Abstract
Pre-harvest sprouting (PHS) remains a long-standing problem for the production of barley (Hordeum vulgare) and wheat (Triticum aestivum) worldwide. Grain dormancy, a key trait for the prevention of PHS, controls the timing of germination. Discovery of the causal sequence polymorphisms (CSPs) that produce naturally occurring variation in dormancy will help improve PHS tolerance. The identification of CSPs for dormancy remains difficult, especially for barley and wheat, because they are the last major cereals to have their genomes sequenced. However, recent work has identified several important CSPs that play pivotal roles in fine-tuning the dormancy levels in barley and wheat cultivars. This review summarizes these recent advances, which can be directly applied in breeding programs to improve PHS tolerance. These recent findings indicate the possibility that barley and wheat cultivars grown in East Asia, where much rain falls during the harvest season, will be rich sources of alleles that confer strong dormancy, since these cultivars have been selected to cope with the regional climate. The newly discovered dormant alleles will be useful for improving PHS tolerance around the world, just as Reduced-height (Rht) alleles from Japanese wheat varieties contributed to yield increases for the Green Revolution.
Keywords: barley, wheat, pre-harvest sprouting, seed dormancy, QTL
Introduction
Pre-harvest sprouting (PHS), a phenomenon where seeds germinate on the plant before harvest, is a major problem around the world, especially for barley (Hordeum vulgare) and wheat (Triticum aestivum), the fourth and second most widely produced cereals. PHS causes devastating damage to barley and wheat production because yields are reduced and the resulting grain is of lower quality, leading to major economic losses.
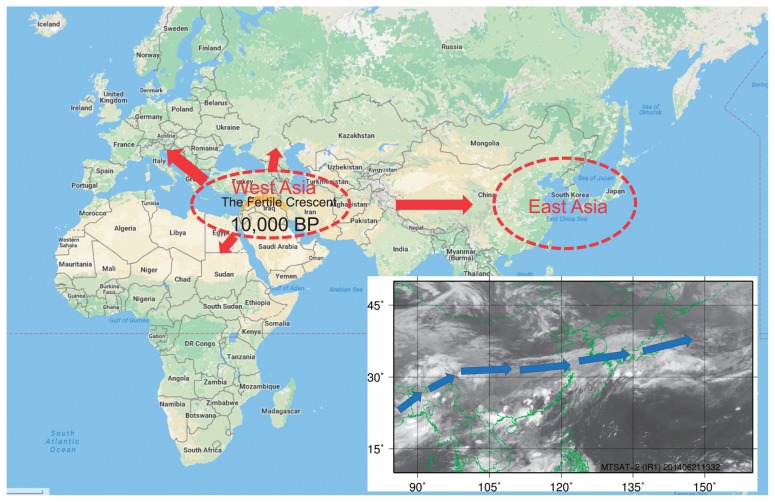
Barley and wheat originated in the Fertile Crescent (Kilian et al. 2009), whose Mediterranean climate is characterized by hot, dry summers and cool, moist winters. In this region, little rain falls during the harvest season (usually around June), so PHS is rare. However, since the end of the last major glacial period about 10,000 years ago, the distribution of barley and wheat cultivars has expanded across the world and today, barley and wheat are cultivated in more than 100 countries. In some parts of these countries, these grains are cultivated in climates where rain tends to fall during the harvest season, and PHS damage has been reported, including Japan and the major wheat-producing countries of China, India, the USA, Canada, Germany, and Australia (Derera 1990, Liu et al. 2016, J.-R. Wang, personal communication 2016). Therefore, PHS remains a problem that must be solved to prevent economic losses and improve food security. Breeding programs have improved PHS tolerance in barley and wheat varieties, and the resulting varieties are much more tolerant to PHS than older ones, but the level of tolerance is not yet sufficient to prevent PHS completely (Fig. 1).
Fig. 1.
Pre-harvest sprouting (PHS) barley and wheat varieties. In pre-harvest sprouting (PHS), grains germinate on the spike after rain during the harvest season. The images show PHS in barley varieties (A: two-row malting barley; B: six-row barley) grown in Tochigi, Japan, in 2014, and PHS in wheat varieties (C and D) grown in Hokkaido, Japan, in 2016. In A, only white roots emerged from grains, In B, C, and D, green shoots emerged from grains.
A key trait for the prevention of PHS is grain dormancy, which is the suppression of the germination of grains (or seeds), even under favorable (wet) conditions (Finkelstein et al. 2008, Nonogaki 2014, Rodríguez et al. 2015). Dormancy is a quantitative trait regulated by multiple genes, and is strongly influenced by environmental conditions. From an agricultural perspective, it is desirable for cultivars to have grains that remain dormant until harvest to prevent PHS; however, the dormancy should quickly disappear after harvest to allow the grains to germinate rapidly and almost simultaneously at sowing (or, in the case of malting barley, at malt production). To select genotypes with this ideal pattern of dormancy, it is necessary to understand the genetic regulatory mechanisms underlying this phenomenon. This review summarizes recent advances in the understanding of quantitative trait loci (QTL) for grain dormancy in barley and wheat.
Analysis of QTL for grain dormancy in barley and wheat: towards cloning the causal genes
QTL analysis is a powerful tool in the genetic analysis of quantitative traits (Tanksley 1993). The first reports of a QTL for grain dormancy in barley and wheat were published in the early 1990s (Anderson et al. 1993, Ullrich et al. 1992). Since then, dormancy QTL have been found on all chromosomes in both barley and wheat (Flintham et al. 2002, Kulwal et al. 2010, Mares and Mrva 2014, Nakamura et al. 2017). Interestingly, among them, only a few dormancy QTL have large effects. Barley has two major dormancy QTL, SD1 and SD2, and wheat has several dormancy QTL, including QPhs.ocs-3A.1 and Phs1 (Gong et al. 2014, Mori et al. 2005, Torada et al. 2005, 2008).
Genetic markers for these major QTL would be valuable for breeders because the QTL are likely to express their effects under different environmental conditions (Tanksley 1993). Therefore, it is important to identify the causal sequence polymorphisms (CSPs) for these major QTL to develop markers for selection of the QTL. For such analyses, fine mapping of the genes with precisely annotated genomic sequences is indispensable. However, compared with model plants such as Arabidopsis thaliana and rice (Oryza sativa), and even with other major crops such as corn (Zea mays) or soybean (Glycine max) (IRGSP 2005, Schmutz et al. 2010, Schnable et al. 2009, The Arabidopsis Genome Initiative 2000), it was much more difficult to decode the barley and wheat genome sequences, because they are much larger (approximately 5 Gb and 17 Gb, respectively) and contain a lot of repeated sequences. In addition, wheat is allohexaploid, making the determination of its genome sequence even more challenging. Although the barley reference genome sequence was published recently (Mascher et al. 2017) and the wheat reference genome sequence v.1.0 is available in 2017 from the International Wheat Genome Sequencing Consortium (https://www.wheatgenome.org/), and will likely be published soon (Callaway 2017), the lack of a reference genomic sequence for barley or wheat had made it much more difficult to identify the causal genes for QTL responsible for dormancy, even those with large effects. Moreover, due to the complex nature of seed dormancy, to date, even in model plants, only a few causal genes for dormancy QTL have been identified, e.g. DELAY OF GERMINATION 1 (DOG1) in Arabidopsis, and Sdr4 in rice (Bentsink et al. 2006, Sugimoto et al. 2010). However, dormancy is so important for improving PHS tolerance in barley and wheat, it is necessary to identify the natural allelic variants that affect dormancy, so that these alleles can be used in barley and wheat breeding programs.
