Abstract
Background
Cutaneous myiasis is the infestation of the skin by larvae (maggots) of the order Diptera (two winged). Being an imported and sporadic illness, furuncular myiasis often poses a diagnostic challenge to the treating physician. This traditionally endemic entity is being more frequently reported worldwide as ‘vacation’ disease in travellers returning from these regions. However, there is a paucity of large scale study, especially on individuals occupationally stationed for longer periods of time in these endemic geographic locations.
Methods
Sixteen Indian male patients with cutaneous furuncular myiasis presenting to dermatology outpatient department at a tertiary care field hospital deployed in a United Nations peacekeeping mission in Central Africa were studied for clinical presentation, sites involved, larvae/maggot extracted, period of resolution and complications if any.
Results
Average age of patients was 29 years. The average duration of infestation was 4 days. All lesions were found to be confined to sites over body normally covered with clothing, commonest being anterior abdomen in 9 (56.25%) patients followed by chest in 6 (37.5%) patients. The lesion count was also highest on anterior abdomen with 39 lesions. The average time to resolution following extraction of larvae (Cordylobia anthropophaga) was 6 days.
Conclusion
The purpose of this study was to familiarize oneself with an endemic infestation which often masquerades itself as pyoderma to the naïve physician, more so in an imported case or more importantly, an ‘exported’ health care professional.
Keywords: Cordylobia anthropophaga, Diptera, Furuncular myiasis, Larvae
Introduction
Cutaneous myiasis is the infestation of the skin by larvae (maggots) of the Arthropod order of Diptera. Based on clinical presentation, cutaneous myiasis can either be furuncular, wound or migratory. Furuncular myiasis is commonly caused by Dermatobia hominis (Bot fly) and Cordylobia anthropophaga (Tumbu fly) and as the name suggests, are characterized by boil like lesions.1, 2 Cutaneous myiasis though endemic, assumes unique importance, in that it is the fourth most common travel associated skin disease among travellers to these regions.3
Materials and methods
Sixteen Indian male and otherwise healthy patients (aged between 26 and 35 years) with cutaneous furuncular myiasis presenting to dermatology outpatient department at a tertiary care field hospital deployed in a United Nations peacekeeping mission in Central Africa over a period of 10 months between July 2015 and April 2016 were examined and treated. The patients were studied for duration of these lesions, sites involved, larvae (maggots) extracted, period of resolution and complications if any. History of red raised lesions or boils with movement within and/or serous exudate was elicited from all patients, as also that of spontaneous or expressed larvae from these lesions. All patient were disrobed completely and examined in natural light for skin coloured or erythematous papules, nodules or furuncular lesions with or without central open puncta and serous discharge. Lesions infested with live larvae were only considered for lesion count. Patients completely or partially treated at referral clinics were excluded from the study. All patients received similar management which included single session or serial extraction of all larvae (maggots) under local anaesthesia by making a cruciate incision over open puncta or sites of serous exudate. All larvae extracted were subjected to parasitological examination by pathologist. Skin biopsy of representative lesion was taken from two patients for histopathological examination. All patient received follow-up treatment with Oral Ampicillin 500 mg and clavulanic acid 125 mg three times a day, oral Paracetamol 500 mg twice a day for pain relief for 7 days. Daily wound dressing was done for all treated lesions till healing and thereafter daily topical application of 2% Mupirocin ointment for another 7 days. Patients were reviewed again at day seven and 1 month later.
