Abstract
Patients with acute leukemia (AL) are predisposed to develop infections including tuberculosis (TB). The risk is specifically higher in patients from TB endemic areas. Patients (≥12 years) with AL treated between January-2014 to January-2017 who developed TB were reviewed. Patients were classified into three groups: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML) and acute promyelocytic leukemia (APML) and a systematic analysis of clinical features and outcomes was conducted. Over the study period, 26 patients of AL developed TB. The median time to diagnosis of TB was 8 weeks (0–432 weeks) following the diagnosis of AL and it was comparable between the three leukemia groups. The diagnosis of TB required alteration of anti-leukemia therapy in 26.9% patients and rescheduling in another 42.3% patients. Therapy alteration/rescheduling were more frequent in patients with AML as compared to ALL and APML (p < 0.03, <0.04). Disseminated TB was more common in AML patients (p < 0.016). ATT could be successfully administered in 86.9% patients with improvement of TB. The incidence of ATT induced hepatitis was 34.9%. Mortality was directly attributable to TB in 10% patients. Managing tuberculosis remains a challenge during treatment of acute leukemia. With this analysis, we advocate for a need of early suspicion and evaluation for TB in patients receiving treatment for acute leukemia. Rescheduling and or alteration of anticancer therapy due to TB is associated with significantly higher mortality. Therefore, in carefully selected cases, antileukemia therapy should continue after starting ATT as early as possible.
Keywords: Acute leukemia, Tuberculosis, Anti tubercular therapy, Acid fast bacilli, Chemotherapy
Introduction
According to the Global TB report published by the World Health Organization (WHO), the incidence of Tuberculosis (TB) in 2015 was 10.4 million and the number of deaths attributable to TB were 1.4 million [1]. The estimated incidence of TB in India in 2015 was 2.8 million [1]. Patients with acute leukemia represent an immunocompromised population with innate, humoral as well as cellular immune-paresis [2]. These patients are therefore, vulnerable for acquiring new infections and reactivation of latent infections including TB. Managing TB poses unique challenges in patients with acute leukemia on diagnostic and therapeutic fronts. Presentation with long standing fever with constitutional symptoms and enlarged lymph nodes, the sine quo non of TB, is often attributed to the hematological malignancy per se in patients of acute leukemia. Despite the high prevalence of TB in general population in developing countries, tubercular re-activation is seldom suspected in patients of acute leukemia, and lesions found on imaging are frequently presumed to be of fungal etiology unless proven otherwise. Furthermore, TB may present with atypical manifestations, leading to a delay in diagnosis in these patients which may facilitate the transmission of TB to other immuno-compromised patients often being treated in the vicinity [2]. The presence of cytopenias further precludes the use of invasive diagnostic modalities to obtain a definitive evidence for TB. On a therapeutic front, institution of anti-tubercular therapy (ATT) in patients receiving antileukemia therapy raises significant concerns such as high pill burden, cumulative toxicity and multiple drug interactions (e.g. additive hepatotoxic effect of vincristine with isoniazid, overlapping hepatotoxicity of antitubercular therapy with azoles and high dose cytarabine, decreased effect of imatinib with rifampin, increased levels of doxorubicin with isoniazid) [3–5]. We describe our experience of managing 26 patients of acute leukemia with tuberculosis highlighting the above diagnostic and therapeutic challenges.
