Abstract
Anaphylaxis is a systemic and hypersensitive allergic reaction caused by various triggers such as environmental, food, drug, and insects. The aim of this study was to identify the prevalence, triggers, and clinical severity of anaphylaxis in 2 emergency departments (EDs) in Saudi Arabia.
A cross-sectional study based on a screening of medical records was conducted between January 2015 and August 2017, to identify confirmed cases of anaphylaxis. Patient characteristics were age, sex, previously known allergies, and the triggering allergens. The clinical severity was measured on the basis of the anaphylaxis international assessment tool (mild, moderate, severe). Factors associated with triggers and severities were identified.
The period prevalence of anaphylaxis among ED admissions was 0.00026%. Pediatric cases (age 1–16 years) were 98 (60.9%), while adults (age 17–40 years) were 63 (39.1%). Triggers of anaphylaxis were food 63 (39.1%), insects 62 (38.5%), drugs 28 (17.4%), and environmental 8 (5.0%). Mild symptoms were observed in 41 (25%) of the sample, while moderate and severe symptoms were observed in 116 (72%) and 4 (3%) of the cases, respectively. Adults were 1.25 times more likely to endure drug allergy rather than food allergy, than pediatrics with adj.P = .015. ED admissions in summer season were 1.29 less likely to be due to drug allergy rather than insect allergy, compared with admissions in winter season, adj.P = .01. Cases with known allergy were 1.72 times less likely to endure drug allergy rather than food allergy, compared with those with unknown allergy, adj.P = .001. Adults were 4.79 more likely to endure severe symptoms than pediatrics with adj.P = .001.
Although the prevalence of anaphylaxis was higher in pediatrics, yet the disease was more severe in adults. Special consideration should be paid to anaphylaxis triggered by insect bites in summer, and food allergy among cases with unknown allergy upon ED admission.
Keywords: adults, anaphylaxis, drug, food, insects., pediatrics, severity
1. Introduction
Anaphylaxis is a systemic and hypersensitive allergic reaction in which histamine and other vasoactive mediators are released in the body. According to the guidelines by World Allergy Organization for the assessment and management of anaphylaxis, clinical features can vary between patients. Further, the reactions have been classified into 4 grades, ranging from mild (only skin reactions), to moderate (respiratory, cardiovascular, or gastrointestinal involvement), to severe (hypoxia, hypotension, or neurologic compromise reactions), or even death.[1] The reaction pattern might be uniphasic (resolves by treatment), biphasic (resolves by treatment, then reoccurs), or protracted (not responsive to treatments).[2] The diagnostic criterion of anaphylaxis is primarily based on the observed clinical signs and symptoms.[3] Laboratory tests may be requested to confirm anaphylaxis, as Tryptase enzyme level exceeds 11.4 μg/L at a 2-hour peak after the event.[4] In rare cases when Tryptase levels remain high, anaphylaxis may progress to cutaneous or even systematic mastocytosis.[5] In a study conducted in a large city in Saudi Arabia, there were 238 reported cases of anaphylaxis in a 2-year period.[6]
Food allergies are more common among children and adolescents, whereas insect and drug-induced allergies are more prevalent in adults.[7] A Turkish study reported that the most common cause of anaphylaxis in children was food (40.1%), and that there was a significant male predominance among children younger than 10 years of age.[8] Similarly, a study in Saudi Arabia found that 46% of the patients in the examined cases of anaphylaxis related to food allergies were under the age of 18 years.[6] In addition to the type of trigger, patients’ pre-existing conditions, such as asthma, make them susceptible to a higher risk of anaphylaxis. One American study reported that the number of asthmatic patients with anaphylaxis admitted to emergency departments (EDs) over a period of 10 years was 5.2 times higher than that for nonasthmatics.[9]
Although a reliable clinical prediction model for anaphylaxis is a priority, one can only be developed by researching and determining the factors associated with this emergency disorder. First, identifying high-risk groups aids in spreading awareness among the community as a primary health care initiative. Second, the risk relationship between various types of triggers (environmental, drug, food, and insects) has not been fully addressed in the extant prediction model. In addition, the prevalence and pattern of anaphylaxis might differ between geographical regions, climates, environments, and even races, as each human population derives its immune response profile from its environment.[10] Thus, it is also important to research emergent cases of anaphylaxis in countries with 2 seasons (summer and winter) and a dry desert climate, where sandstorms are common. The Saudi population has high rates of consanguineous marriages,[11] and the literature has linked anaphylaxis to a genetic influence to a certain degree.[12] At present, limited studies have been conducted on populations with such distinctive features. Therefore, the purpose of this study was to investigate the prevalence of anaphylaxis, to identify the factors that trigger it, and determine its clinical severity at 2 EDs at 1 of the largest tertiary care facilities in the Middle East.
