Abstract
Background
Manual Mycobacterium growth indicator tube (MGIT) was evaluated for isolation and drug susceptibility testing (DST) of Mycobacterium tuberculosis (MTB) for its implementation in laboratories with low and medium volume.
Methods
1018 consecutive clinical specimens were processed using manual MGIT and conventional Lowenstein–Jensen (LJ) culture. Results obtained for culture positivity were analyzed taking combined reference of positivity by either solid or liquid culture. All positive cultures were identified and DST to first line drugs was performed by manual MGIT and 1% proportional method on LJ media. Performance of manual MGIT for DST was compared to conventional DST on LJ media.
Result
Of the total 220 culture positive samples 93.9% were isolated in MGIT while 75.7% in LJ taking combined reference of positivity by either solid or liquid culture. Turn around time for isolation of MTB was significantly less for MGIT as compared to LJ. There was good agreement between manual MGIT and 1% proportional method on LJ media for DST to first line drugs. Turnaround time from inoculation to DST results for smear positive and smear negative cases using manual MGIT was 20.2 and 30.1 days respectively. The total cost for isolation, identification and DST in manual MGIT for smear positive and smear negative cases was INR 2350 and INR 2700 respectively.
Conclusion
It is feasible to implement manual MGIT in low to medium volume laboratory that already has experience with culture provided adequate biosafety measures and appropriate training of laboratory staff are taken care of.
Keywords: Liquid culture, Mycobacterium growth indicator tube, Tuberculosis
Introduction
Tuberculosis (TB) remains the leading infectious disease in developing countries. India has the highest burden of TB in the world with an estimated 2.2 million incident cases out of a global incidence of 9.6 million in the year 2014. The problem is further complicated by emergence of drug resistant strains. Worldwide, 3.3% of newly diagnosed TB cases and 20% of previously treated TB cases are estimated to have multidrug-resistant TB (MDR-TB).1 Laboratory confirmation of TB and drug susceptibility testing (DST) is essential to ensure that patients are correctly diagnosed and appropriately treated.
Smear microscopy for acid-fast bacilli (AFB) is the most commonly used diagnostic test for TB in developing countries. The test, although rapid, cheap and easy to perform, lacks sensitivity, cannot distinguish viable from nonviable bacteria and does not provide any information on drug resistance. Culture of Mycobacterium tuberculosis (MTB) remains the gold standard for both diagnosis and DST. Conventional culture on solid media like Lowenstein–Jensen (LJ) medium, while cheap and simple, have the major disadvantage of being very slow requiring 20–56 days for diagnosis and further 4–6 weeks for DST. Therefore, solid culture results often have limited or no impact on patient management.2
Culture in liquid media (e.g. 7H9 Middlebrook media) is more sensitive and faster than conventional solid media. In 2007, the WHO endorsed use of liquid culture technology3 but due to high cost and complexity of commercial automated liquid culture systems its use is limited to few referral laboratories in developing countries. Mycobacteria growth indicator tube – MGIT (Becton-Dickinson, Sparks, MD) commercially available with 4 ml of 7H9 Middlebrook broth base and a fluorescent sensor is intended for the manual isolation and DST of MTB. Few studies done worldwide have found manual MGIT to have comparable results as that of automated liquid culture systems for primary isolation and DST of MTB.4, 5, 6 The method earlier being used to detect fluorescence in manual MGIT tube was transillumination with a 365 nm UV light, e.g. a Wood's lamp. This was quite cumbersome and is now replaced by a handheld fluorescence reader (BACTEC MicroMGIT) which makes the reading of tubes quite simple and objective.
There is no published study on performance of manual MGIT using MicroMGIT reader. The methodology holds promise for low and medium volume laboratories of developing countries, processing up to 10 mycobacterial samples per day, who cannot afford costly equipment and infrastructure required for automated systems. This study was therefore undertaken to compare the recovery rate and time for isolation and DST of MTB from clinical samples by manual MGIT vs the conventional LJ media and evaluate the feasibility for its implementation for use in laboratories with low and medium volume.
Material and methods
This prospective diagnostic study was conducted at a large tertiary care hospital of northern India from December 2013 to March 2015. Pulmonary and extrapulmonary samples received in the laboratory from out patient department (OPD) and inpatients for mycobacteriology culture and DST were included in the study. Samples from patients suspected to have relapse or treatment failure were also included.