Methods
A hospital database search was done to identify adult cases of acute leukemia who developed TB during the course of their therapy or on follow up between a study duration of January 2014 to January 2017 who were of age more than 12 years. In total we found 26 cases of TB complicating the course of acute leukemia. All the medical records of the identified cases were retrieved from the central records department. A systemic analysis of characteristics pertaining to acute leukemia, treatment regimen, chemotherapy response, organs involved with TB, mode of diagnosis and treatment response to ATT was conducted. TB was labeled as “definitive” if it was proven with demonstration of acid fast bacilli on modified Ziehl–Neelsen staining or positivity for tubercular DNA using Gene Xpert MTB-Rif technique on clinical samples (sputum, body fluids, image guided fine needle aspirate or antemortem/postmortem biopsy). Disseminated tuberculosis was defined as evidence of tubercular involvement in blood or bone marrow, from a liver biopsy specimen or from 2 non contiguous organs in a single patient [6] TB was labeled as “clinico-radiologic” if the diagnosis was based on clinical symptoms and characteristic radiologic findings (cavitation, lymphadenopathy, effusion) together with response to ATT and no evidence of alternative bacterial or fungal etiology based on cultures or serologic tests. Response to ATT was defined as resolution of fever and radiologic evidence of resolution of lesions after institution of ATT. The diagnosis of acute leukemia was based on bone marrow aspirate morphology, flowcytometry and molecular markers. Patients were classified into three groups ALL, AML and APML using standard WHO criteria [7]. Patients who did not receive any antileukemia therapy were excluded from the analysis. Patients with ALL received either modified BFM 90 protocol or combination of vincristine, steroids and/or tyrosine kinase inhibitors followed by standard maintenance as frontline regime. Patients of AML received combination therapy of anthracycline with cytarabine {7 + 3/High Dose Ara-C-Mitoxantrone (HAM), High Dose Ara-C (HiDAC} or hypomethylating agents (HMA). APML patients received combination of All trans retinoic acid with arsenic tri oxide (ATO/ATRA). All patients with APML received 1 mg/kg of prednisolone for prophylaxis of differentiation syndrome for initial 3 weeks. Details of salvage regimes and allo-HSCT were recorded. A delay of planned antileukemic therapy schedule by more than 2 weeks due to TB was defined as “rescheduling” and a change in planned chemotherapeutic regimen due to TB was taken “alteration”. The details of ATT received (duration, hepatotoxicity) were recorded. ATT induced hepatitis was defined as ALT (alanine transferase) >5 upper limit of normal or ALT > 3 times ULN in the presence of symptoms or twice the ULN of bilirubin after ruling out viral hepatitis and other competing causes [8]. Details regarding the cause of mortality whether attributable to leukemia, TB or alternative etiology were recorded.
Results
Baseline Characteristics
Diagnosis of Leukemia
We identified a total of 26 patients with acute leukemia who were also diagnosed with TB in the period of study. Five patients had ALL, 12 had AML, 7 had APML, and 2 had mixed phenotypic acute leukemia (MPAL). As the patients of MPAL were treated on an ALL based chemotherapeutic regime, the patients of ALL and MPAL were included in the same group for the purpose of statistical analysis. The baseline characteristics of the patients and all 3 types of leukemia are given in Tables 1 and 2, respectively.
Table 1.
Baseline patient characteristics
| Patient characteristic | Entire group (n = 26) |
|---|---|
| Number of cases | 26 |
| Median age (years, range) | 29.5 (14–66) |
| Gender (male:female) | 17:9 |
| Median interval between leukemia and TB diagnosis (weeks, range) | 8 (0–432) |
| Impact of TB on leukemia therapy | |
| None | 11 (42.3%) |
| Therapy alteration | 7 (26.9%) |
| Therapy rescheduling | 11 (42.3%) |
| Disseminated TB | 8 (30.8%) |
| Most common organ involved by TB | Lung (80.8%) |
| Outcome of TB | |
| Improved | 20 (76.9%) |
| Inadequate therapy | 3 (11.5%) |
| Death | 1 (3.8%) |
| On-going ATT | 2 (7.7%) |
| ATT induced hepatitis | 9 (34.6%) |
| Neutropenia at the time of TB diagnosis | 21 (80.8%) |
TB tuberculosis, ATT anti tubercular therapy
Table 2.
