Abstract
Background:
Previous studies have evaluated the diagnostic value of serum intercellular adhesion molecule-1 (ICAM-1) in patients with endometriosis, but the results remained inconsistent. This meta-analysis aimed to clarify the overall diagnostic accuracy of serum ICAM-1 for endometriosis.
Methods:
A systematic search was performed of the Cochrane clinical trials database, PubMed, and Embase prior to December 2017. Sensitivity, specificity, and other measures were pooled and determined to evaluate the accuracy of serum ICAM-1 in the diagnosis of endometriosis. Summary receiver operating characteristic curve (SROC) analysis with calculation of the area under curve (AUC) was performed to summarize the overall test performance.
Results:
Nine studies including 878 patients were eligible for inclusion in the analysis. The summary estimates of sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio (95% confidence interval) were 0.65 (0.52–0.75), 0.78 (0.62–0.89), 3.0 (1.7–5.2), 0.45 (0.34–0.60), and 7 (3–13), respectively, and the AUC of the overall analysis was 0.76 (0.72–0.80). Subgroup analysis showed that the summary sensitivity and specificity for patients of Asian ethnicity was increased compared with those of Caucasian ethnicity. The summary specificity was increased in studies with a high ICAM-1 cut-off value compared to those with a low cut-off value. No publication bias was found across the studies.
Conclusions:
Our results suggest that serum ICAM-1 has moderate diagnostic accuracy for endometriosis, while the diagnostic accuracy is higher in patients of Asian ethnicity compared with those of Caucasian ethnicity.
Keywords: diagnosis, endometriosis, intercellular adhesion molecule-1, meta-analysis
1. Introduction
Endometriosis is defined by the presence of endometrial-like tissue located outside the normal uterine cavity, causing a chronic inflammatory condition; it is estimated to occur in 6% to 10% of women of reproductive age.[1,2] Currently, surgical approaches, such as laparoscopy followed by histological confirmation, remains the gold standard method to establish a definitive diagnosis, but this approach is invasive and causes long delays before reaching the diagnosis.[3] While nonsurgical approaches such as trans-vaginal sonography and pelvic magnetic resonance imaging have several advantages, their diagnostic accuracy is affected by variation in clinical experience, and they cannot replace surgery as the gold standard.[4,5]
To date, several serum biomarkers, such as CA125, CA199, and follistatin, have been used for the detection of endometriosis, but the diagnostic accuracy of these biomarkers is variable across many studies.[6,7] Thus, a reliable serum biomarker is still needed. Cell adhesion molecules are cell surface proteins that mediate adherence between cells and the extracellular matrix.[8] Intercellular adhesion molecule-1 (ICAM-1) has been investigated in endometriosis. Previous studies showed that its level in serum or peritoneal fluid is increased in patients with endometriosis compared to healthy controls. Also, it has been suggested that it has a role in regulation of endometrial cell proliferation, activation, motility, chemotaxis, adhesion, morphogenesis, and implantation.[9,10]
The diagnostic accuracy of serum ICAM-1 in endometriosis has been investigated in some studies, but the results of these analyses were variable.[11–14] Possible reasons for such inconsistency in the results of these studies could be small sample size, differences in patient ethnicity, or different cut-off values used. Therefore, in this study, we conducted a meta-analysis of all eligible studies to clarify the diagnostic accuracy of serum ICAM-1 for endometriosis.
2. Methods
2.1. Search strategy and study selection
This meta-analysis was conducted according to the guidelines proposed by the Human Genome Epidemiology Network for the systematic review of genetic-association studies and followed PRISMA guidelines.[15] We systematically searched the Cochrane clinical trials database, Medline (PubMed), Embase, Web of Science, and Chinese National Knowledge Infrastructure (CNKI) database to identify relevant studies from inception to December 2017. References within the identified articles were also searched manually. Search terms included “endometriosis,” “intercellular adhesion molecule 1,” “ICAM-1,” “sICAM-1,” “diagnosis,” and “serum.” The search was limited to studies involving humans but not those limited by language.
