Abstract
Introduction
Advancing age is a strong risk factor for adverse outcomes across multiple disease processes. However, septic surgical and trauma patients are unique in they incur two or more inflammatory insults. The effects of advanced age on sepsis pathophysiology and outcomes remain unclear.
Methods
We performed a single center, prospective observational cohort study of Surgical ICU patients with severe sepsis/septic shock. Peripheral blood was collected for genomic, cytokine and biomarker analysis at 0.5, 1, 4, 7, 14, 21 and 28 days after sepsis onset. Based on sensitivity analysis, cohorts were defined as “young” (<55 years) and “aged” (≥55 years). We compared age-defined cohorts to determine differences in patient characteristics, biomarker profiles and clinical outcomes.
Results
The cohort included 173 patients with severe sepsis (n=93; 53.8%) or septic shock (n=80; 46.2%), with a mean age of 60.9 (±14.5) years. Intra-abdominal sepsis was the leading source (n=81; 46.8%), followed by NSTI (n=33, 19.1%) and pneumonia (n=30; 17.3%). Aged patients had a higher comorbidity burden, but were otherwise similar to the young cohort. The aged cohort had a higher severity of early physiologic derangement (median APACHE II, 23 vs 18, p=0.002), greater incidence of multiple organ failure (MOF; 64.3% vs 40.4%, p=0.006), and hospital mortality (15.9% vs 2.1%, p=0.016). Six-month mortality was significantly higher in the aged as compared to young cohort (31% vs 9%, p=0.003). Aged septic patients biomarker trajectories suggestive of persistent immunosuppression (Absolute lymphocyte count, sPDL-1) and catabolism (Urine 3MH-Cr ratio, IGF, IGF1BP3, albumin) out to 28 days after sepsis.
Conclusions
Aged, critically ill surgical patients have greater organ dysfunction, and incidence of adverse clinical outcomes after sepsis. Biomarker profiles suggest an immunophenotype of persistent immunosuppression and catabolism. Advanced age may necessitate novel therapeutic strategies to promote multi-system organ recovery and improve survival after sepsis.
Level of Evidence
Level II, prognostic
Keywords: Sepsis, chronic critical illness, immunosuppression, catabolism
BACKGROUND
Advancing age is a well-known risk factor for increased morbidity and mortality across a wide-range of disease processes, including traumatic injury, sepsis and acute critical illness.(1–3) In addition to clinically obvious factors such as increasing incidence and severity of comorbidities and frailty, the aging process has also been shown to have a significant effect on the innate immune system. The phenomenon of “inflammaging”, which is a state of low-grade chronic systemic inflammation associated with physiologic aging, has been extensively described.(4, 5) Additionally, the innate immune response after shock states is known to differ between the young and the aged. Previous work has shown that advanced age in patients with hemorrhagic shock is associated with an aberrant leukocyte genomic response, abnormal myelopoeisis, and dysfunctional innate immunity.(1, 6) Similar findings are found in pre-clinical models of sepsis, but the mechanism, magnitude and persistence of immune dysfunction after sepsis has yet to be fully elucidated.(7)
We have shown previously that shock states after both severe injury and sepsis are associated with a similar phenotype of persistent inflammation, immunosuppression and catabolism.(8–12) However, the effect of age on the innate immune response in critically ill patients after sepsis remains incompletely defined. Therefore, we sought to further characterize the role of advancing age on the innate immune response to sepsis and its association with adverse clinical outcomes. Prior to undertaking an exploratory analysis of an ongoing prospective cohort study of surgical ICU patients with sepsis, we hypothesized that advanced age would be associated with a biomarker immunophenotype consistent with an abnormal innate immune response, a delayed trajectory of immunologic recovery, and would be associated with poor clinical outcomes, including increased number and severity of organ dysfunction, increased intensive care resource utilization, and higher inpatient mortality. Additionally, given the continuing trend of decreasing inpatient mortality after sepsis, we hypothesized that in sepsis inpatient survivors, those with advanced age would have higher rates of discharge disposition to resource intensive facilities, known to be associated with dismal long-term outcomes.
METHODS
Study design, inclusion/exclusion criteria and enrollment
This prospective observational cohort study consecutively enrolled trauma and surgical intensive care unit (ICU) patients that were admitted with, or subsequently developed sepsis over a four and a half year period (2012–2016) at a quaternary academic medical and Level One trauma center (UF Health - Gainesville, Florida, U.S.A.). This study was approved by the institutional review board of the University of Florida and registered with clinicaltrials.gov (NCT02276417). Overall program study design and protocols for the Sepsis and Critical Illness Center (SCIRC) research program have been published for further reference.(13) Additional methodologic details relevant to this analysis are also available in Supplemental Digital Content (SDC 1). Sepsis screening, diagnosis, resuscitation and management was performed with uniform evidence-based sepsis management protocols supplemented by computerized clinical decision support (CCDS) to ensure timely and standardized intensive care.(13)
Inclusion criteria for this analysis was the same as for the overall SCIRC cohort and included the following: 1) age ≥ 18 years; 2) clinical diagnosis of severe sepsis, or septic shock as defined by 2001 consensus guidelines (14); and 3) entrance into the ICU sepsis clinical management protocol. All study patients subsequently underwent final clinical adjudication in prospective fashion by the physician investigators at weekly program adjudication and retention meetings. At that time, those whose clinical picture did not subsequently support a diagnosis of sepsis were excluded from further participation in the study and not included in subsequent analyses.(13)
Exclusion criteria consisted of any of the following: 1) refractory shock (death <24 hours from sepsis protocol initiation) or inability to achieve source control; 2) pre-admission expected lifespan <3 months; 3) patient/proxy not committed to aggressive management; 4) severe CHF (NYHA Class IV); 5) Child-Pugh Class C liver disease or pre-liver transplant; 6) known HIV with CD4+ count <200 cells/mm3; 7) patients receiving chronic corticosteroids or immunosuppressive agents, including organ transplant recipients; 8) pregnancy; 9) institutionalized patients; 10) inability to obtain informed consent within 96 hours of enrollment; 11) chemotherapy or radiotherapy within 30 days; 12) severe traumatic brain injury; and 13) spinal cord injury resulting in permanent sensory and/or motor deficits. These criteria were utilized to focus on a population likely to survive the initial insult of sepsis in order to study the subsequent effects on the innate immune response, and whose severe comorbidities or severe functional injuries (i.e. TBI/SCI) would not be the primary determinant of subsequent clinical outcomes.
