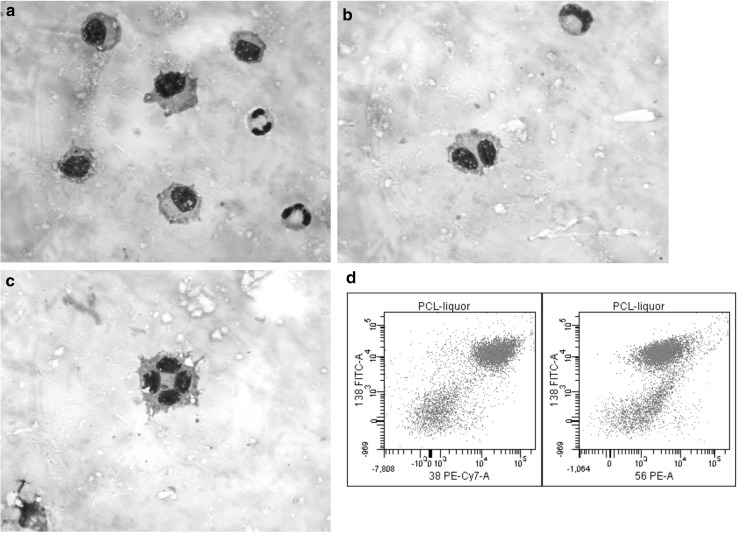
A 46-year old woman was diagnosed as having primary plasma cell leukemia (pPCL), with 4000/μl circulating plasma cells, massive bone marrow plasma cells infiltration and t(4;14),del 1p,1q gain. She was treated with bortezomib, liposomal doxorubicin and dexamethasone with immunofixation negative complete response. However, just before starting peripheral stem cell mobilization therapy, she experienced right facial nerve palsy. Column and cerebral magnetic resonance imaging (MRI) showed no apparent central nervous system (CNS) involvement. However, lumbar puncture and cerebro-spinal fluid (CSF) cytospin documented meningeal infiltration of CD138+ CD56+ CD38+ plasma cells (Fig. 1). A very small IgG/λ paraprotein in serum and 10% of bone marrow plasma cells were also found. Intrathecal administrations of methotrexate, cytarabine and dexamethasone with cranial-lumbar radiotherapy were given, obtaining CSF plasma cells disappearance and complete resolution of neurological symptoms. The initial therapeutic program was re-started.
Fig. 1.
Meningeal infiltrating plasma cells in CSF (a, b, and c) co-expressing CD138, CD56 and CD38 (d)
pPCL is a rare and very aggressive disease with poor prognosis. Meningeal localization is rare, but the exact prevalence is unknown because of reported cases are limited. A high number of circulating plasma cells seems to correlate with an increased incidence of CNS involvement. This case highlights on the need to consider CNS prophylaxis and investigation in pPCL patients with neurological symptoms or simply at risk.
Compliance with Ethical Standards
Conflict of interest
All Authors have no conflict of interest to disclose.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from the patient.