All procedures requiring biosafety precautions were performed as per recommended guidelines for mycobacterial work.7 The laboratory staff was well trained in biosafety procedures and used protective clothing (gloves, cap gown, etc.) and respiratory protection (N 95 masks) at all times. Sample processing, smear preparation, inoculation of media, identification and DST, were performed in a Class II Biosafety Cabinet. The samples and smears after processing were discarded in 5% phenol and inoculated culture media were autoclaved in order to achieve complete disinfection prior to final disposal.
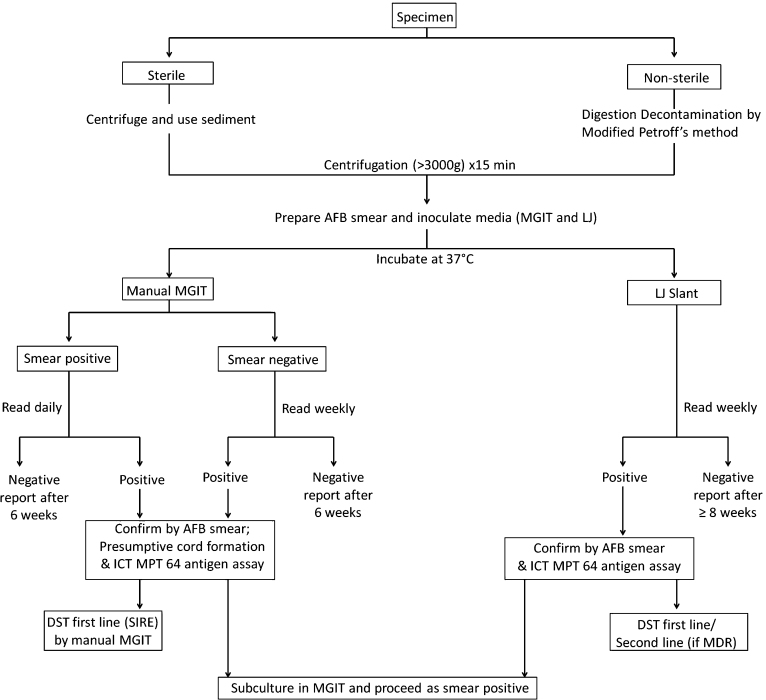
A total of 1018 consecutive clinical specimens (781 sputum, 121 bronchoalveolar lavage, 29 bronchoscopic biopsies, 15 fine needle aspiration cytology (FNAC) aspirates, 9 pus, 57 body fluid aspirates and 6 urine) were received in the laboratory for mycobacterial culture and DST. Specimens were collected in a sterile 15 ml falcon tube with a screw cap. All sterile specimens were concentrated by centrifugation at 3000 × g for 30 min while all nonsterile specimens were digested and decontaminated by the Modified Petroffs N-acetyl-l-cysteine (NALC)-NaOH method before concentration. The pellet obtained was re-suspended in sterile phosphate buffer saline to get a final volume of 2 ml. Resuspended pellet was used for making smears and for inoculation of LJ slopes and MGIT tubes. We inoculated 0.5 ml of the processed specimen into MGIT, and 0.2 ml onto LJ medium slant. All inoculated media were incubated at 37 °C. Positive smears were graded from 1+ to 4+ on Ziehl Neelsen (ZN) stain as per quantitative reporting procedure recommended by Centre for Disease Control and Prevention (CDC).8
Commercially available manual MGIT products (Becton-Dickinson, Sparks, MD) were used in the study. Before inoculation, 4 ml MGIT tubes (Catalogue number-245113) were supplemented with 0.5 ml OADC (oleic–albumin–dextrose–catalase) (Catalogue number-245116) and 0.1 ml of reconstituted PANTA (polymyxin B, amphotericin B, nalidixic acid, trimethoprim and azlocillin) (Catalogue number-245114) as instructed by the manufacturer. MGIT tubes have an oxygen quenching fluorescent compound embedded in silicon at the bottom to detect microbial growth. The amount of fluorescence emitted is inversely proportional to the oxygen level in the culture medium, indicating the consumption of oxygen by growing microorganisms.