Patient demographics, comorbidities, sepsis diagnosis and severity adjudication and clinical outcomes were manually collected in prospective fashion. Additionally, raw clinical data from the electronic medical record (EMR), including information on patient laboratory results, vital signs, medications, and information related to hospital and SICU admission and discharge were directly uploaded to an analytical database by the University of Florida Health Integrated Data Repository (IDR).(13)
Patient cohort and outcomes classification
Age-based cohorts were designated a priori as ‘young’ (<55 years) or ‘aged’ (≥55 years) based on previous age-related outcomes data after severe trauma patients admitted to surgical ICUs.(1, 3) Subsequent sensitivity analysis confirmed this dichotomization as optimal for differentiation of clinical outcomes and biomarker profiles in this sepsis population (see Results). Primary clinical outcomes included hospital mortality, ICU length of stay (LOS), incidence and severity of multiple organ failure (MOF), clinical trajectory and discharge disposition. Clinical trajectory was defined as ‘early death’, ‘rapid recovery’ (RAP), or ‘chronic critical illness’ (CCI). CCI was defined as an ICU LOS greater than or equal to 14 days with evidence of persistent organ dysfunction, determined using components of the Sequential Organ Failure Assessment (SOFA) score (SDC 1).(13) Rapid recovery (RAP) patients were those who did not meet criteria for CCI or early death (death <14 days after sepsis protocol onset). Discharge disposition was classified based on known association with long-term outcomes as either ‘good’ (Home, home with health care service, or rehabilitation facility), or ‘poor’ (Long-term acute care facility [LTAC]), skilled nursing facility [SNF], another acute care hospital, hospice or inpatient death).
Biomarker analyses
For this prospective cohort study, a set of a priori immune biomarkers were proscribed prior to study onset based on the cohort study’s underlying mechanistic hypotheses regarding persistent inflammation, immunosuppression and catabolism after sepsis (SDC1).(13) Based on preliminary data, a focused set of peripheral biomarkers were selected from the overall sampling panel for this age-focused analysis, including (IL-6, IL-8, TNF-α, C-reactive protein [CRP]), immunosuppression (absolute lymphocyte count [ALC], IL-10 and soluble programmed death ligand one [sPD-L1]), and catabolism (insulin growth factor 1 [IGF1], insulin-like growth factor binding protein 3 [IGFBP3], albumin) at 12 hours, one, four, seven, 14 days, and weekly thereafter while hospitalized. Biomarker analyses were performed utilizing the MILLIPLEX® Multiplex (Merck KGaA, Darmstadt, Germany) and Luminex MAGPIX® (Luminex corp., Austin, Texas, U.S.A.) systems. Additionally, urine was collected at these time points to determine 3-methylhistidine (3-MH/Cr) to creatinine ratios as a measure of protein catabolism. 3-MH/Cr analyses were performed by Heartland Assays (Metabolic Technologies Inc., Ames, Iowa, U.S.A.).(13)
Statistical Analysis
We present data as either frequency and percentage, or mean and standard deviation, or median and 25th/75th percentiles. We utilized Fisher’s exact and Kruskal–Wallis tests for comparison of categorical and continuous variables, respectively. We compared measured biomarkers using non-parametric rank tests of medians to determine significant differences between groups at each time point. Biomarker trajectories were modeled via generalized estimating equations (GEE) with Poisson variance assumption and log link to determine differences in the trajectory of means between groups over time. Six-month survival analysis was performed using the Kaplan-Meier method and Log-rank test. All significance tests were two-sided, with p-value ≤0.05 considered statistically significant. We applied a post-hoc Benjamini & Hochberg procedure to the clinical outcome variables to control for false discovery rate (FDR) less than 5 percent.(15, 16) Briefly, the individual p values are placed in order, from smallest to largest. The smallest p value has a rank of i=1, then next smallest has i=2, etc. Each individual p value is compared to its Benjamini-Hochberg critical value, (i/m)Q (q-value) where i is the rank, m is the total number of tests, and Q is the false discovery rate (i.e. 0.05). The largest p value that has p<q is significant, and all of the P values smaller than it are also significant, even the ones that aren’t less than their Benjamini-Hochberg critical value. We performed all statistical analyses with SAS (v.9.4, Cary, NC).
