Abstract
PURPOSE
To investigate the relationship between central corneal thickness (CCT) and diabetes disease severity among patients with diabetic peripheral neuropathy (DPN) compared to controls.
METHODS
In this cross-sectional study, 34 participants were examined. DPN status was assessed by clinical examination, nerve conduction studies and quantitative sensory testing. All participants underwent a comprehensive eye examination that included intraocular pressure (IOP) measured by Goldmann applanation tonometry. CCT was measured using ultrasound pachymetry, and the thickness of the corneal layers was assessed using corneal confocal microscopy. Association of CCT and DPN was examined using analysis of variance.
RESULTS
Among the 34 participants, there were 9 controls, 16 cases with mild DPN and 9 cases with severe DPN. CCT was significantly increased in the DPN groups compared to controls (P = 0.0003). Mean CCT among controls was 552.7 ± 29.2 μm, compared to 583.4 ± 25.0 μm in the mild DPN group and 613.3 ± 28.8 μm in the severe DPN group. Additionally, the stromal thickness differed significantly between the three study groups (P = 0.045). The mean stromal thickness among controls was 439.5 ± 23.5 μm compared to 478.9 ± 37.5 μm in the mild DPN group and 494.5 ± 39.1 μm in the severe DPN group.
CONCLUSION
This study demonstrates that CCT increases with DPN severity due to an increase in stromal thickness. The CCT increase associated with DPN has important clinical implications including glaucoma progression, keratoconus susceptibility and IOP assessment, and should be accounted for when evaluating patients with diabetes.
Keywords: Central Corneal thickness, Diabetes, Diabetic peripheral neuropathy
INTRODUCTION
Diabetes mellitus (DM) causes numerous metabolic, structural and functional changes in various organs. Diabetic peripheral neuropathy (DPN) is one of the most prevalent chronic diabetes complications.1 Although DPN rates are very low in patients with early type 1 diabetes, these rates are higher in patients with newly diagnosed type 2 diabetes, and increase with diabetes duration as a function of glucose control and other risk factors including blood pressure and blood lipid levels.1–3 DPN results from progressive damage and loss of myelinated and unmyelinated nerve fibers causing pain, numbness and tingling.1 In severe cases, DPN can lead to complications like foot ulceration and amputation.
In addition to well-recognized ocular complications of DM, such as diabetic retinopathy, cataract progression and neovascular glaucoma, DM impacts multiple ocular tissues including the cornea. Corneal manifestations in diabetes include recurrent corneal erosion, punctate keratopathy, persistent epithelial defect, increased susceptibility to ulceration, reduced corneal sensation, and neurotrophic ulceration.4, 5
There are also changes in central corneal thickness (CCT) among patients with diabetes. Several studies report an increase in CCT among patients with diabetes.6–11 Increased CCT has clinical implications in IOP assessment, glaucoma progression and protection against ectasia.12, 13 While previous studies have established an association between CCT and DM, none have evaluated the relationship between DPN severity and CCT despite the known associations of DPN with ocular manifestations.14–16
The purpose of this study was to test the hypothesis that there is a direct association between CCT and diabetic neuropathy severity. This study assessed the corneal thickness in association with DPN severity in a well-defined cohort with DPN compared to controls. Furthermore, this study was designed to identify the layer of cornea most affected by DPN status in an effort to consider the mechanism underlying the increased CCT reported in prior studies.
MATERIALS AND METHODS
Study Design: This was a cross-sectional study performed at the University of Michigan, Kellogg Eye Center.
The study was approved by the institutional review board at the University of Michigan, and written informed consent was obtained from each subject prior to testing. This study adhered to the tenets of the Declaration of Helsinki.
Study Participants were recruited from the outpatient clinics of the Division of Metabolism, Endocrinology and Diabetes at the University of Michigan Health System and included in three groups: subjects with diabetes and mild DPN, subjects with diabetes and severe DPN and healthy controls. Main inclusion criteria were: age greater than or equal to 18 years, presence of diabetes defined by the American Diabetes Association for the group with diabetes17 and having at least some evidence of DPN for the group with diabetes.