The fluorescence was detected with the help of a handheld MicroMGIT fluorescence reader (Catalogue number-445923). The reader measures 9.2 cm × 14.5 cm × 12.0 cm (W × D × H) and operates on a standard 9 volt battery (Fig. 1). A calibration tube (Catalogue number-441049) included with the instrument was used to calibrate it before taking readings of the inoculated tubes. The smear positive specimen MGIT tubes were read daily while smear negative specimen MGIT tubes were read weekly till they became positive or for a maximum of six weeks.
Fig. 1.
BACTEC MicroMGIT fluorescence reader.
LJ medium slants were examined weekly for 8 weeks for the visible appearance of colonies. On the day of detection, all positive liquid and solid media were examined by ZN stain to confirm the presence of acid-fast bacteria (AFB). Cultures found AFB positive by microscopy were further identified by presumptive cord formation in liquid media and immunochromatographic test for detection of MPT 64 Antigen (SD Bioline TB Ag MPT64 rapid kit). For all cultures identified as MTB complex DST was performed on MGIT for SIRE (streptomycin, Isoniazid (INH), rifampicin and ethambutol) as per manufacturers guidelines and on LJ media by the 1% proportion method according to Standard Operating procedures based on revised national tuberculosis control program (RNTCP).7
Results
Out of total 1018 specimens, 118 (11.6%) samples were found to be smear positive and 900 (88.4%) samples were smear negative. Of the 118 smear positive specimens 104 (88.1%) were also culture positive by MGIT and/or LJ reference culture method while 13 (11.4%) were culture negative. Of the 900 smear negative specimens 116 were culture positive. A total of 768 smear negative samples were also culture negative by both the methods. Results for one smear positive sample and 16 smear negative samples were inconclusive as both solid and liquid cultures were contaminated and excluded from the final analysis. Contamination rate for MGIT liquid culture and LJ solid culture was 8.4% and 5.2% respectively.
A total of 220 (21.6%) samples were found to be culture positive taking combined reference of positivity by either solid or liquid culture. Amongst pulmonary samples culture positivity was 22.1% while in extrapulmonary samples culture positivity was 16.1% (Table 1). 781 (76.7%) samples were culture negative by both the reference methods. Of the total 220 culture positive samples 198 (90%) were MTB complex while 18 (8.2%) isolates were of non-tuberculous mycobacteria (NTM) and 4 (1.8%) isolates had mixed growth of MTB complex and NTM (Table 2). All the mixed isolates were obtained in MGIT liquid culture and presence of MTB complex in them was confirmed by molecular tests – line probe assay – The GenoType® Mycobacterium CM/AS assay (Hain Lifescience GmbH, Nehren, Germany). 138 (69.7%) samples were positive for MTB growth by both the culture methods. An additional 48 (24.2%) samples were positive only by MGIT liquid culture and 12 (6.1%) samples were positive for MTB only by LJ solid culture. Of the 14 MGIT negative LJ positive cases 8 were negative by MGIT while 6 samples had either contaminants or NTM in MGIT.
Table 1.
Culture positivity according to sample types (n = 1018).
| Number | Culture positive | % | |
|---|---|---|---|
| Pulmonary | |||
| Sputum | 781 | 192 | 24.6 |
| BAL | 121 | 9 | 7.4 |
| Biopsies (TBLB, endobronchial) | 29 | 5 | 17.2 |
| Extra-pulmonary | |||
| FNAC | 15 | 3 | 20.0 |
| Pus | 9 | 4 | 44.4 |
| Body fluid aspirates | 57 | 7 | 12.3 |
| Others (CSF, urine, bone marrow, tissue) | 6 | 00 | 0 |
TBLB – transbronchial lung biopsy, FNAC – fine needle aspiration cytology, CSF – cerebrospinal fluid.
Table 2.
Culture results of manual MGIT liquid and LJ solid culture.
| MGIT only | MGIT and LJ | LJ only | Total (MGIT/LJ) | |
|---|---|---|---|---|
| MTB complex | 48 | 138 | 12 | 198 |
| NTM | 11 | 5 | 2 | 18 |
| Mix MTB and NTM | 4 | 0 | 0 | 4 |
| Total | 63 | 143 | 14 | 220 |
Of the 198 cultures positive for MTB complex, 138 samples positive both by liquid manual MGIT and solid LJ culture methods were included in head to head analysis for time to positivity (TTP). Table 3 shows TTP in days as per smear grading by liquid MGIT and solid LJ culture methods. Smear status had a significant effect on time to culture positivity in both MGIT and LJ culture (p value <0.0001, 95% confidence interval).