RESULTS
Cohort demographics, sepsis diagnosis and severity
One hundred and seventy-three ICU patients that were admitted with, or developed, severe sepsis/septic shock were consecutively enrolled over the study period. Overall and age-defined cohort characteristics are shown in SDC2 and Table 1. The overall study population represents a relatively older aged cohort of patients (mean age, 60.9 years), with a significant comorbidity burden (median Charlson comorbidity score, 4) and severe physiologic derangement at 24 hours after sepsis onset (median APACHE II score, 22) (SDC2). The most common locations from which patients were admitted to the ICU included the operating room (42%), direct ICU inter-facility transfer (22%), and the emergency department (21%). The majority of patients were admitted to the hospital with an acute, infection-related diagnosis, had intra-abdominal infection as the septic source, and approximately 65 percent underwent a source control procedure (Table 1, SDC2). Preliminary sensitivity analyses revealed that age dichotomization at 55 years maximized differentiation in clinical outcomes and a priori selected biomarker profiles between ‘young’ and ‘aged’ cohorts for subsequent analyses.
Table 1.
Age-defined cohort baseline characteristics
| Age ≥ 55 (n=126) | Age < 55 (n=47) | p-value | |
|---|---|---|---|
| Male, n (%) | 72 (57.1) | 29 (61.7) | 0.61 |
| Age in years, mean ± SD | 68.2 ± 7.8 | 41.3 ± 9.2 | NA |
| Race, n (%) | 0.35 | ||
| Caucasian (White) | 115 (91.3) | 43 (91.5) | |
| African American | 9 (7.1) | 2 (4.3) | |
| Asian | 1 (0.8) | 1 (2.1) | |
| Pacific Islander | 0 (0) | 1 (2.1) | |
| Other | 1 (0.0) | 0 (0) | |
| BMI, median (25th, 75th) | 28.8 (24.2, 34.9) | 27.5 (24.1, 37.2) | 0.78 |
| Number of comorbidities, n (%) | 0.0003 | ||
| 0 | 22 (17.5) | 20 (42.6) | |
| 1 | 32 (25.4) | 15 (31.9) | |
| 2 | 30 (23.8) | 8 (17.0) | |
| ≥3 | 42 (33.3) | 4 (8.5) | |
| Charlson comorbidity index, median (25th, 75th) | 5 (4, 7) | 1 (0, 2) | 0.0001 |
| APACHE II, median (25th, 75th) | 23 (17, 27) | 18 (13, 23) | 0.0023 |
| Inter-facility hospital transfer, n (%) | 62 (49.2) | 20 (42.6) | 0.50 |
| Hospital Admission Diagnosis, n (%) | 0.31 | ||
| Intra-abdominal sepsis | 26 (20.6) | 9 (19.1) | |
| NSTI | 17 (13.5) | 13 (27.7) | |
| Acute vascular disease - aortic/mesenteric | 16 (12.7) | 1 (2.1) | |
| Planned surgical procedure | 14 (11.1) | 7 (14.9) | |
| Trauma | 9 (7.1) | 7 (14.9) | |
| Surgical Site Infection | 8 (6.3) | 2 (4.3) | |
| Necrotizing pancreatitis | 7 (5.6) | 2 (4.3) | |
| Pneumonia | 4 (3.2) | 1 (2.1) | |
| UTI | 4 (3.2) | 2 (4.3) | |
| Acute vascular disease - extremity | 2 (1.6) | 0 (0) | |
| Other - non-infectious | 13 (10.3) | 2 (4.3) | |
| Other - acute infection | 6 (4.8) | 1 (2.1) | |
| Delayed sepsis (onset >2 days after admission), n (%) | 45 (35.7) | 15 (31.9) | 0.72 |
| Sepsis severity, n (%) | 0.0598 | ||
| Severe sepsis | 62 (49.2) | 31 (66.0) | |
| Septic shock | 64 (50.8) | 16 (34.0) | |
| Primary sepsis diagnosis, n (%) | 0.33 | ||
| Intra-abdominal sepsis | 62 (49.2) | 19 (40.4) | |
| NSTI | 18 (14.3) | 15 (31.9) | |
| Pneumonia | 23 (18.3) | 7 (14.9) | |
| UTI | 10 (7.9) | 2 (4.3) | |
| Surgical Site Infection | 6 (4.8) | 2 (4.3) | |
| Empyema | 3 (2.4) | 0 (0) | |
| Bacteremia/CLABSI | 3 (2.4) | 0 (0) | |
| Other | 1 (0.8) | 1 (2.1) | |
| Source control procedure, n (%) | 81 (64.3) | 31 (66.0) | 0.86 |
SD, standard deviation; CLABSI, central line-associated bloodstream infection; NSTI, necrotizing soft tissue infection; UTI, urinary tract infection.
Young and aged cohorts were similar with regards to baseline characteristics with the exception of the aged cohort carrying a higher comorbidity burden (Table 1). The aged cohort exhibited a clinically, but not statistically, significant higher incidence of vasopressor dependent septic shock (51% vs 34%, p=0.0598, Table 1). At 24 hours after sepsis protocol initiation, the aged also cohort had a higher degree of physiologic derangement as measured by APACHE II score (Table 1).