2.2. Inclusion and exclusion criteria
Included studies had to include: the diagnosis of endometriosis based on histopathology obtained by surgery or laparoscopy; data on both the sensitivity and specificity of serum ICAM-1 for the diagnosis of endometriosis, or sufficient data reported to calculate the sensitivity and specificity. Studies were excluded if they were review articles or case reports. We also excluded studies that used peritoneal fluid to measure ICAM-1 levels.
2.3. Data extraction and quality assessment
The extracted data of each study included: first author name, year of publication, country, design of study; type of sample; age of participants; number of patients with endometriosis and controls; stage of endometriosis; testing method for ICAM-1; cut-off level for ICAM-1; sensitivity and specificity data. The methodological quality of the studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) criteria. This tool is an evidence-based quality assessment tool developed for use in systematic reviews of studies of diagnostic accuracy,[16] with a maximum score of 14. Two reviewers independently judged the eligibility of studies. Disagreements between reviewers were resolved by consensus.
2.4. Statistical analyses
The standard methods recommended for meta-analyses of diagnostic tests were conducted in this study.[17] The following summary diagnostic measures, including sensitivity and specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) with the corresponding 95% confidence interval (CI) were calculated for each study. The Cochran Q and the inconsistency index (I2) were used to assess the threshold effect as an important component of the source of variation between studies. I2 values <25% indicated mild heterogeneity, I2 values between 25% and 50% indicated moderate heterogeneity, and I2 values >50% indicated significant heterogeneity. A fixed-effects model (Mantel–Haenszel method) was used to calculate parameters when there was less heterogeneity; otherwise, a random-effects model (DerSimonian and Laird) was used.
A summary receiver operating characteristic curve (SROC) was established and described as the area under the SROC curve (AUC) with its Q∗-point representing the maximal joint sensitivity and specificity.[17,18] If heterogeneity existed, sensitivity analysis was used to check the robustness of the results by omitting a study in turn. Subgroup analysis was carried out by dividing the studies according to different ethnicities and cut-off values. Publication bias was tested using Deek's funnel plot asymmetry test.[19] All statistical analyses were performed using Stata 11.2 software (Stata Corp, College Station, TX). All statistical tests were 2-sided and a P value <.05 was considered statistically significant.
3. Results
3.1. Literature selection process
The primary literature search using the search terms retrieved 22 articles. After reading the titles and abstracts, 8 studies were excluded because they were case reports or reviews. The remaining 14 studies were given detailed evaluation, and 4 studies were excluded for the following reasons: 2 studies without sufficient data even after contacting the authors, and 2 that used peritoneal fluid to test ICAM-1 levels. Consequently, 9 studies[11–14,20–25] with 878 participants were included the present meta-analysis. Figure 1 shows the flow chart of the article selection process.
Figure 1.