Around the year 2000, several research groups around the world began trying to identify causal genes for the major dormancy QTL of barley and wheat. After more than a decade of hard work, these studies eventually revealed that Alanine aminotransferase (AlaAT) is the causal gene for the major grain dormancy QTL Qsd1 (SD1) in barley (Sato et al. 2016), and that Mitogen-activated Protein Kinase Kinase 3 (MKK3) is the causal gene for the major grain dormancy QTL Qsd2-AK (SD2) in barley and Phs1 in wheat (Nakamura et al. 2016, Torada et al. 2016). Moreover, in the course of these studies, Mother of FT and TFL1 (MFT) was found as the causal gene for the major wheat grain dormancy QTL QPhs.ocs-3A.1 (Nakamura et al. 2011).
AlaAT is the causal gene for the major dormancy QTL Qsd1 in barley
In barley, Qsd1 was identified as a single major dormancy QTL using doubled haploid lines derived from ‘Haruna Nijo’ and the wild barley accession ‘H602’ (Hori et al. 2007). Haruna Nijo is a malting barley, and has very weak dormancy, with germination rates that are always close to 100%. By contrast, H602 is a wild progenitor of cultivated barley (H. vulgare ssp. spontaneum), and has very strong dormancy with a germination rates that are always close to 0% at harvest. As a single major QTL, Qsd1 has an extremely strong effect on dormancy, explaining more than 50% of the phenotypic variation in dormancy levels between these two parental lines (Hori et al. 2007).
AlaAT was identified as the causal gene for the Qsd1 QTL using map-based cloning (Sato et al. 2016). AlaAT (EC 2.6.1.2), also known as ALT, interconverts the amino acid residues glutamate and alanine by transferring an amino group from alanine to oxaloacetate to form pyruvate and glutamate (Welch 1972). Mammalian studies have revealed that AlaAT is crucial in gluconeogenesis and amino acid metabolism and this familiar enzyme is used as a marker of liver function (Felig 1973). Almost all living organisms have AlaAT, including Archaea, implying that it is ancient and highly conserved (Sakuraba et al. 2004). In plants, AlaAT is involved in nitrogen assimilation, protein synthesis, and carbon metabolism (Duff et al. 2012). However, the mechanism underlying the role of AlaAT in the regulation of dormancy remains unknown. One hypothesis is that AlaAT might alter grain color, which has been suggested to affect dormancy (Debeaujon et al. 2000, Flintham 2000, Himi and Noda 2005). In barley, proanthocyanidin accumulation in the testa of developing grains was reported to affect grain dormancy (Himi et al. 2012). Chalcone is an intermediate metabolite made during the synthesis of proanthocyanidin. Free alanine residues in the embryo are used to make chalcone for pigmentation synthesis in the seed coat of Brassica napus (Wang et al. 2016). Thus, different AlaAT genotypes might affect dormancy through regulation of grain color; however, so far, there is no evidence to suggest that AlaAT is involved in the regulation of chalcone biosynthesis in barley.
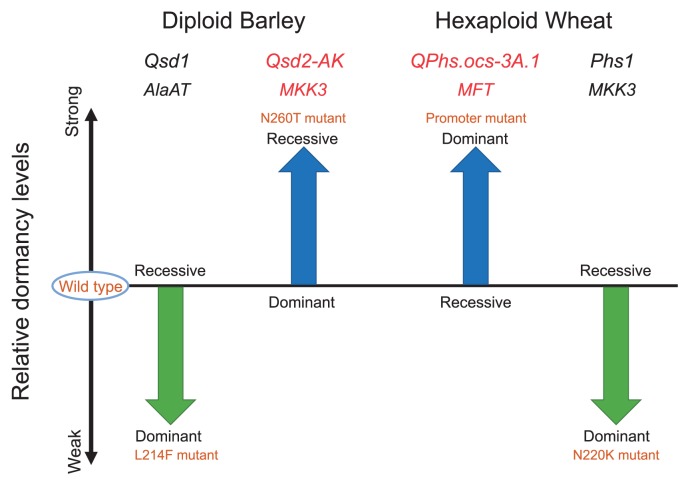
Association analysis between AlaAT genotypes and dormancy phenotypes has suggested that a single nucleotide polymorphism (SNP) G642C at exon 9 of AlaAT could be a CSP for dormancy. This SNP causes substitution of the 214th amino acid residue from leucine (L) in the dormant allele to phenylalanine (F) in the non-dormant allele in AlaAT (Table 1), though the effect of this substitution on the function of AlaAT remains unknown. The L214F substitution site is not close to the active site, the cofactor binding site, or the junction zone of the two polypeptides of the dimer (Sato et al. 2016). It has been speculated that the region is more likely to be involved in dimer formation, or in binding another regulatory protein. The F214 non-dormant mutation allele is dominant (Fig. 2) (Sato et al. 2009, 2016). This mutation is thought to have occurred in West Asia soon after domestication, resulting in the transition from strong to weak dormancy in cultivated barley (Sato et al. 2016).
Table 1.
Major QTL for grain dormancy in barley and wheat
| Diploid barley | Hexaploid wheat | |||
|---|---|---|---|---|
| Dormancy QTL | Qsd1 | Qsd2-AK | QPhs.ocs-3A.1 | Phs1 |
| Chromosome | 5HC | 5HL | 3AS | 4AL |
| Causal gene | AlaAT | MKK3 | MFT | MKK3 |
| Tissue expression | Embryo | All organs (low levels) | Embryo Coleorhiza | All organs (low levels) |
| CSP | G642C | A779C | T(−222) C | C660A |
| Mutation site | Coding sequence (L214F) | Coding sequence (N260T) | Promoter sequence (A-motif) | Coding sequence (N220K) |
| Mutation function | ND | Reducing kinase activity | Maintaining expression | ND |
A-motif, a 6-bp palindromic sequence (TACGTA); C, centromere; CSP, causal sequence polymorphism (wild type to mutant); L, long arm; Low levels, gene expression levels are low and stable; ND, not determined; QTL, quantitative trait locus/loci; S, short arm.
Fig. 2.
Dominance and relative effects of mutant alleles on dormancy. The relative levels of dormancy caused by each mutation are compared with those of the wild-type allele. The starting and the ending points (heads) of the arrows show the relative dormancy levels of the wild-type allele and the mutant allelele of each gene, respectively. The arrow’s length represents the qualitative effect of each QTL. Blue arrows show increasing dormancy levels produced by the mutations. Green arrows show decreasing dormancy levels produced by the mutaions. Red and black letters of the QTL and gene names indicate the mutations were estimated to have originated in East Asia and West Asia, respectively.
MKK3 is the causal gene for the major dormancy QTL Qsd2-AK in barley
Using recombinant inbred lines (RILs) from the old Japanese varieties ‘Azumamugi (Az)’ and ‘Kanto Nakate Gold’ (KNG), a single major dormancy QTL, Qsd2-AK, was found at the SD2 locus. Az, which was bred from Japanese landraces (Sameri et al. 2006), is a six-row barley variety that is used for food and has strong dormancy. KNG is a two-row malting barley with weak dormancy and is derived from a malting variety ‘Golden Melon’, which was imported from northern Europe to Japan in 1881 (Sameri et al. 2006, Takahashi 1980). Recently, using map-based cloning, the causal gene for the QTL Qsd2-AK was identified as MKK3 (Nakamura et al. 2016).