Organ dysfunction and clinical outcomes
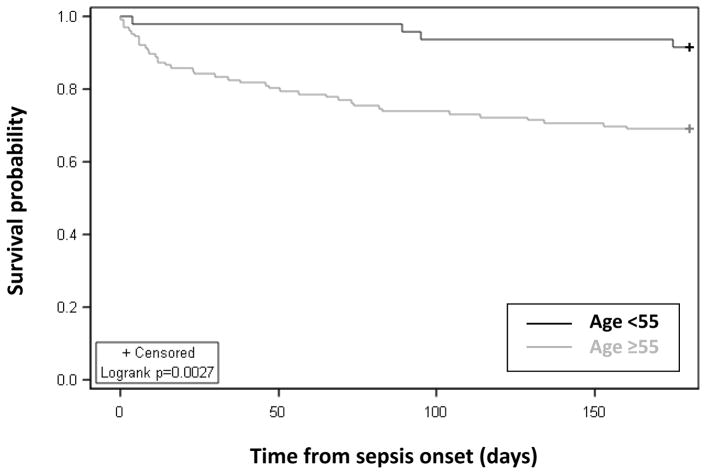
Overall in-hospital mortality for the study population of critically ill sepsis patients was 12 percent (SDC3). While having similar ICU and hospital length of stay, the aged cohort had significantly higher incidence and severity of multiple organ dysfunction (Table 2). Inpatient mortality was significantly higher in the aged (16%) as compared to the young (2%) cohort (Table 2). As compared to the young cohort, the aged cohort had a significantly higher percentage of “poor” discharge dispositions (LTAC, SNF, another hospital, hospice) known to be associated with poor long-term outcomes, as opposed to discharge to home or a rehabilitation facility (Table 2). The incidence of 6-month mortality in the aged was over three times greater than that of the young cohort (31% vs 9%, p=0.0027; Figure 1), and double the initial in-hospital mortality rate (inpatient, 31%; vs 6-month, 16%).
Table 2.
Clinical outcomes of young vs aged cohort
| Age ≥ 55 (n=126) | Age < 55 (n=47) | p-value | q-value1 | |
|---|---|---|---|---|
| Hospital mortality, n (%) | 20 (15.9) | 1 (2.1) | 0.016* | 0.0373* |
| ICU LOS, median (25th, 75th) | 13 (6, 21) | 11 (5, 26) | 0.70 | 0.73 |
| Hospital LOS, median (25th, 75th) | 18 (11, 34) | 21 (8, 36) | 0.61 | 0.71 |
| Maximum SOFA score, median (25th, 75th) | 9 (7, 12) | 7 (5, 9) | 0.0009* | 0.0042* |
| MOF incidence2, n (%) | 81 (64.3) | 19 (40.4) | 0.0057* | 0.02* |
| Organ system dysfunction2, n (%) | ||||
| Pulmonary | 71 (56.4) | 21 (44.7) | 0.23 | 0.36 |
| CNS | 72 (57.1) | 23 (48.9) | 0.39 | 0.50 |
| Cardiovascular | 63 (50.0) | 13 (27.7) | 0.01* | 0.028* |
| Hepatic | 8 (6.4) | 2 (4.3) | 0.73 | 0.73 |
| Coagulation | 10 (7.9) | 1 (2.1) | 0.29 | 0.41 |
| Renal | 32 (25.4) | 6 (12.8) | 0.10 | 0.18 |
| Clinical trajectory, n (%) | 0.062 | 0.12 | ||
| CCI/Early death | 74 (58.7) | 20 (42.6) | ||
| Rapid recovery | 52 (41.3) | 27 (57.5) | ||
| Discharge disposition, n (%) | 0.0001* | 0.0007* | ||
| “Good” disposition | 42 (33.3) | 31 (66.0) | 0.0001* | 0.0007* |
| Home | 13 (10.3) | 13 (27.7) | ||
| Home with healthcare services | 19 (15.1) | 16 (34.0) | ||
| Rehab | 10 (7.9) | 2 (4.3) | ||
| “Poor” disposition | 84 (66.7) | 16 (34.0) | 0.0001* | 0.0007* |
| LTAC | 25 (19.8) | 6 (12.8) | ||
| SNF | 25 (19.8) | 4 (8.5) | ||
| Another Hospital | 8 (6.4) | 5 (10.6) | ||
| Hospice | 6 (4.8) | 0 (0) | ||
| Death | 20 (15.9) | 1 (2.1) | ||
Benjamini & Hochberg correction for multiple comparisons,
represents statistical significance at false discovery rate <5%.
Incidence of organ system failure onset during hospitalization as defined by modified SOFA criteria, see Methods and SDC1.
SOFA, sequential organ failure assessment score; MOF, multiple organ failure by SOFA criteria; CCI, chronic critical illness (>14 day ICU LOS with ongoing organ dysfunction); Rehab, inpatient rehabilitation facility; LTAC, long-term acute care facility; SNF, skilled nursing facility.
Figure 1.
6-month survival of aged compared to young sepsis patients.