Table 3.
Time to positivity (TTP) in days by liquid MGIT vs solid LJ culture methods (n = 138).
| Smear result | n | MGIT | LJ |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| 4+ | 28 | 7.3 ± 2.7 | 21.9 ± 4.6 |
| 3+ | 24 | 9.0 ± 4.2 | 25.9 ± 5.2 |
| 2+ | 25 | 12.5 ± 4.4 | 30.2 ± 8.0 |
| 1+ | 13 | 15.5 ± 4.9 | 33.4 ± 9.9 |
| All smear +ve | 90 | 10.4 ± 4.9 | 26.9 ± 7.8 |
| Smear negative | 48 | 20.2 ± 6.5 | 37.3 ± 9.1 |
| All smears | 138 | 13.8 ± 7.2 | 30.5 ± 9.1 |
p value <0.0001, 95% confidence interval for all grades of smears.
DST was performed for SIRE drugs on only one positive culture of each patient. A total of 113 DSTs were performed though 138 mycobacterial isolates were positive by both the methods, rest being duplicate culture positive samples. Table 4 shows comparison of DST results of manual MGIT with reference 1% proportional method on LJ media. Statistical analysis of performance of manual MGIT DST for SIRE drugs in comparison to 1% proportional method on LJ media is shown in Table 5.
Table 4.
Comparison of drug susceptibility results of manual MGIT with reference 1% proportional method on LJ media (n = 113).
| LJ |
|||
|---|---|---|---|
| MGIT | Sensitive | Resistance | |
| Streptomycin | Sensitive | 86 | 5 |
| Resistance | 3 | 19 | |
| INH | Sensitive | 89 | 1 |
| Resistance | 4 | 19 | |
| Rifampicin | Sensitive | 93 | 1 |
| Resistance | 2 | 17 | |
| Ethambutol | Sensitive | 83 | 4 |
| Resistance | 6 | 20 | |
Table 5.
Statistical analysis of performance of manual MGIT DST for SIRE drugs in comparison to 1% proportional method on LJ media (95% confidence interval).
| Streptomycin | INH | Rifampicin | Ethambutol | |
|---|---|---|---|---|
| Sensitivity | 96.6% | 95.7% | 97.9% | 93.3% |
| (90.46–99.30%) | (89.35–98.82%) | (92.60–99.74%) | (85.90–97.49%) | |
| Specificity | 79.2% | 95.0% | 94.4% | 83.3% |
| (57.85–92.87%) | (75.13–99.87%) | (72.71–99.86%) | (62.62–95.26%) | |
| PPV | 94.5% | 98.9% | 98.9% | 95.4% |
| (87.64–98.19%) | (93.96–99.97%) | (94.21–99.97%) | (88.64–98.73%) | |
| NPV | 86.4% | 82.6% | 89.5% | 76.9% |
| (65.09–97.09%) | (61.22–95.05%) | (66.86–98.70%) | (56.35–91.03%) | |
| Kappa (κ) value | 0.78 | 0.86 | 0.90 | 0.74 |
PPV – positive predictive value.
NPV – negative predictive value.
Discussion
The vast majority of TB cases go undiagnosed in developing countries due to non-availability of appropriate laboratory facilities. While liquid media are known to be more sensitive and rapid for isolation of mycobacteria, most laboratories in developing countries still rely on solid egg based LJ media for MTB culture. CDC recommends combined use of both liquid and solid media for maximum recovery of MTB.9, 10 Modified Middlebrook 7H9 broth is the preferred liquid medium for MTB culture7 and is used in various commercially available automated TB culture systems like MGIT 960/320 systems (Becton-Dickinson, Sparks, MD). MGIT tubes containing 7 ml of Modified Middlebrook 7H9 broth are incubated and monitored for increasing fluorescence every 60 min in these automated systems. But they are prohibitively expensive for implementation in small and medium volume laboratories.