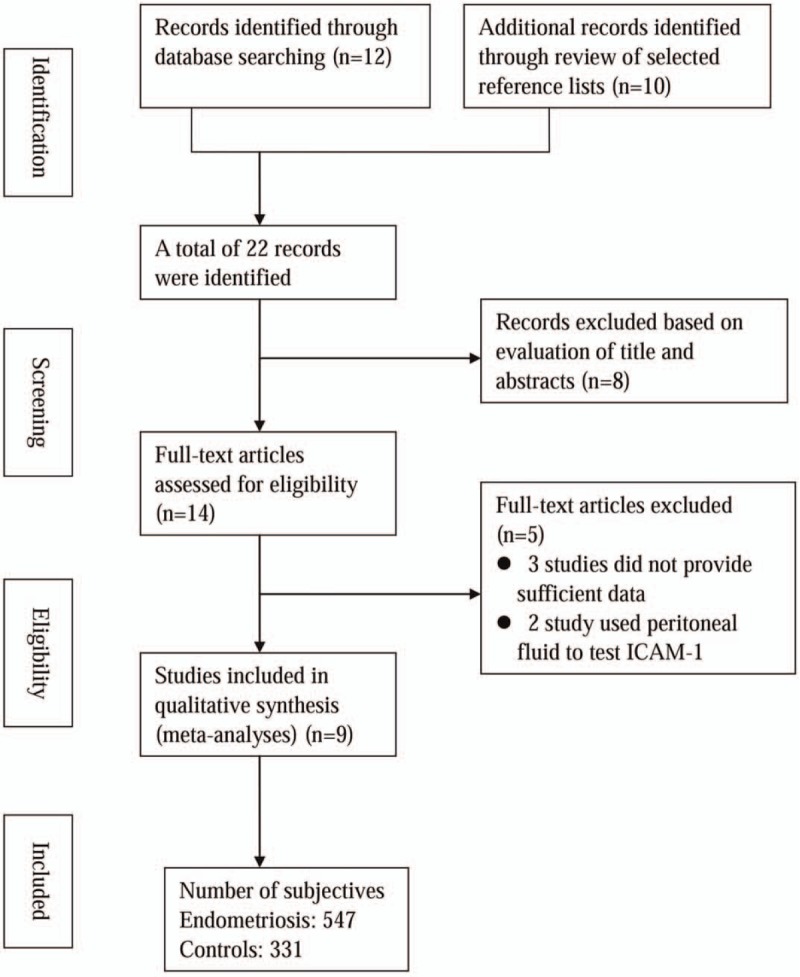
Flow diagram of the articles selection process.
3.2. Characteristics and quality of the studies
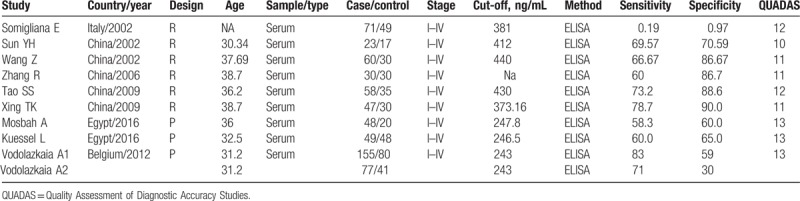
Table 1 reports the clinical characteristics of included studies and the QUADAS scores. Briefly, all the included studies used laparoscopy followed by histological examination as the gold standard to diagnose endometriosis. They all used the ELISA method to test the serum ICAM-1 levels. The stage of endometriosis ranged from I to IV. Patients from 5 studies were of Caucasian ethnicity, the other 5 studies included patients of Asian ethnicity. One study[13] had 2 datasets, which we notated as Vodolazkaia A1 and Vodolazkaia A2 in the analysis. The QUADAS scores ranged from 8 to 12.
Table 1.
Characteristics of the included studies.
3.3. Overall analysis and sensitivity analysis
The overall analysis for the 14 studies showed that the summary sensitivity, specificity, PLR, NLR, and DOR of serum ICAM-1 levels in the diagnosis of endometriosis were 0.65 (0.52–0.75), 0.78 (0.62–0.89), 3.0 (1.7–5.2), 0.45 (0.34–0.60), and 7 (3–13), respectively. There was a significant heterogeneity in the summary sensitivity (I2 = 91.11%, P < .01) and specificity (I2 = 88.78%, P < .01) (Fig. 2). The AUC of SROC was 0.76 (0.72–0.80), suggesting moderate diagnostic accuracy (Fig. 3).
Figure 2.