DPN presence and severity were assessed using nerve conduction studies and quantitative sensory testing (QST) by a multidisciplinary team using a standardized and validated protocol.18 Participants were classified as having mild DPN if they had the following: (1) an abnormal neurological examination performed by a board-certified neurologist confirmed by abnormal QST and; (2) the presence of one mild abnormal attributes (of amplitude, latency, F-wave or nerve conduction velocity) in one or more separate nerves among the median, peroneal and sural nerves in nerve conduction studies (NCS).
Participants were classified as having severe DPN if they had a combination of neuropathic symptoms and signs of distal sensorimotor polyneuropathy with two or more of the following: absent distal sensation (as diagnosed by a neurological examination from a board-certified neurologist and abnormal QST), unequivocally decreased or absent ankle reflexes, and severe abnormalities in NCS in two or more separate nerves among the median, peroneal and sural nerves as described above.
Exclusion criteria for all participants were: 1) any systemic neuropathy other than DPN; 2) history of corneal disease or any eye surgery; 3) any neurodegenerative condition, such as Parkinson’s disease or multiple sclerosis; 4) history of stroke or cancer; 5) history of spinal stenosis or previous back surgery; and 6) not willing or able to provide consent.
Ophthalmologic evaluations: All participants underwent a comprehensive ophthalmological examination. Visual acuity was recorded using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart.19 Intraocular pressure (IOP) was measured using Goldmann applanation tonometry. The CCT was measured under topical anesthesia using an ultrasound pachymeter (DGH-550 PACHETTE 2, DGH Technology, Inc., Exton, PA).
Corneal confocal microscopy (CCM) was performed using the Heidelberg Retina Tomograph-2 (HRT-2) Rostock cornea module (Heidelberg Engineering, Germany) to determine the corneal layer thickness and endothelial cell count.20 A drop of proparacaine hydrochloride 0.5% was given, followed by a small amount of Genteal® eye gel (Novartis, East Hanover, NJ). The participant placed his/her head in a headrest for stabilization and was instructed to look straight ahead. Confocal images were captured in sequence mode at 8 frames per second to allow imaging of the entire corneal thickness. CCM was performed in the right eye only. Hence only the right eye data were used for analyses in this study.
Statistical Analyses
Demographic characteristics were analyzed by computing frequencies and percentages for categorical variables and employing a Chi-square test to examine differences between groups. Mean and standard deviation were calculated for continuous variables and mean differences among groups were evaluated using analysis of variance (ANOVA) for variables including age, body mass index, HbA1c levels, and the Student t-test for duration of diabetes. The effects of DPN status on CCT were analyzed using one-way ANOVA. Tukey’s honest significant difference (HSD) was used for pairwise group comparisons. All statistical analyses were performed using SAS 9.3 (Cary, North Carolina).
RESULTS
Thirty-four participants with valid data sets were included in these analyses (9 controls, 16 participants with mild DPN and 9 participants with severe DPN). The characteristics of the participants are shown in Table 1. As expected, patients with severe DPN had longer diabetes duration and worse glucose control as documented by HbA1c levels compared with those with mild DPN. In addition, both groups with diabetes had higher BMI.
Table 1.