2. Materials and method
2.1. Study design
This cross-sectional study was conducted between January 2015 and August 2017 through a review of medical records of patients who were admitted to the EDs of King Abdulaziz Medical city and King Abdullah Specialist Children Hospital. The EDs at these 2 settings receive 60,000 to 80,000 annual admissions of varying illnesses and severity, and are well staffed with qualified teams of physicians, specialists, and consultants. These settings are located in the Central region of Saudi Arabia (bed capacity >1200) and mainly provide health care services to the community of Saudi National Guards, their dependents, and hospital employees. As a governmental institution, emergency services are also provided free of charge to the public community, so they also receive cases from all regions in Saudi Arabia. The climate in the central region can be described as a harsh dry desert climate (111 mL rain water per year), frequent sandstorms, and 2 seasons with extreme temperatures between 1oC in winter and 49oC in summer.
2.2. Data collection
A group of 5 well-trained data collectors, mainly medical students, were accountable for screening the medical records and obtaining the data of interest. Additional 2 persons were assigned to validate the collected data and fulfill any missing items. Eligibility criteria for subject selection were being of any age group, admitted to the ED within the study period, and with a definite diagnosis of anaphylaxis. Anaphylaxis was confirmed by emergency medical consultants based on the involvement of 2 or more systems, together with respiratory or cardiovascular symptoms, and elevated Tryptase enzyme levels (above 11.4 μg/L). Cases with fever/viral exanthema and drug eruption were excluded. Cases where the type of trigger is not specified or those with more than 1 type of trigger were also excluded. Other forms of allergies such as allergic rhinitis, sinusitis, asthma, and conjunctivitis were also not included in this study.
Medical records contained the detailed medical history as reported by the patients and/or their companions. These included patient characteristics such as age (pediatrics <17 years and adults ≥17 years),[13] sex, previous history of asthma or allergy, season of event that is summer (April to September) versus winter (October to March). Anaphylaxis event characteristics assessed were type of trigger (insect bites, environmental, food, and drugs) and route of exposure (oral, inhalations, parenteral, skin). Outcomes were period prevalence, clinical severity based on the anaphylaxis international assessment tool; mild (only skin reactions), moderate (respiratory, cardiovascular, or gastrointestinal involvement), severe (hypoxia, hypotension, or neurologic compromise reactions), or unfortunate death.[1] Other outcomes included pattern of event (uniphasic vs biphasic), referral rate to specialist, medications given, and in-hospital admission.
2.3. Data analysis and management
Data were entered and analyzed by using the SPSS statistical software (IBM, SPSS Inc, NY). The period prevalence of anaphylaxis was calculated by dividing the number of events over the number of ED admissions within the study period ∗100. Descriptive statistics such as mean, standard deviation, as well as frequency and percentage, were used to describe various continuous and categorical variables, respectively. Identification of any age group differences was made by testing various exposures using Pearson Chi-square. Multinomial logistic regression was performed to model the relationship between the predictors (gender, age group, season, asthmatic status, allergy known prior ED admission) and 4 levels of outcomes (types of triggers). The reference group was the drug trigger. Moreover, a cumulative odds ordinal regression was constructed to identify which of the variables (gender, age, asthmatic status, and type of trigger) were predictors of 3 ordinal levels of severity (mild, moderate, severe). These models passed the required assumptions and contained the intercepts that significantly improved the fit between the model and data and they also both passed the goodness of fit. P value was statistically significant at <.05.
2.4. Ethical consideration
Ethical approval of this study was obtained from the Institutional Review Board (RSS 17/022) at the Saudi Ministry of National Guards Health Affairs. No patient informed consent was obtained, as it was a secondary analysis from medical records.
