Abstract
Euthyroid sick syndrome (ESS) is commonly observed in various acute and chronic illness as risk factor for mortality in patients with severe diseases, with lower triiodothyronine (T3) and free triiodothyronine (fT3).
To explore the relationship between disease severity and thyroid function in critically ill Chinese patients with ESS.
A total of 51 patients admitted to intensive care unit were examined to determine acute physiology and chronic health assessment II (APACHE II) scores within 24 hours of admission; thyroid function tests (TSH, fT3, fT4, tT3, tT4) and rT3 levels were determined on the second day. Based on the test results, patients were divided into euthyroid (n = 13), decreased fT3 or fT4 (n = 17), and decreased TSH (n = 21) groups. APACHE II scores and thyroid function were compared between the 3 groups. Furthermore, the relationship between the severity of disease and euthyroid sick syndrome was assessed.
Out of 51 patients, 38 were men and 13 were women [mean age (± SD): 60.39 (± 19.32) years; range, 15–88 years]. APACHE II scores and rT3 levels were increased in all the 3 groups (P > .05). APACHE II scores showed a positive correlation with rT3 (P = .004, r = 0.379).
Critically ill Chinese patients with ESS have a poor health state. Higher rT3 values are associated with severe disease.
Keywords: disease severity, euthyroid sick syndrome, thyroid function
1. Introduction
Euthyroid sick syndrome (ESS) (also referred to as nonthyroidal illness syndrome) is characterized by higher reverse triiodothyronine (rT3) levels. The condition is commonly associated with acute and chronic morbid conditions, fasting state, protein calorie malnutrition, general surgery, trauma, myocardial infarction, and chronic renal failure. In mild illness, the condition is associated with lower serum levels of triiodothyronine (T3), free triiodothyronine (fT3). In severe illness, there is additional lowering of thyroxine (T4), free thyroxine (fT4), and thyrotropin (TSH) levels.[1,2]
ESS was shown to be a risk factor for mortality in patients with severe diseases.[3] Moreover, ESS was shown to be significantly associated with disease severity of patients with heart failure, respiratory failure, liver failure, acute renal failure, and community acquired pneumonia,[4–13] which was reflected in the prolonged duration of stay in the intensive care unit (ICU).[14,15] Previous studies on the management of ESS have been largely inconclusive.[1,2] A better understanding of ESS is a key imperative.
In this study, we categorized patients with ESS who were admitted to ICU into various groups based on the severity of ESS, and compared the APACHE II scores of these patients; the objective was to assess the relationship between severity of the disease and euthyroid sick syndrome. Further, we discuss the significance and strategy of intervention for ESS in critically ill patients.
2. Methods
2.1. Ethical statement
This study was approved by the ethical committee at the People's Hospital of Wuxi (WXCL2016-072). Written informed consent was obtained from all patients or their attendants prior to their enrolment in the study.
2.2. Study population
This was a prospective cohort study.
The inclusion criteria were:
Patients admitted to the ICU at The People's Hospital of Wuxi for acute illness between January 2016 and July 2016.
The exclusion criteria were
-
1.
patients with endocrinological disorders such as disorders of thyroid, adrenal and pituitary glands, hypothalamus disease;
-
2.
patients with brain injury;
-
3.
patients with autoimmune disease;
-
4.
patients with diseases that require long-term hormone therapy.
2.3. Thyroid function
Fasting venous blood samples were obtained from all enrolled patients for thyroid function test (TFT) on the second day, including TSH, fT3, fT4, total triiodothyronine (tT3), total thyroxine (tT4), and rT3. Specifically, TSH, fT3, fT4, tT3, and tT4 were tested using BECKMAN COULTER (chemiluminescence). Normal reference levels were: TSH (0.49–4.91 mU/L); fT3 (3.28–6.47 pmol/L); fT4 (7.64–16.03 pmol/L); tT3 (1.01–2.48 nmol/L); tT4 (69.97–152.52 nmol/L).
2.4. rT3
rT3 was tested using Beijing North Institute of Biotechnology (normal reference level: 20–64 ng/dL).
