Abstract
Background
Iron deficiency anemia (IDA) in cyanotic congenital heart disease (CCHD) and its association with cyanotic spells has been documented in literature. However, Indian data especially in the pediatric age group is scarce. This study was conducted to find out the prevalence of IDA in this population.
Methods
An observational study was conducted in a tertiary care hospital. Children with CCHD in the age group of birth–12 years were included in the study. Hematological parameters of these patients were determined and compared. An assessment of the incidence of cyanotic spells in the iron-deficient and iron non-deficient children was also done. Data analysis was done using Fischer's exact test.
Results
The prevalence of IDA was 47.06% in the study population. The study also showed that hemoglobin and hematocrit levels were paradoxically higher in the iron-deficient group as compared to the non-deficient, though the iron studies revealed the iron deficiency. The incidence of cyanotic spells was higher in the iron-deficient group. The mean corpuscular volume (MCV), red cell distribution width (RDW), serum ferritin, serum iron, total iron binding capacity (TIBC), and transferrin saturation (TS) values were the parameters, which were found to be statistically significant to differentiate the study groups.
Conclusion
The prevalence of IDA in children with CCHD was found to be high. Iron-deficient group had an increased frequency of cyanotic spells as compared to the non-deficient group, which was statistically significant.
Keywords: Iron deficiency anemia, Cyanotic spells, Cyanotic congenital heart disease
Introduction
Cyanotic congenital heart disease (CCHD) is congenital heart defect with right to left shunting of desaturated blood. This results in decreased oxygen saturation in the systemic circulation which acts as a trigger for increase in erythropoietin production and secondary erythropoiesis in an effort to maintain tissue oxygenation.1 The resultant polycythemia and hyperviscosity manifests clinically as thromboembolic events in the children with CCHD.1, 2 Iron deficiency anemia (IDA) is commonly encountered in children of CCHD. IDA aggravates hyperviscosity symptoms due to the presence of microcytic erythrocytes not amenable to deformation in the microcirculation. Thus, presence of IDA in these children further increases their chances of morbidity in the form of cerebrovascular events and cyanotic spells.3 Polycythemia causes hemoglobin and hematocrit to rise and the otherwise normal values for age are unable to reflect the iron-deficient status of these children.
This study is an attempt to look at the prevalence of iron deficiency in Indian children with CCHD and to find out the prevalence of cyanotic spells in the subsets of iron-deficient and iron non-deficient children with CCHD.
Material and methods
The study was an observational study conducted in pediatric OPD of a tertiary care center in Western India over 18 months. Informed consent from parents and institutional ethical committee clearance was obtained.
Inclusion criteria
All children with CCHD were diagnosed on 2D-Echocardiography.
Exclusion criteria
The children were excluded if they had undergone definitive surgery, received iron supplements in the previous three months, had systemic involvement to explain the cause of anemia e.g. chronic kidney disease, hemolytic anaemia. A total of 51 children were included in study.
The demographic profile, clinical data including frequency of cyanotic spells and the results from 2-D Echocardiography were chronicled. Thereafter, these children underwent complete blood count including red blood cell indices and reticulocyte count and serum iron studies including serum ferritin, total iron binding capacity (TIBC) and transferrin saturation. Based on Serum Ferritin levels the children were divided into iron deficient and non-deficient group. IDA was diagnosed by a serum ferritin concentration of less than 12 ng/mL in children less than 5 years and 15 ng/mL in children more than 5 years and 30 ng/mL in children with infection.4, 5, 6, 7
Sample collection and estimation methods
Two ml of blood was collected in an EDTA vacutainer for CBC, red cell indices and PBS and the sample was tested within four hours of collection. The CBC and red cell indices were done on Beckman Coulter five point differential automated hematology analyzer. The values for Hemoglobin (Hb), Mean Corpuscular Volume (MCV), and Total Iron-Binding Capacity (TIBC) are directly derived and rest were calculated. 3 ml of blood was taken in sterile vacutainer for serum iron studies and the serum separated at room temperature. The sample was preserved at −10 °C till it was run. Serum ferritin was done by ELISA by the Bio Rad ELISA reader model 680. Serum Iron was done by Bathophenanthroline method and the transferrin saturation (TS) was calculated.
Statistics
The data was recorded in a predecided format. This was fed into an excel sheet by the authors and verified by a co-author. The data was analysed using SPSS. The Fischer's exact test was used to find the various associations among the cases and a p-value of ≤0.05 was considered statistically significant.