3. Results
3.1. Anaphylaxis event characteristics
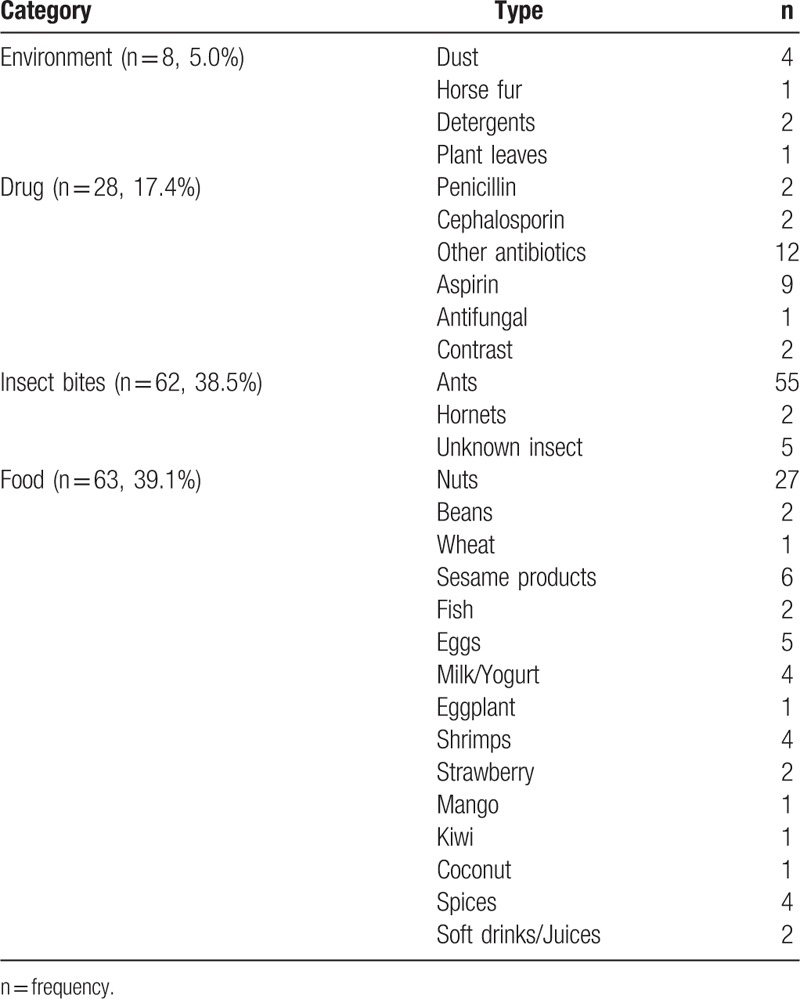
Between January 2015 and August 2017, 161 cases were identified suffering of anaphylaxis out of 617,401 ED admissions, with a prevalence of 0.00026%. Excluded cases are presented in Fig. 1. No cases of cutaneous or systematic mastocytosis were identified. There were 98 (60.9%) pediatrics with a mean of age ± standard deviation 6.6 ± 4.4 years, while adults were 63 (39.1%) with mean of age ± standard deviation 28.4 ± 6.4 years. Prevalence of anaphylaxis in pediatrics was 0.0005%, significantly higher Risk ratio = 3.16 (95% CI 2.30–4.33) than the prevalence in adults, which was 0.0002%, P < .001. Among the 161 cases, almost equal gender distribution was observed, in which males constituted 53.7% versus 46.3% for females. Season variations were observed between winter season 58 (36%) and summer season 103 (64%). Those with a previous history of asthma were 29 (18%), while those with a previous history of allergy were 68 (42.2%). Triggers of anaphylaxis included environmental triggers 8 (5.0%), insect bites 62 (38.5%), food 63 (39.1%), and drug 28 (17.4%). Table 1 summarizes all types of triggers identified in the study samples. Routes of exposure were oral 85 (52.8%), inhalation 4 (2.5%), parenteral 6 (3.7%), bites 62 (38.5%), and skin contact 4 (2.5%).
Figure 1.
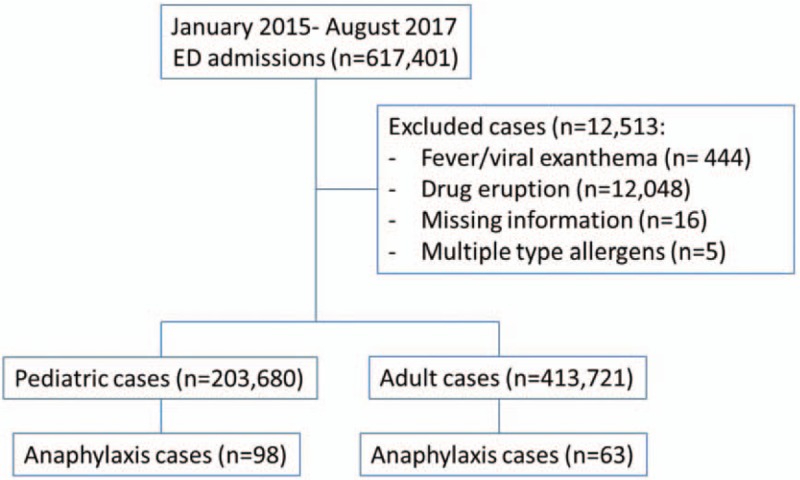
Eligible case enrolled in study.
Table 1.
Triggers of anaphylaxis.