2.5. APACHE II
APACHE II is a severity-of-disease classification system that was first developed by Knaus et al. It is used for assessment of patients within 24 hours of admission to an ICU. An integer score (range: 0–71) is computed based on several co-variates. Higher scores correspond to more severe disease and a higher risk of death.[16,17]
APACHE II was used as an indicator of the severity of disease. The highest APACHE II score within the first 24 hours of ICU stay was used for the purpose of this analysis.
2.6. Group
Thyroid hemostasis is regulated via a complex feedback mechanism. Under normal circumstances, decrease in serum T3 and T4 levels triggers an increase in TSH levels via a feedback loop in order to normalize the levels of T3 and T4. According to the results of TFTs, the patients were categorized into 3 groups.
When T3 and T4 levels are decreased without a concomitant increase in TSH level, it blocks the ability to alleviate the reduction in T3 and T4 levels. Such patients were assigned to Decreased fT3 or fT4 group (decreased fT3 or fT4 level, but TSH in the normal range).
Further aggravation of ESS leads to reduction in TSH levels below the normal reference levels, a state which is characterized by the inability to respond to reduced T3 and T4 feedback. Such patients were assigned to Decreased TSH group (decreased TSH level, with fT4 or fT3 levels decreased or within the normal range).
Patients with TSH, fT3, and fT4 levels within the normal range were assigned to the Euthyroid group.
2.7. Statistical method
SPSS 19.0 (IBM Corporation) was used for statistical analysis. Continuous variables are expressed as mean ± standard deviation (SD). Between-group differences were assessed by one-way analysis of variance (ANOVA). The relationship between 2 continuous variables was assessed with Pearson correlation analysis. P < .05 was considered statistically significant.
3. Results
3.1. Basic information
A total of 51 patients [38 men and 13 women; mean age (±SD): 60.39 (±19.32) years; range, 15–88] were included in the study.
The clinical diagnoses included severe acute pancreatitis (3 patients), acute cholangitis (3 patients), mesenteric vascular thrombosis (4 patients), acute peritonitis (11 patients), multiple injuries (8 patients), severe pneumonia (7 patients), acute respiratory distress syndrome (6 patients), acute exacerbation of chronic obstructive pulmonary disease (4 patients), severe asthma (1 patient), cardiac arrest (6 patients), acute left heart failure (2 patients), acute myocardial infarction (1 patient), cerebrovascular accident (3 patients), gastrointestinal bleeding (5 patients), acute liver failure (2 patients), and acute kidney injury (7 patients).
3.2. Comparison of thyroid function
Based on the TFT results, 13 patients were included in the euthyroid group, 17 patients in the decreased fT3 or fT4 group and 21 patients in the decreased TSH group.
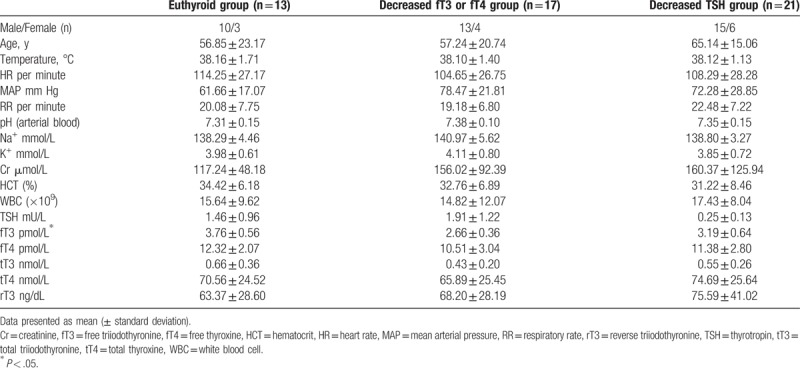
The mean (±SD) fT3 levels in the 3 groups were 3.76 (±0.56) pmol/L, 2.66 (±0.36) pmol/L, and 3.19 (±0.64) pmol/L, respectively (P < .05). Other clinical characteristics are summarized in Table 1. Between-group differences with respect to TSH, fT4, tT3, and tT4 levels were not statistically significant.
Table 1.
Patient characteristics and thyroid function status in the 3 study groups.
