vasospasm of 60%, a power of 0.80, and an α level of 0.05, approximately 96 patients were required. Considering this assumption and review of the medical records, the study period was set as mentioned above. Comparisons of outcomes by the anesthetic agents were performed using Chi-squared or Fisher exact where appropriate. Continuous variables were analyzed by the Student t test or the Mann-Whitney U test after assessing normality and were presented as the means ± standard deviations (SD) or as medians with interquartile ranges (IQRs) as appropriate. As only the incidence of vasospasm defined by TCD was significantly different between the groups, binary logistic regression analysis was used to investigate the associations of covariates with TCD-evident vasospasm. Independent variables were age, sex, Fisher grade, Hunt-Hess grade, lumbar drainage, intracerebral hemorrhage, and hypertension, which were known to be significantly related with the vasospasm.[18] The effects of anesthetic agents on the occurrence of TCD-evident vasospasm were evaluated after adjusting for these factors using multivariate logistic regression analyses with backward selection. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. The reference groups for calculation of ORs were the use of desflurane, male, Hunt-Hess grade 1 to 3, Fisher grade 1 to 2, with no use of lumbar drainage, no intracerebral hemorrhage, and no history of hypertension. Results are presented as the mean ± SD or as medians with IQRs, as appropriate. A P value <.05 was considered statistically significant.
4. Results
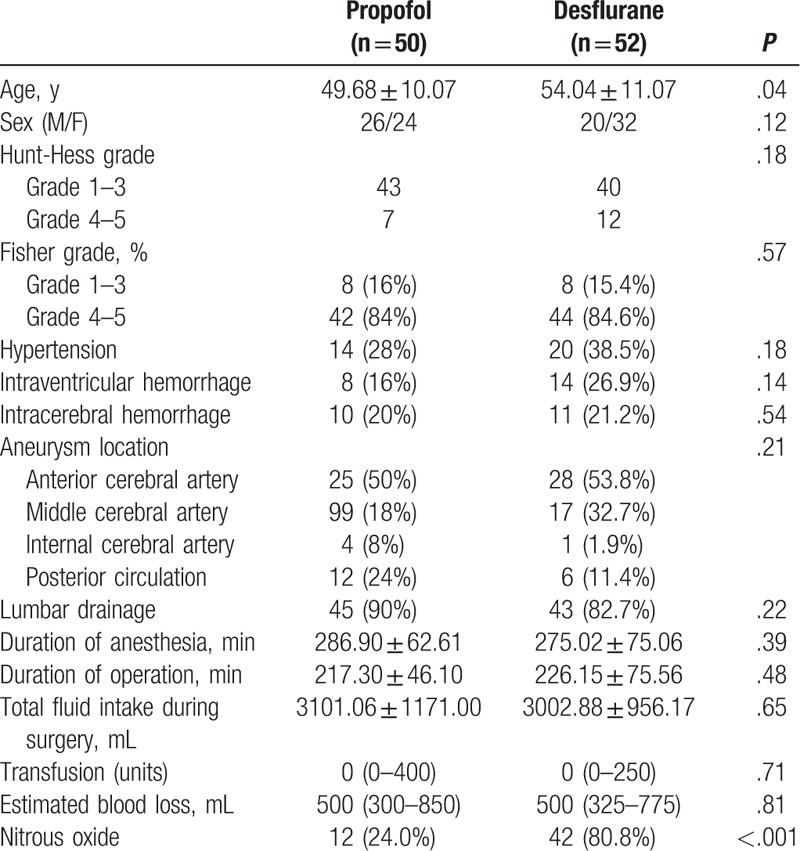
A total of 350 patients underwent aneurysm clipping with the diagnosis of SAH between July 2008 and September 2015. In total, 248 patients were excluded from the analysis. Finally, this study included 102 patients; 50 received propofol anesthesia and 52 received desflurane anesthesia. Average patient age was lower in the propofol group than in the desflurane group, otherwise, no significant differences in the patient demographics or perioperative clinical details were observed, such as Hunt–Hess grade, Fisher grade, the presence of hypertension, or lumbar drainage (Table 1).
Table 1.
Patient demographics and perioperative characteristics.