3.2. Outcome characteristics
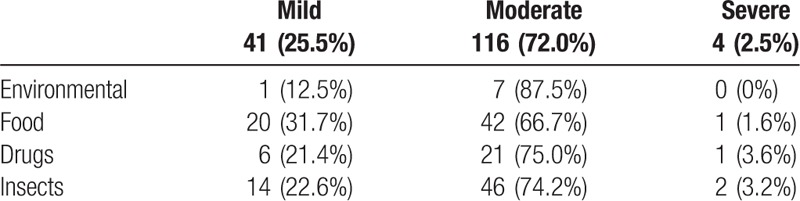
Almost 41 (25%) of the cases were classified as level 1 severity (mild), 116 (72%) were level 2 (moderate), and 4 (3%) were level 3 (severe). No death cases were reported. Table 2 describes the severity level of anaphylaxis associated with various triggers. Majority of cases, that is, 151 (93.8%) were uniphasic, while 10 (6.2%) were biphasic and protracted. In-hospital admission rate was 53 (32.9%) and the rate of referral to specialist was 60 (37.3%). The rates of epinephrine administered in the EDs were 143 (88.8%), steroids 149 (92.5%), and antihistamine 156 (96.9%).
Table 2.
Severity levels in association with various anaphylaxis triggers.
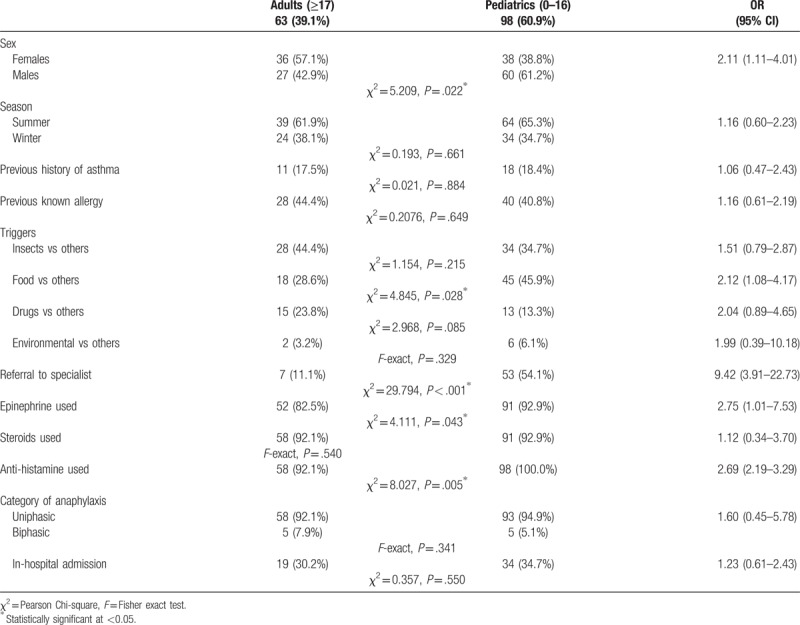
Age group differences were tested in a series of bivariate analysis as indicated in Table 3. It showed that within the pediatric group, males were significantly at a higher risk for anaphylaxis (61.2%) than females (38.8%), while within the adult group, females (57.1%) were at a higher risk than males (42.9%), P = .022. Pediatrics with anaphylaxes were significantly more likely to be referred to a specialist, that is, 53 (54.1%) compared with adults 7 (11.1%), P < .001. Pediatrics were also significantly more likely to receive epinephrine 91 (92.9%), compared with adults 52 (82.5%), P = .043. Pediatrics were also significantly more likely to receive antihistamine 98 (100%), compared with adults 58 (92.1%), P = .005.
Table 3.
Age group differences in relation to anaphylaxis event characteristics and its outcomes.
3.3. Predictors of anaphylaxis triggers and clinical severity
Although the prevalence of anaphylaxis was higher among pediatrics, more clinical severity was observed in adults as shown in Fig. 2. Multinomial logistic regression showed that adults were 1.25 times more likely to suffer from drug allergy rather than food allergy, than pediatrics, adj.P = .015. ED admissions in summer season are 1.29 less likely to be due to drug allergy rather than insect allergy, compared with winter season admissions, adj.P = .01. Cases with known allergy were 1.72 times less likely to endure drug allergy rather than food allergy, compared with those with unknown allergy, adj. P = .001 (Table 4). Ordinal logistic regression showed that adults were 4.79 more likely to endure severe symptoms than pediatrics, adj.P = .001, (Table 5).
Figure 2.
