Abstract
Background
The differentiation between the causes of cervical lymphadenopathy is of paramount importance as these have different modalities of treatment with varying prognosis. The aim of this study was to evaluate the efficacy of B Mode and colour Doppler ultrasound (CDUS) to differentiate between benign and metastatic lymph nodes.
Methods
100 patients of clinically palpable lymph nodes were evaluated with B Mode and CDUS. B Mode assessment included short-long (S:L) axis ratio, hilum, nodal border, echogenicity, intranodal necrosis and ancillary features. CDUS assessment included distribution of vascularity, resistive index (RI) and pulsatility index (PI). Statistical analysis was carried out with histopathological or cytological diagnosis as gold standard.
Results
B-Mode US correctly diagnosed 22/25 (88%) of the reactive lymph nodes giving it a sensitivity of 88% and specificity of 97.3%. Colour Doppler US diagnosed 23/25 (92%) reactive lymph nodes with a sensitivity of 92% and specificity of 97.3%. B-Mode underdiagnosed one case each of granulomatous disease and metastasis as reactive node while CDUS missed out two cases of granulomatous disease as reactive lymph node.
Conclusion
Individual parameters of B Mode when used alone were not found to be very effective in differentiating benign and malignant lymph nodes. However features of B-Mode combined together as well as color Doppler ultrasound, help in the detection of reactive lymph nodes and can be used as a diagnostic tool with good accuracy. However, they cannot be used as a diagnostic method for metastatic or tubercular nodes and cytopathology/histopathology remains the gold standard in such situations.
Keywords: Cervical lymphadenopathy, B Mode, Colour Doppler ultrasound (CDUS)
Introduction
Pathological cervical lymph nodes are encountered in neoplastic as well as non-neoplastic conditions. Clinical examination remains important in the evaluation of cervical lymphadenopathy in day to day practice. However, clinical examination is frequently inaccurate in distinguishing benign from malignant causes which is crucial in prognostication and further treatment planning.1, 2 Imaging plays an important role in the evaluation of cervical lymphadenopathy especially in distinguishing benign from malignant etiology. Almost all diagnostic imaging modalities (Ultrasound, CT, MRI) have been found to have superior diagnostic accuracy as compared to physical examination. Ultrasound (USG) has long been utilized to assess cervical lymph nodes. The addition of color Doppler has further sharpened the diagnostic accuracy.3 Advantages of USG include wider accessibility, availability of high frequency probes with greater resolution, no radiation issues and real time examination in multiple planes with feasibility for USG guided aspiration cytology/histopathology whenever needed.3
Ahuja et al. reported an accuracy of 96.8% for B mode USG in differentiating benign from malignant cervical lymphadenopathy.4 With the addition of Color Doppler, Yousem et al. reported an improved accuracy over B mode USG in evaluating cervical lymphadenopathy.5 Although there are a large number of studies describing and correlating USG and Doppler features of benign as well as malignant cervical lymphadenopathy with cytological/histopathological examinations, there is paucity of literature on the use of USG in predicting benign versus malignant etiology and thereby reducing the need for cytology/histological examination. The present study aimed at diagnosing and predicting etiology (benign vs malignant) in fresh cases of clinically palpable undiagnosed cervical lymphadenopathy based on established standard criteria on B mode as well as Color Doppler studies. The study also attempted to find out if USG features can be used to reduce the need of cytology/histopathology examination in select cases.
Material and methods
This cross sectional descriptive study was carried out from September 2012 to August 2014 at Department of Radiodiagnosis and Imaging of a tertiary care and teaching hospital. The study was approved by institutional ethics committee.
Study population: Patients with visible or clinically palpable neck nodes referred for ultrasonography from indoor or outdoor services of the hospital formed the study group.
Sample size: One hundred patients (100) of clinically palpable neck nodes were included for the final study. The sample size was calculated by comparing the sensitivity of ultrasound (reactive lymph node ∼ 88%) with FNAC (reactive lymph node ∼ 99%) at 90% power and at 5% level of significance. The minimum sample size thus calculated was 103. Sample size for this study was taken as 100.
Inclusion criteria: All consecutive, non-repetitive patients with clinically palpable neck swelling referred for ultrasonography were taken as the study group.
Exclusion criteria: All previously treated or diagnosed patients, patients who were lost to follow-up or who did not undergo cytology/histopathology were excluded from the study.
Informed consent was taken from all the patients as per WHO format.