MGIT products intended for manual use are also commercially available from the same manufacturer. Manual MGIT tubes contains 4 ml of Modified Middlebrook 7H9 broth which is supplemented with OADC and PANTA prior to inoculation. The tubes are incubated at 37 °C and read manually using a hand held Fluorescence Reader. At the time of tube positivity, there are approximately 104–107 CFU/ml of mycobacteria present. As per manufacturer's instruction for DST, a positive MGIT tube should be used the day after it first becomes positive, up to and including three days following initial positivity. A tube which has been positive longer than four days should be subcultured to a fresh MGIT tube and used from one to three days following positivity in that MGIT tube.8 We therefore made a protocol as shown in Fig. 2 for our laboratory and recommend the same for any MTB laboratory with low and medium volume.
Fig. 2.
Protocol for MTB culture by LJ and manual MGIT followed in the study.
In our study we resorted to daily reading of smear positive MGIT culture tube so that DST can be performed within stipulated period after confirmation and identification. All smear negative MGIT culture positive cases were subcultured once again in a fresh MGIT tube after identification for DST as they were being read only weekly. Depending upon the workload of laboratories smear negative tubes may also be read daily. Similarly MGIT negative LJ positive growth can also be subcultured in fresh MGIT tube for DST. These subculture tubes are read daily and further processed as smear positive MGIT tubes.
In our study, isolation rate for MTB was significantly higher in liquid MGIT (93.9%) than the solid LJ culture (75.7%) taking combined reference of positivity by either solid or liquid culture as gold standard (p value <0.0001, 95% confidence interval). A study done in a high throughput tertiary care centre in India on pulmonary and extra pulmonary samples has also demonstrated high recovery rate in MGIT in comparison to conventional LJ method.11 However, neither MGIT nor LJ medium was able to recover all the mycobacterial isolates on their own in this study. We have therefore taken combined reference of positivity by either solid or liquid culture as gold standard as also recommended by CDC for maximum recovery of MTB.9, 10
TTP using manual MGIT was 10.4, 20.2 and 13.8 days, respectively, for smear positive, smear negative and all smears as compared to 26.9, 37.3 and 30.5 days, respectively, using LJ culture. The cumulative percentages of smear positive culture positive cases at days 7, 14, and 21 were 32.8%, 85%, and 96.6%, respectively, in MGIT liquid culture; and 0%, 5.3%, and 40.6% in LJ solid culture while that of smear negative culture positive cases at days 7, 14, and 21 were 3.7%, 35.8%, and 74.3%, respectively, in MGIT liquid culture; and 0%, 0%, and 8.3% in LJ solid culture. Smear status had a significant effect on time to culture positivity in culture. Compared to other published studies11, 12, 13 the TTP for MGIT is comparatively higher in our study. This is due to the fact that those studies were done using automated liquid culture systems which continuously monitor the fluorescence in the MGIT tubes while we resorted to daily reading of smear positive specimen tubes and weekly reading of smear negative specimen tubes owing to high workload.
The emergence of drug resistant strains of MTB has made it essential that accurate and timely drug susceptibility results of all culture positive cases are provided for appropriate treatment and control of the disease. In our study, we obtained good agreement between manual MGIT and 1% proportional method on LJ media for susceptibility testing to first line antituberculous drugs. The agreement between manual MGIT and 1% proportional method was 97.7% for rifampicin and 95.6% for isoniazid. We could correctly identify all MDR cases using manual MGIT. The manual MGIT system also performed well for streptomycin and ethambutol when compared to the 1% proportion method with sensitivity, i.e. ability to detect true resistance being 96.2% and 93.1% respectively; although the specificity, i.e. ability to detect true susceptibility was low for both drugs (79.7% and 81.5% respectively). Similar correlation between manual MGIT and LJ susceptibility results have been reported in other studies.4, 5, 6 The discrepant results of DST could be due to improper concentration of inoculum, presence of clumps of bacteria in inoculum or due to presence of mixed population of susceptible and resistant mycobacteria within the inoculum.
CDC recommends turnaround time of 21 days and 28 days for MTB culture/identification and DST, respectively.14 In our study turnaround time from inoculation to drug susceptibility results for smear positive and smear negative cases using manual MGIT was 20.2 and 30.1 days respectively. The overall mean turnaround time for mycobacterial culturing and DST using manual MGIT was 23.6 days in comparison to 61.2 days for LJ medium.