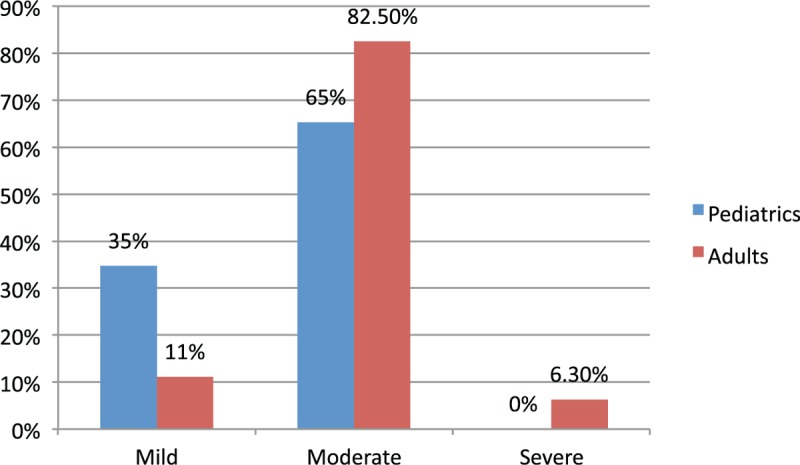
Severity of clinical presentation of anaphylaxis.
Table 4.
Multinomial logistic regression showing factors associated with type 1 trigger compared with other triggers.
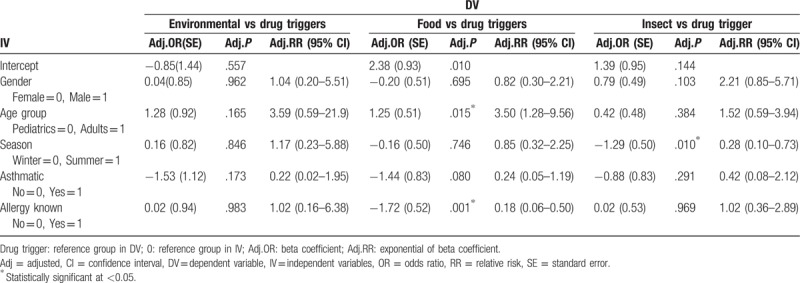
Table 5.
Cumulative odds ordinal logistic regression model to predict the risk of clinical severity adjusting for event characteristics.
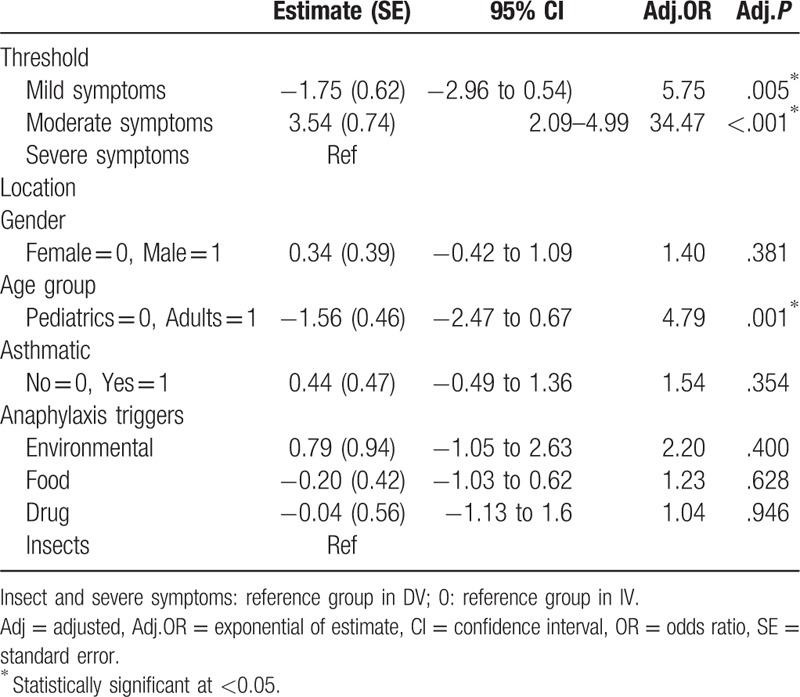
4. Discussion
As anaphylaxis remains a great public health concern, investigating the factors associated with it is of great significance. The prevalence of anaphylaxis in pediatrics was significantly higher than that in adults, which contradicts a recent report by the World Allergy Organization, which stated that anaphylaxis is higher in adults than in pediatrics.[13,14] However, the prevalence in Australia was equal in both adults and pediatrics, probably due to the distinct desert environment of Saudi Arabia. In countries with humid or tropical climates, different types of insects are prevalent and plant pollens travel around, both of which may contribute to anaphylaxis.[15] This was reflected in the findings of the current study, in which the environmental triggers were found to be the most common in adults, while food was the most common trigger in pediatrics. It is also congruent with the results reported in the literature.[13]
One of the environmental triggers in the setting was insects, which were apparently influenced by climate characteristics. Most of the anaphylaxis events occurred in the summer (64%), during which insects tend to be more active, foraging for food and seeking cooler temperature indoors.[16] Systemic allergic reactions to insect stings affect up to 5% of the population during their lifetime, with only 2% who might develop cardiorespiratory arrests.[17,18] These rates vary between countries, as 40 deaths are reported annually in the US, while 16 to 18 in France.[19]
The risk of developing anaphylaxis will never be zero, and the severity of its signs/symptoms will vary depending on various confounding factors.[20] Interestingly, in this study, anaphylaxis was found to be more prevalent in pediatrics, though the results illustrated that adults endured more severe clinical presentations than pediatrics (adj. OR = 4.79). This finding was comparable to that reported by a clinical review study that stated that the odds of severe reactions are 9-fold for adults compared with children.[21] Cardiac and lung diseases, such as asthma, have also been known to increase the clinical severity of anaphylaxis, as 75% of asthmatic patients were reported to present with severe or fatal anaphylaxis.[22] Although it was not statistically significant in this study, the findings indicated that an asthmatic is 1.54 times more likely to experience severe symptoms than a nonasthmatic patient.