Results
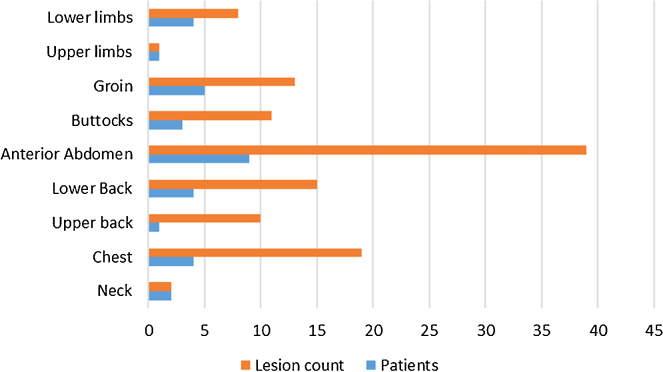
Sixteen male patients aged between 26 and 35 years (mean age 29.12 years) presenting with complaints suggestive of cutaneous furuncular myiasis were examined for characteristic lesions and managed with extraction of larvae/maggots from these lesions. The clinical patient profile including age, sites involved, lesion count, onset/duration, resolution post-treatment and complications if any are shown comprehensively in Table 1. The longest duration of infestation noticed by the patients was 7 days and shortest 2 days (mean 3.81 days). All lesions were found to be confined to sites over body normally covered with clothing, i.e. neck, chest, anterior abdomen, back, buttocks and proximal upper and lower limbs. Chart 1 displays the distribution of lesions in patients as well as their frequency as per site involved. Most common site involved was anterior abdomen in 9 (56.25%) patients followed by chest in 6 (37.5%) patients (Fig. 1). The lesion count was also highest on anterior abdomen with 39 lesions, followed by that on the chest with 19 lesions. No mucosal sites including eyes, nose, ears, oral cavity or genitalia were involved. The average time to resolution following extraction was 5.75 days. There was a solitary case with cellulitis over anterior abdomen as complication. Skin biopsy of the two index cases revealed the larvae of C. anthropophaga in the dermis surrounded by intense inflammatory reaction composed of predominantly neutrophils as well as eosinophils and few histiocytes (Fig. 2a) and a high power view (400×) of an intact larvae revealed appendages with acellular cuticle, underlying skeletal muscle fibres and developing eggs in situ (Fig. 2b).
Table 1.
Patient profile.
| S. no. | Age (in years) | Sex | Site | Larvae extracted | Onset/duration (in days) | Clinical resolution (in days) | Complications |
|---|---|---|---|---|---|---|---|
| 1. | 32 | Male | Buttocks | 3 | 6 | 4 | Nil |
| 2. | 28 | Male | Lower back and groin | Lower back (4) and groin (2), Total – 6 |
3 | 7 | Nil |
| 3. | 27 | Male | Chest and anterior abdomen | Chest (2) and anterior abdomen (2), Total – 4 |
5 | 5 | Nil |
| 4. | 29 | Male | Anterior abdomen | 6 | 7 | 6 | Nil |
| 5. | 29 | Male | Right shoulder and arm, chest, upper back and groin | Right shoulder (1) and arm (2), chest (5), upper back (10) and groin (6), Total – 24 |
6 | 8 | Nil |
| 6 | 32 | Male | Anterior abdomen, lower back, buttocks | Anterior abdomen (3), lower back (1), buttocks (3), Total – 7 |
4 | 7 | Nil |
| 7. | 26 | Male | Right buttock and right thigh | Right buttock (1) and right thigh (2), Total – 3 |
2 | 5 | Nil |
| 8. | 30 | Male | Anterior abdomen, lower back, neck | Anterior abdomen (8), lower back (5), neck (2), Total – 15 |
4 | 10 | Cellulitis |
| 9. | 28 | Male | Anterior abdomen, left thigh | Anterior abdomen (1), left thigh (2), Total – 3 |
2 | 4 | Nil |
| 10. | 35 | Male | Buttocks and groin | Buttocks (4) and groin (2), Total – 6 |
3 | 5 | Nil |
| 11. | 29 | Male | Neck, Chest, anterior abdomen, groin | Neck (1), chest (7), anterior abdomen (6), groin (2), Total – 18 |
5 | 8 | Nil |
| 12. | 24 | Male | Anterior abdomen and lower back | Anterior abdomen (5) and lower back (5), Total – 10 |
4 | 6 | Nil |
| 13. | 30 | Male | Neck and chest | Neck (1) and chest (1), Total – 2 |
2 | 3 | Nil |
| 14. | 28 | Male | Chest, anterior abdomen and left thigh | Chest (2), anterior abdomen (4) and left thigh (2), Total – 8 |
2 | 5 | Nil |
| 15. | 32 | Male | Groin and left thigh | Groin (1) and left thigh (2), Total – 3 |
2 | 3 | Nil |
| 16. | 27 | Male | Chest and ant abdomen | Chest (2) and ant abdomen (4), Total – 6 |
4 | 6 | Nil |
Chart 1.
Distribution and frequency of lesions.
Fig. 1.
