Supplemental Digital Content is available in the text
Keywords: lung cancer, NSCLC, spontaneous regression, spontaneous remission, untreated
Abstract
Rationale:
Spontaneous regression of non-small cell lung cancer is exceptionally rare.
Patient concerns:
Treatment-related toxicity.
Diagnoses:
We report a case of a patient diagnosed with locally advanced non-small cell lung cancer.
Interventions:
The patient declined potentially curative treatment, and did not receive any anti-cancer treatment.
Outcomes:
He has survived more than two years since his initial diagnosis, maintaining his good performance status. Serial imaging with computed tomography scans showed tumour regression and near-complete resolution of his disease.
Lessons:
Spontaneous regression of non-small cell lung cancer, by virtue of its scarcity, has not been well-studied and is poorly understood. Further studies are required, in order to clarify the mechanisms by which spontaneous regression occurs, and possibly identify new targets for cancer treatment.
1. Introduction
Patients with locally advanced nonsmall cell lung cancer (NSCLC) have a poor prognosis despite receiving standard treatment,[1] which may include various combinations of surgery, radiation therapy and chemotherapy.[2] Other patients that are deemed unfit for, or decline, all forms of cancer treatment typically have a prognosis measured in months.[3] Spontaneous regression of NSCLC is an exceptionally rare phenomenon, and the underlying mechanisms by which it occurs are as yet unclear. We report a case of a patient that was diagnosed with locally advanced NSCLC and declined all anticancer treatment, but whose cancer regressed spontaneously, and has remained quiescent >2 years after initial diagnosis.
2. Case report
A 77-year-old man initially presented with intermittent right upper chest pain of a month's duration. This was reported as being mild and not requiring analgesia. He had no significant past medical history and had a good performance status. He was a lifelong nonsmoker, and denied experiencing other associated symptoms like cough, dyspnoea, haemoptysis, anorexia, loss of weight, headache, or dizziness. His physical examination was normal apart from reduced breath sounds at the right upper zone.
A chest x-ray showed a right middle zone lesion, and a subsequent computed tomography (CT) scan of the thorax and liver revealed a 5.8 × 5.0 cm mass in the anterior segment of the upper lobe of the right lung, abutting the transverse fissure and infiltrating the lateral chest wall with erosion of the third rib (Fig. 1). There was also a 2.0 × 1.7 cm enlarged right hilar node. He underwent a bronchoscopy with endobronchial ultrasound and transbronchial biopsies. A tumor was seen arising from the anterior segment of the right upper lobe. Histopathological examination confirmed the diagnosis of a poorly differentiated NSCLC with marked nuclear pleomorphism (Fig. 2), with no targetable mutations found on further testing.
Figure 1.
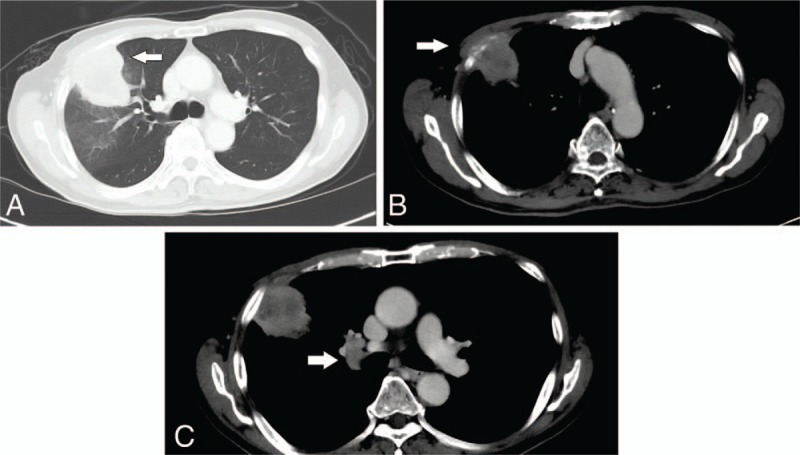
Computed tomography (CT) images at presentation. (A) Primary tumor (white arrow) in the lung window. (B) Involvement of the right third rib (white arrow). (C) Enlarged right hilar lymph node (white arrow).
Figure 2.
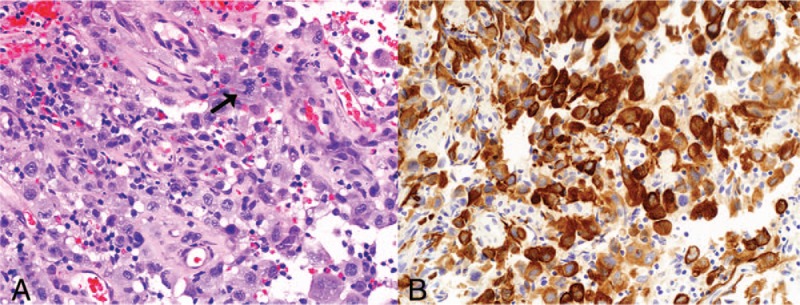
Biopsy of tumor at initial presentation. (A) Sheets of infiltrating pleomorphic tumor cells, with atypical mitoses (arrow) (hematoxylin and eosin stain, original magnification ×200). (B) The tumor cells demonstrate strong diffuse positive staining with AE1/3 immunostain (original magnification ×200).