The estimated cost of consumables for primary isolation in manual MGIT was Indian Nation Rupee (INR) 350 per sample. The total cost for isolation, identification and DST in manual MGIT for smear positive and smear negative cases was INR 2350 and INR 2700 respectively. This was substantially higher than LJ culture, which costs INR 50 per sample for isolation and INR 1000 for identification and DST to first line drugs. Although the cost is significantly high but the use of MGIT for the isolation of MTB is mainly justified by increased sensitivity and detection rate with reduced detection time as compared to solid culture media. MGIT cultures reduce the delays in obtaining results to days rather than weeks.
Short shelf life of lyophilized reagents used in manual MGIT could be a major limitation for its implementation in low volume laboratories. Commercially available PANTA antibiotic mixture vial is to be reconstituted in 3 ml of sterile distilled water and is sufficient for at least 25 MGIT tubes. The reconstituted mixture stored at 2–8 °C is to be used within 72 h or up to six months if stored at −20 °C or colder. Similarly SIRE drug susceptibility kit is sufficient for 30–40 tests and is to be stored in a similar fashion. Once thawed the mixture is to be used immediately and unused portion is to be discarded. This could add significantly to the cost of isolation and DST in manual MGIT.
Biosafety is a concern as any liquid TB culture media pose a great biohazard than solid media because of the risk of spillage, aerosolization and large mycobacterial load. But any laboratory already doing culture and drug sensitivity testing for TB on solid media which also requires manipulations of mycobacterial suspensions should be equipped with minimum Class II Type Biosafety Cabinet. Implementation of manual MGIT in these laboratories would help to rapidly isolate and detect MTB in primary culture and to test susceptibility to the primary anti-TB drugs.
Another limitation of the manual MGIT for implementation in laboratories of low and medium volume is that appropriate training of laboratory technician is required. MGIT requires careful processing and handling of specimens. The training should emphasize on strict implementation of SOPs for digestion and decontamination, biosafety measures and proper handling of culture material to minimize cross-contamination. The present study showed a contamination rate of 8.4% for MGIT and 5.2% with LJ medium. The contamination rate in the present study is similar to that reported in literature.15, 16 In our laboratory maximum contamination was observed in the initial phase of the study and then the practice of dedicated decontamination and asepsis was strengthened and continuously monitored leading to better results.
Middlebrook 7H9 liquid media used in MGIT is very sensitive and as a result is prone to contamination not only by non-mycobacterial organisms but also by NTM. A rapid method for differentiation of NTM from MTB complex is therefore required for all positive cultures before putting up DST. In our laboratory we use combined presumptive cord formation and immunochromatographic test for detection of MPT 64 Antigen for identification of MTB complex as suggested in recently published studies.17 These tests offer advantage of being rapid and are quite sensitive and specific when combined,17 but further adds to the cost of primary isolation in manual MGIT.
This study was conducted in a medium volume laboratory where up to 10 TB cultures were performed daily. We could implement manual MGIT system quite effectively in our laboratory for primary isolation and primary DST. Positive MTB culture and primary DST results were generally available within 28 days of specimen receipt. Implementation of Manual MGIT system is feasible in low to medium volume laboratories that already have experience with culture. These laboratories can get advantage of increased sensitivity and reduced delays of liquid culture media by using manual MGIT system. Higher cost of reagents involved is justified as it contributes significantly to improved patient management.
Conflicts of interest
The authors have none to declare.
Acknowledgements
This paper is based on Armed Forces Medical Research Committee Project No. 4404/2013 granted by the office of the Directorate General Armed Forces Medical Services and Defence Research Development Organization, Government of India.