Characteristics of the study population
| Controls (n =9) | Mild DPN (n=16) | Severe DPN (n=9) | P | |
|---|---|---|---|---|
|
Age (years) Mean (SD) |
43.9 (10.2) | 52.0 (12.8) | 55.4 (9.2) | 0.1a |
|
Sex N (%) |
0.053b | |||
| Male | 3 (33.33%) | 10 (62.5%) | 8 (88.89%) | |
| Female | 6 (66.67%) | 6 (37.5%) | 1 (11.11%) | |
|
Race N (%) |
0.813b | |||
| White | 7 (77.78%) | 12 (75%) | 7 (77.78%) | |
| Black | 0 (0%) | 2 (12.50%) | 1 (11.11%) | |
| Other | 2 (22.22%) | 2 (12.50%) | 1 (11.11%) | |
|
BMI (kg/m2) Mean (SD) |
22.7 (3.3) | 36.8 (6.2) | 31.9 (4.9) | <0.0001a |
|
HbA1c (%) Mean (SD) |
5.4 (0.2) | 7.9 (1.1) | 8.09 (1.5) | 0.0006a |
|
Duration of DM (years) Mean (SD) |
N/A | 7.6 (3.8) | 11.9 (5.2) | 0.09c |
|
Type of DM N (%) |
0.15b | |||
| Type 1 | - | 6 (37.5%) | 1 (11.1%) | |
| Type 2 | - | 10 (62.5%) | 8 (88.9%) | |
|
Blood Pressure (mmHg) Mean (SD) |
||||
| Systolic | 105.8 (8.3) | 115.4 (15.7) | 124.5 (25.7) | 0.29a |
| Diastolic | 65.8 (6.2) | 72.6 (12.4) | 77.3 (8.04) | 0.16a |
|
Blood Lipids (mg/dL) Mean (SD) |
||||
| HDL | 59.6 (15.4) | 60.2 (12.8) | 32.1 (8.6) | 0.001a |
| LDL | 99.1 (15.6) | 109.4 (22.7) | 89.9 (52.2) | 0.65a |
| Triglycerides | 126 (135.7) | 70.4 (26.5) | 206.6 (152.3) | 0.20a |
| Total Cholesterol | 178.9 (16.7) | 183.2 (26.0) | 156.1 (50.3) | 0.35a |
Analysis of Variance
Chi-square
Student’s T-test
Mean CCT and stromal thickness among the three study groups are summarized in Table 2. Evaluation of CCT measurements showed a statistically significant increase (ANOVA P = 0.0003) from controls to mild and severe DPN (Figure 1). Tukey’s HSD revealed that mean CCT was significantly increased in both the mild and the severe group compared to controls. Additionally, Tukey’s HSD also showed a significant increase in CCT from mild to severe DPN cases. (P < 0.05).
Table 2.
Mean central corneal thickness (CCT) and stromal thickness measurements among the controls, mild diabetic peripheral neuropathy (DPN) and Severe DPN Cases.
| Eye | Control Mean (SD) |
Mild DPN Mean (SD) |
Severe DPN Mean (SD) |
P * |
|---|---|---|---|---|
| CCT (μm) | 552.7 ± 29.2 | 583.4 ± 25.0 | 613.3 ± 28.8 | 0.0003 |
| Stromal thickness (μm) | 439.5 ± 23.5 | 478.9 ± 37.5 | 494.5 ± 39.1 | 0.045 |
Analysis of Variance
Figure 1. Boxplot representing central corneal thickness (μm) by ultrasound pachymetry among controls, mild diabetic peripheral neuropathy (DPN) and severe DPN cases.