Only 1.6% of the cases of food allergies in this study experienced severe symptoms, while 66.7% experienced moderate symptoms and 31.7% experienced mild symptoms. These figures were higher than those reported in the literature, which stated that up to 20% of cases of food anaphylaxis may present without even cutaneous symptoms.[23] Normally, the leading causative food allergens are seafood and shellfish (34.8%),[24] while in this study setting, they were mainly beans, nuts, and seeds (31.7%) in comparison to seafood (3.7%). This can be explained by the study's geographical location,[25] which was inland, so the population might not be as likely to consume fish as those living in coastal cities.
Adults were 1.25 times more likely to suffer from drug allergies rather than food allergies than pediatrics. However, 1 study stated that among children and adolescents who suffered from anaphylaxis, the trigger was predominantly (75%) drug hypersensitivity.[26] Food allergies in adults are often under reported, as people usually self-adjust their diet on the basis of experiencing unpleasant signs/symptoms after a meal. Sensitivity to food allergens might emerge among infants and toddlers, who have just started experimenting with various foods for the first time in their lives, with a worldwide prevalence of 1% to 10%.[27] This explains why food allergies in this study were more prevalent among pediatrics than adults. High-risk individuals will probably become hypervigilant about avoiding food allergens throughout their childhood, which lessens the rate of food allergies in adulthood. Accidental exposure to food allergens is pretty common in children, mainly due to improper food labeling and a lack of adult supervision.
Generally, anaphylaxis presents with classic, yet progressive, signs and symptoms that might escalate to the feeling of dying or even actual death. This immunological response is neither pathogenic nor specific to age, gender, or race, and its clinical severity varies. One study claimed that the clinical severity of drug-induced anaphylaxis of the majority of patients could be classified as mild (22%), moderate (45%), or severe (33%).[26] In this study, the clinical severity associated with drug allergies was less severe than that reported in the literature. In 1 systemic review paper, drug allergy rates were higher among adults mainly for antibiotics, nonsteroidal ant-inflammatory drugs, and anesthetics.[28] Their results support the current study findings in which adults were 1.25 times more likely to suffer from drug allergies rather than food allergies. It is noteworthy that the rate of biphasic/protracted anaphylaxis in this setting was 6.2%, which was comparable to the findings in the literature that ranged from 10% to 23%.[29,30]
4.1. Limitations
Although this study provided significant findings, the small sample size may have limited its statistical power. Accordingly, the most suitable statistical tests were utilized to identify factors associated with triggers and severity of allergic reactions. Pooling of data about anaphylaxis from multicenters in national data bases will reinforce the generalizability of such findings. The low prevalence in this setting could be an indicator that not all allergic cases in the targeted population had received medical treatments in the addressed EDs, but rather resolved either spontaneously or by using over-the-counter drugs at home or sought help at the primary health care clinics. Findings in this study can be generalized to populations residing in similar climates such that in the neighboring Gulf countries, African countries, some USA states such as Arizona, cities proximal to the Australian outback, and so on. Furthermore, Saudi Arabia has been known to be a common work destination for expatriates from all over the world, driven by the higher paid salaries and low taxes. Findings in this paper might be of interest for those planning to move and reside in Saudi Arabia, with or without their families. One of the potentially associated factors with anaphylaxis is having a family history of allergies, yet it was not addressed in this study due to the fact that the initial data were obtained and recorded for clinical, rather than research purposes. Another limitation is the fact that most of the published studies focused on anaphylaxis among specific populations or that was associated with certain triggers, with very little attention having been paid to generating a universal language or coding to diagnose anaphylaxis.