Equipment used: Ultrasound examination was performed using linear transducer (7–10 MHz, Logiq P5, GE Health care, USA).
Parameters studied in B-Mode ultrasound: Distribution of lymph nodes, size, shape, short to long axis (S/L) ratio, hilum (absence or presence), nodal echogenicity (hypo/hetero/hyperechoic), nodal border (sharp/unsharp), nodal calcification and necrosis and ancillary features (matting/soft tissue edema/both).6, 7
Parameters studied in Color Doppler Ultrasound (CDUS): Vascularity (peripheral/central/mixed), resistive index (RI), pulsatility index (PI).8
Criteria of nodes on USG/Color Doppler:
Reactive LN: A lymph node was considered reactive if the node is oval in shape, hypoechoic with presence of central echogenic hilum, had unsharp borders, had no nodal calcification or ancillary features like necrosis or matting. On CDUS, a lymph node was considered reactive if central vascularity was maintained. No definite cut off was considered in RI and PI values.
Metastatic LN: A lymph node was considered metastatic if the node was round, hyper or hypoechoic with loss of central echogenic hilum, had sharp borders, and had central necrosis or intranodal calcification. On CDUS, a lymph node was considered malignant if central vascularity was lost with presence of peripheral vascularity. No definite cut off was considered in RI and PI values.
Tuberculosis was considered if nodes had absent hilum, unsharp nodal border, hypoechoic echotexture, intranodal necrosis, had matting with peripheral vascularity. Lymphoma: Lymphomatous nodes were diagnosed if nodes were round in shape with absent hilum, sharp nodal borders, hypoechoic echotexture with intranodal reticulation, absence of ancillary features and mixed vascularity.6, 7, 8
Statistical analysis: B-Mode and CDUS findings were compared with the cytological/histopathological findings. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and inter-observer variability (kappa) were calculated. Receiver operating characteristic (ROC) curves were drawn for cut-off values of RI and PI of reactive, tubercular, lymphomatous and metastatic lymph nodes. Statistical analysis was done using SPSS (Statistical Package for Social Sciences) Version 19.0. p-Value was calculated for each of the criteria separately using Chi Square test or Fisher's exact test as appropriate. p-Value < 0.05 was considered statistically significant.
Results
One hundred and twelve untreated and previously undiagnosed patients with visible or clinically palpable cervical lymphadenopathy were examined with B-mode, color and spectral Doppler analysis over a period of two years from September 2012 to August 2014. Out of the 112 patients examined, 05 patients were lost to follow up and 07 patients did not undergo cytopathological or histopathological examination (HPE). Hence, these 12 patients were excluded from the study (Fig. 1).
Fig. 1.
Flowchart showing selection of study population.
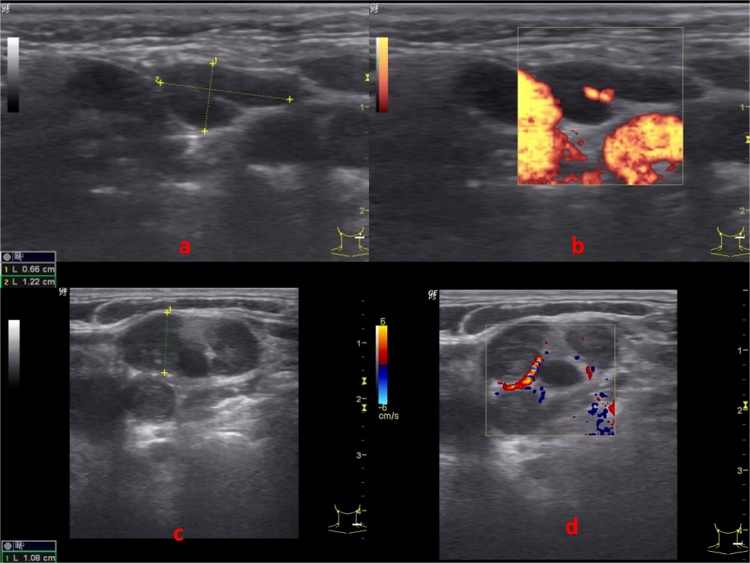
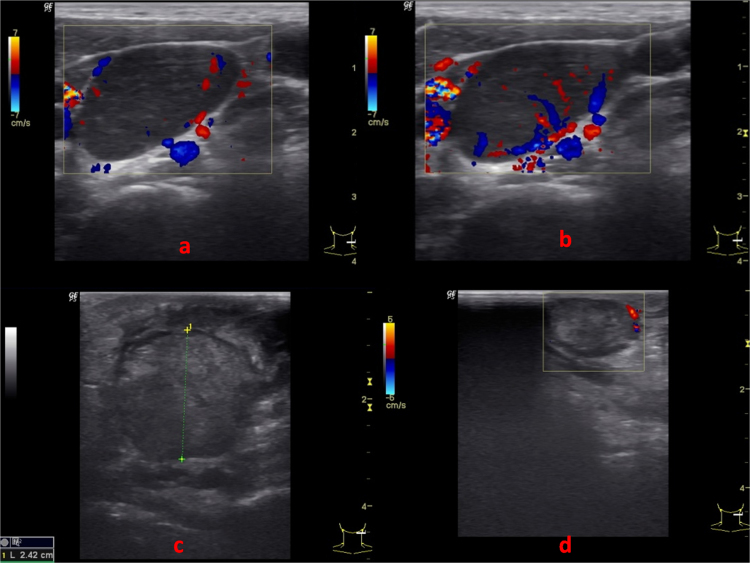