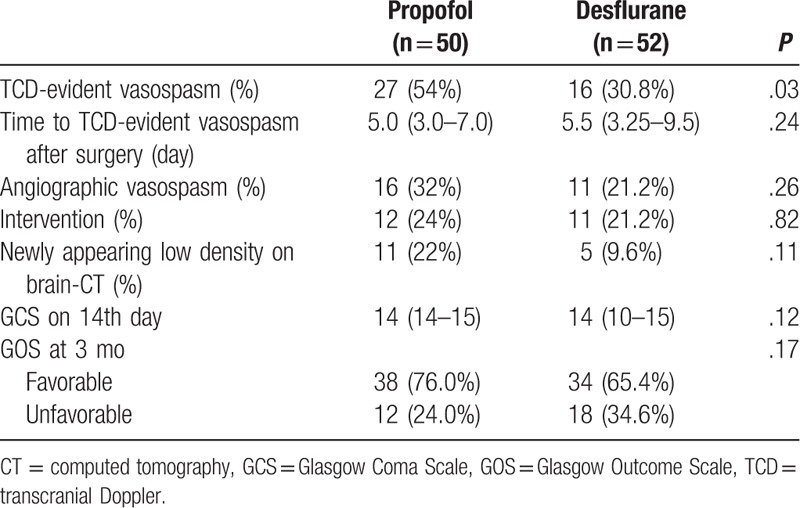
Patients who intraoperatively received propofol had higher incidence of TCD-evident vasospasm than those that received desflurane (54% vs 30.8%, P = .027) (Table 2). The median onset of vasospasm was similar; 5.0 (3.0–7.0) days in propofol group and 5.5 (3.25–9.5) days in desflurane group after surgery. The incidence of angiographic vasospasm, interventions to treat cerebral vasospasms, and newly appearing low density assessed on brain CT did not differ significantly between the groups. There was no significant difference in GCS on day 14 after surgery and GOS at 3 months between the 2 groups.
Table 2.
The incidence of cerebral vasospasm and clinical outcomes.
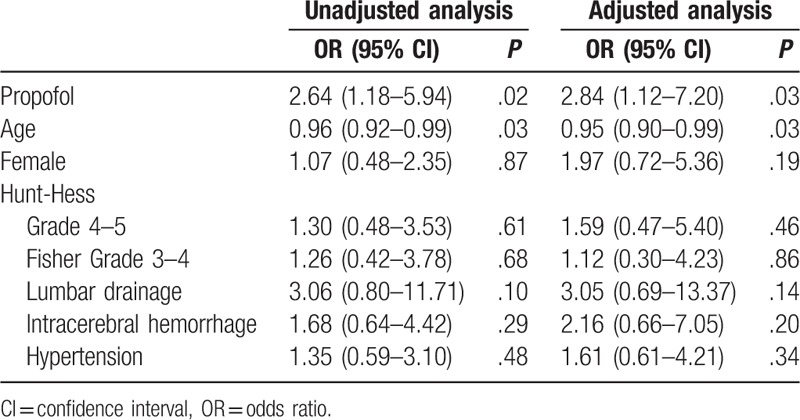
Univariate analyses showed that the use of propofol anesthesia (OR, 2.64; 95% CI, 1.18–5.94; P = .02) and younger age (OR, 0.96; 95% CI, 0.92–0.99; P = .03) was associated with TCD-evident vasospasm. Multivariate logistic regression analyses showed that the occurrence of TCD-evident vasospasm was higher (adjusted OR, 2.84; 95% CI, 1.12–7.20; P = .03) in the propofol group than in the desflurane group after adjusting for age, sex, Hunt-Hess grade, Fisher grade, lumbar drainage, intracerebral hemorrhage, and hypertension (Table 3).
Table 3.
Unadjusted and adjusted odds ratios for clinical vasospasm.
5. Discussion
Our results show that there were no significant differences in the incidence of angiographic vasospasms or cerebral infarctions, whereas desflurane anesthesia was associated with a lower incidence of TCD-evident vasospasms compared to propofol anesthesia. Although the patients in the propofol group were younger than those in the desflurane group, the effects of anesthetic agent were still associated with TCD-evident vasospasms after adjusting for confounding factors including age.