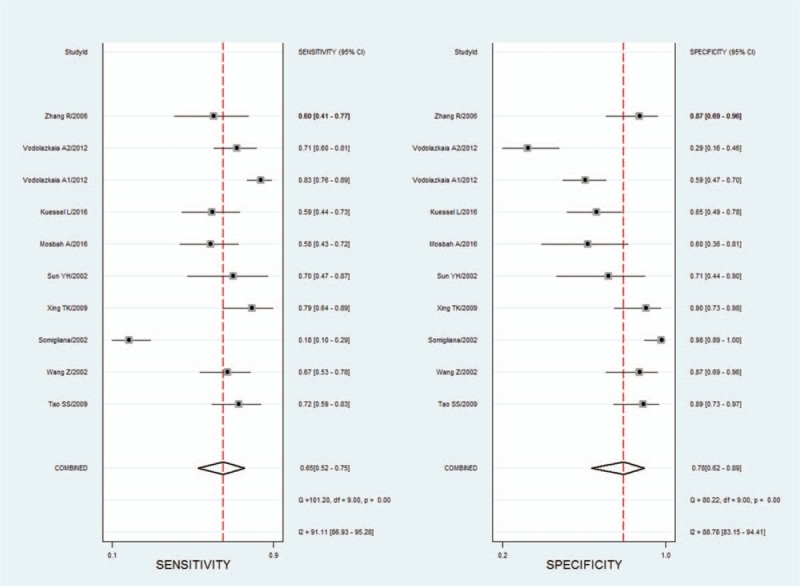
Summary sensitivity and specificity forest graphs for ICAM-1 in diagnosis of endometriosis. ICAM-1 = intercellular adhesion molecule-1.
Figure 3.
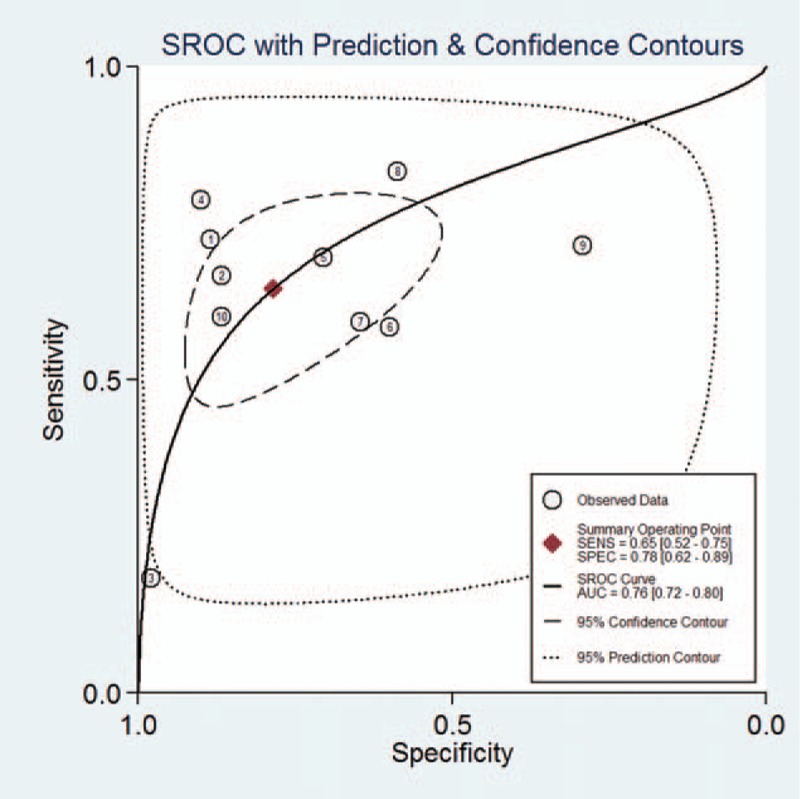
SROC curve graph for ICAM-1 in diagnosis of endometriosis. ICAM-1 = intercellular adhesion molecule-1.
Because there was a significant heterogeneity in the summary sensitivity and specificity, therefore we performed a sensitivity analysis by excluding studies in turn. The results showed that the heterogeneity of summary sensitivity and specificity was decreased, and the summary sensitivity and specificity did not change greatly compared with the overall results, suggesting robustness of the overall results.