The statistically significant (p value < 0.05) correlates with reactive lymph nodes were found to be oval shape, presence of hilum, unsharp nodal border, absence of ancillary features, absence of intranodal necrosis and central vascularity (Fig. 2a and b; Table 1). In ROC curves drawn for RI and PI of reactive lymph nodes, cut off value of PI ≤ 1.5 was found to be statistically significant. No significant cut off value was obtained for RI. Statistically significant association with tuberculous lymph nodes were absent hilum, unsharp nodal border, hypoechoic echotexture, intranodal necrosis, presence of ancillary features and peripheral vascularity (Fig. 2c and d; Table 1). Lymphomatous nodes (Fig. 3a and b; Table 1) were characterized by round shape, absent hilum, sharp nodal borders, hypoechoic echotexture with intranodal reticulation, absence of ancillary features and mixed vascularity (p value < 0.05). For metastatic lymph nodes, round shape (S/L ratio ≥ 0.5), hypoechoic echotexture, soft tissue edema and peripheral vascularity (Fig. 3c and d; Table 1) were found to be statistically significant parameters. For lymphomatous nodes cut off RI > 0.7 was found to be statistically significant. ROC curves drawn for RI and PI did not show significant cut-off values for metastatic or tuberculous nodes.
Fig. 2.
(a) USG B mode and (b) CDUS (Power Doppler) of cervical LN showing oval shape with echogenic hilum, unsharp nodal border, hypoechoic appearance and no ancillary features. No intra-nodal necrosis or calcification noted. CDUS shows central vascularity. USG features suggest reactive LN. The same was confirmed on FNAC. (c and d) USG B mode and color Doppler showing lymph node with loss of echogenic hilum, sharp nodal border, heteroechoic appearance with matting. No intranodal necrosis or calcification noted. CDUS shows peripheral vascularity. On USG, impression was of tubercular lymph node which was confirmed on FNAC.
Table 1.
Various criteria used to differentiate between different types of lymph nodes.
| S.No. | US/CDUS features | Reactive (n = 25) | Tuberculosis (n = 28) | Metastases (n = 25) | Lymphoma (n = 22) |
|---|---|---|---|---|---|
| 1. | Shape | Oval (23) p < 0.001 |
Round (23) p = 0.094 |
Round (22) p < 0.001 |
Round (22) p < 0.001 |
| 2. | Hilum | Present (25) p < 0.001 |
Absent (27) p = 0.001 |
Absent (25) p = 0.024 |
Absent (22) p = 0.001 |
| 3. | Nodal border | Unsharp (21) p < 0.001 |
Sharp (11) p = 0.265 |
Sharp (16) p = 0.165 |
Sharp (19) p < 0.001 |
| 4. | Echogenicity | Hypoechoic (25) p = 0.067 |
Hypoechoic (22) p = 0.039 |
Hypoechoic (17) p = 0.002 |
Hypoechoic (22) p = 0.131 |
| 5. | Nodal calcification | – | – | 1 | – |
| 6. | Intranodal necrosis | Absent (25) p = 0.003 |
Present (14) p < 0.001 |
Present (6) p = 0.572 |
Absent (22) p = 0.005 |
| 7. | Ancillary features | ||||
| (a) Soft tissue edema | 0 | 2 | 7 | 1 | |
| (b) Matting | 0 | 14 | 1 | 0 | |
| (c) Both | 0 | 5 | 1 | 0 | |
| p < 0.001 | p = 0.001 | p = 0.004 | p < 0.001 | ||
| 8. | Pseudo cystic appearance with reticulation | – | – | – | 18 (81%) |
| 9. | Vascularity | ||||
| (a) Central | 23 | 1 | 0 | 0 | |
| (b) Peripheral | 0 | 14 | 12 | 8 | |
| (c) Mixed | 2 | 5 | 7 | 14 | |
| (d) Absent | – | 8 | 6 | 0 | |
| p = 0.002 | p < 0.001 | p < 0.001 | p < 0.001 |
Fig. 3.