MKK3 functions in the well-known, conserved mitogen-activated protein (MAP) kinase cascade signal transduction pathway. In general, a MAP kinase cascade consists of three modules: a MAP kinase kinase kinase (MAP3K), a MAP kinase kinase (MAP2K), and a MAP kinase (MAPK). In response to external signals, the MAP kinase cascade successively transmits the signals via phosphorylation to downstream factors, such as transcription factors, to control cell growth and proliferation. Plants have many more of these modules than animals; being sessile, plants must respond appropriately to environmental signals to survive. Plants such as Arabidopsis and rice have about 80 MAP3Ks, 10 MAP2Ks, and 20 MAPKs (Ichimura et al. 2002). MAP3K signals converge on the intermediate module MAP2Ks; thus MAP2Ks play pivotal roles in the MAP kinase cascade. MKK3 is a member of the MAP2K family with a unique structure, having a kinase domain and nuclear transport factor 2 (NTF2) domain together in the same MAP2K. MKK3 is present in all photosynthetic eukaryotes, which means its origin can be traced back at least 800 million years to when single-celled algae first emerged (Ramanan et al. 2016). MKK3 has been reported to function in several signaling pathways such as those involved in pathogen resistance (Dóczi et al. 2007), jasmonic acid signaling (Takahashi et al. 2007), wounding signals (Takahashi et al. 2011), osmotic stress and abscisic acid (ABA) signaling (Zhang et al. 2012), and blue light signaling (Sethi et al. 2014). Recent Arabidopsis studies have revealed that MKK3 functions in an ABA-activated MAPK cascade comprising the MAP3Ks MAP3K17 and MAP3K18, the MAP2K MKK3, and the MAPKs MPK1, 2, 3, 7, and 14 (Colcombet et al. 2016, Danquah et al. 2015, Matsuoka et al. 2015). This cascade is involved in the ABA stress signaling pathway. The plant hormone ABA has a role in the repression of germination (Nambara et al. 2010). These results suggest that, in barley, MKK3 is involved in the regulation of grain dormancy through control of the ABA signaling pathway. A splice junction mutant of MKK3 that can not produce normal MKK3 protein showed a delayed-germination phenotype (Nakamura et al. 2016). Therefore, these results indicate that barley MKK3 functions as a negative regulator of ABA.
In MKK3, the CSP for Qsd2-AK is thought to be an adenine (A) residue at the 779th position of exon 7 of the non-dormant KNG allele, which is mutated to a cytosine (C) in the dormant Az allele (Table 1). This nucleotide change causes a non-synonymous amino acid substitution in MKK3 in the evolutionarily conserved 260th amino acid residue, changing asparagine (N) to threonine (T). This N260T substitution reduces the kinase activity of MKK3 (Nakamura et al. 2016). Thus, the N260T-substituted dormant MKK3 allele cannot efficiently transmit phosphorylation signals for germination, delaying germination and conferring the dormant phenotype. These results explain why the dormant MKK3 allele is recessive (Fig. 2).
MKK3 is expressed in all organs. It participates in the regulation of not only dormancy, but also various abiotic stress response mechanisms such as drought tolerance through the ABA-activated MAPK cascade. Therefore, MKK3 deficiency should affect not only grain characteristics, but also other phenotypes, such as stomata closure (Zeilcourt et al. 2016). This may be why natural null mutation alleles of MKK3 have not yet been found in barley. The N260T mutation has only a partial effect in decreasing kinase activity and may not be sufficient to severely affect other stress responses or important agricultural traits so that cultivars with the SNP can grow normally in the field.
MFT is the causal gene for the major dormancy QTL QPhs.ocs-3A.1 in wheat
MFT was identified as the causal gene for the major dormancy QTL QPhs.ocs-3A.1 in wheat (Nakamura et al. 2011). This QTL was detected at the terminal region of the short arm of chromosome 3A using RILs from ‘Zenkouji komugi’ (Zen) and ‘Chinese Spring’ (CS) (Mori et al. 2005, Osa et al. 2003). Zen was selected from a gamma-ray mutant of Igachikugo-Oregon and released in 1969 (Hoshino et al. 2000). Zen is known for its strong dormancy (Miura et al. 1997, Osanai and Amano 1993). By contrast, CS is a popular variety widely used for genetic studies worldwide, with far weaker dormancy compared to Zen. CS is thought to have originated from a landrace in the Sichuan region of south China (Sears and Miller 1985). A previous study revealed the existence of genetic similarities between CS and landraces in southwest Japan (Kobayashi et al. 2016), indicating that Zen and CS have similar genetic backgounds.
MFT was first identified in a transcriptome analysis of the effect of temperature on grain dormancy in wheat (Nakamura et al. 2011). In barley and wheat, dormancy levels fluctuate depending on the temperature during grain development; for example, lower temperatures during grain development lead to higher dormancy levels (Nakazono et al. 2013, Rodríguez et al. 2015). Microarray analysis showed that the MFT transcript level was higher in embryos that developed at lower temperatures, compared with those that developed at higher temperatures. MFT function was confirmed via transient assays, which showed that embryos that expressed MFT at high levels (under the control of the maize ubiquitin promoter) were unable to germinate. MFT was located at the peak of QPhs.ocs-3A.1. Furthermore, transgenic complementation analysis showed that introduction of the Zen MFT allele into CS led to higher expression of MFT and increased dormancy levels of the CS transformants. Comparison of genomic sequences of Zen and CS identified a SNP responsible for the dormancy attributed to QPhs.ocs-3A.1 in the MFT promoter region.
MFT is a member of the phosphatidylethanolamine binding protein (PEBP) family, which functions in diverse signaling pathways involved in growth and differentiation in bacteria, animals, and plants (Wickland and Hanzawa 2015). In plants, the PEBP family is divided into three subfamilies: Flowering locus T (FT), Terminal Flower 1 (TFL1), and MFT. All are thought to function as switches for growth phase transitions in the plant life cycle. FT and TFL1 are well-known regulators that determine the timing of flowering; i.e., the phase transition from vegetative growth to reproductive growth. MFT is thought to be an ancestral gene for FT and TFL1 because mosses only have an MFT homolog. MFT functions as a switch for germination, another important phase transition from embryonic growth to post-embryonic growth. MFT functions as a suppressor of germination in wheat (Nakamura et al. 2011). Subsequent studies suggested that MFT has the same function in Arabidopsis (Penfield and MacGregor 2017).
The CSP for QPhs.ocs-3A.1 is a nucleotide substitution from T in CS to C in Zen, 222 bp upstream from the MFT initiation codon (–222 SNP) (Table 1). The –222 SNP forms a palindromic sequence (A-motif) in the MFT promoter. T in CS is the wild-type conserved allele and C is the mutated allele, which truncates the palindromic sequence. This nucleotide change is presumed to be the cause for the differenential MFT expression during seed development in Zen. In CS, the expression level of MFT is reduced in the final stage of seed development, thus allowing mature grains to germinate. However, in the case of the mutated dormant MFT allele, MFT expression is not reduced (Nakamura et al. 2011). One plausible explanation for this is that some transcription factors, such as bZIP transcription factors, bind the palindromic sequence to stop MFT expression at the final stage of grain development. However, the nucleotide change prevents or decreases the binding of these factors and MFT continues to be expressed, even during the last, mature stage of seed development. Thus, MFT continues to function as a repressor of germination at the mature stage, and in the mature, imbibed grains. Therefore, cultivars with the dormant MFT allele have higher levels of seed dormancy.