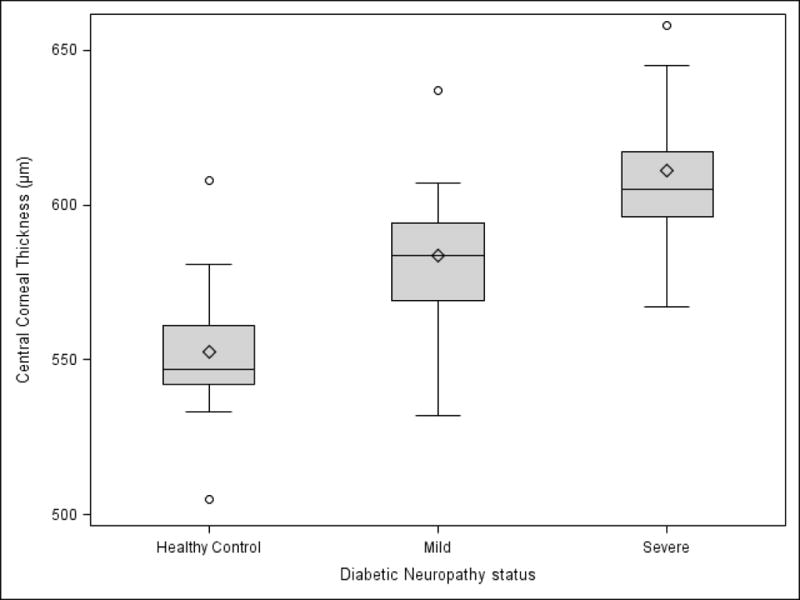
The diamond represents the mean. The center line in the box represents the median. The shaded box represents the interquartile range. Circles represent the outliers. The whiskers, upper and lower, represent the distance between the maximum observation and upper quartile, minimum observation and lower quartile respectively.
In an effort to define the corneal regions most affected by the DPN status, confocal microscopy was performed. Stromal thickness showed a significant increase (ANOVA P = 0.045) in the mild and severe DPN groups compared to controls (Figure 2). In contrast, the endothelial (ANOVA P = 0.257) and epithelial thicknesses (ANOVA P = 0.840) were not different among the three groups. The endothelial cell count did not differ significantly between the three study groups (Table 2, ANOVA P = 0.861).
Figure 2. Boxplot of Stromal thickness (μm) by confocal microscopy among controls, mild diabetic peripheral Neuropathy (DPN) and severe DPN cases.
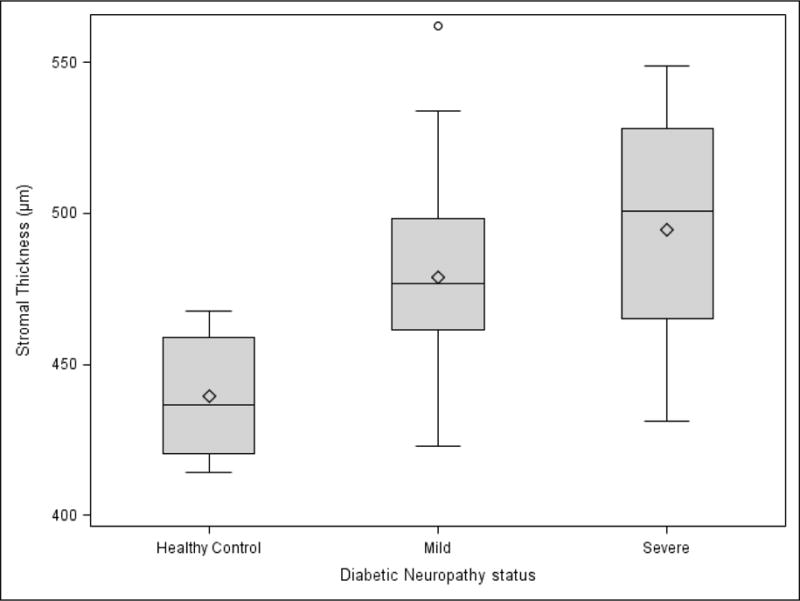
The diamond represents the mean. The center line in the box represents the median. The shaded box represents the interquartile range. Circles represent the outliers. The whiskers, upper and lower, represent the distance between the maximum observation and upper quartile, minimum observation and lower quartile respectively.
The mean IOP among controls was 13.7 ± 2.0 mmHg, while the mild and severe DPN groups had a mean IOP of 14.8 ± 4.0 mmHg and 15.1 ± 2.2 mmHg respectively. Though mean IOP increased from controls to mild DPN and severe DPN, this was not statistically significant (ANOVA P = 0.581).