An incremental trend was observed in rT3 levels (euthyroid group < decreased fT3 or fT4 group < decreased TSH group) (Fig. 1).
Figure 1.
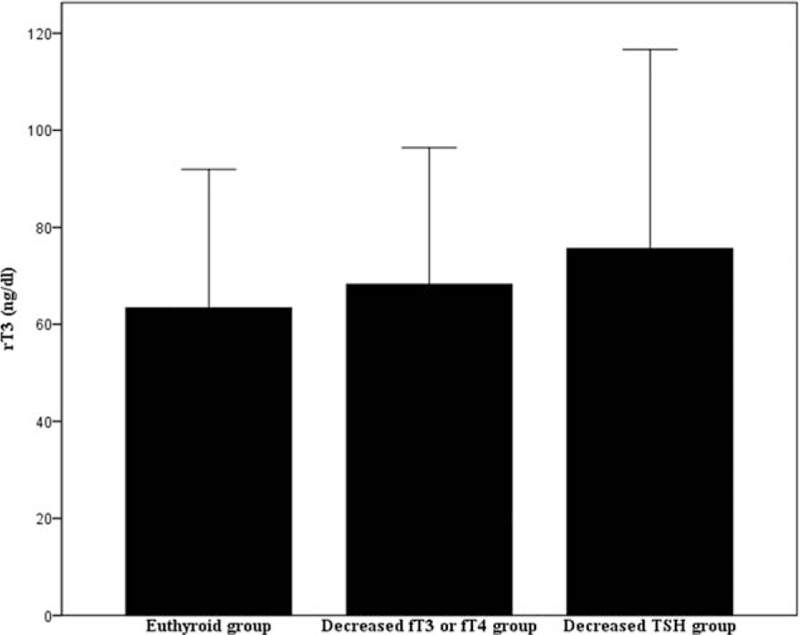
rT3 levels in the 3 study groups.
3.3. Relationship between APACHE II score and thyroid function
The mean (±SD) APACHE II score for the euthyroid, decreased fT3 or fT4, and decreased TSH groups were 13.23 (±3.56), 14.12 (±7.42), and 16.19 (±5.15), respectively, which implies that the APACHE II scores showed an increasing trend with increase in the severity of the disease. However, the between-group differences were not statistically significant (P > .05).
We further examined the relationship between the APACHE II scores and specific thyroid indices. The APACHE II scores showed a positive correlation with rT3 levels (P = .004, r = 0.379), which suggests that higher APACHE II scores were associated with higher rT3 levels. However, a negative correlation of APACHE II score with TSH, tT3, and tT4 levels was observed (P < .01). No statistically significant correlation of APACHE II score with fT3 and fT4 levels was observed (P > .05). The correlation between APACHE II scores and various thyroid function indices are shown in Table 2. The scatter diagrams are shown in Figure 2.
Table 2.
Correlation between APACHE II score and thyroid function status.
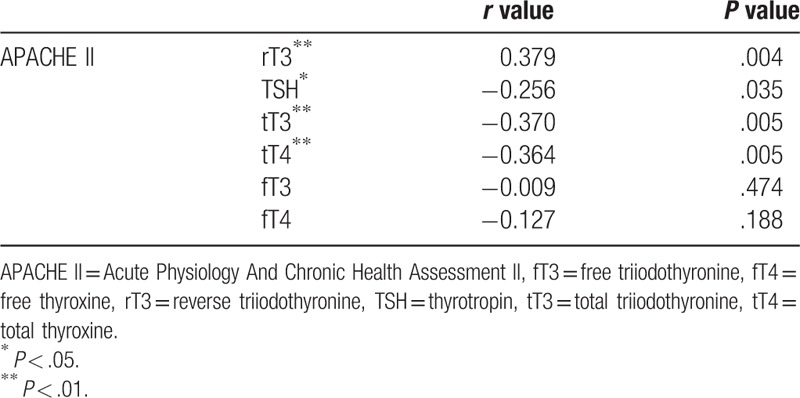
Figure 2.