Inflammation, immunosuppression and catabolism biomarkers
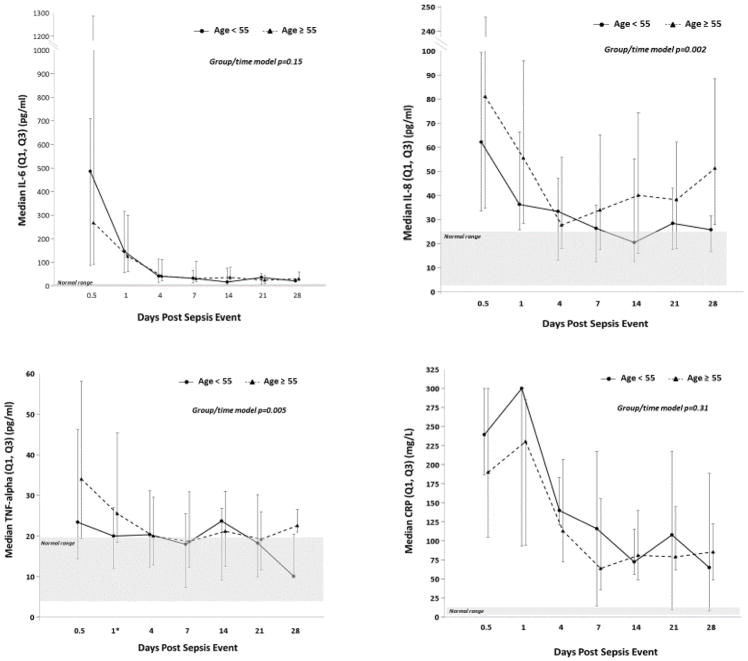
Select biomarker profiles of inflammation, immunosuppression and catabolism are shown in Figure 2. As sampling was limited to alive inpatients, missing data points increased over time due to discharges, LTAC/SNF transfers and deaths, and are listed along with full biomarker data in SDC 4. In both young and aged cohorts, levels of IL-6, IL-8, and TNFα were significantly and persistently elevated above the normal range of healthy controls up to 28 days after sepsis onset, consistent with large and sustained innate inflammatory response to infection (Figure 2). Both young and aged cohorts exhibited a similar, robust and sustained acute phase response after sepsis, as measured by CRP levels (Figure 2). Time-dependent modeling revealed modest, but statistically significant differences between young and aged cohort inflammatory biomarker trajectories over time (Figure 2). IL-6 levels were similar between groups in both early response to sepsis and overall 28-day trajectory (Figure 2). IL-8, IL-10 and TNFα showed modestly increased elevations early after sepsis, with slightly slower trend towards normalization over time (Figure 2, SDC 4). There were no significant differences between young and aged cohorts in the magnitude or trajectory of circulating recruitment chemokines, including GM-CSF, G-CSF, MCP1, MIP-1α, and SDF1 (SDC 4). Interestingly, IP-10, a known biomarker associated with chronic inflammatory conditions, was stably elevated from baseline to 28 days in the aged cohort, and may be suggestive of pre-existing and chronic low-level inflammation (SDC 4).
Figure 2.
Circulating inflammatory biomarker analysis of aged compared to young sepsis patients.
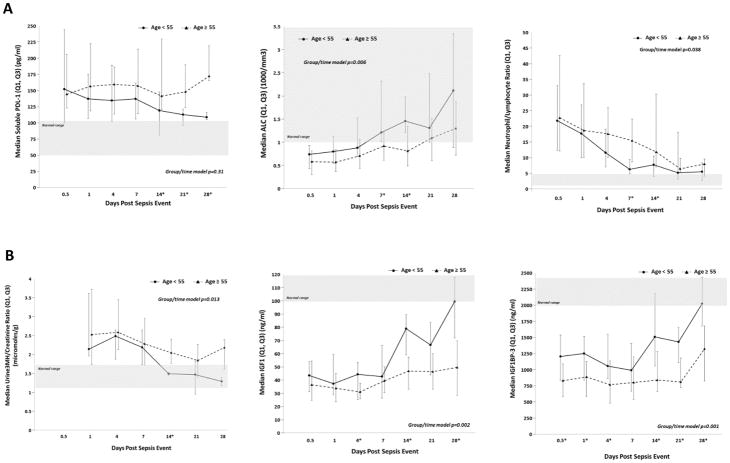
Biomarker trajectories associated with immune suppression (sPDL-1, ALC) were consistent with a persistent state of immunosuppression out to 28 days after sepsis, in both young and aged cohorts (Figure 3a). However, soluble PDL-1 levels remained persistently elevated in the aged cohort out to 28 days after sepsis, whereas the young cohort established a trajectory towards normalization. Additionally, cellular markers of immunosuppression were different between the young and aged cohorts. While both cohorts exhibited significantly decreased absolute lymphocyte counts after sepsis, young subjects returned to normal levels by day 14, whereas aged individuals had persistent lymphopenia out to 28 days after sepsis onset. Also, the aged cohort had a significantly higher neutrophil to lymphocyte ratio (a marker of systemic inflammation and adaptive immune suppression) between 1–2 weeks after sepsis (Figure 3a).
Figure 3.
Circulating immunosuppression and catabolism biomarker analysis of aged compared to young sepsis patients.