As there was no evidence of metastatic disease on bone scan or CT brain, he was staged as having T3N1M0 (Stage IIIA) disease. Radical (curative) intent radiation therapy with concurrent chemotherapy was recommended, but the patient declined. Palliative radiation therapy was also discussed and declined by the patient. He instead opted to alter his diet, increasing his intake of fruit and vegetables, and take up exercise.
Upon review at about 3 months after his diagnosis, he reported resolution of his chest pain. A CT scan repeated then showed a significant reduction in the size of the right upper lobe mass, now measuring 3.8 × 2.7 cm but still invading the right anterior chest wall with associated erosion of the third rib (Fig. 3). The right hilar node also demonstrated reduction in size now measuring 2.2 × 1.4 cm.
Figure 3.

Computed tomography (CT) images 3 months after diagnosis, showing reduction in size. (A) Primary tumor (white arrow) in the lung window. (B) Involvement of the right third rib (white arrow). (C) Enlarged right hilar lymph node (white arrow).
Serial scans done at 6, 9, 12, and 18 months after initial diagnosis continued to show further decrease in size of the lesions (2.0 × 1.2 cm, 1.1 × 1.0 cm, 0.8 × 0.6 cm, and 0.6 × 0.5 cm respectively). The most recent CT scan of the thorax and liver, done 24 months after diagnosis, shows a stable focus of soft tissue density at the site of the primary tumor, with associated scarring of the adjacent lung parenchyma and tethering of the adjacent pleura and sclerotic changes at the right anterior third rib (Fig. 4). There are no enlarged right hilar lymph nodes, and no other lymphadenopathy or metastases.
Figure 4.
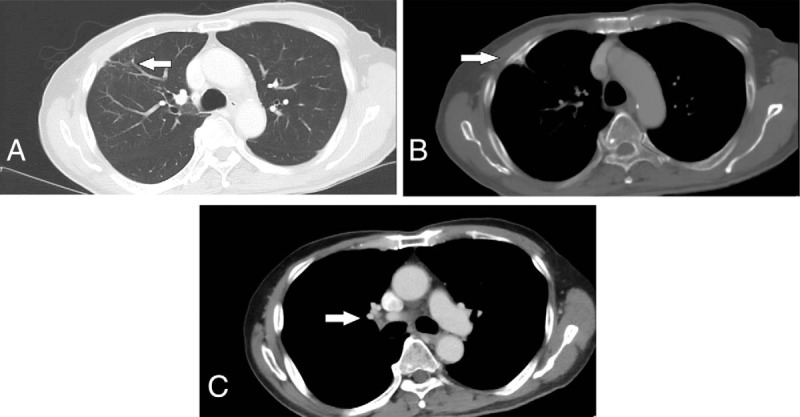
Computed tomography (CT) images 24 months after diagnosis, showing near-complete resolution. (A) Initial site of primary tumor with residual soft tissue density (white arrow). (B) Residual sclerotic changes at the right third rib (white arrow). (C) Right hilar lymph node not enlarged (white arrow).
The Case Report Timeline summarises the consultations and investigations undergone by the patient, and the interventions performed for him (Supplemental Digital Content). Written consent was obtained from the patient for this case report.
3. Discussion
Spontaneous regression (SR) of cancer has been defined as the partial or complete disappearance of a malignant tumor, diagnosed on microscopic examination of tissues, in the absence of treatment or in the presence of therapy considered inadequate to exert a significant influence on the disease.[4] With this definition, SR is an uncommon phenomenon, estimated to occur in one in 60,000 to 100,000 patients.[5] While SR has been documented to occur more frequently in certain malignancies such as renal cell carcinoma, neuroblastoma, and malignant melanoma,[6] SR of NSCLC is very rare, with only 11 reported cases in the English literature of advanced NSCLC undergoing SR from 1950 to 2004.[7]
Kumar et al[8] sought to refine this definition by excluding patients who had received any systemic therapy, defined as chemotherapy, radioablative techniques or chemoembolization. Applying this revised criteria to literature published between January 1951 and December 2008, out of the 782 articles written on “spontaneous regression of thoracic lesions,” he identified 76 cases of spontaneous regression of thoracic malignancy, of which only 2 were primary lung cancers.[9,10]
The postulated mechanisms for SR include immune modulation after infection or injury, interaction between neuropsychological and immunological systems, hormonal mechanisms and differentiation back to normal.[11] Of these, hormonal mechanisms are unlikely to be the cause in our patient, as there was no hormonal manipulation employed. Tumor differentiation back to normal is also unlikely to be a factor in the present case, being mainly reported in germ-cell or paediatric tumors.