DISCUSSION
Our results demonstrated a progressive increase in CCT in patients with more severe DPN, which is consistent with previous studies on corneal thickness in diabetes.6–11 Our data showed an 11% increase in corneal thickness among severe DPN compared to controls, a 5.6% increase in corneal thickness among mild DPN compared to the controls, and a 4.9% increase in CCT among severe DPN cases compared to mild cases. Based on the confocal microscopy results, this increase in CCT can be attributed to an increase in the corneal stromal thickness with increasing DPN severity. In contrast to previous studies that reported an increase in IOP among patients with diabetes,21, 22 IOP results in this study did not differ among the three groups. This discrepancy could be due to lower power associated with the sample size in this study.
DM is characterized by chronic hyperglycemia which has been shown to cause non-enzymatic glycation of several tissues.23 This glycation reaction leads to parallel changes in the nervous system and ocular tissues. Two postulated mechanisms for increase in CCT among patients with diabetes are collagen cross-linking and stromal hydration. Regarding the first mechanism of collagen cross-linking, collagen fibers are known to undergo glycation under chronic hyperglycemic conditions.24, 25 The corneal stroma, being rich in collagen, is a target of nonenzymatic glycation and accumulation of advanced glycation end products leading to subsequent collagen cross-linking.26, 27 The collagen cross-linking could explain the protective effect of diabetes on keratoconus susceptibility and progression.28 Regarding the second mechanism of endothelial pump dysfunction, decreased endothelial cell density and altered endothelial structure leading to stromal hydration and corneal edema has been suggested as yet another mechanism underlying thicker corneas among patients with diabetes.9, 10, 29, 30 In the small sample of participants in our study, endothelial cell count did not differ among the cases and controls. Additionally, visual acuity among the controls and DPN cases were not significantly different (Table 3) indicative of lack of clinically significant corneal edema among the cases. Hence, we propose collagen cross-linking as the possible mechanism for thicker corneas among patients with diabetes. Future studies are needed to elucidate the molecular changes in the cornea and to identify the causative mechanism for thicker corneas among patients with diabetes.
Table 3.
Visual acuity distribution using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart among the controls, mild diabetic peripheral neuropathy (DPN) and severe DPN Cases. (Fisher’s exact test P = 0.14)
| Visual Acuity (OD) |
Control N (%) |
Mild DPN N (%) |
Severe DPN N (%) |
|---|---|---|---|
| 20/20 | 2 (22.2%) | 3 (18.8%) | 0 (0%) |
| 20/25 | 5 (55.6%) | 6 (37.5%) | 2 (22.2%) |
| 20/32 | 2 (22.2%) | 3 (18.8%) | 6 (66.7%) |
| 20/40 | 0 (0%) | 3 (18.8%) | 0 (0%) |
| <20/40 | 0 (0%) | 1 (6.3%) | 1 (11.1%) |
CCT is an important factor for assessing the risk of open-angle glaucoma. While a thinner cornea is an independent risk factor for open-angle glaucoma, a thicker cornea offers protection against glaucoma progression.13, 31 Thus thicker corneas among populations with diabetes may be viewed as potentially “protective” but associated with an overestimation of IOP. Results from health care claims data and meta-analysis demonstrate that diabetes is a risk factor for glaucoma.32, 33 While it is clear that hyperglycemia has detrimental effects on retinal vasculature as a mechanism of diabetic retinopathy34, 35 there is a lack of detailed studies on the hyperglycemic effects on other ocular tissues.
The strengths of this study include meticulous phenotyping of DPN status using objective measures including electrophysiology studies and clinical examination by a multidisciplinary team. To the best of our knowledge, this was the first study to examine the corneal thickness from a DPN severity framework and also to isolate the stromal thickness measurements among patients with diabetes. The main limitation of this study was a small sample size that restricts the ability to detect smaller effects or differences. Lack of an additional control group consisting of patients with diabetes but without DPN is another limitation, as this would have provided another population in which to evaluate the effect of DPN on CCT. However, observation of a significant CCT difference between mild and severe DPN as well as between normal controls and these two DPN groups is suggestive that DPN may play a role in CCT. Adjusting for additional factors such as BMI, HbA1c, and HDL that could potentially confound the association between CCT and DPN was not possible due to numerous missing data. Temporality of association and the causal relationship could not be established due to the cross-sectional nature of this study.