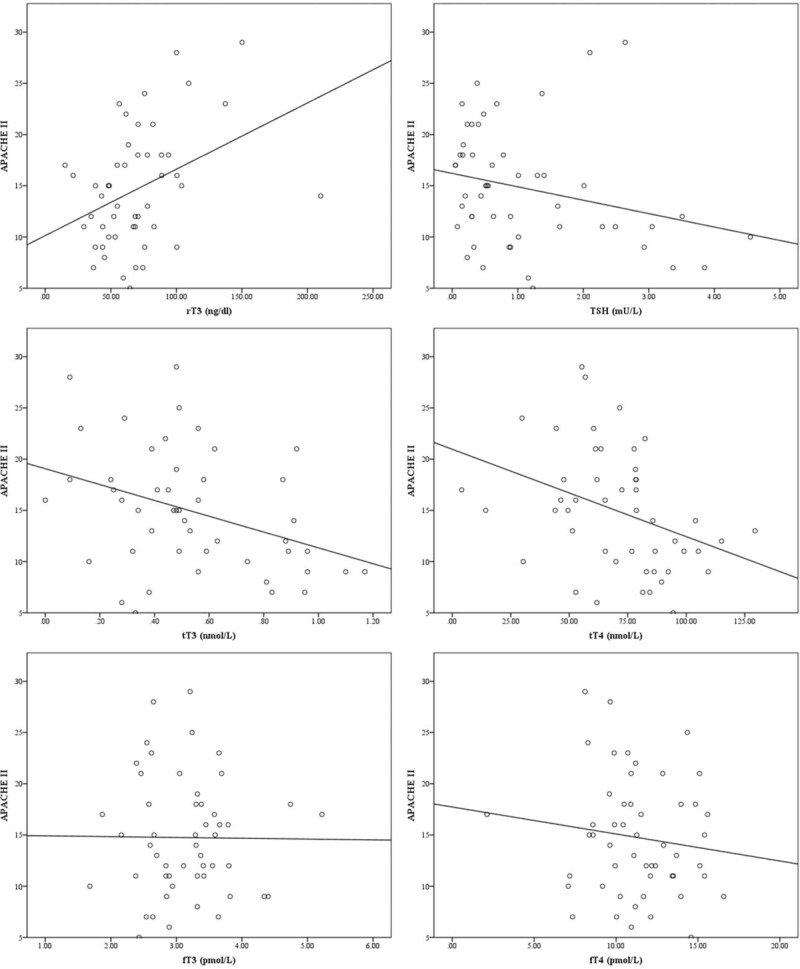
Scatter diagrams showing correlation between APACHE II score and thyroid function status. APACHE II = acute physiology and chronic health assessment II.
4. Discussion
Previous studies have shown that patients with ESS are more likely to have more serious disease state as compared to that of patients with normal thyroid function. Further, thyroid function in the majority of patients was shown to recover with remission of the primary disease.[2] Generally, ESS is a common finding in critically ill patients, even in the absence of observable thyroid pathology. Changes in thyroid hormone metabolism and ability of tissues or cells for uptake of thyroid hormones contribute to impaired thyroid function during critical illness, including decreased T3 and increased rT3. However, TSH and T4 levels tend to decrease with the progression of illness.
In this study, APACHE II scores of patients with ESS were higher than those of patients with normal thyroid function. Further, the scores increased with development of symptomatic ESS in patients with decreased TSH levels, which reflects a more serious disease state among critically ill patients. Therefore, the severity of ESS seems to aggravate with progression of the primary disease.
From the view of hypothalamic-pituitary-thyroid-target organ axis, low fT3 and fT4 levels reflect thyroid and target organ changes; however, low TSH level also reflects changes in pituitary and hypothalamus, which is indicative of worse health state. Therefore, ESS decompensation is more evident in low TSH states, which requires more cautious intervention. Previous studies[18–20] have shown that increased levels of rT3 may be related to decreased deiodinase activity, which indicates that this may be a target for intervention for ESS.
However, most reports seem to suggest that ESS is a self-protective mechanism in critical illnesses (such as fever, tachycardia) rather than being a disease per se, owing to the resolution of ESS with primary disease remission. However, no clear consensus has emanated from clinical studies of ESS.[21] In addition, no conclusive evidence of the benefit of ESS intervention has been demonstrated in clinical studies. Therefore, many scholars suggest that treatment of the primary disease takes precedence over ESS intervention.