The surgical oncologist William Coley observed a patient's tumor regress following infection with erysipelas in the 1890s, and was convinced that the infection was responsible.[12] He developed his Coley's toxins, a vaccine made from 2 killed bacteria (Streptococcus pyogenes and Serratia marcescens), in a bid to induce the same tumor regression. While he did not achieve great success with this rudimentary form of immunotherapy then, our understanding of cancer immune modulation and evasion of the immune system has advanced a great deal since. Several case reports have linked cytokine release from viral or bacterial infections to SR of some haematological malignancies,[13,14] theorising that these cytokines induce inflammatory necrosis or T-cell mediated apoptosis. Another case report has suggested a role for the Mycobacterium tuberculosis that was isolated from the patient's sputum in stimulating the immune system.[15]
Our patient did not have any evidence of intercurrent infections; hence immune modulation through infection is not likely to be the trigger for SR in our patient. Ogawa et al[16] have suggested that an invasive procedure, such as a biopsy of the tumor, may be a stimulus that serves to initiate the process of SR, and this remains a possibility in our patient.
Interaction between neuropsychological and immunological systems has been put forth as another prospective mechanism for SR. This theory suggests that positive neuropsychological changes can allow the immunological system to better identify and/or eliminate the malignant cells. Early studies are inconclusive,[17,18] but with a better understanding of the underlying immune mechanisms malignant cells use to evade the immune system, perhaps future studies may be more enlightening.
4. Conclusion
Spontaneous regression of nonsmall cell lung cancer is a recognized, albeit exceedingly rare, phenomenon. As we have yet to elucidate the mechanisms by which this occurs, additional research is required. Further studies should ideally incorporate collection of tumor tissue and patient blood samples, and utilize genetic and molecular assays to characterize these patients, their tumors, and the tumor microenvironment. In so doing, we may yet shed light on this rare phenomenon, and perhaps open the door to new treatments.
Author contributions
Conceptualization: Kiat Huat Ooi, Timothy Cheo, Cheng Nang Leong.
Writing – original draft: Kiat Huat Ooi, Timothy Cheo, Gwyneth Shook Ting Soon, Cheng Nang Leong.
Supplementary Material
Footnotes
Abbreviations: CT = computed tomography, NSCLC = nonsmall cell lung cancer, SR = spontaneous regression.
The authors have no funding or conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Santana-Davila R, Martins R. Treatment of stage IIIA non-small-cell lung cancer: a concise review for the practicing oncologist. J Oncol Pract 2016;12:601–6. [DOI] [PubMed] [Google Scholar]
- [2].Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5 suppl):e314S–40S. [DOI] [PubMed] [Google Scholar]
- [3].Wao H, Mhaskar R, Kumar A, et al. Survival of patients with non-small cell lung cancer without treatment: a systematic review and meta-analysis. Syst Rev 2013;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Everson TC, Cole WH. Spontaneous Regression of Cancer: A Study and Abstract of Reports in the World Medical Literature and of Personal Communications Concerning Spontaneous Regression of Malignant Disease. Philadelphia: Saunders; 1966. [Google Scholar]
- [5].Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol 1981;17:201–9. [DOI] [PubMed] [Google Scholar]
- [6].Challis GB, Stam HJ. The spontaneous regression of cancer. A review of cases from 1900 to 1987. Acta Oncol 1990;29:545–50. [DOI] [PubMed] [Google Scholar]
- [7].Gladwish A, Clarke K, Bezjak A. Spontaneous regression in advanced non-small cell lung cancer. BMJ Case Rep 2010;2010:bcr0720103147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kumar T, Patel N, Talwar A. Spontaneous regression of thoracic malignancies. Resp Med 2010;104:1543–50. [DOI] [PubMed] [Google Scholar]
- [9].Eckhart PW. Disappearing lung lesion. Med J Aust 1983;1:104. [PubMed] [Google Scholar]
- [10].Sperduto P, Vaezy A, Bridgman A, et al. Spontaneous regression of squamous cell lung carcinoma with adrenal metastasis. Chest 1988;94:887e9. [DOI] [PubMed] [Google Scholar]
- [11].Kappauf H, Gallmeier WM, Wünsch PH, et al. Complete spontaneous remission in a patient with metastatic non-small-cell lung cancer. Ann Oncol 1997;8:1031–9. [DOI] [PubMed] [Google Scholar]
- [12].Jessy T. Immunity over inability: the spontaneous regression of cancer. J Nat Sci Biol Med 2011;2:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Drobyski WR, Qazi R. Spontaneous regression in non-Hodgkin's lymphoma: clinical and pathogenetic considerations. Am J Hematol 1989;31:138–41. [DOI] [PubMed] [Google Scholar]
- [14].Ifrah N, James JM, Viguie F, et al. Spontaneous remission in adult acute leukemia. Cancer 1985;56:1187–90. [DOI] [PubMed] [Google Scholar]
- [15].Choi SM, Go H, Chung DH, et al. Spontaneous regression of squamous cell lung cancer. Am J Respir Crit Care Med 2013;188:e5–6. [DOI] [PubMed] [Google Scholar]
- [16].Ogawa R, Watanabe H, Yazaki K, et al. Lung cancer with spontaneous regression of primary and metastatic sites: a case report. Oncol Lett 2015;10:550–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Spiegel D, Bloom JR, Kraemer HC, et al. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet 1989;2:888–91. [DOI] [PubMed] [Google Scholar]
- [18].Goodwin PJ, Leszcz M, Ennis M, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med 2001;345:1719–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