Although Zen was derived from Igachikugo-Oregon treated with 60Co, Igachikugo-Oregon already has the –222 SNP mutation (Nakamura et al. 2015). Thus, the mutation did not come from the ganma-ray mutagenesis of Igachikugo-Oregon. The naturally occurring mutation (–222 SNP) in the Zen variety functions as a dominant allele in hexaploid wheat (Fig. 2) and is a very important functional SNP for improving PHS tolerance in wheat. Liu et al. (2013) found two mutations at 646 bp and 666 bp (+646 and +666 mutations, respectively) in MFT, which they refer to as TaPHS1. The +646 and +666 mutations generate a mis-splicing event and a premature stop codon, respectively. Detected using another parental combination of ‘Rio Blanco’ and ‘NW97S186’, these mutations were found to be CSPs for dormancy in the QTL Qphs.pseru-3AS (Liu et al. 2013). These two mutations were also found in diploid and tetraploid wheat, indicating that these mutations might have been selected for during domestication of diploid and tetraploid wheat, to decrease dormancy levels of cultivated wheat (Liu et al. 2015). These CSPs are loss-of-function mutations of MFT, and thus are recessive non-dormant alleles. Although information about these CSPs is useful to exclude them from breeding programs to develop new varieties, they are not useful for conferring higher levels of dormancy. The gain-of-function SNP –222 does increase dormancy levels and is therefore useful for improving PHS tolerance.
MKK3 is also a causal gene for the major dormancy QTL Phs1 in wheat
A major QTL for dormancy in wheat has been consistently detected on chromosome 4A using various parental combinations (Flintham et al. 2002, Kato et al. 2001, Mares et al. 2005, Torada et al. 2005). The same QTL has been given four different names by four different research groups: Qphs.ocs.1, Phs, Phs1, and Phs-A1. In this review, the QTL is referred to as Phs1, as designated by Torada et al. (2016).
During the evolution of hexaploid wheat, a complex chromosomal rearrangement occurred on chromosome 4A (Devos et al. 1995). A segment of the terminal region of chromosome 5A translocated to chromosome 4A at the diploid level. Comparative genome analysis indicated that the Phs1 region in wheat might correspond to the SD2 locus in barley (Flintham et al. 2002, Gao et al. 2003, Li et al. 2004). Torada et al. (2016) recently also identified MKK3 as the causal gene for Phs1, providing direct evidence that Phs1 in wheat is an ortholog of SD2 in barley. However, the CSP in Phs1 is different from that in Qsd2-AK in barley (Table 1). For Phs1, the CSP is a nucleotide substitution from the wild-type nucleotide C at the 660th position in the dormant allele, to an A in the non-dormant allele. This causes a non-synonymous substitution from the evolutionarily conserved asparagine (N) to lysine (K) in the 220th amino acid residue of the kinase domain. Interestingly, in wheat, the recessive dormant allele has a wild-type, conserved N220, but the dominant, non-dormant allele has a mutated K220 (Fig. 2). This is in contrast to barley, in which the mutant allele is dormant and recessive, and the wild-type allele is non-dormant and incompletely dominant. To date, how the N220K substitution makes the MKK3 allele dominant and non-dormant remains unknown. This mutation should cause a gain-of-function change in MKK3. Since the N220K substitution occurs in the kinase domain, we suspect that the mutation might affect the kinase function of MKK3.
East Asia is a nursery for natural mutations that increase PHS tolerance
Mutations in the four known barley and wheat dormancy genes have differing effects on grain dormancy levels. As summarized in Fig. 2, mutations in barley MKK3 and wheat MFT increase dormancy levels, but mutations in barley AlaAT and wheat MKK3 decrease dormancy. The origin of these mutations was deduced from genomic sequence analysis of worldwide collections of wild and cultivated barley and wheat cultivars (Nakamura et al. 2015, 2016, Sato et al. 2016, Shorinola et al. 2017). Interestingly, these geographical studies indicated that the mutations that increase dormancy levels occurred in East Asia, and the mutations that decreased dormancy levels occurred in West Asia.
Analysis of the genomic sequences of wild and cultivated barley identified the direct ancestral allele of the dormant Az MKK3 allele in barley (Nakamura et al. 2016). The ancestral allele has the same genomic sequence as the dormant Az MKK3 allele, except for the functional SNP that causes the N260T amino acid substitution (Nakamura et al. 2016). The ancestral gene has the non-mutated, original nucleotide (A) and therefore, the allele has the evolutionarily conserved amino acid N260. The ancestral allele sequence was found in both wild and cultivated barley. Cultivars collected from Iran, China, and Korea have the ancestral allele sequence, indicating that it traveled from the Fertile Crescent to the northeast of China. Since the dormant MKK3 allele in barley is mainly distributed in and around East Asia, this indicates that the N260T substitution occurred in East Asia.
Investigation of the geographical distribution of the −222 SNP in the promoter of MFT showed that a lot of wheat varieties in Japan have the dormant MFT allele, but the non-dormant allele dominates in other regions (Nakamura et al. 2015). In this study, only a few varieties from China and Korea were investigated, so the possibility remains that investigation of more wheat varieties from these regions might find more varieties with the dormant MFT allele. However, this result suggests that the dormant MFT allele is predominantly distributed at least in Japan or perhaps in East Asia.
The study of MFT alleles examined approximately 400 European varieties and found only two with the dormant MFT allele (Nakamura et al. 2015). One of these is ‘Ardito’, a variety derived from a cross between the line ‘Selezione 21’ and the Japanese variety ‘Akakomugi’ by the Italian plant breeder Strampelli in 1913 (Salvi et al. 2013). At that time, Akakomugi had two unique, attractive traits for improving lodging tolerance and avoiding drought stress during grain filling: short straw, and extremely early maturation caused by the genes Rht8 and Ppd-D1, respectively. Akakomugi also has the dormant MFT allele (Nakamura et al. 2015). Thus, we can deduce that the dormant MFT allele in Ardito came from Akakomugi, together with Rht8 and Ppd-D1. Lines from this cross soon became well-known in other countries, notably Argentina and China (Salvi et al. 2013). This indicates that some of the dormant MFT alleles in modern Chinese varieties originated in this lineage.
The cultivation of barley and wheat reached East Asia about four thousand years ago (Fig. 3; Crawford 2006, Feldman 2000, Kidder 1993, Zhang 1983). The climate in East Asia is very different from that of West Asia. The Asian Monsoon, one of the largest-scale seasonal winds in the world, blows from eastern Africa and Madagascar to Japan approximately between May and July (Fig. 3), bringing warm, humid air to East Asia. A lot of rain falls in East Asia around barley and wheat harvest time, which can induce PHS in cultivars with low dormancy, suggesting that when the cultivars reached East Asia, genotypes better adapted to the local climate were selected. Thus, spontaneous mutations for the dormant alleles in wheat MFT and barley MKK3 have been selected and prevailed predominantly in East Asia, and East Asia has acted as a nursery for PHS tolerance genes. Genetic analysis of East Asian cultivars is therefore useful for finding natural mutations to increase dormancy levels for PHS tolerance.
Fig. 3.