(a and b) CDUS images of lymph node showing mixed (peripheral as well as central) vascularity with hypoechoic pseudocystic appearance. US features were suggestive of lymphomatous node which was confirmed on histopathology. (c) B Mode and (d) CDUS images of lymph node showing rounded appearance with absent hilum, sharp nodal border, isoechoic appearance and adjacent soft tissue edema. No intranodal necrosis or calcification noted. CDUS shows peripheral vascularity. Impression on US was of metastatic lymph node which was confirmed on FNAC as metastasis from adenocarcinoma.
For metastatic lymph nodes, the combined B-mode features had a sensitivity of 68%, specificity of 73%, PPV of 45.9% and NPV of 87.3%. Kappa coefficient for inter-observer variability was 0.36 (fair agreement). CDUS features had a sensitivity of 60%, specificity of 82.7%, PPV of 53.6% and NPV of 86.1% and kappa value of 0.41 (moderate agreement) (Table 2). For tubercular lymph nodes, the combined B-mode features had a sensitivity of 64.3%, specificity of 91.7%, PPV of 75%, NPV of 86.8% and kappa value of 0.59 (moderate agreement). CDUS had a sensitivity of 53.6%, specificity of 80.6%, PPV of 51.7% and NPV of 81.7% with Kappa coefficient of 0.34 (fair agreement) (Table 3). For lymphomatous lymph nodes, combined B-mode features had sensitivity, specificity, PPV, NPV and Kappa coefficient of 59.1%, 97.4%, 86.7%, 89.4% and 0.64 (good agreement) respectively and that of CDUS features were 50%, 91%, 61.1%, 86.6% and 0.64 (good agreement) respectively (Table 4). For reactive nodes, B-mode features had a sensitivity of 88%, specificity of 97.3%, PPV of 91.6% and NPV of 96% with Kappa coefficient as 0.87 (very good agreement). CDUS has sensitivity, specificity, PPV, NPV and Kappa coefficient of 92%, 97.3%, 92%, 97.3% and 0.89 (very good agreement) respectively (Table 5). Therefore it can be assumed that FNAC/histopathological examination could have been avoided in majority (>90%) of the patients with sonographic features of reactive lymph nodes. At best, this subset of patients can be put on regular follow up.
Table 2.
Correlation of metastasis with B-Mode and CDUS features.
| Metastasis | Metastasis on Histopath |
Total | Sensitivity (95%CIa) | Specificity (95%CI) | PPV | NPV | Kappa | ||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||||
| B Mode | Positive | 17 | 20 | 37 | 68.00 (46.50–85.05%) | 73.33 (61.86–82.89%) | 45.95 | 87.30 | 0.36 |
| Negative | 8 | 55 | 63 | ||||||
| Color Doppler | Positive | 15 | 13 | 28 | 60.00 (38.67–78.87%) | 82.67 (72.19–90.43%) | 53.57 | 86.11 | 0.41 |
| Negative | 10 | 62 | 72 | ||||||
CI – confidence interval.
Table 3.
Correlation of tuberculous nodes with B-Mode and CDUS features.
| Tuberculosis | Tuberculosis on Histopath |
Total | Sensitivity (95%CIa) | Specificity (95%CI) | PPV | NPV | Kappa | ||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||||
| B Mode | Positive | 18 | 6 | 24 | 64.29 (44.07–81.36%) | 91.67 (82.74–96.88%) | 75.00 | 86.84 | 0.59 |
| Negative | 10 | 66 | 76 | ||||||
| Color Doppler | Positive | 15 | 14 | 29 | 53.57 (33.87–72.49%) | 80.56 (69.53–88.94%) | 51.72 | 81.69 | 0.34 |
| Negative | 13 | 58 | 71 | ||||||
CI – confidence interval.
Table 4.