Biomarkers of protein catabolism revealed a state of increased protein catabolism after sepsis in all patients. However, the aged cohort exhibited evidence of a state of prolonged, persistent catabolism as compared to the young cohort. While the urine 3-MH/Cr ratio normalized in the young cohort by day 14, it remained persistently elevated in the aged cohort (Figure 3b). Additionally, the insulin growth factor/growth hormone axis (as measured by IFG1/IGFBP3) remained persistently suppressed out to 28 days in the aged as compared to the young cohort (Figure 3b). While pre-albumin levels increased over time in both cohorts, albumin levels were higher in the young cohort at 21 days after sepsis onset than in the aged (SDC 4).
DISCUSSION
Based upon ongoing improvements in critical care organ support and implementation of evidence-based clinical protocols, inpatient mortality after sepsis continues to decline.(2, 17, 18) While this may at first appear to be a story of success, the long-term mortality and function of sepsis survivors remains unclear. As survivorship increases, CCI (>14 days ICU LOS with ongoing organ dysfunction) is now common (19% incidence) after severe trauma, and is the predominant trajectory (49%) of surgical intensive care unit patients with sepsis.(3, 11) It is therefore crucial to understand the risk factors, underlying mechanisms and long-term outcomes associated with the development of CCI after these systemic, pro-inflammatory insults.
Advanced age is a known risk factor across a broad range of medical conditions. We have recently shown that advancing age is an independent predictor of inpatient mortality and poor discharge disposition amongst those that initially survive both severe trauma and surgical sepsis.(3, 11) In this current study, we found that surgical ICU patients older than 55 years of age appear to have more profound shock, greater severity of organ dysfunction, and higher incidence of adverse clinical outcomes after sepsis. Inpatient mortality in the aged was nearly 8-fold higher than those under age fifty-five. While age-associated inpatient mortality after sepsis has been thoroughly reported, the long-term mortality of aged sepsis survivors is incompletely understood. Two-thirds of patients over the age of 55 had an inpatient disposition (LTAC, SNF, inpatient facility, hospice) known to be associated with high 1 to 2 year mortality rates.(19, 20) Additionally, we were able to show through prospective follow-up that while inpatient mortality after sepsis in the aged is nineteen percent, mortality jumps to a striking 31 percent at six months. The reasons behind these dismal outcomes in the aged after sepsis are surely multi-factorial, including a higher burden of chronic comorbidities. Additionally, there is some truth in the adage that “age is merely a number”. Chronologic age as a predictor is very likely a reflection of its high correlation with underlying physiologic frailty which is probably the true factor driving these poor outcomes.(20–22) Unfortunately, we did not measure specific frailty metrics in this study population. However, we have incorporated baseline and serial follow-up frailty measurements into an ongoing prospective cohort study (NCT02276417).
While the adverse outcomes seen in advancing age after sepsis are multi-factorial, we have shown evidence here that there are clear age-dependent differences in the physiologic and innate immunologic responses after sepsis. Both young and aged patients mounted a robust pro-inflammatory response early after sepsis, with prolonged elevation of circulating inflammatory cytokines above the level of healthy, normal controls for up to 28 days after sepsis onset (Figure 2). This is consistent with other recent findings documenting a state of prolonged, dysfunctional inflammation after sepsis.(10, 23, 24) While the differences in the initial inflammatory response as measured by circulating pro-inflammatory biomarkers were modest at best, there is clear differentiation between young and aged individuals regarding resolution of biomarker profiles consistent with post-sepsis immunosuppression and catabolism. Aged individuals show a clear trend of failure to resolve both circulating (sPDL-1) and cellular biomarker profiles (ALC) consistent with a state of persistent immunosuppression (Figure 3).(25, 26) There is now an increasing literature base noting striking similarities between cancer and post-sepsis immunosuppression through mechanisms such as the programmed death ligand (PDL) signaling pathway.(11, 27) Additionally, secondary infections have been shown to be predictors of the development of CCI and increased mortality in surgical ICU patients.(11, 28, 29) As the development of CCI after sepsis is known to be associated with poor hospital discharge disposition and high 6-month mortality, breaking the cycle of persistent immunosuppression after sepsis is an attractive target for interventional trials of immune checkpoint inhibitors.(30, 31) Accordingly, a multi-center phase III clinical trial investigating the effects of blockade of the PD-1 pathway after sepsis has recently begun enrollment (NCT02960854).
Sarcopenia and muscle wasting is another common clinical manifestation of persistent critical illness, is postulated to be associated with both systemic and local inflammation, and likely contributes to poor functional outcomes as a barrier to successful physical rehabilitation.(32, 33) Recently, we have shown evidence that a biomarker profile of persistent protein catabolism can be seen in surgical ICU patients that go on to develop CCI.(12) Here we have shown further evidence that a persistent state of protein catabolism after sepsis, and that this fails to resolve in the aged (Figure 3b). This suggests that dysfunctional inflammation after sepsis may be closely linked to CCI-associated sarcopenia, and supports the hypothesis that a multi-modality approach to interventional therapies will be necessary.