Patient characteristics according to the type of leukemia
| Patient characteristic | ALL/MPAL (N = 7) | AML (N = 12) | APML (N = 7) | p value |
|---|---|---|---|---|
| Median age (years, range) | 28 (16–43) | 44.5 (14–66) | 29 (23–55) | 0.244 |
| Gender | 5M:2F | 8M:4F | 4M:3F | 0.847 |
| Median interval between TB and leukemia diagnosis (weeks, range) | 24 (0–77) | 13 (0–432) | 5 (0–68) | 0.605 |
| Impact of TB on therapy | ||||
| None | 2 (28.6%) | 2 (16.7%) | 7 (100%) | 0.001 |
| Alteration | 1 (14.3%) | 6 (50%) | – | 0.041 |
| Rescheduling | 4 (57.1%) | 7 (58.3%) | – | 0.030 |
| Disseminated TB | 0 (0%) | 7 (58.3%) | 1 (14.3%) | 0.016 |
| Extra-pulmonary TB | 2 (28.6%) | 7 (58.3%) | 1 (14.3%) | 0.134 |
| AFB positivity | 3 (42.9%) | 6 (50%) | 5 (71.4%) | 0.527 |
| Outcome of TB | 0.135 | |||
| Improved | 4 (57.1%) | 10 (83.3%) | 6 (85.7%) | |
| Inadequate therapy | 1 (14.3%) | 2 (16.7%) | – | |
| Undergoing therapy | 2 (28.6%) | – | – | |
| Death | – | – | 1 (14.3%) | |
| ATT induced Hepatitis | 3 (42.9%) | 5 (41.7%) | 1 (14.3%) | 0.416 |
| Mortality | 1 (14.3%) | 8 (66.67%) | 1 (14.3%) | 0.024 |
ALL acute lymphoblastic leukemia, MPAL mixed phenotypic acute leukemia, AML acute myeloid leukemia, APML acute promyelocytic leukemia, TB tuberculosis, AFB acid fast bacilli, ATT anti tubercular therapy
Diagnosis of Tuberculosis
The most common organs involved with TB were lungs followed by mediastinal lymph nodes, which were seen in 80.8 and 69.2% of patients respectively. Details of various organs involved with TB are described in Table 3. Seven patients were diagnosed as disseminated tuberculosis, 6 out of them had AML and 1 had APML. The diagnosis of tuberculosis was “definitive” in 76.9% of the patients, with acid fast bacilli (AFB) demonstrated in 53.8% of patients, and only Gene Xpert/TB PCR was positive in 23.1% of patients. The diagnosis in the remaining patients (23.1%) was “clinico-radiological”.
Table 3.
Frequency and distribution of organ involvement with TB in patients with acute leukemia
| Organs | Total (n, %) | ALL (n, %) | AML (n, %) | APML (n, %) |
|---|---|---|---|---|
| Lungs | 21 (80.8) | 4 (57.1) | 10 (83.3) | 7 (100) |
| Mediastinal nodes | 18 (69.2) | 4 (57.1) | 9 (75) | 5 (71.4) |
| Cervical node | 5 (19.2) | 1 (14.3) | 4 (33.3) | – |
| Pleura | 11 (42.3) | 3 (42.9) | 5 (41.7) | 3 (42.9) |
| Pericardium | 2 (7.7) | – | 1 (8.3) | 1 (14.3) |
| Liver | 3 (11.5) | – | 3 (25) | – |
| Spleen | 2 (7.7) | – | 2 (16.7) | – |
| Skeleton | 2 (7.7) | – | 2 (16.7) | – |
| Breast | 1 (3.8) | – | 1 (8.3) | – |
| Ileocecum | 2 (7.7) | – | 1 (8.3) | 1 (14.3) |
| Abdominal nodes | 1 (3.8) | – | 1 (8.3) | – |
Treatment of TB and Leukemia
A total of 9 patients developed ATT induced hepatitis. In patients with acute leukemia who were undergoing chemotherapy and had developed TB, the presence of fever and clinical suspicion of active infection required additional investigations and temporary discontinuation of anti-leukemia therapy. This led to rescheduling of chemotherapy in 11 (42.3%) patients. In 26.9% patients, physicians felt that risk of flare/dissemination of TB precluded the use high intensity chemotherapy and led to “alteration” of chemotherapeutic regimen.
Clinical Profile and Outcome of Patients of ALL with TB
In the subgroup of seven patients who were diagnosed with ALL/MPAL with TB, the median interval of diagnosis of TB from the diagnosis of ALL was 28 weeks (range 0–77 weeks). The individual details of therapy and outcome of leukemia and TB are summarized in Appendix 1. While 6 of these patients were treated with modified BFM 90 chemotherapy protocol, one of them received only vincristine and steroids for induction due to financial constraints. Three patients (42.8%) were positive for t (9:22) and received tyrosine kinase inhibitors along with chemotherapy. The development of TB led to the rescheduling of therapy in 4 patients (57.1%), while they were in maintenance phase (due to active infection in 3 and development of ATT induced hepatitis in 1 patient). Alteration of therapy was done in one patient (14.3%) where anthracycline and cyclophosphamide was omitted for initial 3 weeks from induction phase due to risk of flare of TB. Four patients (57.1%) developed TB during the induction phase of chemotherapy, 2 at the time of initial presentation with leukemia, 1 during third week of induction and 1 in the salvage phase of induction for relapsed leukemia. Remaining three patients were diagnosed with TB during the first year of maintenance therapy. In 6 (85.7%) patients, TB was limited to the lungs, mediastinal lymph nodes and pleura, while one patient (14.3%) had cervical tubercular lymphadenitis. None of these patients had disseminated tuberculosis. Four of the patients (57.1%) had response to ATT while 1 patient discontinued therapy on her own due to excessive GI toxicity (nausea, vomiting).