Migration of barley and wheat cultivars, and a satellite image taken in June in East Asia. BP, before present. The blue arrow shows the direction of winds in the Asian monsoon. The satellite infrared image was taken by the Multifunctional Transport Satellite-2 (MTSAT-2; Himawari-7) in 2014. Green lines show country borders and the world map was obtained from Google Maps.
Application of the PHS tolerance genes for breeding programs
Causal genes for the four major dormancy QTL in barley and wheat are now available for marker-assisted selection to improve PHS tolerance in barley and wheat (Table 1).
As Yanagisawa et al. (2005) showed in wheat, breeders can develop lines with excellent tolerance to PHS by accumulating natural mutations. However, to develop elite varieties, breeders must also optimize important agricultural traits such as yield, lodging tolerance, and disease resistance. If there is a gene that has deleterious effects on an important agricultural trait closely linked to a dormancy QTL gene, the linkage drag between them could cause a problem for traditional plant breeding approaches. However, in such a case, if we have a marker for the dormancy QTL gene, we can break the linkage using the marker. CSPs themselves might have pleiotropic, deleterious effects on other important agricultural traits. In this case, detailed analysis of the effect of CSPs on gene functions and plant traits will reveal the full effects of the CSPs. This knowledge will inform efforts to utilize the CSPs in breeding programs.
CSP data for the four genes are now ready to use for marker-assisted selection of PHS tolerance genes in breeding programs. Identification of the genes involved in known dormancy QTL provides insight into the uneven distribution of dormancy alleles in cultivars around the world, or even in subregions of the world. For example, wheat varieties in the northern part of Japan do not have the dormant MFT allele (Chono et al. 2015, Nakamura et al. 2015) because they are mainly from the lineage of high quality wheat varieties introduced from North America and Europe (Kobayashi et al. 2016). However, transferring the dormant allele from varieties in southern Japan into varieties in northern Japan can improve PHS tolerance in northern Japanese varieties. This strategy has already shown promising results.
Outside of Japan, few varieties have the dormant MFT allele, so incorporating it into other varieties seems a promising strategy to improve PHS tolerance, in much the same way as Rht8 from the Japanese wheat variety Akakomugi, or Rht1 and Rht2 from Norin10 were introduced into other varieties to develop high yields around the world, before and during the Green Revolution (Hedden 2003, Salvi et al. 2013).
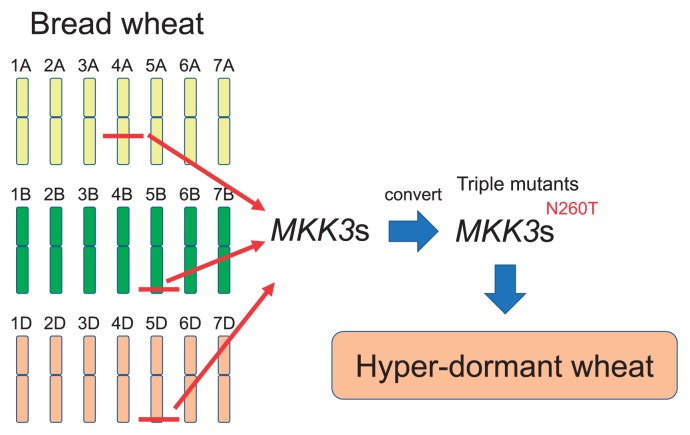
The discovery of a recessive dormant allele of the major dormancy QTL Qsd2-AK in barley has indicated the possibility of developing novel strategies to improve PHS tolerance in wheat using recessive alleles (Fig. 4). In hexaploid wheat, the N260T mutation is expected to give rise to a strongly dormant allele of MKK3, but it has not been selected because the allohexaploid nature of wheat masks the recessive phenotype. Introducing the recessive mutation into all of the functional wheat homoeologs of MKK3 will be a novel way to increase seed dormancy in wheat.
Fig. 4.
New strategy for improving PHS tolerance of wheat using the N260T mutation in MKK3. Allohexaploid wheat has A, B, and D sub-genomes. If we could introduce the N260T mutation into all homoeologous MKK3 genes, the triple mutant could express its phenotype of increased dormancy, providing a new avenue for developing hyper-dormant wheat cultivars. Red horizontal lines show the locations of the homoeologous MKK3 genes on each wheat chromosome.
Another challenge to be solved is how to collect or create specific mutations that are used in breeding programs. It is easier to find knockout mutations of genes than to find specific mutations, but in this case, because MKK3 is also important in ABA stress signaling pathways (Colcombet et al. 2016), null mutations of MKK3 carry a high risk of harmful pleiotropic effects on growth or stress tolerance. Developing new ways to efficiently identify specific mutations is the key to success. Of the four mutations identified, this strategy can be specifically applied to the Qsd2-AK mutation because it is the only one known so far to be recessive and to increase dormancy levels.
Conclusions and perspectives
Causal genes for four major grain dormancy QTL in barley and wheat have been identified, increasing our understanding of the regulatory mechanisms of grain dormancy in barley and wheat. From a practical perspective, as a recent paper showed that the MFT dormant allele can be used to improve PHS tolerance in durum wheat (Triticum turgidum ssp. durum (Desf.) Husn.) (Kato et al. 2017), identification of these genes paves the way for marker-assisted selection to confer PHS tolerance, and for increasing dormancy levels of hexaploid wheat using the recessive MKK3 dormant allele. These results have informed strategies for breeding of grain dormancy or PHS tolerance in barley and wheat. However, this is just the beginning of the study on natural mutations in grain dormancy in barley and wheat. There are other known dormancy QTL in barley and wheat (Cao et al. 2016, Gong et al. 2014, Hickey et al. 2012, Nakamura et al. 2017, Somyong et al. 2014) for which the causal genes remain to be identified. Future QTL studies in model plants such as Arabidopsis and rice will reveal more causal genes (Bentsink et al. 2010, Marzougui et al. 2012), and with the rapid progress of studies in seed dormancy and germination (Nonogaki 2017), findings will no doubt facilitate the identification of other causal genes for dormancy QTL in barley and wheat, and eventually, breeding of PHS tolerant barley and wheat cultivars will be realized.
Acknowledgments
The author thanks Professor K. Onishi at Obhiro University of Agriculture and Veterinary Medicine and Dr T. Kato at Tochigi Prefecture Experimental Station for the photos of PHS, and Dr K. Toyoshima at Chiba University for the satellite image photo. The author thanks Professor C. Li at Murdoch University for critical reading of the manuscript and Professor J.-R. Wang at Sichuan Agriculture University for information on the recent state of PHS damage in China. This work was financially supported by grants from the Japanese Ministry of Agriculture, Forestry and Fisheries (Genomics for Agricultural Innovation, TRC-1002 and TRG-1002, and Genomics-based Technology for Agricultural Improvement, TRS-1001).