Correlation of lymphomatous nodes with B-Mode and CDUS features.
| Lymphoma | Lymphoma on Histopath |
Total | Sensitivity (95%CIa) | Specificity (95%CI) | PPV | NPV | Kappa | ||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||||
| B Mode | Positive | 13 | 2 | 15 | 59.09 (36.35–79.29%) | 97.44 (91.04–99.69%) | 86.67 | 89.41 | 0.64 |
| Negative | 9 | 76 | 85 | ||||||
| Color Doppler | Positive | 11 | 7 | 18 | 50.00 (28.22–71.78%) | 91.03 (82.38–96.32%) | 61.11 | 86.59 | 0.64 |
| Negative | 11 | 71 | 82 | ||||||
CI – confidence interval.
Table 5.
Correlation of reactive nodes with B-Mode and CDUS features.
| Reactive | Reactive on Histopath |
Total | Sensitivity (95%CIa) | Specificity (95%CI) | PPV | NPV | Kappa | ||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||||
| B Mode | Positive | 22 | 2 | 24 | 88.00 (68.78–97.45%) | 97.33 (90.70–99.68%) | 91.67 | 96.05 | 0.87 |
| Negative | 3 | 73 | 76 | ||||||
| Color Doppler | Positive | 23 | 2 | 25 | 92.00 (73.97–99.02%) | 97.33 (90.70–99.68%) | 92.00 | 97.33 | 0.89 |
| Negative | 2 | 73 | 75 | ||||||
CI – confidence interval.
Discussion
Cervical lymphadenopathy is a commonly encountered clinical condition with varying etiologies ranging from innocuous benign reactive lymphadenopathy to sinister and malignant conditions like lymphoma and metastases.1, 2, 9 It is extremely important to have an etiological diagnosis for prognostication and further treatment planning. Although, cytological/histopathological examination is the gold standard for accurate etiological diagnosis of cervical lymphadenopathy, various imaging modalities like USG, CT scan and MRI have also been found to be accurate in characterizing cervical lymph nodes.10, 11, 12
Sonographic features of benign and malignant cervical lymph nodes have been well described in the literature.6, 7, 8
B-Mode features
Shape of lymph node: A lymph node with S/L ratio < 0.5 is oval in shape and >0.5 indicates round node. An oval node is indicative of benign pathology, while malignant lymph nodes tend to be round.13, 14, 15, 16, 17, 18 Apart from the shape and size, eccentric cortical hypertrophy, due to focal tumor infiltration, has also been described as a useful indicator to identify metastatic lymph nodes.16 The present study showed similar results with statistically significant correlation between S/L ratio < 0.5 and reactive lymph nodes, while S/L ratio ≥ 0.5 was seen to be statistically significant for metastatic and lymphomatous nodes. However, no significant correlation was seen between S/L ratio and tubercular lymph nodes in the present study.
Hilum characteristics: Several studies have reported that metastatic and lymphomatous nodes show loss of echogenic hilum.19, 20, 21, 22 On the other hand, few studies have also shown that echogenic hilum may be found in malignant nodes.14, 17, 23 In the present study, statistically significant correlation was found between presence of echogenic hilum and reactive lymph nodes, while absence of hilum had significant correlation with tubercular, lymphomatous and metastatic lymph nodes. None of the patients with histopathologically proven lymphomatous or metastatic nodes were found to have an intact hilum. All the patients with reactive lymph nodes were seen to have an intact echogenic hilum. Thus, the present study shows similar findings as described by Ahuja et al. and Ishii et al.19, 20
Lymph node border: Sharp borders have been usually associated with metastatic lymph nodes while benign lymph nodes are seen to have unsharp borders.24 However, ill-defined borders may be seen in metastatic lymph nodes in advanced stages indicating capsular spread.25 Unsharp borders may also be seen in tubercular nodes because of edema and active infiltration of surrounding tissues.23 The present study showed similar results with no significant correlation between nodal border and tubercular as well as metastatic lymph nodes. However, statistically significant correlation was seen between sharp borders and lymphomatous nodes as well as between unsharp borders and reactive lymph nodes.
Echogenicity of lymph node: Metastatic lymph nodes are generally hypoechoic in relation to the adjacent muscles.14, 23 Lymphomatous as well as reactive lymph nodes also tend to be hypoechoic.7 In the present study, significant association was found between nodal hypoechogenicity and metastatic as well as tubercular lymph nodes. However, no correlation was found between echogenicity and reactive as well as lymphomatous lymph nodes.