There are several limitations to this study which warrant discussion. As a single center study at a large referral quaternary medical center, these finding will need replication amongst a broader population of critically ill septic patients. Additionally, while we have shown biomarker patterns and trajectories consistent with the persistent inflammation, immunosuppression and catabolism syndrome (PICS) with notable differences in magnitude and permanence between the young and aged, further characterization of immunologic functional deficits are required. We chose to identify the trend of PICS phenotype across multiple mechanistically overlapping biomarkers in order to avoid the limitations of the choice of a single, possibly flawed selection of an individual biomarker. With this choice, we accepted the increased possibility of a single false positive result. Thus individual biomarker findings at any given time point, or their use in prediction of outcomes, must be interpreted with caution. We currently have ongoing studies that will investigate this with functional immune effector cell assays to demonstrate functional immunosuppression, and radiographic and biopsy analyses to confirm sarcopenia and its association with long-term physical function. We also acknowledge that our sepsis severity and inclusion criteria are based on 2001 consensus definitions, as this study was designed prior to publication of consensus Sepsis-3 criteria. Compared to Sepsis-3 criteria, this study likely enrolled a subset of patients of lower organ dysfunction severity at time of sepsis onset. However, we would argue that any infection in the ICU setting is of significant clinical significance and likely to detrimentally contribute to the already perturbed innate immune response. Regardless, we do not believe the choice of consensus criteria for classification purposes affects the outcomes of this analysis of clinically significant infection in the surgical ICU.
In summary, we have shown that there are significant differences between young and aged patients in the physiologic and innate immune response of septic critically ill surgical patients. Aged, critically ill patients have more profound shock, greater organ dysfunction, and incidence of adverse clinical outcomes after sepsis. The 6-month mortality of aged septic patients is strikingly dismal, and 3 times higher than the rate of inpatient mortality. Based upon circulating biomarker patterns, aged septic patients show evidence of an immunophenotype of persistent inflammation, immunosuppression and catabolism.
Supplementary Material
Acknowledgments
Supported, in part, by National Institute of General Medical Sciences (NIGMS) grants: R01 GM-040586 and R01 GM-104481 (LM), R01 GM-113945 (PE), and P50 GM-111152 (FM, SB, LM, PE, BB) awarded by the National Institute of General Medical Sciences (NIGMS). Support was also provided by National Institute on Aging (NIA) grant R03 AG056444 (SB). In addition, this work was supported, in part, by a postgraduate training grant T32 GM-008721 (LM, JS) in burns, trauma, and perioperative injury by NIGMS. The authors have no relevant conflicts of interest to disclose.
The authors would like to acknowledge the staff of the University of Florida Sepsis and Critical Illness Research Center for their invaluable contributions, including Jennifer Lanz, Ruth Davis, Jillianne Brakenridge, Ashley McCray, Bridget Baisden, Ricky Ungaro, Dina Nacionales, Marvin Dirain, Angela Avery, Tabitha Johns and Ada Malcolm.
Footnotes
This paper was presented at the 76th Annual Meeting of AAST and Clinical Congress of Acute Care Surgery and awarded the AAST Peter C. Canizaro Award, September 13–16, 2017. Baltimore, MD.
Author contributions: PE, JS, AB, AM, LM, FM, and SB contributed to the conception and design of this project. TO, ZW, GG, BB, LM, FM and SB performed data analysis and interpretation. All authors (JS, PE, TO, ZW, GG, AB, BB, AM, PE, LM, FM, SB) contributed in drafting the manuscript and/or revising it critically for intellectual content.
References
- 1.Vanzant EL, Hilton RE, Lopez CM, Zhang J, Ungaro RF, Gentile LF, Szpila BE, Maier RV, Cuschieri J, Bihorac A, et al. Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care. 2015;19:77. doi: 10.1186/s13054-015-0788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–90. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mira JC, Cuschieri J, Ozrazgat-Baslanti T, Wang Z, Ghita GL, Loftus TJ, Stortz JA, Raymond SL, Lanz JD, Hennessy LV, et al. The Epidemiology of Chronic Critical Illness After Severe Traumatic Injury at Two Level-One Trauma Centers. Crit Care Med. 2017;45(12):1989–96. doi: 10.1097/CCM.0000000000002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 5.Melton DW, Roberts AC, Wang H, Sarwar Z, Wetzel MD, Wells JT, Porter L, Berton MT, McManus LM, Shireman PK. Absence of CCR2 results in an inflammaging environment in young mice with age-independent impairments in muscle regeneration. J Leukoc Biol. 2016;100(5):1011–25. doi: 10.1189/jlb.3MA0316-104R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nacionales DC, Szpila B, Ungaro R, Lopez MC, Zhang J, Gentile LF, Cuenca AL, Vanzant E, Mathias B, Jyot J, et al. A Detailed Characterization of the Dysfunctional Immunity and Abnormal Myelopoiesis Induced by Severe Shock and Trauma in the Aged. J Immunol. 2015;195(5):2396–407. doi: 10.4049/jimmunol.1500984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nacionales DC, Gentile LF, Vanzant E, Lopez MC, Cuenca A, Cuenca AG, Ungaro R, Li Y, Baslanti TO, Bihorac A, et al. Aged mice are unable to mount an effective myeloid response to sepsis. J Immunol. 