References
- 1.20th ed. World Health Organisation; Geneva: 2015. Global Tuberculosis Report 2015 [Internet] Available from: http://www.who.int/tb/publications/global_report/en/ [cited 26 April 2016] [Google Scholar]
- 2.Stall N., Rubin T., Michael J.S. Does solid culture for tuberculosis influence clinical decision making in India? Int J Tuberc Lung Dis. 2011;15(5):641–646. doi: 10.5588/ijtld.10.0195. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization; Geneva: 2007. The Use of Liquid Medium for Culture and DST [Internet] Available from http://www.who.int/tb/laboratory/policy_liquid_medium_for_culture_dst/en/ [cited 26 April 2016] [Google Scholar]
- 4.Adjers-Koskela K., Katila M.L. Susceptibility testing with the manual mycobacteria growth indicator tube (MGIT) and the MGIT 960 system provides rapid and reliable verification of multidrug-resistant tuberculosis. J Clin Microbiol. 2003;41(3):1235–1239. doi: 10.1128/JCM.41.3.1235-1239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goloubeva V., Lecocq M., Lassowsky P., Matthys F., Portaels F., Bastian I. Evaluation of mycobacteria growth indicator tube for direct and indirect drug susceptibility testing of Mycobacterium tuberculosis from respiratory specimens in a Siberian prison hospital. J Clin Microbiol. 2001;39(4):1501–1505. doi: 10.1128/JCM.39.4.1501-1505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palaci M., Ueki S.Y., Sato D.N., Da Silva Telles M.A., Curcio M., Silva E.A. Evaluation of mycobacteria growth indicator tube for recovery and drug susceptibility testing of Mycobacterium tuberculosis isolates from respiratory specimens. J Clin Microbiol. 1996;34(3):762–764. doi: 10.1128/jcm.34.3.762-764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Central Tuberculosis Division . Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; New Delhi: 2009. Culture of Mycobacterium tuberculosis and Drug Susceptibility Testing on Solid Medium. [Google Scholar]
- 8.Siddiqi S.H., Rusch-Gerdes S. Foundation for Innovative New Diagnostics; Geneva: 2007. MGIT Procedure Manual. [Google Scholar]
- 9.Pfyffer G.E. Mycobacterium: general characteristics, laboratory detection and staining procedures. In: Murray P.R., Baron E.J., Jorgensen J.H., Landry M.L., Pfaller M.A., editors. Manual of Clinical Microbiology. 9th ed. American Society for Microbiology Press; Washington, DC: 2007. pp. 543–572. [Google Scholar]
- 10.Metchock B.G., Nolte F.S., Wallace R.J. Mycobacterium. In: Murray P.R., editor. Manual of Clinical Microbiology. 7th ed. American Society for Microbiology Press; Washington, DC: 1999. pp. 399–437. [Google Scholar]
- 11.Rodrigues C., Shenai S., Sadani M. Evaluation of the BACTEC MGIT 960 TB system for recovery and identification of M. tuberculosis complex in high through put tertiary care centre. Indian J Med Microbiol. 2009;27(3):217–221. doi: 10.4103/0255-0857.53203. [DOI] [PubMed] [Google Scholar]
- 12.Rishi S., Sinha P., Malhotra B., Pal M. A comparative study for the detection of mycobacteria by BACTEC MGIT 960, Lowenstein Jensen media and direct AFB smear examination. Indian J Med Microbiol. 2007;25(4):383–386. doi: 10.4103/0255-0857.37344. [DOI] [PubMed] [Google Scholar]
- 13.Giampaglia C.M., Martins M.C., Vieira G.B. Multicentre evaluation of an automated BACTEC 960 system for susceptibility testing of Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2007;11(9):986–991. [PubMed] [Google Scholar]
- 14.Shinnick T.M., Iademarco M.F., Ridderhof J.C. National plan for reliable tuberculosis laboratory services using a systems approach. Recommendations from CDC and the Association of Public Health Laboratories Task Force on Tuberculosis Laboratory Services. MMWR Recomm Rep. 2005;54(RR 6):1–12. [PubMed] [Google Scholar]
- 15.Somoskovi A., Kodmon C., Lanstos A. Comparison of recoveries of Mycobacterium tuberculosis using the automated BACTEC MGIT 960 System, BACTEC 460 TB System and Lowenstein–Jensen Medium. J Clin Microbiol. 2000;38(6):2395–2397. doi: 10.1128/jcm.38.6.2395-2397.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna B.A., Ebrahimzadeh A., Elliott B. Multicenter evaluation of the BACTEC MGIT 960 system for recovery of mycobacteria. J Clin Microbiol. 1999;37(3):748–752. doi: 10.1128/jcm.37.3.748-752.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar N., Agarwal A., Dhole T.N., Sharma Y.K. Rapid identification of Mycobacterium tuberculosis complex in clinical isolates by combining presumptive cord formation and MPT64 Antigen Immunochromatographic Assay. Indian J Tuberc. 2015;62(2):86–90. doi: 10.1016/j.ijtb.2015.04.007. [DOI] [PubMed] [Google Scholar]