Results
Fifty one children participated in the study with mean age of 4 years (Range 3 months–9 yrs). These included thirty four male (66.67%). The demographic details have been highlighted in the table (Table 1). Thirty eight of fifty one children had tetrology of Fallot.
Table 1.
Age-wise distribution of the study population.
| Age | No of cases | Percent |
|---|---|---|
| Birth–6 months | 16 | 31.37 |
| 6 months–1 year | 15 | 29.41 |
| 1 year–6 years | 18 | 35.29 |
| 6 years–12 years | 2 | 3.92 |
| Total | 51 | |
Twenty four of fifty one children (47.06%) were iron deficient, 95% CI [34.05, 60.48] and 52.94% were non-deficient, 95% CI [39.52, 65.95]. Seven out of fifty-one children (13.72%) had a history of cyanotic spells, 95% CI [6.81, 25.72]. There was a significant association between iron deficiency and cyanotic spells (p = 0.042) (Table 2). The iron deficient group had a higher prevalence (12%) of cyanotic spells, 95% CI [12.00, 44.90] as compared to the non-deficient group (3.7%), 95% CI [0.66, 18.28].
Table 2.
Comparison of prevalence of cyanotic spells in iron-deficient and iron non-deficient group.
| Cyanotic spells |
Total | ||
|---|---|---|---|
| Yes | No | ||
| IDA | |||
| Yes | 6 | 18 | 24 |
| No | 1 | 26 | 27 |
| Total | 7 | 44 | 51 |
p = 0.042; odds ratio = 8.67.
The hematological parameters of the iron-deficient and non-deficient groups were compared. The Hb and hematocrit were higher in the iron-deficient group as compared to the non-iron deficient group. The mean serum ferritin was 7.63 ng/mL (SD 3.4) in the iron-deficient group as compared to 33.53 ng/mL (SD 10.51) in non-iron deficient group. The serum iron and TS were lower and the TIBC higher in the iron-deficient group. The group with cyanotic spells and the group with no cyanotic spells were compared. Among the various haematological parameters MCV, RDW, Serum Ferritin, TIBC, TS and Serum Iron were detected to be significantly associated with the incidence of cyanotic spells. The mean serum ferritin in children with cyanotic spells was much lower (5.32 ng/mL) than children who did not have spells (23.89 ng/mL) (Table 3, Table 4).
Table 3.
Comparison of hematological parameters of iron-deficient and non-deficient group.
| IDA | N | Mean | SD | p-value | |
|---|---|---|---|---|---|
| Hb | Present | 24 | 14.13 | 1.97 | 0.012 |
| Absent | 27 | 12.87 | 1.34 | ||
| PCV | Present | 24 | 43.08 | 6.45 | 0.004 |
| Absent | 27 | 38.41 | 4.02 | ||
| TLC | Present | 24 | 9910.42 | 2890.25 | 0.616 |
| Absent | 27 | 9543.70 | 2190.29 | ||
| Pl Count | Present | 24 | 2.60 | 0.83 | 0.739 |
| Absent | 27 | 2.53 | 0.68 | ||
| TRBC | Present | 24 | 6.36 | 1.36 | <0.001 |
| Absent | 27 | 4.86 | 0.53 | ||
| Retics | Present | 24 | 1.01 | 0.15 | 0.404 |
| Absent | 27 | 1.07 | 0.37 | ||
| MCV | Present | 24 | 68.50 | 5.92 | <0.001 |
| Absent | 27 | 79.39 | 9.02 | ||
| MCH | Present | 24 | 23.23 | 2.33 | 0.001 |
| Absent | 27 | 26.26 | 3.44 | ||
| MCHC | Present | 24 | 33.03 | 1.71 | 0.018 |
| Absent | 27 | 34.22 | 1.74 | ||
| RDW | Present | 24 | 18.38 | 2.08 | <0.001 |
| Absent | 27 | 11.70 | 1.68 | ||
| Serum ferritin | Present | 24 | 7.63 | 3.40 | <0.001 |
| Absent | 27 | 33.53 | 10.61 | ||
| Serum iron | Present | 24 | 68.17 | 10.91 | <0.001 |
| Absent | 27 | 96.44 | 16.17 | ||
| TIBC | Present | 24 | 371.50 | 51.61 | <0.001 |
| Absent | 27 | 287.30 | 46.47 | ||
| TS | Present | 24 | 18.92 | 4.77 | <0.001 |
| Absent | 27 | 34.82 | 10.70 | ||
Values given in bold are statistically significant.
Table 4.