5. Conclusion
The prevalence of anaphylaxis in 2 Middle Eastern EDs showed that although the prevalence of anaphylaxis reaction was higher in pediatrics, the reactions were more severe in adults. Anaphylaxis in pediatrics was more common in males yet in adults the prevalence was higher in females. Food was the most common trigger of anaphylaxis in pediatrics. Findings also showed a poor referral rate of anaphylactic cases to allergy immunologists.
5.1. Recommendations
The concept of prevention, rather than the clinical prediction or management, of anaphylaxis is of great importance. Any person classified as a high-risk individual because they reside in a high-risk environment, have a family history of allergies, and/or have a known allergy should receive a sensitivity profile as part of primary or secondary prevention measures. Prompt recognition of anaphylaxis symptoms is vital, as these conditions require immediate clinical management to avoid a drastic deterioration to more life-threatening conditions. It is also crucial for health care practitioners to increase awareness and ensure proper education on the self-administration of epinephrine injections and emphasize that those experiencing a reaction should report directly to the nearest health facility. Emergency hotlines ought to be accessible in houses, schools, and public areas so that any person suspecting the early signs of anaphylaxis can receive proper instructions. It is also crucial for all health care professionals to follow the international guidelines for the treatment and management of anaphylaxis, including referring cases to allergy immunologists.
Acknowledgment
The authors would like to thank King Abdullah International Medical Research Center/King Saud bin Abdulaziz University for Health Sciences, Saudi Ministry of National Guard Health Affairs, for providing support during the conduction of this project. This project was part of the 9th research summer school. We would also like to thank Dr. Ibraheem Bushnak for his efforts in making this program a success.
Author contributions
Conceptualization: Raghad Alkanhal, Ibrahim Alhoshan, Sadal Aldakhil, Nouf Alromaih, Nesrin Alharthy, Adel F Almutairi.
Data curation: Raghad Alkanhal, Ibrahim Alhoshan, Sadal Aldakhil, Nouf Alromaih.
Formal analysis: Mahmoud Salam, Adel F Almutairi.
Investigation: Raghad Alkanhal, Ibrahim Alhoshan, Sadal Aldakhil, Nouf Alromaih, Nesrin Alharthy.
Methodology: Raghad Alkanhal, Ibrahim Alhoshan, Sadal Aldakhil, Nouf Alromaih, Nesrin Alharthy, Mahmoud Salam, Adel F Almutairi.
Project administration: Raghad Alkanhal, Ibrahim Alhoshan, Sadal Aldakhil, Nouf Alromaih, Adel F Almutairi.
Resources: Adel F Almutairi.
Supervision: Adel F Almutairi.
Validation: Mahmoud Salam, Adel F Almutairi.
Visualization: Mahmoud Salam, Adel F Almutairi.
Writing – original draft: Mahmoud Salam, Adel F Almutairi.
Writing – review & editing: Mahmoud Salam, Adel F Almutairi.
Footnotes
Abbreviation: ED = emergency departments.
The author(s) of this work have nothing to disclose.
The authors declare that there are no conflict of interests.
References
- [1].Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol 2004;114:371–6. [DOI] [PubMed] [Google Scholar]
- [2].Golden DB. Patterns of anaphylaxis: Acute and late phase features of allergic reactions. In Novartis Foundation Symposium. Vol. 257. 2004. p. 101–110. (Novartis Foundation Symposium). [PubMed] [Google Scholar]
- [3].Tejedor-Alonso MA, Moro-Moro M, Múgica-García MV. Epidemiology of anaphylaxis: contributions from the last 10 years. J Investig Allergol Clin Immunol 2015;25:163–75. [PubMed] [Google Scholar]
- [4].National Clinical Guideline Centre. Patterns of Anaphylaxis: Acute and Late Phase Features of Allergic Reactions. London, UK: National Institute for Health and Care Excellence; 2014: https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0068956/pdf/PubMedHealth_PMH0068956.pdf. Accessed June 2018. [Google Scholar]
- [5].Cohen SS, Skovbo S, Vestergaard H, et al. Epidemiology of systemic mastocytosis in Denmark. Br J Haematol 2014;166:521–8. [DOI] [PubMed] [Google Scholar]
- [6].Sheikh F, Amin R, Rehan Khaliq AM, et al. First study of pattern of anaphylaxis in a large tertiary care hospital in Saudi Arabia. Asia Pac Allergy 2015;5:216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee S-Y, Ahn K, Kim J, et al. A multicenter retrospective case study of anaphylaxis triggers by age in Korean children. Allergy Asthma Immunol Res 2016;8:535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Civelek E, Erkoçoğlu M, Akan A, et al. The etiology and clinical features of anaphylaxis in a developing country: nationwide survey. Asian Pac J Allergy Immunol 2017;35:212–9. [DOI] [PubMed] [Google Scholar]