However, ESS is a manifestation of compensatory mechanism in critically ill patients. If the original disease cannot be alleviated, or gets further aggravated over a short period of time, the effect of compensatory mechanism will lead to overt thyroid decompensation, and the resultant hypometabolic state is liable to aggravate the primary disease and create a vicious cycle. Identification of means to break this vicious cycle is a key focus of ESS treatment research.
Thyroid-related hormones play a key role in the regulation of metabolism of various organs and tissues as well as in the occurrence and development of various diseases, which further explains the observed heterogeneity in the disease spectrum of ESS,[22] that is, similar thyroid function test results are seen in different diseases.[23,24] Therefore, the target for therapeutic intervention needs to be individualized based on the different impact of hormones on the body.
4.1. Study limitations
Our study has several limitations. First, the sample size of patients was too small and may have introduced an element of bias, considering the heterogeneity with respect to the primary disease.
Secondly, the assessment of disease severity in the study was based on APACHE II score (<24 hours of admission); therefore, longer follow-up of patients will provide a more valid assessment of prognosis.
Third, the thyroid function status was determined at the morning of the second day of admission, at which point the thyroid function status may not fully correspond to the worst APS score; this may have influenced the observed correlation between thyroid function and APACHE II score.
Fourth, the difference in APACHE II scores between the euthyroid and decreased TSH groups was not statistically significant as the timing of TFT may not have corresponded to the most serious state of ESS patients. This may have resulted in the assignment of more patients to the euthyroid or decreased fT3 or fT4 groups, rather than to the decreased TSH group.
However, the disease severity did show a positive correlation with the severity of ESS. An appropriate intervention for ESS may potentially open new vistas for the treatment of patients with severe diseases.
Author contributions
Conceptualization: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Data curation: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Formal analysis: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Funding acquisition: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Investigation: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Methodology: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Project administration: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Resources: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Software: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Supervision: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Validation: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Visualization: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Writing – original draft: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Writing – review & editing: Yi-Feng Wang, Jun-Feng Heng, Jie Yan, Liang Dong.
Footnotes
Abbreviations: APACHE II = acute physiology and chronic health assessment II, ESS = euthyroid sick syndrome, fT3 = free triiodothyronine, fT4 = free thyroxine, ICU = intensive care unit, rT3 = reverse triiodothyronine, T3 = triiodothyronine, T4 = thyroxine, TFT = thyroid function test, TSH = thyrotropin, tT3 = total triiodothyronine, tT4 = total thyroxine.
This work was supported by The Natural Science Foundation of China (81400054) and The Natural Science Foundation of Jiangsu Province (BK20140122).
The authors have no conflicts of interest to disclose.
References
- [1].Golombek SG. Nonthyroidal illness syndrome and euthyroid sick syndrome in intensive care patients. Semin Perinatol 2008;32:413–8. [DOI] [PubMed] [Google Scholar]
- [2].Mebis L, Van den Berghe G. Thyroid axis function and dysfunction in critical illness. Best Pract Res Clin Endocrinol Metab 2011;25:745–57. [DOI] [PubMed] [Google Scholar]
- [3].Munoz-Ramirez Mdel R, Ortega-Valdez CA, Murillo-Heredia E. Euthyroid sick syndrome as a risk factor for mortality in critically ill patients. Med Clin (Barc) 2016;146:414–5. [DOI] [PubMed] [Google Scholar]