Necrosis: Intra-nodal necrosis has been described as a feature of metastatic and tuberculous lymph nodes.23, 26 Regardless of the size of lymph nodes, the presence of intranodal necrosis is considered as a pathological feature.26 The present study showed statistically significant correlation between presence of intranodal necrosis and tubercular nodes and between absence of necrosis and reactive as well as lymphomatous nodes. No significant correlation was found between necrosis and metastatic nodes.
Ancillary features: Features like matting and adjacent soft tissue edema have been described to be a common feature of tuberculous lymph nodes.2, 18, 23 Metastatic lymph nodes may also show soft tissue edema due to invasion into surrounding soft tissue suggestive of extracapsular spread.27 In this study, statistically significant correlation was found between presence of these features and metastatic as well as tubercular lymph nodes. Absence of these features was significantly associated with reactive and lymphomatous nodes.
Color Doppler ultrasound features
Vascularity: Benign or reactive nodes have central vascularity or may appear avascular, but rarely show peripheral vascularity.23, 24 On the other hand, metastatic lymph nodes show peripheral or mixed vascularity.7, 9 Approximately 60–90% of lymphomatous lymph nodes tend to have mixed (both peripheral and central) vascularity.7 The present study showed similar results with significant correlation found between peripheral vascularity and malignant as well as tubercular nodes. Central vascularity had significant correlation with reactive lymph nodes. Mixed vascularity i.e. both peripheral and central vascularity was found to be statistically significant for lymphomatous lymph nodes.
Doppler indices: The role of vascular resistance in differentiating malignant and benign lymph nodes is debatable because of inconsistent findings. Some reports mention that vascular resistance of metastatic lymph nodes is higher than that of reactive nodes, however others have suggested that metastatic lymph nodes show lower or similar vascular resistance as compared to benign nodes.7, 26 It is generally considered that RI and PI of lymphomatous nodes are higher than those of normal, reactive and tuberculous nodes but lower than metastatic lymph nodes.7 In the present study, PI ≤ 1.5 showed a high sensitivity for reactive lymph nodes, with fair accuracy of this criteria as a diagnostic test. For lymphomatous nodes, cut-off RI > 0.7 showed high specificity. The cut-off values for RI and PI for other nodes did not show good diagnostic accuracy.
Role of B mode and CDUS features of cervical lymphadenopathy in minimizing FNAC/biopsy
For metastatic lymph nodes, the combined B-mode features had a sensitivity of 68%, specificity of 73%, PPV of 45.9% and NPV of 87.3%. Kappa coefficient for inter-observer variability was 0.36. CDUS features had a sensitivity of 60%, specificity of 82.7%, PPV of 53.6% and NPV of 86.1% and kappa value of 0.41 (Table 2). This shows that combined B-mode features have a reasonable sensitivity and specificity to detect metastatic nodes. However, since kappa coefficient of B-Mode features and histopathological findings is 0.36, the diagnostic accuracy is fair. On the other hand, combined CDUS features show a high specificity and reasonable sensitivity to detect metastatic nodes with moderate accuracy (Kappa coefficient 0.41). Hence, though CDUS can be used as a predictor of metastatic nodes, neither B-mode nor CDUS can be used as a substitute for histopathology/cytology as a diagnostic test.
For tubercular lymph nodes, the combined B-mode features had a sensitivity of 64.3%, specificity of 91.7%, PPV of 75% and NPV of 86.8% and CDUS had a sensitivity of 53.6%, specificity of 80.6%, PPV of 51.7% and NPV of 81.7% with Kappa coefficient of 0.34 (Table 3). This shows that B-mode as well as CDUS has good specificity but lacks sensitivity to detect tubercular lymph nodes. The diagnostic accuracy of B-Mode ultrasound is moderate (kappa value 0.59), while that of CDUS is fairly low. Hence, though B-mode can be used as a predictor of tubercular lymph nodes, neither B-mode nor CDUS can replace cytology/histopathology as a diagnostic test.
For lymphomatous lymph nodes, combined B-mode features had a sensitivity, specificity, PPV, NPV and Kappa coefficient of 59.1%, 97.4%, 86.7%, 89.4% and 0.64 respectively and that of CDUS features were 50%, 91%, 61.1%, 86.6% and 0.64 respectively (Table 4). This shows that B-mode as well as color Doppler has got high specificity and poor sensitivity to detect lymphoma nodes with substantial accuracy. Hence, both these tests can be used to rule out lymphoma with significant accuracy. However, these tests cannot be used to diagnose lymphomatous nodes due to their low sensitivity and cannot replace cytology/histopathology.