- [9].Iribarren C, Tolstykh IV, Miller MK, et al. Asthma and the prospective risk of anaphylactic shock and other allergy diagnoses in a large integrated health care delivery system. Ann Allergy Asthma Immunol 2010;104:371–7. [DOI] [PubMed] [Google Scholar]
- [10].Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc R Soc B 2015;282:20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].El-Hazmi M, Al-Swailem A, Warsy A, et al. Consanguinity among the Saudi Arabian population. J Med Genetics 1995;32:623–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].BenShoshan M, Clarke A. Anaphylaxis: past, present and future. Allergy 2011;66:1–4. [DOI] [PubMed] [Google Scholar]
- [13].World Allergy Organization. Anaphylaxis: Global Overview: World Allergy Organization; 2017. Available at: http://www.worldallergy.org/professional/allergic_diseases_center/anaphylaxis/anaphylaxisglobal.php. Accessed October 26, 2017. [Google Scholar]
- [14].Jiang N, Yin J, Wen L, et al. Characteristics of anaphylaxis in 907 Chinese patients referred to a tertiary allergy center: a retrospective study of 1,952 episodes. Allergy Asthma Immunol Res 2016;8:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Asthma and Allergy Foundation of America. Anaphylaxis: A Severe Allergic Reaction. Landover, MD: Asthma and Allergy Foundation of America; 2007. Available at: http://www.aafa.org/page/anaphylaxis-severe-allergic-reaction.aspx. Accessed October 26, 2017. [Google Scholar]
- [16].Jayatilaka P, Narendra A, Reid SF, et al. Different effects of temperature on foraging activity schedules in sympatric Myrmecia ants. J Exp Biol 2011;214:2730–8. [DOI] [PubMed] [Google Scholar]
- [17].Ludman SW, Boyle RJ. Stinging insect allergy: current perspectives on venom immunotherapy. J Asthma Allergy 2015;8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Golden D. Stinging insect allergy. Am Fam Phys 2003;67:2541–6. [PubMed] [Google Scholar]
- [19].Jerschow E, Lin RY, Scaperotti MM, et al. Fatal anaphylaxis in the United States, 1999–2010: temporal patterns and demographic associations. J Allergy Clin Immunol 2014;134:1318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sarinho E, Lins MdGM. Severe forms of food allergy. J Pediatr (Versão em Português) 2017;93:53–9. [DOI] [PubMed] [Google Scholar]
- [21].Castells M. Diagnosis and management of anaphylaxis in precision medicine. J Allergy Clin Immunol 2017;140:321–33. [DOI] [PubMed] [Google Scholar]
- [22].Turner PJ, Jerschow E, Umasunthar T, et al. Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol 2017;5:1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mansoor DK, Sharma HP. Clinical presentations of food allergy. Pediatr Clin North Am 2011;58:315–26. [DOI] [PubMed] [Google Scholar]
- [24].Lee S-H, Ban G-Y, Jeong K, et al. A retrospective study of Korean adults with food allergy: differences in phenotypes and causes. Allergy Asthma Immunol Res 2017;9:534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Burger J, Gochfeld M, Batang Z, et al. Fish consumption behavior and rates in native and non-native people in Saudi Arabia. Environ Res 2014;133:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cavkaytar O, Karaatmaca B, Cetinkaya PG, et al. Characteristics of drug-induced anaphylaxis in children and adolescents. Allergy Asthma Proc 2017;38:56–63. [DOI] [PubMed] [Google Scholar]
- [27].Chin B, Chan ES, Goldman RD. Early exposure to food and food allergy in children. Can Fam Phys 2014;60:338–9. [PMC free article] [PubMed] [Google Scholar]
- [28].Sousa-Pinto B, Fonseca JA, Gomes ER. Frequency of self-reported drug allergy: a systematic review and meta-analysis with meta-regression. Ann Allergy Asthma Immunol 2017;119:362–73. e2. [DOI] [PubMed] [Google Scholar]
- [29].Confino-Cohen R, Goldberg A. Allergen immunotherapy–induced biphasic systemic reactions: incidence, characteristics, and outcome: a prospective study. Ann Allergy Asthma Immunol 2010;104:73–8. [DOI] [PubMed] [Google Scholar]
- [30].Scranton SE, Gonzalez EG, Waibel KH. Incidence and characteristics of biphasic reactions after allergen immunotherapy. J Allergy Clin Immunol 2009;123:493–8. [DOI] [PubMed] [Google Scholar]