In conclusion, our study confirms increased corneal thickness in patients with diabetes. More importantly, our findings demonstrate an increased CCT that is confined to the stroma. The mechanisms of this stromal-mediated CCT change are currently not fully understood. Further studies are needed to fill this gap in knowledge to fully understand the spectrum of homeostatic tissue adaptation in early hyperglycemia to late pathophysiological consequences of uncontrolled diabetes.
Acknowledgments
FUNDING DISCLOSURE:
This project was funded by the Michigan Diabetes Research Center through NIH P60DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases. RPB is also supported by grant NIH/NIDDK-1-R01-DK-107956-01 and NIH/NIDDK 1UC4DK101108.RPB is supported by a departmental grant NIH/NHLBI-1R01HL102334-01 from Research to Prevent Blindness, New York, NY.
Footnotes
CONFLICT OF INTEREST: The authors declare that there is no conflict of interest.
Presented in part by AM at Association for Research in Vision and Ophthalmology Annual Meeting 2012.
References
- 1.Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40:136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Martin CL, Albers JW, Pop-Busui R, et al. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:31–8. doi: 10.2337/dc13-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bikbova G, Oshitari T, Tawada A, et al. Corneal changes in diabetes mellitus. Curr Diabetes Rev. 2012;8:294–302. doi: 10.2174/157339912800840479. [DOI] [PubMed] [Google Scholar]
- 5.Misra SL, Braatvedt GD, Patel DV. Impact of diabetes mellitus on the ocular surface: a review. Clin Exp Ophthalmol. 2016;44:278–88. doi: 10.1111/ceo.12690. [DOI] [PubMed] [Google Scholar]
- 6.Su DH, Wong TY, Wong WL, et al. Diabetes, hyperglycemia, and central corneal thickness: the Singapore Malay Eye Study. Ophthalmology. 2008;115:964–968. doi: 10.1016/j.ophtha.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Goldich Y, Barkana Y, Gerber Y, et al. Effect of diabetes mellitus on biomechanical parameters of the cornea. J Cataract Refract Surg. 2009;35:715–9. doi: 10.1016/j.jcrs.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Storr-Paulsen A, Singh A, Jeppesen H, et al. Corneal endothelial morphology and central thickness in patients with type II diabetes mellitus. Acta Ophthalmol. 2014;92:158–60. doi: 10.1111/aos.12064. [DOI] [PubMed] [Google Scholar]
- 9.Briggs S, Osuagwu UL, AlHarthi EM. Manifestations of type 2 diabetes in corneal endothelial cell density, corneal thickness and intraocular pressure. J Biomed Res. 2015;30 doi: 10.7555/JBR.29.20140075. [DOI] [PubMed] [Google Scholar]
- 10.El-Agamy A, Alsubaie S. Corneal endothelium and central corneal thickness changes in type 2 diabetes mellitus. Clin Ophthalmol. 2017;11:481–486. doi: 10.2147/OPTH.S126217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galgauskas S, Laurinavičiūtė G, Norvydaitė D, et al. Changes in choroidal thickness and corneal parameters in diabetic eyes. Eur J Ophthalmol. 2016;26:163–7. doi: 10.5301/ejo.5000677. [DOI] [PubMed] [Google Scholar]
- 12.Woodward MA, Blachley TS, Stein JD. The Association Between Sociodemographic Factors, Common Systemic Diseases, and Keratoconus: An Analysis of a Nationwide Heath Care Claims Database. Ophthalmology. 2016;123:457–65. doi: 10.1016/j.ophtha.2015.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 14.DeMill DL, Hussain M, Pop-Busui R, et al. Ocular surface disease in patients with diabetic peripheral neuropathy. Br J Ophthalmol. 2016;100:924–928. doi: 10.1136/bjophthalmol-2015-307369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz RO, Peters MA, Sobocinski K, et al. Diabetic keratopathy as a manifestation of peripheral neuropathy. Am J Ophthalmol. 1983;96:368–71. doi: 10.1016/s0002-9394(14)77829-8. [DOI] [PubMed] [Google Scholar]