3.4. Subgroup analysis
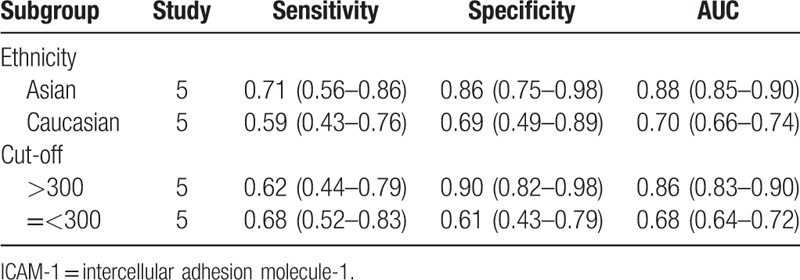
To further explore the source of significant heterogeneity among the summary sensitivity and specificity, we performed a subgroup analysis by dividing the studies according to different ethnicities and ICAM-1 cut-off levels. We found that the summary sensitivity and specificity in patients of Asian ethnicity was increased compared with patients of Caucasian ethnicity. We then divided the studies into high (>300) and low (=<300) ICAM-1 cut-off levels, and the found that the summary specificity was enhanced in studies with high cut-off levels for ICAM-1 compared to those with low cut-off levels, but the summary sensitivity showed no obvious difference between studies using high and low cut-off levels for ICAM-1. This suggests that ethnicity was a factor affecting the diagnostic accuracy of ICAM-1 (Table 2).
Table 2.
Subgroup analysis of the diagnostic accuracy of ICAM-1 in endometriosis.
3.5. Publication bias
Publication bias was assessed using Deek's test, which indicated that there was no significant publication bias across the included studies (P = .887) (Fig. 4)
Figure 4.
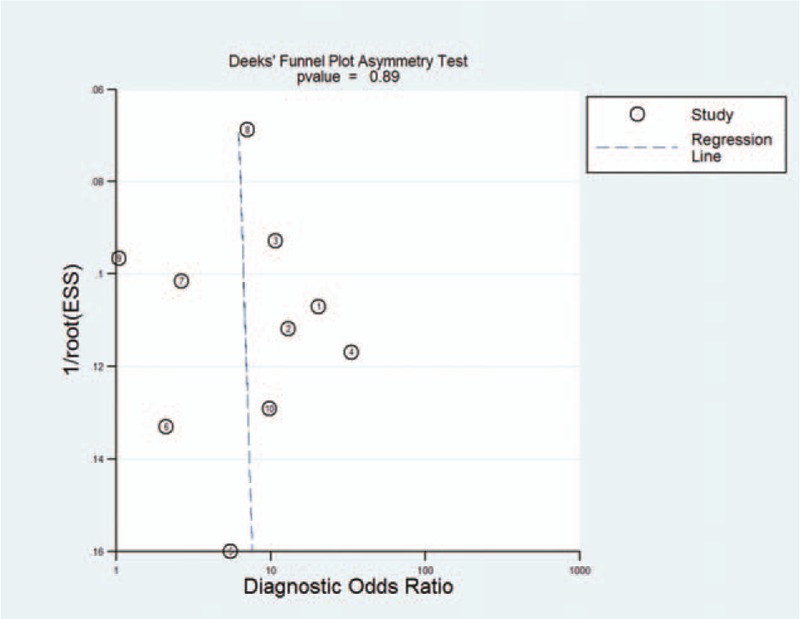
Publication bias plotted graph of included studies by Deek's test.
4. Discussion
The present study investigated the diagnostic accuracy of serum ICAM-1 for endometriosis using the meta-analysis method. We found that serum ICAM-1 has moderate diagnostic accuracy for endometriosis. Compared to previous individual studies, our meta-analysis combined the data of 9 studies with a larger sample size, thus providing greater power to provide reliable estimates. In addition, this study revealed the impact of ethnicity on the diagnostic accuracy of ICAM-1 for endometriosis, which has not been previously reported. Furthermore, the lack of significant publication bias suggests the robustness of our analysis. Concerning the rapid increase in studies on the diagnosis of endometriosis and the inconsistency of previous studies, our findings are particularly meaningful to clinicians.