Literature Cited
- Anderson, J.A., Sorrells, M.E. and Tanksley, S.D. (1993) RFLP analysis of genomic regions associated with resistance to preharvest sprouting in wheat. Crop Sci. 33: 453–459. [Google Scholar]
- Bentsink, L., Jowett, J., Hanhart, C.J. and Koornneef, M. (2006) Cloing of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc. Natl. Acad. Sci. USA 103: 17042–17047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentsink, L., Hanson, J., Hanhart, C.J., Blankestijn-de Vries, H., Coltrane, C., Keizer, P., El-Lithy, M., Alonso-Blanco, C., de Andrés, M.T., Reymond, M.et al. (2010) Natural variation for seed dormancy in Arabidopsis is regulated by additive genetic and molecular pathways. Proc. Natl. Acad. Sci. USA 107: 4264–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway, E. (2017) Small group scoops international effort to sequence huge wheat genome. Nature News, doi: 10.1038/nature.2017.22924. [DOI] [Google Scholar]
- Cao, L., Hayashi, K., Tokui, M., Mori, M., Miura, H. and Onishi, K. (2016) Detection of QTLs for traits associated with pre-harvest sprouting resistance in bread wheat (Triticum aestivum L.). Breed. Sci. 66: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chono, M., Matsunaka, H., Seki, M., Fujita, M., Kiribuchi-Otobe, C., Oda, S., Kojima, H. and Nakamura, S. (2015) Molecular and genealogical analysis of grain dormancy in Japanese wheat varieties, with specific focus on MOTHER OF FT AND TFL1 on chromosome 3A. Breed. Sci. 65: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombet, J., Sözen, C. and Hirt, H. (2016) Convergence of multiple MAP3Ks on MKK3 identifies a set of novel stress MAPK modules. Front. Plant Sci. 7: 1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford, G.W. (2006) Prehistoric plant domestication in East Asia. In: Cowan, C.W. and Watson P.J. (eds.) The origins of agriculture: An international perspective, University of Alabama Press, pp. 7–38. [Google Scholar]
- Danquah, A., Zélicourt, A., Boudsocq, M., Neubauer, J., Frey, N.F., Leonhardt, N., Pateyron, S., Gwinner, F., Tamby, J.-P., Oritiz-Masia, D.et al. (2015) Identification and characterization of an ABA-activated MAP kinase cascade in Arabidopsis thaliana. Plant J. 82: 232–244. [DOI] [PubMed] [Google Scholar]
- Debeaujon, I., Léon-Kloosterziel, K.M. and Koornneef, M. (2000) Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 122: 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derera, N.F. (1990) A perspective of sprouting research. In: Ringlud, K., Mosleth E. and Mares D.J. (eds.) Fifth international symposium on pre-harvest sprouting, Westview Press, Boulder, pp. 3–11. [Google Scholar]
- Devos, K.M., Dubcovsky, J., Dvořák, J., Chinoy, C.N. and Gale, M.D. (1995) Structural evolution of wheat chromosomes 4A, 5A, and 7B and its impact on recombination. Theor. Appl. Genet. 91: 282–288. [DOI] [PubMed] [Google Scholar]
- Dóczi, R., Brader, G., Pettkó-Szandtner, A., Rajh, I., Djamei, A., Pitzschke, A., Teige, M. and Hirt, H. (2007) The Arabidopsis mitogen-activated protein kinase kinase MKK3 is upstream of group C mitogen-activated protein kinases and participates in pathogen signaling. Plant Cell 19: 3266–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff, S.M.G., Rydel, T.J., McClerren, A.L., Zhang, W., Li, J.Y., Sturman, E.J., Halls, C., Chen, S., Zeng, J., Peng, J.et al. (2012) The enzymology of alanine aminotransferase (AlaAT) isoforms from Hordeum vulgare and other organisms, and the HvAlaAT crystal structure. Arch. Biochem. Biophys. 528: 90–101. [DOI] [PubMed] [Google Scholar]
- Feldman, M. (2000) Origin of cultivated wheat. In: Alin, P.B. and William J.A. (eds.) The World Wheat Book: A Hstory of Wheat Breeding, TEC and DOC, London, pp. 3–56. [Google Scholar]
- Felig, P. (1973) The glucose-alanine cycle. Metab. Clin. Exp. 22: 179–207. [DOI] [PubMed] [Google Scholar]
- Finkelstein, R., Reeves, W., Ariizumi, T. and Steber, C. (2008) Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 59: 387–415. [DOI] [PubMed] [Google Scholar]
- Flintham, J.E. (2000) Different genetic components control coat-imposed and embryo-imposed dormancy in wheat. Seed Sci. Res. 10: 43–50. [Google Scholar]
- Flintham, J., Adlam, R., Bassoi, M., Holdsworth, M. and Gale, M. (2002) Mapping genes for resistance to sprouting damage in wheat. Euphytica 126: 39–45. [Google Scholar]
- Gao, W., Clancy, J.A., Han, F., Prada, D., Kleinhofs, A. and Ullrich, S.E. (2003) Molecular dissection of a dormancy QTL region near the chromosome7 (5H) L telomere in barley. Theor. Appl. Genet. 107: 552–559. [DOI] [PubMed] [Google Scholar]
- Gong, X., Li, C., Zhou, M., Bonnardeaux, Y. and Yan, G. (2014) Seed dormancy in barley is dictated by genetics, environments and their interactions. Euphytica 197: 355–368. [Google Scholar]
- Hedden, P. (2003) The genes of the Green Revolution. Trends Genet. 19: 5–9. [DOI] [PubMed] [Google Scholar]
- Hickey, L.T., Lawson, W., Arief, V.N., Fox, G., Franckowiak, J. and Dieters, M.J. (2012) Grain dormancy QTL identified in a doubled haploid barley population derived from two non-dormant parents. Eyphytica 188: 113–122. [Google Scholar]
- Himi, E. and Noda, K. (2005) Red grain colour gene (R) of wheat is a Myb-type transcription factor. Euphytica 143: 239–242. [Google Scholar]
- Himi, E., Yamashita, Y., Haruyama, N., Yanagisawa, T., Maekawa, M. and Taketa, S. (2012) Ant28 gene for proanthocyanidin synthesis encoding the R2R3 MYB domain protein (Hvmyb10) highly affects grain dormancy in barley. Euphytica 188: 141–151. [Google Scholar]
- Hori, K., Sato, K. and Takeda, K. (2007) Detection of seed dormancy QTL in multiple mapping populations derived from crosses involving novel barley germplasm. Theor. Appl. Genet. 115: 869–876. [DOI] [PubMed] [Google Scholar]
- Hoshino, T., Kato, K. and Ueno, K. (2000) Japanese wheat pool. In: Bonjean, A.P. and Angus W.J. (eds.) The World Wheat Book: A History of Wheat Breeding, TEC and DOC, London, pp. 703–726. [Google Scholar]
- Ichimura, K., Shinozaki, K., Tena, G., Sheen, J., Henry, Y., Champion, A., Kresis, M., Zhang, S., Hirt, H., Wilson, C.et al. (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci. 7: 301–308. [DOI] [PubMed] [Google Scholar]
- IRGSP (International Rice Genome Sequencing Project) (2005) The map-based sequence of the rice genome. Nature 436: 793–800. [DOI] [PubMed] [Google Scholar]
- Kato, K., Nakamura, W., Tabiki, T. and Miura, H. (2001) Detection of loci controlling seed dormancy on group 4 chromosomes of wheat and comparative mapping with rice and barley genomes. Theor. Appl. Genet. 102: 980–985. [Google Scholar]