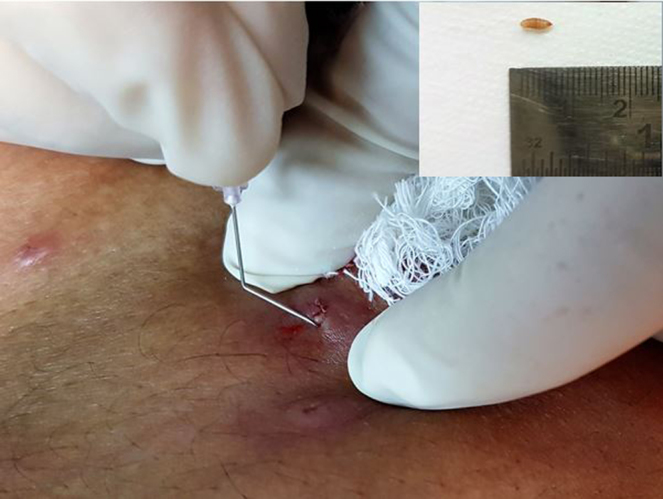
Extraction of larvae (maggot) from one representative lesion with help of a cruciate incision and bent hypodermic needle. Inset shows extracted larvae of Tumbu fly (Cordylobia anthropophaga).
Fig. 2.
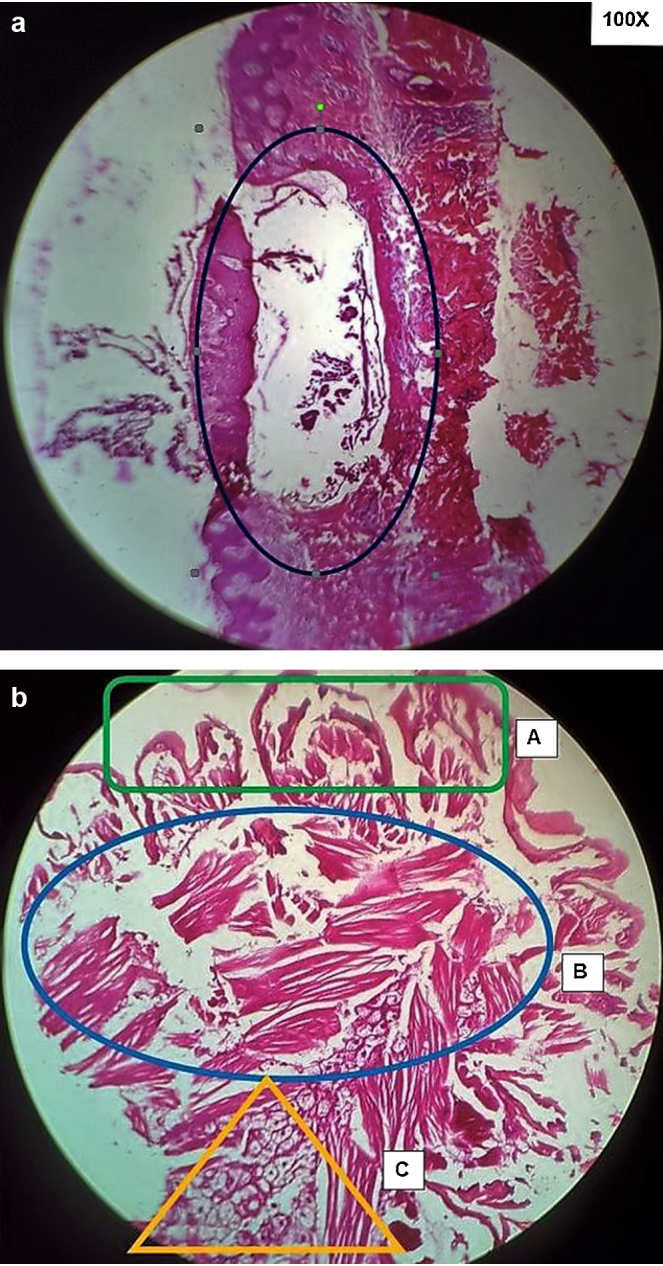
(a) H&E section (100×) showing in situ larvae (maggot) within dermis and consequent surrounding inflammation. (b) Internal structure of larvae (400×) showing (A) appendages with thick acellular cuticle and (B) underlying layer of well-developed skeletal muscle and (C) eggs in situ.