To date, it is not possible to make a definitive diagnosis of endometriosis based on symptoms, clinical examination, sonography, imaging techniques, or blood tests. In addition, laparoscopic inspection with histological confirmation is an invasive approach that results in delayed diagnosis and increased risk of complications.[3] Therefore, identification of a noninvasive, rapid approach for the diagnosis of endometriosis with high specificity and sensitivity is crucial to improve treatment of patients with endometriosis. Since ICAM-1 is increased in many diseases, including endometriosis,[13] several studies have assessed the diagnostic accuracy of serum or peritoneal fluid ICAM-1 for the diagnosis of endometriosis. However, the diagnostic accuracy of serum ICAM-1 for endometriosis in these studies was markedly variable, with sensitivity ranging from 19% to 83% and specificity ranging from 30% to 97%.[11,13,14,21] These inconsistent results are unreliable and required further analysis.
In the present study, we combined the data of serum ICAM-1 levels from published articles, which included 547 patients with endometriosis and 331 controls, and found the ICAM-1 has moderate diagnostic accuracy for endometriosis. Because significant heterogeneity was identified among the summary sensitivity and specificity, we conducted sensitivity analysis and subgroup analysis to identify the source of heterogeneity. In the sensitivity analysis, after removing each study in turn, heterogeneity was reduced, but the summary sensitivity and specificity did not change significantly. Then, in the subgroup analysis, we found that the summary sensitivity and specificity in patients of Asian ethnicity was increased compared to patients of Caucasian ethnicity, indicating that differences in ethnicity may lead to differences in diagnostic accuracy. The subgroup analysis showed that a high cut-off value of ICAM-1 has high specificity, but the sensitivity was not significantly affected by cut-off value. Taken together, these results indicate that ICAM-1 has moderate diagnostic accuracy for endometriosis, but the diagnostic accuracy was much higher in patients of Asian ethnicity compared with those of Caucasian ethnicity, and the diagnostic specificity increased with an increased ICAM-1 cut-off value.
Although this study has several advantages compared with previous investigations, several limitations should be noted. First, the significant heterogeneity across studies might undermine the credibility of the results and conclusions, although we found that ethnicity was the source of heterogeneity. Second, some factors are considered to be associated with the pathogenesis of endometriosis, but due to limitations in the available data, we did not analyze some factors that might affect diagnostic accuracy of ICAM-1. Third, all the included studies were observational in design, and the possibility of selection bias should be acknowledged. Fourth, although we included a large number of patients compared with those in individual studies, the sample size remained relatively small. Therefore, our results should be interpreted and extrapolated with caution.
In conclusion, our meta-analysis demonstrates that serum ICAM-1 has moderate diagnostic accuracy for endometriosis, while the diagnostic sensitivity was much higher in patients of Asian ethnicity compared with those of Caucasian ethnicity, and the diagnostic specificity was increased with a higher ICAM-1 cut-off value. However, a large sample size study that evaluates for potential confounders is needed to further validate its diagnostic accuracy.
Author contributions
Conceptualization: Rong Li, Ying Qiu.
Formal analysis: Rong Li.
Funding acquisition: Rong Li.
Methodology: Rong Li.
Validation: Ying Qiu.
Writing – original draft: Rong Li.
Writing – review & editing: Rong Li, Ying Qiu.
Footnotes
Abbreviations: ICAM-1 = intercellular adhesion molecule-1, PLR = positive likelihood ratio, NLR = negative likelihood ratio, DOR = diagnostic odds ratio, SROC = summary receiver operating characteristic curve, AUC = area under curve, QUADAS = Quality Assessment of Diagnostic Accuracy Studies.
Funding: This study was supported by Scientific Project of Guangxi Health and Family Planning Commission (No.Z20170107).
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
The authors have no conflicts of interest to disclose.