For reactive nodes, B-mode features had a sensitivity of 88%, specificity of 97.3%, PPV of 91.6% and NPV of 96% with Kappa coefficient as 0.87. CDUS has sensitivity, specificity, PPV, NPV and Kappa coefficient of 92%, 97.3%, 92%, 97.3% and 0.89 respectively (Table 5). This proves that both B-mode and CDUS can be used to detect reactive lymph nodes with almost perfect accuracy. Hence, if patients are detected to have features of reactive lymph nodes on B-mode and CDUS, the need for cytopathogy/histopathology can be reduced.
Conclusion
Individual parameters of B-Mode USG when used alone are not very effective in differentiating benign and malignant cervical lymph nodes. However all features of B Mode combined together with color Doppler findings help in the precise diagnosis of reactive lymph nodes and can be used as a diagnostic tool for such lymph nodes with good accuracy. In these select cases, immediate cytological/histopathological examination can be avoided. These patients can be periodically followed up. Also, sonographic findings have high specificity to rule out lymphomatous nodes. However, sonography cannot be used for diagnosing metastatic and tubercular nodes with a high degree of accuracy. Cytopathology/histopathology will remain the gold standard in such situations.
Conflicts of interest
The authors have none to declare.
References
- 1.Lee Y.Y., Van Tassel P., Nauert C., North L.B., Jing B.S. Lymphomas of the head and neck: CT findings at initial presentation. Am J Roentgenol. 1987;149(3):575–581. doi: 10.2214/ajr.149.3.575. [DOI] [PubMed] [Google Scholar]
- 2.Reede D.L., Bergeron R.T. Cervical tuberculous adenitis: CT manifestations. Radiology. 1985;154(3):701–704. doi: 10.1148/radiology.154.3.3969473. [DOI] [PubMed] [Google Scholar]
- 3.Phelps P.D. The pharynx and larynx: the neck. In: Sutton D., editor. 7th ed. vol. 2. Churchill Livingstone; New Delhi: 2003. pp. 1489–1517. (Text Book of Radiology and Imaging). [Google Scholar]
- 4.Ahuja A.T., Ying M., Ho S.Y. Ultrasound of malignant cervical lymph nodes. Cancer Imaging. 2008;8(1):48–56. doi: 10.1102/1470-7330.2008.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yousem D.M., Grossman R.I. 3rd ed. Mosby Elsevier; St. Louis: 2010. Neuroradiology. [Google Scholar]
- 6.Ying M., Ahuja A. Sonography of neck lymph nodes: Part I: Normal lymph nodes. Clin Radiol. 2003;58:351–358. doi: 10.1016/s0009-9260(02)00584-6. [DOI] [PubMed] [Google Scholar]
- 7.Ahuja A., Ying M. Sonography of neck lymph nodes: Part II: abnormal lymph nodes. Clin Radiol. 2003;58(5):359–366. doi: 10.1016/s0009-9260(02)00585-8. [DOI] [PubMed] [Google Scholar]
- 8.Ahuja A.T., Ying M. Sonographic evaluation of cervical lymph nodes. Am J Radiol. 2005;184(5):1691–1699. doi: 10.2214/ajr.184.5.01841691. [DOI] [PubMed] [Google Scholar]
- 9.Baatenburg de Jong R.J., Rongen R.J., Lameris J.S., Harthoorn M., Verwoerd C.D., Knegt P. Metastatic neck disease: palpation vs ultrasound examination. Arch Otolaryngol Head Neck Surg. 1989;115(6):689–690. doi: 10.1001/archotol.1989.01860300043013. [DOI] [PubMed] [Google Scholar]
- 10.Sun J., Li B., Li C.J. Computed tomography versus magnetic resonance imaging for diagnosing cervical lymph node metastasis of head and neck cancer: a systematic review and meta-analysis. Onco Targets Ther. 2015;8:1291–1313. doi: 10.2147/OTT.S73924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamer F., Ali T. Neck lymph nodes: characterization with diffusion-weighted MRI. Egypt J Radiol Nucl Med. 2012;43(2):173–181. [Google Scholar]