- 16.Achtsidis V, Eleftheriadou I, Kozanidou E, et al. Dry eye syndrome in subjects with diabetes and association with neuropathy. Diabetes Care. 2014;37:e210–1. doi: 10.2337/dc14-0860. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(Suppl. 1):S1–S135. [Google Scholar]
- 18.Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–93. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferris FL, 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- 20.Böhnke M, Masters BR. Confocal microscopy of the cornea. Prog Retin Eye Res. 1999 Sep;18:553–628. doi: 10.1016/s1350-9462(98)00028-7. [DOI] [PubMed] [Google Scholar]
- 21.Yazgan S, Celik U, Kaldırım H, et al. Evaluation of the relationship between corneal biomechanic and HbA1C levels in type 2 diabetes patients. Clin Ophthalmol. 2014;8:1549–53. doi: 10.2147/OPTH.S67984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheler A, Spoerl E, Boehm AG. Effect of diabetes mellitus on corneal biomechanics and measurement of intraocular pressure. Acta Ophthalmol. 2012;90(6):e447–51. doi: 10.1111/j.1755-3768.2012.02437.x. [DOI] [PubMed] [Google Scholar]
- 23.Negre-Salvayre A, Salvayre R, Augé N, et al. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal. 2009;11:3071–109. doi: 10.1089/ars.2009.2484. [DOI] [PubMed] [Google Scholar]
- 24.Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984;81:583–7. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Kaji Y, Usui T, Oshika T, et al. Advanced glycation end products in diabetic corneas. Invest Ophthalmol Vis Sci. 2000;41:362–8. [PubMed] [Google Scholar]
- 27.Zou C, Wang S, Huang F, et al. Advanced glycation end products and ultrastructural changes in corneas of long-term streptozotocin-induced diabetic monkeys. Cornea. 2012;31(12):1455–9. doi: 10.1097/ICO.0b013e3182490907. [DOI] [PubMed] [Google Scholar]
- 28.Naderan M, Naderan M, Rezagholizadeh F, et al. Association between diabetes and keratoconus: a case-control study. Cornea. 2014;33:1271–3. doi: 10.1097/ICO.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 29.Shenoy R, Khandekar R, Bialasiewicz A, et al. Corneal endothelium in patients with diabetes mellitus: a historical cohort study. Eur J Ophthalmol. 2009;19:369–75. doi: 10.1177/112067210901900307. [DOI] [PubMed] [Google Scholar]
- 30.Anbar M, Ammar H, Mahmoud RA. Corneal Endothelial Morphology in Children with Type 1 Diabetes. J Diabetes Res. 2016;7319047 doi: 10.1155/2016/7319047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt JD. Corneal thickness in glaucoma screening, diagnosis, and management. Curr Opin Ophthalmol. 2004;15:85–9. doi: 10.1097/00055735-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Newman-Casey PA, Talwar N, Nan B, et al. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology. 2011;118:1318–26. doi: 10.1016/j.ophtha.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou M, Wang W, Huang W, et al. Diabetes mellitus as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. PLoS One. 2014;9:e102972. doi: 10.1371/journal.pone.0102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durham JT, Herman IM. Microvascular modifications in diabetic retinopathy. Curr Diab Rep. 2011;11:253–64. doi: 10.1007/s11892-011-0204-0. [DOI] [PubMed] [Google Scholar]
- 35.Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (Lond) 2009;23:1496–508. doi: 10.1038/eye.2009.108. [DOI] [PubMed] [Google Scholar]