References
- [1].Giudice LC. Clinical practice. Endometriosis. N Engl J Med 2010;362:2389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Falcone T, Lebovic DI. Clinical management of endometriosis. Obstet Gynecol 2011;118:691–705. [DOI] [PubMed] [Google Scholar]
- [3].Bouaziz J, Dotan Z, Zajicek M, et al. Laparoscopic findings associated with bladder endometriosis are correlated with disease severity. J Laparoendosc Adv Surg Tech A 2017;27:1245–50. [DOI] [PubMed] [Google Scholar]
- [4].Nisenblat V, Prentice L, Bossuyt PM, et al. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev 2016;7:CD012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bazot M, Darai E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril 2017;108:886–94. [DOI] [PubMed] [Google Scholar]
- [6].Burney RO. Biomarker development in endometriosis. Scand J Clin Lab Invest Suppl 2014;244:75–81. discussion 80. [DOI] [PubMed] [Google Scholar]
- [7].Gupta D, Hull ML, Fraser I, et al. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016;4:CD012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jung WC, Jang YJ, Kim JH, et al. Expression of intercellular adhesion molecule-1 and e-selectin in gastric cancer and their clinical significance. J Gastric Cancer 2012;12:140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bedaiwy MA, Falcone T, Sharma RK, et al. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod 2002;17:426–31. [DOI] [PubMed] [Google Scholar]
- [10].Kim KH, Lee EN, Park JK, et al. Curcumin attenuates TNF-alpha-induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and proinflammatory cytokines in human endometriotic stromal cells. Phytother Res 2012;26:1037–47. [DOI] [PubMed] [Google Scholar]
- [11].Mosbah A, Nabiel Y, Khashaba E. Interleukin-6, intracellular adhesion molecule-1, and glycodelin A levels in serum and peritoneal fluid as biomarkers for endometriosis. Int J Gynaecol Obstet 2016;134:247–51. [DOI] [PubMed] [Google Scholar]
- [12].Somigliana E, Vigano P, Candiani M, et al. Use of serum-soluble intercellular adhesion molecule-1 as a new marker of endometriosis. Fertil Steril 2002;77:1028–31. [DOI] [PubMed] [Google Scholar]
- [13].Vodolazkaia A, El-Aalamat Y, Popovic D, et al. Evaluation of a panel of 28 biomarkers for the non-invasive diagnosis of endometriosis. Hum Reprod 2012;27:2698–711. [DOI] [PubMed] [Google Scholar]
- [14].Wang Z. Soluble intracellular adhesion molecule in endometriosis. Chin J Mod Med 2003;13:53–5. [Google Scholar]
- [15].Sagoo GS, Little J, Higgins JP. Systematic reviews of genetic association studies. Human Genome Epidemiology Network. PLoS Med 2009;6:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Deville WL, Buntinx F, Bouter LM, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2002;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993;12:1293–316. [DOI] [PubMed] [Google Scholar]
- [19].Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol Sep 2005;58:882–93. [DOI] [PubMed] [Google Scholar]
- [20].Tao SS. The application of ultrasonograph and serum sICAM -1 in diagnosis of ovarian endometriomas. Chin Pri Health Care 2009;23:86–7. [Google Scholar]
- [21].Zhang R. Soluble intercellular adhesion molecule 1 in serum and peritoneal fluid on patients with endometriosis. Med J Wuhan Univ 2006;27:97–9. [Google Scholar]
- [22].Xing KT. Serum sICAM -1 combine ultrasonograph in the diagnosis of endometriomas. J Pract Med 2009;25:214–5. [Google Scholar]
- [23].Yan GY, Zheng MB, Zhang HW, et al. Expression of soluble intracellular adhesion molecule in peritoneal fluid on patients of sterility with endometriosis. Chin J Reprode Health 2004;15:229–32. [Google Scholar]
- [24].Sun YH, GY H. Measurements of soluble intercellular adhesion molecule-1 and soluble interleukin-2 receptor and their significance in 23 patients with endometriosis. Chin J Pract Gynecol Obstet 2002;18:179–80. [Google Scholar]
- [25].Kuessel L, Wenzl R, Proestling K, et al. Soluble VCAM-1/soluble ICAM-1 ratio is a promising biomarker for diagnosing endometriosis. Hum Reprod 2017;32:770–9. [DOI] [PubMed] [Google Scholar]