- [4].Angelousi AG, Karageorgopoulos DE, Kapaskelis AM, et al. Association between thyroid function tests at baseline and the outcome of patients with sepsis or septic shock: a systematic review. Eur J Endocrinol 2011;164:147–55. [DOI] [PubMed] [Google Scholar]
- [5].Galusova A, Pauliny M, Majek M, et al. Dynamic neuroendocrine changes in critically ill patients with polytrauma. Neuro Endocrinol Lett 2015;36:498–503. [PubMed] [Google Scholar]
- [6].Okayama D, Minami Y, Kataoka S, et al. Thyroid function on admission and outcome in patients hospitalized for acute decompensated heart failure. J Cardiol 2015;66:205–11. [DOI] [PubMed] [Google Scholar]
- [7].Silva-Tinoco R, Carrasco Ortiz O, Castillo-Martinez L, et al. Persistence of thyroid hormones disorders in chronic heart failure outpatients: “heart hypothyroidism”. Int J Cardiol 2013;167:2359–60. [DOI] [PubMed] [Google Scholar]
- [8].Nafae RM, Mohammed MA, Morsi AF. Thyroid function in respiratory failure patients. Egypt J Chest Dis Tuberc 2014;63:513–21. [Google Scholar]
- [9].Agiasotelli D, Alexopoulou A, Vasilieva L, et al. Low free T3 levels are related to early mortality in patients with decompensated cirrhosis and acute-on chronic liver failure. J Hepatol 2014;61:1446–7. [DOI] [PubMed] [Google Scholar]
- [10].Iglesias P, Olea T, Vega-Cabrera C, et al. Thyroid function tests in acute kidney injury. J Nephrol 2013;26:164–72. [DOI] [PubMed] [Google Scholar]
- [11].Liu J, Wu X, Lu F, et al. Low T3 syndrome is a strong predictor of poor outcomes in patients with community-acquired pneumonia. Sci Rep 2016;6:22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yasar Z, Kirakli C, Cimen P, et al. Is non-thyroidal illness syndrome a predictor for prolonged weaning in intubated chronic obstructive pulmonary disease patients? Int J Clin Exp Med 2015;8:10114–21. [PMC free article] [PubMed] [Google Scholar]
- [13].Yasar ZA, Kirakli C, Yilmaz U, et al. Can non-thyroid illness syndrome predict mortality in lung cancer patients? A prospective cohort study. Horm Cancer 2014;5:240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Plikat K, Langgartner J, Buettner R, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism 2007;56:239–44. [DOI] [PubMed] [Google Scholar]
- [15].Bello G, Pennisi MA, Montini L, et al. Nonthyroidal illness syndrome and prolonged mechanical ventilation in patients admitted to the ICU. Chest 2009;135:1448–54. [DOI] [PubMed] [Google Scholar]
- [16].Saleh A, Ahmed M, Abdel-Lateif A. Comparison of the mortality prediction of different ICU scoring systems (APACHE II and III, SAPS II, and SOFA) in acute respiratory distress syndrome patients. Chest 2016;149:A147. [Google Scholar]
- [17].Zhou XY, Ben SQ, Chen HL, et al. A comparison of APACHE II and CPIS scores for the prediction of 30-day mortality in patients with ventilator-associated pneumonia. Int J Infect Dis 2015;30:144–7. [DOI] [PubMed] [Google Scholar]
- [18].Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol 2010;205:1–3. [DOI] [PubMed] [Google Scholar]
- [19].de Vries EM, Fliers E, Boelen A. The molecular basis of the non-thyroidal illness syndrome. J Endocrinol 2015;225:R67–81. [DOI] [PubMed] [Google Scholar]
- [20].Wajner SM, Maia AL. New insights toward the acute non-thyroidal illness syndrome. Front Endocrinol (Lausanne) 2012;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Peeters RP. Non thyroidal illness: to treat or not to treat? Ann Endocrinol (Paris) 2007;68:224–8. [DOI] [PubMed] [Google Scholar]
- [22].Van den Berghe G. Non-thyroidal illness in the ICU: a syndrome with different faces. Thyroid 2014;24:1456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Loh TP, Tee JC, Tee NW, et al. Association between thyroid function tests and anti-thyroid peroxidase (TPO) antibodies in pregnancy. Endocrine 2016;53:865–7. [DOI] [PubMed] [Google Scholar]
- [24].Suzuki Y, Matsushita K, Seimiya M, et al. Paradoxical effects of thyroid function on glomerular filtration rate estimated from serum creatinine or standardized cystatin C in patients with Japanese Graves’ disease. Clin Chim Acta 2015;451:316–22. [DOI] [PubMed] [Google Scholar]