- Kato, K., Maruyama-Funatsuki, W., Yanaka, M., Ban, Y. and Takata, K. (2017) Improving preharvest sprouting resistance in durum wheat with bread wheat genes. Breed. Sci. 67: 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder, J.E. Jr. (1993). The earliest societies in Japan. In: Brown, D.M. (ed.) The Cambridge History of Japan, Vol. 1: Ancient Japan, Cambridge University Press, pp. 48–107. [Google Scholar]
- Kilian, B., Özkan, H., Pozzi, C. and Salamini, F. (2009) Domestication of the Triticeae in the fertile cresent. In: Feuillet, C. and Mühlbauer G.J. (eds.) Genetics and Genomics of the Triticeae (Plant Genetics and Genomics: Crops and Models), Springer, New York, pp. 81–119. [Google Scholar]
- Kobayashi, F., Tanaka, T., Kanamori, H., Wu, J., Katayose, Y. and Handa, H. (2016) Characterization of a mini core collection of Japanese wheat varieties using single-nucleotide polymorphisms generated by genotyping-by sequencing. Breed. Sci. 66: 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulwal, P.L., Mir, R.R., Kumar, S. and Gupta, P.K. (2010) QTL analysis and molecular breeding for seed dormancy and pre-harvest sprouting tolerance in bread wheat. J. Plant Biol. 37: 59–74. [Google Scholar]
- Li, C., Ni, P., Francki, M., Hunter, A., Zhang, Y., Schibeci, D., Li, H., Tarr, A., Wang, J., Cakir, M.et al. (2004) Genes controlling seed dormancy and pre-harvest sprouting in a rice-wheat-barley comparison. Funct. Integr. Genomics 4: 84–93. [DOI] [PubMed] [Google Scholar]
- Liu, C., Ding, F., Hao, F., Yu, M., Lei, H., Wu, X., Zhao, Z., Guo, H., Yin, J., Wang, Y.et al. (2016) Reprogramming of seed metabolism facilitates pre-harvest sprouting resistance of wheat. Sci. Rep. 6: 20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., Sehgal, S.K., Li, J., Lin, M., Trick, H.N., Yu, J., Gill, B.S. and Bai, G. (2013) Cloning and characterization of a critical regulator for pre-harvest sprouting in wheat. Genetics 195: 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., Sehgal, S.K., Lin, M., Li, J., Trick, H.N., Gill, B.S. and Bai, G. (2015) Independent mis-splicing mutation in TaPHS1 causing loss of preharvest sprouting (PHS) resistance during wheat domestication. New Phytol. 208: 928–935. [DOI] [PubMed] [Google Scholar]
- Mares, D.J., Mrva, K., Cheong, J., Williams, K., Watson, B., Storile, E., Sutherland, M. and Zou, Y. (2005) A QTL located on chromosome 4A associated with dormancy in white- and red-grained wheats of diverse origin. Theor. Appl. Genet. 111: 1357–1364. [DOI] [PubMed] [Google Scholar]
- Mares, D.J. and Mrva, K. (2014) Wheat grain preharvest sprouting and late maturity alpha-amylase. Planta 240: 1167–1178. [DOI] [PubMed] [Google Scholar]
- Marzougui, S., Sugimoto, K., Yamanouchi, U., Shimono, M., Hoshino, T., Hori, K., Kobayashi, M., Ishiyama, K. and Yano, M. (2012) Mapping and characterization of seed dormancy QTLs using chromosome seqment substitution lines in rice. Theor. Appl. Genet. 124: 893–902. [DOI] [PubMed] [Google Scholar]
- Mascher, M., Gundlach, H., Himmelbach, A., Beier, S., Twardziok, S.O., Wicker, T., Radchuk, V., Dockter, C., Hedley, P.E., Russell, J.et al. (2017) A chromosome conformation capture ordered sequence of the barley genome. Nature 544: 427–433. [DOI] [PubMed] [Google Scholar]
- Matsuoka, D., Yasufuku, T., Furuya, T. and Nanmori, T. (2015) An abscisic acid inducible Arabidopsis MAPKKK, MAPKKK18 regulates leaf senescence via its kinase activity. Plant Mol. Biol. 87: 565–575. [DOI] [PubMed] [Google Scholar]
- Miura, H., Fukuda, Y. and Sawada, S. (1997) Expression of seed dormancy in diallel F1 and F2 seed of wheat ripened under a controlled environment. J. Genet. Breed. 51: 195–200. [Google Scholar]
- Mori, M., Uchino, N., Chono, M., Kato, K. and Miura, H. (2005) Mapping QTLs for grain dormancy on wheat chromosome 3A and the group 4 chromosomes, and their combined effect. Theor. Appl. Genet. 110: 1315–1323. [DOI] [PubMed] [Google Scholar]
- Nakamura, S., Abe, F., Kawahigashi, H., Nakazono, K., Tagiri, A., Matsumoto, T., Ustugi, S., Ogawa, T., Handa, H., Ishida, H.et al. (2011) A wheat homolog of Mother of FT and TFL1 acts in the regulation of germination. Plant Cell 23: 3215–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, S., Chono, M., Stehno, Z., Holubec, V., Morishige, H., Pourkheirandish, M., Kanamori, H., Wu, J., Matsumoto, T. and Komatsuda, T. (2015) Diversification of the promoter sequences of wheat Mother of FT and TFL1 on chromosome 3A. Mol. Breed. 35: 164. [Google Scholar]
- Nakamura, S., Pourkheirandhish, M., Morishige, H., Kubo, Y., Nakamura, M., Ichimura, K., Seo, S., Kanamori, H., Wu, J., Ando, T.et al. (2016) Mitogen-activated Protein Kinase Kinase 3 regulates seed dormancy in barley. Curr. Biol. 26: 775–781. [DOI] [PubMed] [Google Scholar]
- Nakamura, S., Pourkheirandhish, M., Morishige, H., Sameri, M., Sato, K. and Komatsuda, T. (2017) Quantitative trait loci and maternal effects affecting the strong grain dormancy of wild barley (Hordeum vulgare ssp. spontaneum). Front. Plant Sci. 8: 1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazono, K., Ohno, H., Yoshida, H. and Nakagawa, H. (2013) Effects of meterological factors during grain development on pre-harvest sprouting in wheat. Jpn. J. Crop. Sci. 82: 183–191. [Google Scholar]
- Nambara, E., Okamoto, M., Tatematsu, K., Yano, R., Seo, M. and Kamiya, Y. (2010) Abscisic acid and the control of seed dormancy and germination. Seed Sci. Res. 20: 55–67. [Google Scholar]
- Nonogaki, H. (2014) Seed dormancy and germination-emerging mechanisms and new hypotheses. Front. Plant Sci. 5: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki, H. (2017) Seed biology updates―Highlights and new discoveries in seed dormancy and germination research. Front. Plant Sci. 8: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osa, M., Kato, K., Mori, M., Shindo, C., Torada, A. and Miura, H. (2003) Mapping QTLs for seed dormancy and the Vp1 homologue on chromosome 3A in wheat. Theor. Appl. Genet. 106: 1491–1496. [DOI] [PubMed] [Google Scholar]
- Osanai, S. and Amano, Y. (1993) Selection of tolerant lines to low temperature germinability in wheat. In: Walker-Simmons, M.K. and Ried J.L. (eds.) Pre-harvest sprouting in cereals 1992, American Association of Cereal Chemists, St. Paul, Minn, pp. 76–82. [Google Scholar]