Clinical Profile and Outcome of Patients of AML with TB
Among the nine patients who had AML with TB, the median interval for the development of tuberculosis from the diagnosis of acute leukemia was 9 weeks (range 0–432 weeks). The individual details of therapy and outcome of leukemia and TB are summarized in Appendix 2. Eight (66.67%) patients developed TB while they were receiving induction chemotherapy for AML, while TB was diagnosed in the consolidation phase in 2 (16.67%) patients and after the completion of chemotherapy in another 2 patients (16.67%). Ten patients (83.3%) were diagnosed with TB while they were in the neutropenic phase of therapy. Hypomethylating agents were used for the therapy of AML in 7 (58.3%) patients. Of these 7 patients, HMA was used as a bridge to subsequent high dose chemotherapy in 3 patients where active TB precluded high intensity therapy initially. Of the 8 patients who had disseminated TB in our study, 7 patients had a hematological diagnosis of AML. In 2 of these patients, PET CT Scan helped in diagnosing unusual sites of Tuberculosis (breast and hepato-splenic) (Figs. 1, 2) and helped in guiding diagnostic FNAC. In our data disseminated TB was significantly more common in patients of AML as compared to to other forms of acute leukemia.
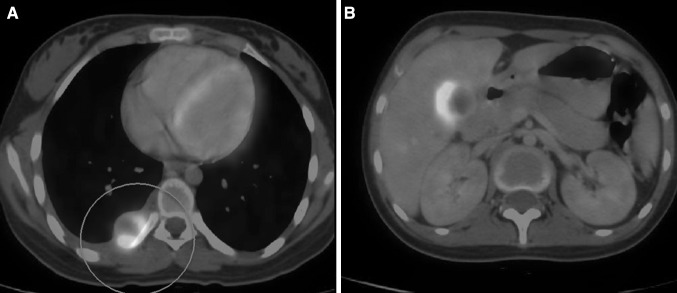
Fig. 1.
a PET-CT scan showing FDG avid nodule at costo-vertebral junction [case-Appendix 2, P2]; b PET-CT scan showing hypodense lesions of T.B. in the liver [case-Appendix 2, P2]
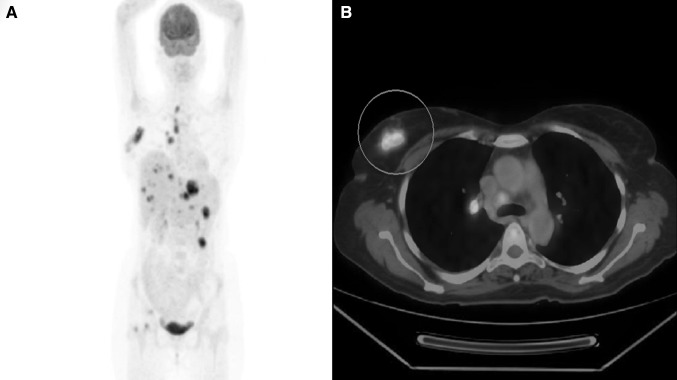
Fig. 2.
a Maximum intensity projection image of PET-CT scan showing FDG avid lesions in mediastinal and abdominal lymphnodes, liver, spleen, and right femoral head [case-Appendix 2, P3]; b PET-CT cross sectional image at the level of carina showing intense FDG avid lesion in right breast [case-Appendix 2, P3]
Clinical Profile and Outcome of Patients of APML with TB
Seven patients of APML developed TB during the study period. The individual details of each patient are summarized in Appendix 3. The median interval for the development of diagnosis of TB from the diagnosis of acute leukemia was 5 weeks (range 0–68 weeks). As per institutional protocol, all 7 patients received ATO + ATRA based therapy along with corticosteroids (1 mg/kg of prednisolone) as a part of prophylaxis for differentiation syndrome and were able to achieve remission. In the APML group six patients were diagnosed with TB during the induction phase of therapy and responded to ATT. All 6 patients continue to be in molecular remission till date. One of the 7 patients in APML group died with febrile neutropenia during maintenance phase (6mercaptopurine, methotrexate maintenance) and was found to have disseminated TB on autopsy. This patient had multiple granulomas with AFB positivity in her bone marrow that contributed to Grade IV neutropenia by exaggerating the toxicity of 6 MP/methotrexate. She thus had a mortality attributable to TB while APML was in morphologic remission.