Discussion
The word myiasis is derived from the Greek word ‘Myia’ meaning a fly. The entity was originally proposed by Hope in 1840. It is defined as infestation of live human & vertebrate animals by larvae (maggots) of the order Diptera (two winged). Myiasis can be classified either on the basis of relationship between the host and the parasite or based on the area of the body infested. The second clinical classification appears more useful and includes cutaneous, ophthalmic, nasopharyngeal, auricular, oral, enteric and urogenital. Of these, cutaneous myiasis is the most common form encountered the world over. Cutaneous myiasis further include furuncular, migratory and wound myiasis based on the type of larvae infesting. Furuncular myiasis is caused by larvae of D. hominis, C. anthropophaga, Cuterebra spp., Wohlfahrtia vigil, and W. opaca.2, 4 Of these, D. hominis ‘Human Botfly’ and C. anthropophaga ‘Tumbu Fly’ are the most common worldwide causing human infestation.1
While D. hominis is native to central and South America, C. anthropophaga is confined to tropics and subtropics of Sub-saharan Africa. The adult female C. anthropophaga lays its eggs over damp soil often contaminated with urine or faeces. Upon hatching, the first instar larvae can survive up to 9 days before they are picked up when clothes are left to dry in these shady areas or unsuspecting victim rests on these shady areas. On contact with skin the larvae penetrate and lodge themselves in the dermis for 8–12 days, following which the third instar larvae fall out and pupate into the soil.2, 3
Most cases of cutaneous furuncular myiasis in literature worldwide have been reported as imported cases as these flies are endemic in certain regions of the world and often picked up by travellers visiting these regions.5, 6, 7 C. anthropophaga is also known as ‘skin maggot fly’, the ‘mango fly’, the ‘putzi fly’, ‘ver du Cayor’ (worm of Cayor) or, most commonly, the ‘tumbu fly’ which in creole language of Sierra Leone translates to “an insect, maggot or earthworm that eats wood, grain, rice, etc”. Infestation with C. anthropophaga occurs all-round the year in endemic areas.2 In the present study all patients were deployed in urban/semi-urban setting of an endemic geographic area of Central Africa. The mean age of patients in the present study was 29 years. However, literature suggests a wide range including infants.8 There is also no sex predilection, though all patients in present study were incidentally males as combatants deployed in a United Nations peacekeeping operation. The average duration of lesions in this study was 4 days which was consistent with referenced literature.2
All patients in this study reported pruritus, pain and movement in all or some of their lesions at some time or the other during the course of their illness. Other cutaneous symptoms reported in literature include ‘prickly heat sensation’, insomnia and even agitation. As was expected, the distribution of lesions was confined to primarily the trunk, buttocks, groins and proximal extremities. These are the sites normally covered with clothing likely to be impregnated with eggs/larvae of C. anthropophaga.2, 3 Other less common sites reported in literature include genitalia and foot.9, 10 The hallmark cutaneous lesion encountered in all subjects was a furuncular lesion with an open puncta and sometimes oozing with serous fluid. Some of the puncta were large enough for the rear end of the larvae to be visualized prior to extraction. Diagnosis is usually essentially clinical and straightforward. However, methods such as submersion of the affected part in water to observe for air bubbles, dermatoscopy and even sonography have been described in less than obvious cases. Diagnosis of larvae of C. anthropophaga is made by its size confined to less than 15 mm and spiracles at the caudal respiratory end emerging from the skin.2, 3 Other differential diagnosis to be entertained in such cases are pyoderma, staphylococcal furunculosis, cutaneous leishmaniasis, dracunculiasis, onchocerciasis, tungiasis, ruptured epidermoid cyst, an abscess, a foreign body reaction, an exaggerated arthropod bite reaction, lymphadenopathy and factitial disorders. Conglomeration of numerous lesions, also referred to as ‘plaque myiasis’ may often mimic lesions of impetigo and herpes zoster as well.1, 2 Only one patient with extensive lesions over the abdomen and groin presented with complication of cellulitis which had to be treated with IV antibiotics. Other complications described in literature include regional lymphadenopathy and fever.2