- Penfield, S. and MacGregor, D.R. (2017) Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot. 68: 819–825. [DOI] [PubMed] [Google Scholar]
- Ramanan, R., Kim, B.-H., Cho, D.-H., Oh, H.-M. and Kim, H.-S. (2016) Algae-bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 34: 14–29. [DOI] [PubMed] [Google Scholar]
- Rodríguez, M.V., Barrero, J.M., Corbineau, F., Gubler, F. and Benech-Arnold, R.L. (2015) Dormancy in cereals (not too much, not so little): about the mechanisms behind this trait. Seed Sci. Res. 25: 99–119. [Google Scholar]
- Sakuraba, H., Kawakami, R., Takahashi, H. and Ohshima, T. (2004) Novel archaeal alanine: glyoxylate aminotransferase from Thermococcus litoralis. J. Bacteriol. 186: 5513–5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi, S., Porfiri, O. and Ceccarelli, S. (2013) Nazareno Strampelli, the ‘Prophet’ of the green revolution. J. Agr. Sci. 151: 1–5. [Google Scholar]
- Sameri, M., Takeda, K. and Komatsuda, T. (2006) Quantitative trait loci controlling agronomic traits in recombinant inbred lines from a cross of oriental- and occidental-type barley cultivars. Breed. Sci. 56: 243–252. [Google Scholar]
- Sato, K., Matsumoto, T., Ooe, N. and Takeda, K. (2009) Genetic analysis of seed dormancy QTL in barley. Breed. Sci. 59: 645–650. [Google Scholar]
- Sato, K., Yamane, M., Yamaji, N., Kanamori, H., Tagiri, A., Schwerdt, J.G., Fincher, G.B., Matsumoto, T., Takeda, K. and Komatsuda, T. (2016) Alanine aminotransferase controls seed dormancy in barley. Nat. Com. 7: 11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz, J., Cannon, S.B., Schlueter, J., Ma, J., Mitros, T., Nelson, W., Hyten, D.L., Song, Q., Thelen, J.J., Cheng, J.et al. (2010) Genome sequence of the palaeopolyploid soybean. Nature 463: 178–183. [DOI] [PubMed] [Google Scholar]
- Schnable, P.S., Ware, D., Fulton, R.S., Stein, J.C., Wei, F., Pasternak, S., Liang, C., Zhang, J., Fulton, L., Graves, T.A.et al. (2009) The B73 maize genome: complexity, diversity, and dynamics. Science 326: 1112–1115. [DOI] [PubMed] [Google Scholar]
- Sears, E.R. and Miller, T.E. (1985) The history of Chinese Spring wheat. Cereal Res. Commun. 13: 261–263. [Google Scholar]
- Sethi, V., Raghuram, B., Sinha, A.K. and Chattopadhyay, S. (2014) A mitogen-activated protein kinase cascade module, MKK3-MPK6 and MYC2, is involved in blue light-mediated seedling development in Arabidopsis. Plant Cell 26: 3343–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorinola, O., Balcárková, B., Hyles, J., Tibbits, J.F.G., Hayden, M.J., Holušova, K., Valárik, M., Distelfeld, A., Torada, A., Barrero, J.M.et al. (2017) Haplotype analysis of the pre-harvest sprouting resistance locus Phs-A1 reveals a causal role of TaMKK3-A in global germplasm. Front. Plant Sci. 8: 1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somyong, S., Ishikawa, G., Munkvold, J.D., Tanaka, J., Benscher, D., Cho, Y.-G. and Sorrells, M.E. (2014) Fine mapping of a preharvest sprouting QTL interval on chromosome 2B in white wheat. Theor. Appl. Genet. 127: 1843–1855. [DOI] [PubMed] [Google Scholar]
- Sugimoto, K., Takeuchi, Y., Ebana, K., Miyao, A., Hirochika, H., Hara, N., Ishiyama, K., Kobayashi, M., Ban, Y., Hattori, T.et al. (2010) Molecular cloing of Sdr4, a regulator involved in seed dormancy and domestication of rice. Proc. Natl. Acad. Sci. USA 107: 5792–5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, F., Yoshida, R., Ichimura, K., Mizoguchi, T., Seo, S., Yonezawa, M., Maruyama, K., Yamaguchi-Shinozaki, K. and Shinozaki, K. (2007) The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 19: 805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, F., Mizoguchi, T., Yoshida, R., Ichimura, K. and Shinozaki, K. (2011) Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis. Mol. Cell 41: 649–660. [DOI] [PubMed] [Google Scholar]
- Takahashi, R. (1980) The origin of a malting barley variety Golden Melon. Japan. J. Breed. 30: 272–275. [Google Scholar]
- Tanksley, S.D. (1993) Mapping polygenes. Annu. Rev. Genet. 27: 205–233. [DOI] [PubMed] [Google Scholar]
- The Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815. [DOI] [PubMed] [Google Scholar]
- Torada, A., Ikeguchi, S. and Koike, M. (2005) Mapping and validation of PCR-based markers associated with a major QTL for seed dormancy in wheat. Euphytica 143: 251–255. [Google Scholar]
- Torada, A., Koike, M., Ikeguchi, S. and Tsutsui, I. (2008) Mapping of a major locus controlling seed dormancy using backcrossed progenies in wheat (Triticum aestivum L.). Genome 51: 426–432. [DOI] [PubMed] [Google Scholar]
- Torada, A., Koike, M., Ogawa, T., Takenouchi, Y., Tadamura, K., Wu, J., Matsumoto, T., Kawaura, K. and Ogihara, Y. (2016) A causal gene for seed dormancy on wheat chromosome 4A encodes a MAP kinase kinase. Curr. Biol. 26: 782–787. [DOI] [PubMed] [Google Scholar]
- Ullrich, S.E., Hayes, P.M., Dyer, W.E., Blake, T.K. and Clancy, J.A. (1992) Quantitative trait locus analysis of seed dormancy in ‘Steptoe’ barley. In: Walker-Simons, M.K. and Ried J.L. (eds.) Preharvest sprouting in Cereals 1992, American Association of Cereal Chemists, St. Paul, MN, pp. 136–145. [Google Scholar]
- Wang, F., He, J., Shi, J., Zheng, T., Xu, F., Wu, G., Liu, R. and Liu, S. (2016) Embryonal control of yellow seed coat locus ECY1 is related to alanine and phenylalanine metabolism in the seed embryo of Brassica napus. G3 (Bethesda) 6: 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch, S.G. (1972) Quantitative differences between the human red cell glutamate-pyruvate transaminase phenotypes. Hum. Hered. 22: 190–197. [Google Scholar]
- Wickland, D.P. and Hanzawa, Y. (2015) The FLOWERING LOCUS T/TERMINAL FLOWER 1 gene family: Functional evolution and molecular mechanisms. Mol. Plant 8: 983–997. [DOI] [PubMed] [Google Scholar]
- Yanagisawa, A., Nishimura, T., Amano, Y., Torada, A. and Shibata, S. (2005) Development of winter wheat with excellent resistance to pre-harvest sprouting and rain damage. Euphytica 143: 313–318. [Google Scholar]
- Zeilcourt, A.D., Colcombet, J. and Hirt, H. (2016) The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 21: 677–685. [DOI] [PubMed] [Google Scholar]
- Zhang, M., Pan, J., Kong, X., Zhou, Y., Liu, Y., Sun, L. and Li, D. (2012) ZmMKK3, a novel maize group B mitogen-activated protein kinase kinase gene, mediates osmotic stress and ABA signal responses. J. Plant Physiol. 169: 1501–1510. [DOI] [PubMed] [Google Scholar]
- Zhang, Y.Z. (1983) Brief summary of the ancient crops excavated in Xinjiang, China. Agricultural Archaeology 1983: 122–126. [Google Scholar]