2014;192(2):612–22. doi: 10.4049/jimmunol.1302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mira JC, Szpila BE, Nacionales DC, Lopez MC, Gentile LF, Mathias BJ, Vanzant EL, Ungaro R, Holden D, Rosenthal MD, et al. Patterns of gene expression among murine models of hemorrhagic shock/trauma and sepsis. Physiol Genomics. 2016;48(2):135–44. doi: 10.1152/physiolgenomics.00072.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, Ungaro R, Davis R, Cuenca AG, Gentile LF, Nacionales DC, Cuenca AL, Bihorac A, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76(1):21–9. doi: 10.1097/TA.0b013e3182ab1ab5. discussion 9–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathias B, Delmas AL, Ozrazgat-Baslanti T, Vanzant EL, Szpila BE, Mohr AM, Moore FA, Brakenridge SC, Brumback BA, Moldawer LL, et al. Human Myeloid-derived Suppressor Cells are Associated With Chronic Immune Suppression After Severe Sepsis/Septic Shock. Ann Surg. 2016 Apr;265(4):827–834. doi: 10.1097/SLA.0000000000001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stortz JA, Murphy TJ, Raymond SL, Mira JC, Ungaro R, Dirain ML, Nacionales DC, Loftus TJ, Wang Z, Ozrazgat-Baslanti T, et al. Evidence for Persistent Immune Suppression in Patients WHO Develop Chronic Critical Illness After Sepsis. Shock. 2018 Mar;49(3):249–258. doi: 10.1097/SHK.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stortz JA, Mira JC, Raymond SL, Loftus TJ, Ozrazgat-Baslanti T, Wang Z, Ghita GL, Leeuwenburgh C, Segal MS, Bihorac A, et al. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. J Trauma Acute Care Surg. 2018 Feb;84(2):342–349. doi: 10.1097/TA.0000000000001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loftus TJ, Mira JC, Ozrazgat-Baslanti T, Ghita GL, Wang Z, Stortz JA, Brumback BA, Bihorac A, Segal MS, Anton SD, et al. Sepsis and Critical Illness Research Center investigators: protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open. 2017 Aug 1;7(7):e015136. doi: 10.1136/bmjopen-2016-015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57(1):289–300. [Google Scholar]
- 16.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annals of Statistics. 2001;29(4):1165–88. [Google Scholar]
- 17.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–7. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 19.Davidson GH, Hamlat CA, Rivara FP, Koepsell TD, Jurkovich GJ, Arbabi S. Long-term survival of adult trauma patients. JAMA. 2011;305(10):1001–7. doi: 10.1001/jama.2011.259. [DOI] [PubMed] [Google Scholar]
- 20.Joseph B, Orouji Jokar T, Hassan A, Azim A, Mohler MJ, Kulvatunyou N, Siddiqi S, Phelan H, Fain M, Rhee P. Redefining the association between old age and poor outcomes after trauma: The impact of frailty syndrome. J Trauma Acute Care Surg. 2017;82(3):575–81. doi: 10.1097/TA.0000000000001329. [DOI] [PubMed] [Google Scholar]
- 21.Joseph B, Pandit V, Zangbar B, Kulvatunyou N, Tang A, O’Keeffe T, Green DJ, Vercruysse G, Fain MJ, Friese RS, et al. Validating trauma-specific frailty index for geriatric trauma patients: a prospective analysis. J Am Coll Surg. 2014;219(1):10–7. e1. doi: 10.1016/j.jamcollsurg.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 23.Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, Mohr AM, Moore FA, Moldawer LL. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit Care Med. 2017;45(2):253–62. doi: 10.1097/CCM.0000000000002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minejima E, Bensman J, She RC, Mack WJ, Tuan Tran M, Ny P, Lou M, Yamaki J, Nieberg P, Ho J, et al. A Dysregulated Balance of Proinflammatory and Anti-Inflammatory Host Cytokine Response Early During Therapy Predicts Persistence and Mortality in Staphylococcus aureus Bacteremia. Crit Care Med. 2016;44(4):671–9. doi: 10.1097/CCM.0000000000001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(5):383–91. doi: 10.1097/SHK.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ha H, Nam AR, Bang JH, Park JE, Kim TY, Lee KH, Han SW, Im SA, Kim TY, Bang YJ, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget. 2016;7(47):76604–12. doi: 10.18632/oncotarget.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med. 2014;371(4):380–3. doi: 10.1056/NEJMcibr1404664. [DOI] [PubMed] [Google Scholar]
- 28.Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, Cuenca AG, Warner E, Wallet SM, Wallet MA, et al. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 2011;186(1):195–202. doi: 10.4049/jimmunol.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Vught LA, Klein Klouwenberg PM, Spitoni C, Scicluna BP, Wiewel MA, Horn J, Schultz MJ, Nurnberg P, Bonten MJ, Cremer OL, et al. Incidence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA. 2016;315(14):1469–79. doi: 10.1001/jama.2016.2691. [DOI] [PubMed] [Google Scholar]
- 30.Patera AC, Drewry AM, Chang K, Beiter ER, Osborne D, Hotchkiss RS. Frontline Science: Defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J Leukoc Biol. 2016;100(6):1239–54. doi: 10.1189/jlb.4HI0616-255R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shindo Y, McDonough JS, Chang KC, Ramachandra M, Sasikumar PG, Hotchkiss RS. Anti-PD-L1 peptide improves survival in sepsis. J Surg Res. 2017;208:33–9. doi: 10.1016/j.jss.2016.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 33.Gruther W, Benesch T, Zorn C, Paternostro-Sluga T, Quittan M, Fialka-Moser V, Spiss C, Kainberger F, Crevenna R. Muscle wasting in intensive care patients: ultrasound observation of the M. quadriceps femoris muscle layer. J Rehabil Med. 2008;40(3):185–9. doi: 10.2340/16501977-0139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.