One large survey of clinical practice in treating SAHs found significant variability among anesthesiologists in practice patterns such as anesthetic management and prevention strategies for postoperative complications.[19] Maintaining an adequate depth of anesthesia, controlling blood pressure to avoid hypotensive episodes, and ensuring adequate cerebral perfusion pressure have been considered important rather than the choice of any specific anesthetic agent. However, our results suggest the possibility that the choice of anesthetic agent itself can influence the occurrence of a cerebral vasospasm. Several evidences in the literature can be one of possible explanations regarding why the incidence of TCD-evident vasospasm was lower in the desflurane group. Wang et al[6] showed significantly reduced plasma ET concentrations during desflurane anesthesia, suggesting that desflurane suppresses the role of ET in the pathogenesis of cerebral vasospasm. In a similar study by the same research group,[5] plasma CGRP concentrations decreased significantly during propofol anesthesia, whereas ET concentrations did not change. Our results are in accordance with these studies, considering these vasoactive effects and the role of ET and CGRP in patients with SAH. The level of ET, a vasoconstrictor, is elevated in the CSF and plasma of patients after an SAH and correlates with the persistence of cerebral vasospasm. CGRP is one of the most potent microvascular vasodilator peptides, and intravenous infusion of CGRP has been investigated as a treatment for vasospasm after an SAH in both animals and humans, with promising results.[20]
The effects of anesthetic agents on CBF, cerebral metabolic rate (CMR), and ICP during surgery have been well documented. Generally, propofol reduces CBF, CMR, and ICP, whereas desflurane increases CBF, decreases CMR, and maintains ICP.[17] The effects of anesthetic agents on cerebral hemodynamics and metabolism have also been investigated in other clinical settings. Regional cerebral oxygenation was measured in patients undergoing surgery in the sitting position. The authors showed that regional cerebral oxygenation decreased less with desflurane than with propofol, suggesting that desflurane preserves cerebral oxygenation better than propofol at clinically equipotent concentrations.[21] It remains unclear whether the effect on cerebral vasomotor tone and CMR of anesthetic agents used during surgery continues until the postoperative period when cerebral vasospasms usually occur, whereas there is growing evidence that even a single operation and anesthesia can influence long-term outcome after surgery in terms of immune modulation and mortality.[22,23]
We found a lower incidence of TCD-evident vasospasm when using desflurane anesthesia compared to propofol anesthesia, but not of angiographic vasospasms or cerebral infarction. Angiographic vasospasm develops in 50% to 70% of patients during the first 2 weeks, but the clinically detected incidence of symptomatic vasospasm is only 20% to 30%.[3] Although angiography is the most accurate and reliable method to detect a vasospasm, it is invasive and difficult to perform readily.[18] Therefore, TCD measurements of MCA MV are commonly used as an indicator of vasospasm after an SAH. In a prior study,[18] TCD velocities >120 cm/s at the MCA showed global accuracy of 81.1% for clinical vasospasm and 77.2% for angiographic vasospasm. A Lindegaard ratio >3.0 predicts global accuracy of 85% for clinical vasospasm and 83.2% for angiographic vasospasm.[18] Although we could not find a significant difference of incidence of vasospasm assessed by angiography, which is a criterion standard to assess vasospasm, a significant difference of TCD-evident vasospasm cannot be ignored as vasospasm is the main cause of mortality and we did not perform a routine angiography in all patients. In our institution, we used a daily TCD routinely to monitor vasospasm, and angiography was used only when a vasospasm was suspected. If angiography was used routinely in this study, we could find a higher incidence of angiographic vasospasm.
It has been traditionally considered that the primary mechanism underlying vasospasm after an SAH is cerebral arterial constriction, which leads to tissue ischemia. On the other hand, an emerging body of evidence suggests that delayed cerebral ischemia is likely to have a multifactorial etiology beyond pure cerebral vasoconstriction. The pathophysiology contributing to delayed cerebral ischemia includes cortical spreading depression (CSD), cerebral autoregulation and inflammation, and oxidative stress, which are also related to the choice of anesthetic.[22,24] CSD is a mass of depolarizations arising spontaneously and propagating around injured lesions in the human brain. Two rat studies[25,26] showed that isoflurane significantly lowers the frequency of CSD compared to propofol, suggesting that the choice of anesthetic affects acute neuronal injury states.