- 12.King A.D., Tse G.M.K., Ahuja A.T. Necrosis in metastatic neck nodes: diagnostic accuracy of CT, MR imaging and US. Radiology. 2004;230(3):720–726. doi: 10.1148/radiol.2303030157. [DOI] [PubMed] [Google Scholar]
- 13.Solbiati L., Cioffi V., Ballarati E. Ultrasonography of the neck. Radiol Clin North Am. 1992;30:941–954. [PubMed] [Google Scholar]
- 14.Ahuja A., Ying M., King W., Metreweli C. A practical approach to ultrasound of cervical lymph nodes. J Laryngol Otol. 1997;111(3):245–256. doi: 10.1017/s0022215100137004. [DOI] [PubMed] [Google Scholar]
- 15.Tohnosu N., Onoda S., Isono K. Ultrasonographic evaluation of cervical lymph node metastases in esophageal cancer with special reference to the relationship between the short to long axis ratio (S/L) and the cancer content. J Clin Ultrasound. 1989;17(2):101–106. doi: 10.1002/jcu.1870170206. [DOI] [PubMed] [Google Scholar]
- 16.Vassallo P., Wernecke K., Roos N., Peters P.E. Differentiation of benign from malignant superficial lymphadenopathy: the role of high resolution US. Radiology. 1992;183(1):215–220. doi: 10.1148/radiology.183.1.1549675. [DOI] [PubMed] [Google Scholar]
- 17.Vassallo P., Edel G., Roos N., Naquib A., Peters P.E. In-vitro high-resolution ultrasonographyof benign and malignant lymph nodes. A sonographic-pathologic correlation. Invest Radiol. 1993;28(8):698–705. doi: 10.1097/00004424-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Ahuja A., Ying M. Grey-scale sonography in assessment of cervical lymphadenopathy: review of sonographic appearances and features that may help a beginner. Br J Oral Maxillofac Surg. 2000;38(5):451–459. doi: 10.1054/bjom.2000.0446. [DOI] [PubMed] [Google Scholar]
- 19.Ahuja A., Ying M., Yang W.T., Evans R., King W., Metreweli C. Theuse of sonography in differentiating cervical Lymphomatous lymph nodes from cervical metastatic lymph nodes. Clin Radiol. 1996;51(3):186–190. doi: 10.1016/s0009-9260(96)80321-7. [DOI] [PubMed] [Google Scholar]
- 20.Ishii J., Fujii E., Suzuki H., Shinozuka K., Kawase N., Amagasa T. Ultrasonic diagnosis of oral and neck malignant lymphoma. Bull Tokyo Med Dent Univ. 1992;39(4):63–69. [PubMed] [Google Scholar]
- 21.Bruneton J.N., Normand F., Balu-Maestro C. Lymphomatous superficial lymph nodes: US detection. Radiology. 1987;165(1):233–235. doi: 10.1148/radiology.165.1.3306785. [DOI] [PubMed] [Google Scholar]
- 22.Ying M.T.C. Department of Optometry and Radiography, The Hong Kong Polytechnic University; Hong Kong: 1996. Ultrasound evaluation of cervical lymph nodes in a Chinese population; p. 235. [M. Phil thesis] [Google Scholar]
- 23.Ying M., Ahuja A.T., Evans R., King W., Metreweli C. Cervical lymphadenopathy: sonographic differentiation between tuberculous nodes and nodal metastases from non-head and neck carcinomas. J Clin Ultrasound. 1998;26(8):383–389. doi: 10.1002/(sici)1097-0096(199810)26:8<383::aid-jcu2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Shozushima M., Suzuki M., Nakasima T., Yanagisawa Y., Sakamaki K., Takeda Y. Ultrasound diagnosis of lymph nodemetastasis in head and neck cancer. Dentomaxillofac Radiol. 1990;19(4):165–170. doi: 10.1259/dmfr.19.4.2097226. [DOI] [PubMed] [Google Scholar]
- 25.Johnson J.T. A surgeon looks at cervical lymph nodes. Radiology. 1990;175(3):607–610. doi: 10.1148/radiology.175.3.2188292. [DOI] [PubMed] [Google Scholar]
- 26.Som P.M. Lymph nodes of the neck. Radiology. 1987;165(3):593–600. doi: 10.1148/radiology.165.3.3317494. [DOI] [PubMed] [Google Scholar]
- 27.Swartz J.D., Yussen P.S., Popky G.L. Imaging of the neck: nodal disease. Crit Rev Diagn Imaging. 1991;31(3–4):413–469. [PubMed] [Google Scholar]