Comparison of hematological parameters in children with and without cyanotic spells.
| Spells | Number of children | Mean | SD | p-value | |
|---|---|---|---|---|---|
| Hb | Present | 7 | 15.37 | 3.20 | 0.118 |
| Absent | 44 | 13.16 | 1.23 | ||
| PCV | Present | 7 | 46.00 | 10.55 | 0.17 |
| Absent | 44 | 39.75 | 4.17 | ||
| TLC | Present | 7 | 10,977.14 | 4077.07 | 0.387 |
| Absent | 44 | 9515.68 | 2188.44 | ||
| Pl Ct | Present | 7 | 2.52 | 1.03 | 0.916 |
| Absent | 44 | 2.57 | 0.71 | ||
| TRBC | Present | 7 | 7.06 | 2.30 | 0.095 |
| Absent | 44 | 5.33 | 0.82 | ||
| Retics | Present | 7 | 0.93 | 0.22 | 0.192 |
| Absent | 44 | 1.06 | 0.30 | ||
| MCV | Present | 7 | 66.83 | 7.69 | 0.025 |
| Absent | 44 | 75.45 | 9.18 | ||
| MCH | Present | 7 | 22.84 | 3.68 | 0.158 |
| Absent | 44 | 25.15 | 3.18 | ||
| MCHC | Present | 7 | 33.30 | 1.97 | 0.61 |
| Absent | 44 | 33.72 | 1.81 | ||
| RDW | Present | 7 | 19.29 | 3.25 | 0.004 |
| Absent | 44 | 14.14 | 3.46 | ||
| Serum ferritin | Present | 7 | 5.32 | 4.00 | <0.001 |
| Absent | 44 | 23.89 | 14.89 | ||
| Serum iron | Present | 7 | 66.29 | 12.04 | 0.004 |
| Absent | 44 | 85.82 | 19.60 | ||
| TIBC | Present | 7 | 406.14 | 54.10 | 0.003 |
| Absent | 44 | 314.32 | 56.86 | ||
| TS | Present | 7 | 16.92 | 4.38 | <0.001 |
| Absent | 44 | 29.00 | 11.53 | ||
Values given in bold are statistically significant.
Discussion
Cyanotic heart defects have generated interest due to the special problems seen in these defects, the complex surgeries and their peri-operative care and recently for the growing population of adults with congenital heart diseases and the attendant problems. In these defects, the right to left shunting of blood results in systemic arterial desaturation. This hypoxia is a stimulus to increased production of erythropoietin resulting in an increase in erythrocyte mass in an attempt to correct the hypoxia.8, 9, 10, 11, 12 However this causes hyperviscosity symptoms to develop which include headache, dizziness, cerebrovascular events. Hyperviscosity and polycythemia cause deformation of RBCs, cell aggregates form and embolisation may be seen. Iron deficiency is an important determinant of the quality of life of children with CCHD. IDA causes further deformation of RBCs due to the rigidity of the erythrocyte membrane and has been implicated in the aggravation of hyperviscosity symptoms.13
There is a paucity of literature discussing iron deficiency among children with CCHD and these studies have shown varying results. In 1990, West et al, have demonstrated that more than one-third of patients with CCHD had iron deficiency.14 In another study done by Olcay et al, the prevalence of IDA was found to be 52.2%.15 In a study done in India by Gaiha et al, a prevalence of 18.18% was reported, however the subjects of this study were adolescents and young adults.16 In this study, we demonstrate that nearly five of ten children with CCHD are iron deficient and these children with iron deficiency are more likely to have cyanotic spells.
The study population was dominated by infants who comprised of half of the total. This is probably because of increasing frequency of ante-natal diagnosis and awareness in the population for the need for early intervention. Two-thirds of the study population comprised of male children. Previous studies have not shown any sex predilection in Tetralogy of Fallot, Double Outlet Right Ventricle (DORV), Truncus Arteriosus (TA), Ebstein's anomaly, Supracardiac Total Anomalous Pulmonary Venous connection (TAPVC) but, males outnumber females in Transposition of the Great Arteries and infracardiac TAPVC by a high ratios.17, 18, 19, 20, 21 In this study a majority of cases were children with Tetrology of Fallot (74.51%). This is an observation similar to other studies on this subject.
Almost half (47.06%) of the children were found to have iron deficiency as determined by their serum ferritin levels. This is similar to the study by Cemile Banu Onur et al and Olcay et al done in children with CCHD but higher than that seen in studies of adults with CCHD.1, 15, 16 However one study in children with CCHD done by West et al also showed much lower prevalence of IDA as compared to the other studies on children.14
The higher prevalence of IDA in our population was probably due to a large subset of infants and toddlers in whom nutritional iron deficiency was coexistent. However, as is exemplified in our study too, the TIBC tends to be low in nutritional iron deficiency as opposed to that seen in the iron deficiency of CCHD.