In our study, the use of nitrous oxide was not similar between group and several animal studies reported that nitrous oxide was capable of augmenting ischemic brain injury or conversely reversing or attenuating the cerebroprotective effects of other anesthetics. However, in the previous large multicenter human study, nitrous oxide use had no overall beneficial or detrimental impact on neurologic or neuropsychological outcomes in a population of patients at risk for ischemic brain injury.[27,28]
Our study had several limitations. First, this study included a small number of patients and was a retrospective single-center design as mentioned above. The concentration, dose and combination of anesthetic agents were not controlled, and we did not analyze the blood pressure or total anesthetic dose used during surgery. In terms of the use, however, patients were managed based on general principles for neurosurgical patients such as maintenance of adequate anesthesia depth and blood pressure. Second, we did not evaluate long-term neurological sequelae, but rather, focused on the effects of the choice of anesthetic agent on the occurrence of cerebral vasospasms during the immediate postoperative period. Nevertheless, our study provides a basis for further randomized controlled studies in a larger patient population to clarify the effects of anesthetic agents on the occurrence of cerebral vasospasms.
6. Conclusions
In this retrospective study, there were no significant differences in the incidence of angiographic vasospasms or cerebral infarctions, whereas desflurane anesthesia was associated with a lower incidence of TCD-evident vasospasms compared to propofol anesthesia. Although this study has limitations as a small retrospective design, our findings highlight the importance of conducting further randomized controlled studies in a larger patient population to clarify the effects of anesthetic agents on the occurrence of cerebral vasospasms.
Author contributions
Conceptualization: Jae Hee Woo, Narae Yang.
Data curation: Jae Hee Woo, Narae Yang, Eui kyo Seo.
Formal analysis: Jae Hee Woo.
Investigation: Jong Hwa Lee, Jae Hee Woo, Hee Jung Baik, Dong Yeon Kim, Ji Seon Chae, Eui kyo Seo.
Supervision: Jong Hwa Lee.
Writing – original draft: Jae Hee Woo.
Footnotes
Abbreviations: CBF = cerebral blood flow, CI = confidence interval, CGRP = calcitonin gene–related peptide, CMR = cerebral metabolic rate, CSD = cortical spreading depression, CT = computed tomography, DSA = digital subtraction angiography, ET = endothelin, GCS = Glasgow Coma Scale, GOS = Glasgow Outcome Scale, ICA = internal carotid artery, ICP = intracranial pressure, IQR = interquartile range, MCA = middle cerebral artery, MV = mean velocities, OR = odds ratio, SAH = subarachnoid hemorrhage, SD = standard deviations, TCI = target-controlled infusion, TCD = transcranial Doppler.
JHW has received grants from the Ewha Womans University Research Grant of 2015.
The authors report no conflicts of interest.
References
- [1].Dorsch NW, King MT. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage Part I: incidence and effects. J Clin Neurosci 1994;1:19–26. [DOI] [PubMed] [Google Scholar]
- [2].Keyrouz SG, Diringer MN. Clinical review: prevention and therapy of vasospasm in subarachnoid hemorrhage. Crit Care 2007;11:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harrod CG, Bendok BR, Batjer HH. Prediction of cerebral vasospasm in patients presenting with aneurysmal subarachnoid hemorrhage: a review. Neurosurgery 2005;56:633–54. [DOI] [PubMed] [Google Scholar]
- [4].Dinsmore J. Anaesthesia for elective neurosurgery. Br J Anaesth 2007;99:68–74. [DOI] [PubMed] [Google Scholar]
- [5].Luo F, Ji N, Zhang S, et al. Changes of endothelin and calcitonin gene-related peptide concentrations in plasma during propofol anesthesia. J Neurosurg Anesthesiol 2009;21:47–50. [DOI] [PubMed] [Google Scholar]
- [6].Wang T, Luo F, Shan R, et al. Changes of endothelin and calcitonin gene-related peptide during desflurane anesthesia in patients undergoing intracranial aneurysm clipping. J Neurosurg Anesthesiol 2004;16:236–9. [DOI] [PubMed] [Google Scholar]
- [7].Juvela S. Plasma endothelin concentrations after aneurysmal subarachnoid hemorrhage. J Neurosurg 2000;92:390–400. [DOI] [PubMed] [Google Scholar]