Surgical management is the most frequently recommended definitive treatment modality, which includes manual extraction by either squeezing out the larvae, enlarging the orifice by incision or excision of the larvae and surrounding tissue. Conservative modalities of management include traditional methods of occlusion of central orifice and suffocation of larvae using porcine lard, strips of bacon, petrolatum jelly, nail polish, beeswax or mineral oil and newer agents such as liquid nitrogen, chloroform, ethyl chloride sprays and Ivermectin.1, 2, 3, 4 Oral Antibiotic cover for secondary bacterial infection and vaccination for Clostridium tetani should also be considered.1 The Tumbu fly lays its eggs in damp soil where the first instar larvae post hatching are picked up by clothing left to dry on such damp and shady areas. This is as opposed to the Human Botfly which uses a insect vector such as blood sucking mosquitoes to carry its eggs to the human host, a phenomenon referred to as ‘phoresis’. Hence, simple preventive measures such as drying of clothes in bright sunlight and proper ironing of clothes which kill the maggots and avoiding resting on damp soil in endemic areas suffices.2, 3
Conclusion
With ever increasing military conflicts across the globe and consequent deployment of peacekeeping forces, it is imperative that the modern day health care providers and commanders are vigil to the perils of endemic diseases and their geographic distribution. The purpose of this study was to familiarize oneself with one such endemic infestation and discuss its presentation and management. The condition by itself does not cause significant morbidity, unless complicated. However, in the authors’ experience, the psychological burden on the patients, owing out of pre-occupation with his ‘worms’ and the man hours lost due to management of this condition assume novel concerns.
Conflicts of interest
The authors were deployed as part of United Nations peacekeeping mission in Central Africa at the time of study.
References
- 1.Burkhart C.N., Burkhart C.G., Morrell D.S. Infestations. In: Bolognia J.L., Jorizzo J.L., Rapini R.P., editors. Bolognia Textbook of Dermatology. 3rd ed. Mosby Elsevier Publishing; Spain: 2012. pp. 1432–1433. [Google Scholar]
- 2.McGraw T.A., Turiansky G.W. Cutaneous myiasis. J Am Acad Dermatol. 2008;58(6):907–926. doi: 10.1016/j.jaad.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Robbins K., Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49(10):1092–1098. doi: 10.1111/j.1365-4632.2010.04577.x. [DOI] [PubMed] [Google Scholar]
- 4.Burns D.A. Diseases caused by arthropods and other noxious animals. In: Burns T., Breathnach S., Griffiths C., Cox N., editors. Rook's Text Book of Dermatology. 8th ed. Blackwell Publishing Ltd.; West Sussex, UK: 2010. 38.8–10. [Google Scholar]
- 5.Frieling U., Nashan D., Metze D. Cutaneous myiasis – a vacation souvenir. Hautarzt. 1999;50(3):203–207. doi: 10.1007/s001050050890. [DOI] [PubMed] [Google Scholar]
- 6.Bardach H., Aspöck H. Furunculoid myiasis due to Cordylobia anthropophaga in a traveler returning from Africa and review of the literature. Z Hautkr. 1981;56(4):216–220. [PubMed] [Google Scholar]
- 7.Logar J., Šoba B., Parač Z. Cutaneous myiasis caused by Cordylobia anthropophaga. Wien Klin Wochenschr. 2006;118(5–6):180–182. doi: 10.1007/s00508-006-0535-z. [DOI] [PubMed] [Google Scholar]
- 8.Edirisinghe J.S., Rajapakse C. Myiasis due to Cordylobia anthropophaga, the ‘Tumbu fly’ in a Sri Lankan infant. Ceylon Med J. 1991;36(3):112–115. [PubMed] [Google Scholar]
- 9.Kovaleva A., Climent P., Bécares C., Martín Azaña M., Irishina N., Goy E. Urogenital Myiasis by Cordylobia anthropophaga. J Pediatr Adolesc Gynecol. 2013;26(6):e123–e125. doi: 10.1016/j.jpag.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Palmieri J., North D., Santo A. Furuncular myiasis of the foot caused by the tumbu fly, Cordylobia anthropophaga: report in a medical student returning from a medical mission trip to Tanzania. Int Med Case Rep J. 2013;6:25. doi: 10.2147/IMCRJ.S44862. [DOI] [PMC free article] [PubMed] [Google Scholar]