All children with iron deficiency had a microcytic hypochromic picture on peripheral blood smear, their red cell distribution width was increased and they had a low MCV. RDW is of prime importance in the diagnosis of iron deficiency. Previous studies have deduced that iron deficiency can be diagnosed with 98% accuracy by evaluating RDW and MCV together.22, 23
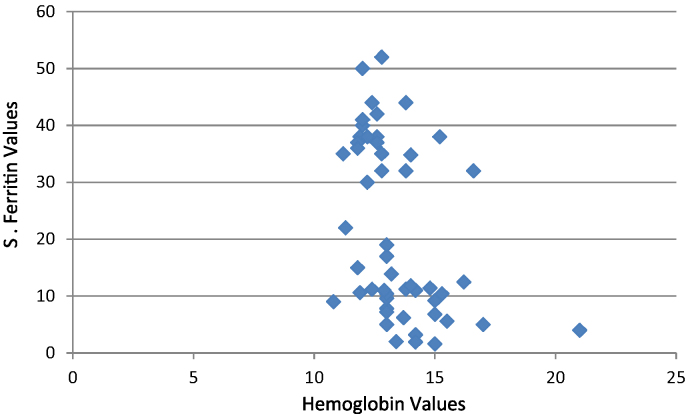
The frequency of cyanotic spells in the iron deficient group was 25% and association between iron deficiency anemia and cyanotic spells were found to be statistically significant (p value −0.042). Children with IDA had eight times the risk of having cyanotic spells compared to the children who were not iron deficient. The mean serum ferritin in children with cyanotic spells was much lower (5.32 ng/mL) than children who did not have spells (23.89 ng/mL). The ferritin levels in children with cyanotic congenital heart disease showed little correlation with the haemoglobin levels (Fig. 1).
Fig. 1.
Correlation graph of hemoglobin and ferritin. X axis: hemoglobin values; Y-axis: serum ferritin values.
Limitations of the study
The present study is limited by a small sample size. Therefore it may not be adequately powered to draw major statistical conclusions. In addition it was carried out at a single tertiary care centre, and hence likely to have a referral bias. Another issue is the study population which also includes children younger than 6 months, which make the mean values of the hematological parameters on the higher side. Another limitation is that since it is a cross-sectional study – the therapeutic effects of iron supplementation though implied have not been demonstrated statistically.
Conclusion
Notwithstanding these limitations, we demonstrate that nearly half of children with CCHD have iron deficiency and would benefit from iron supplementation. IDA is frequently under-diagnosed among children with CCHD, due to the falsely high hemoglobin levels, despite the presence of anaemia. Iron deficiency is associated with increase in incidence of cyanotic spells. It is also associated with hyperviscosity and its attendant problems. Serum ferritin should be done routinely in children with cyanotic congenital heart disease for diagnosis of IDA.
Conflicts of interest
The authors have none to declare.
References
- 1.Onur C.B., Sipahi T., Tavil B. Diagnosing iron deficiency in cyanotic heart disease. Indian J Pediatr. 2003;70(1):29–31. doi: 10.1007/BF02722740. [DOI] [PubMed] [Google Scholar]
- 2.Freidland M., Grant S. Hematocrit, viscosity and cerebral blood flow. Am Heart J. 1979;97:405–407. doi: 10.1016/0002-8703(79)90443-5. [DOI] [PubMed] [Google Scholar]
- 3.Perloff J.K., Roseve M.H. Adults with congenital cyanotic heart disease: hematologic management. Ann Intern Med. 1988;109:406–413. doi: 10.7326/0003-4819-109-5-406. [DOI] [PubMed] [Google Scholar]
- 4.Stanley F.Lo. Reference, intervals for laboratory tests and procedures. In: Kliegman R.M., Stanton B.F., St Geme J.W., Schor N.F., editors. Nelson Textbook of Pediatrics. 20th ed. Elsevier; New Delhi: 2016. pp. 3471–3472. [Google Scholar]
- 5.Brugnara C., Oski F.A., Nathan D.G. Diagnostic approach to the anaemic patients. In: Orkin S.H., Nathan D.G., Ginsburg D., editors. Nathan and Oski's Hematology of Infancy and Childhood. 8th ed. Elsevier; Canada: 2014. p. 460. [Google Scholar]