- [8].Kessler IM, Pacheco YG, Lozzi SP, et al. Endothelin-1 levels in plasma and cerebrospinal fluid of patients with cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Surg Neurol 2005;64(suppl 1):S1:2-5. [DOI] [PubMed] [Google Scholar]
- [9].Lin CL, Jeng AY, Howng SL, et al. Endothelin and subarachnoid hemorrhage-induced cerebral vasospasm: pathogenesis and treatment. Curr Med Chem 2004;11:1779–91. [DOI] [PubMed] [Google Scholar]
- [10].Locatelli M. The importance of substance P and calcitonin gene related peptide as vasodilator neuropeptide during acute phase of experimental posthemorrhagic vasospasm. J Neurosurg Sci 2000;44:186–91. [PubMed] [Google Scholar]
- [11].Vajkoczy P, Meyer B, Weidauer S, et al. Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. J Neurosurg 2005;103:9–17. [DOI] [PubMed] [Google Scholar]
- [12].Park S, Yang N, Seo E. The effectiveness of lumbar cerebrospinal fluid drainage to reduce the cerebral vasospasm after surgical clipping for aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc 2015;57:167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kassell NF, Torner JC, Haley EC, Jr, et al. The international cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J Neurosurg 1990;73:18–36. [DOI] [PubMed] [Google Scholar]
- [14].Frontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke 2009;40:1963–8. [DOI] [PubMed] [Google Scholar]
- [15].Lindegaard KF, Nornes H, Bakke SJ, et al. Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochir (Wien) 1989;100:12–24. [DOI] [PubMed] [Google Scholar]
- [16].Dumont AS, Crowley RW, Monteith SJ, et al. Endovascular treatment or neurosurgical clipping of ruptured intracranial aneurysms: effect on angiographic vasospasm, delayed ischemic neurological deficit, cerebral infarction, and clinical outcome. Stroke 2010;41:2519–24. [DOI] [PubMed] [Google Scholar]
- [17].Priebe HJ. Aneurysmal subarachnoid haemorrhage and the anaesthetist. Br J Anaesth 2007;99:102–18. [DOI] [PubMed] [Google Scholar]
- [18].Gonzalez NR, Boscardin WJ, Glenn T, et al. Vasospasm probability index: a combination of transcranial Doppler velocities, cerebral blood flow, and clinical risk factors to predict cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg 2007;107:1101–12. [DOI] [PubMed] [Google Scholar]
- [19].Velly LJ, Bilotta F, Fabregas N, et al. Anaesthetic and ICU management of aneurysmal subarachnoid haemorrhage: a survey of European practice. Eur J Anaesthesiol 2015;32:168–76. [DOI] [PubMed] [Google Scholar]
- [20].Kokkoris S, Andrews P, Webb DJ. Role of calcitonin gene-related peptide in cerebral vasospasm, and as a therapeutic approach to subarachnoid hemorrhage. Front Endocrinol (Lausanne) 2012;3:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim JY, Lee JS, Lee KC, et al. The effect of desflurane versus propofol on regional cerebral oxygenation in the sitting position for shoulder arthroscopy. J Clin Monit Comput 2014;28:371–6. [DOI] [PubMed] [Google Scholar]
- [22].Woo JH, Baik HJ, Kim CH, et al. Effect of propofol and desflurane on immune cell populations in breast cancer patients: a randomized trial. J Korean Med Sci 2015;30:1503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Landoni G, Greco T, Biondi-Zoccai G, et al. Anaesthetic drugs and survival: a Bayesian network meta-analysis of randomized trials in cardiac surgery. Br J Anaesth 2013;111:886–96. [DOI] [PubMed] [Google Scholar]
- [24].Rowland MJ, Hadjiipavlou G, Jelly M, et al. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth 2012;109:315–29. [DOI] [PubMed] [Google Scholar]
- [25].Kudo C, Toyama M, Boku A, et al. Anesthetic effects on susceptibility to cortical spreading depression. Neuropharmacology 2013;67:32–6. [DOI] [PubMed] [Google Scholar]
- [26].Takagaki M, Feuerstein D, Kumagai T, et al. Isoflurane suppresses cortical spreading depolarizations compared to propofol—implications for sedation of neurocritical care patients. Exp Neurol 2014;252:12–7. [DOI] [PubMed] [Google Scholar]
- [27].McGregor DG, Lanier WL, Pasternak JJ, et al. Effect of nitrous oxide on neurologic and neuropsychological function after intracranial aneurysm surgery. Anesthesiology 2008;108:568–79. [DOI] [PubMed] [Google Scholar]
- [28].Pasternak JJ, McGregor DG, Lanier WL, et al. Effect of nitrous oxide use on long-term neurologic and neuropsychological outcome in patients who received temporary proximal artery occlusion during cerebral aneurysm clipping surgery. Anesthesiology 2009;110:563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