- 6.Nairz M., Theurl I., Wolf D., Weiss G. Iron deficiency or anemia of inflammation? Wiener Medizinische Wochenschrift. 2016;166(October (13–14)):411–423. doi: 10.1007/s10354-016-0505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W., Knovich M.A., Coffman L.G., Torti F.M., Torti S.V. Serum ferritin: past, present and future. Biochim Biophys Acta. 2010;1800:760–769. doi: 10.1016/j.bbagen.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gidding S.S., Stockman J.A., 3rd Erythropoietin in cyanotic heart disease. Am Heart J. 1988;116(July (1 Pt 1)):128–132. doi: 10.1016/0002-8703(88)90260-8. [DOI] [PubMed] [Google Scholar]
- 9.Koeffler H.P., Goldwasser E. Erythropoietin radioimmunoassay in evaluating patients with polycythemia. Ann Intern Med. 1981;94:44–47. doi: 10.7326/0003-4819-94-1-44. [DOI] [PubMed] [Google Scholar]
- 10.Erslev A.J., Caro J. Pure erythrocytosis classified according to erythropoietin titres. Am J Med. 1984;76:57–61. doi: 10.1016/0002-9343(84)90750-2. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal A., Button L.N., Nathan D.G., Meittnen O.S., Nadas A.S. Blood volume changes in cyanotic congenital heart disease. Am J Cardiol. 1971;27:162–167. doi: 10.1016/0002-9149(71)90253-0. [DOI] [PubMed] [Google Scholar]
- 12.Lang’O M.O., Githanga J.N., Yuko-Jowi C.A. Prevalence of iron deficiency in children with cyanotic congenital heart disease seen at Kenyatta National Hospital and Mater Hospital Nairobi. East Afr Med J. 2009;86:S47–S51. doi: 10.4314/eamj.v86i12.62901. [DOI] [PubMed] [Google Scholar]
- 13.Yip R., Mohandas N., Clark M.R., Jain S., Shohet S.B., Dallman P.R. Red cell membrane stiffness in iron deficiency. Blood. 1983;62:99–106. [PubMed] [Google Scholar]
- 14.West W.D., Janet N. Iron deficiency in children with CCHD. J Pediatr. 1990;117:266–268. doi: 10.1016/s0022-3476(05)80544-x. [DOI] [PubMed] [Google Scholar]
- 15.Olcay L., Ozer S. Parameters of iron deficiency in children with CCHD. Pediatr Cardiol. 1996;17:150–154. doi: 10.1007/BF02505204. [DOI] [PubMed] [Google Scholar]
- 16.Gaiha M., Sethi H.P., Sudha R., Arora R., Acharya N.R. A clinic-hematological study of iron deficiency anaemia and its correlation with hyperviscosity symptoms in cyanotic congenital heart disease. Indian Heart J. 1993;45(January–February (1)):53–55. [PubMed] [Google Scholar]
- 17.Elliot L.P., Neufeld H.N., Anderson R.C. Complete transposition of the great vessels. I. An anatomic study of sixty cases. Circulation. 1963;27:1105. [Google Scholar]
- 18.Flyer D.C. Report of the New England Regional Infant Cardiac Program. Pediatrics. 1980;65(suppl):377. [PubMed] [Google Scholar]
- 19.Liebman J., Cullum L., Belloe N.B. Natural history of transposition of great arteries. Circulation. 1969;40:237. doi: 10.1161/01.cir.40.2.237. [DOI] [PubMed] [Google Scholar]
- 20.Gatham G.E., Nadas A.S. Total anomalous pulmonary venous connection: clinical and physiological observations of 75 pediatric patients. Circulation. 1970;42:143. doi: 10.1161/01.cir.42.1.143. [DOI] [PubMed] [Google Scholar]
- 21.Solymar L., Sabel K., Zetterqvist P. Total anomalous pulmonary venous connection in siblings: report on three families. Acta Pediatr Scand. 1987;76:124. doi: 10.1111/j.1651-2227.1987.tb10427.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim S.K., Cheong W.S., Jun Y.H., Choi J.W., Son B.K. Red blood cell indices and iron status according to feeding practices in infants and young children. Acta Paediatr. 1996;85(February (2)):139–144. doi: 10.1111/j.1651-2227.1996.tb13979.x. [DOI] [PubMed] [Google Scholar]
- 23.Choi Y.S., Reid T. Anaemia and red cell distribution width at the 12 months well baby examination. South Med J. 1998;91:372–374. doi: 10.1097/00007611-199804000-00012. [DOI] [PubMed] [Google Scholar]