Clinical Profile of Allo-HSCT Recipients who had Acute Leukemia with TB (Table 4)
Table 4.
Characteristics of patients of acute leukemia with tuberculosis who underwent allogeneic-HSCT
| Number | Primary hematological diagnosis | HSCT conditioning regimen used | Organs involved by TB | Time interval between TB and HSCT (weeks) | Type of HSCT | Impact of TB on HSCT |
|---|---|---|---|---|---|---|
| 1 | AML | Flu-Mel | Lung, mediastinal lymph nodes | 12 | MSD-HSCT | MAC altered to RIC |
| 2 | B ALL | Cy-TBI | Lung, mediastinal lymph nodes | 16 | MSD-HSCT | Rescheduling of HSCT by 2 weeks |
| 3 | Ph + B ALL | Flu-TBI with PTCy | Lung | 16 | Haploidentical HSCT | Rescheduling of HSCT by 6 weeks |
HSCT hematopoietic stem cell transplant, TB tuberculosis, AML acute myeloid leukemia, Flu-Mel fludarabine-melphalan, MSD matched sibling donor, MAC myeloablative conditioning, RIC reduced intensity conditioning, B ALL B cell acute lymphoblastic leukemia, Ph + B ALL Philadelphia positive acute lymphoblastic leukemia, PTCy post-transplant cyclophosphamide, TBI total body irradiation
Among these 26 patients, 3 (11.5%) patients received HSCT. All these three patients were diagnosed with TB prior to Allo-HSCT. Two of the patients had ALL, while one had AML. None of these patients were on ATT while undergoing Allo-HSCT. None of the patients had a recurrence of TB post transplant. None of these patients had a complication post transplant attributable to TB or its therapy. While two of these patients are surviving and are in remission, the patient with AML had a relapse. He underwent a second Allo-HSCT, but died of a CNS relapse of leukemia after 52 weeks of second Allo-HSCT.
Risk Factors Associated with Mortality in Patients of Acute Leukemia with TB (Table 5)
Table 5.
Risk factors for mortality in patients of Acute Leukemia with TB
| Patient characteristics | Died (n = 10) | Alive (n = 16) | p value |
|---|---|---|---|
| Median age (years, range) | 33.5 (14–60) | 29.5 (16–60) | 0.614 |
| Type of leukemia | 0.024 | ||
| ALL/MPAL | 1 | 6 | |
| AML | 8 | 4 | |
| APML | 1 | 6 | |
| Median interval between leukemia and TB diagnosis (weeks, range) | 18.5 | 5 | 0.732 |
| Neutropenia at time of diagnosis of TB | 9 | 12 | 0.617 |
| Extra pulmonary tuberculosis | 6 | 4 | 0.109 |
| Chemotherapy altered/postponed | 9 | 6 | 0.014 |
| Disseminated TB | 4 | 1 | 0.026 |
ALL acute lymphoblastic leukemia, MPAL mixed phenotypic acute leukemia, AML acute myeloid leukemia, APML acute promyelocytic leukemia, TB tuberculosis
In our study cohort, 10 (38.4%) patients with acute leukemia and TB died during the course of treatment. However, the mortality was attributable to TB only in one case. The other patients died due to causes attributable to active leukemia or its therapy. As expected, the risk factors associated with mortality included a diagnosis of AML (p value = 0.024), presence of disseminated tuberculosis (p value = 0.026) and alteration/rescheduling of chemotherapy (p value = 0.014). The presence of extra pulmonary tuberculosis (p value = 0.234) and interval between diagnosis of TB and leukemia (p value = 0.732) were not significantly associated with mortality.
Discussion
Infections in neutropenic patients are the biggest challenge in management of acute leukemia [9]. Acute bacterial and fungal infections are the commonest infection related complications during the therapy for acute leukemia [10, 11]. Tuberculosis as a complication during acute leukemia treatment is rarely suspected, therefore, uncommonly diagnosed as well. In the present era, with trans-continental and intra-continental migration and mixing of populations from low and high prevalence areas for TB, a larger part of the world population remains exposed to MTB related infections [12].
Among the patients suffering from malignancies the incidence of tuberculosis is highest in patients with hematologic malignancies in comparison to solid organ malignancies [13]. Prevalence of tuberculosis in acute leukemia has been variously reported from the range of 22–28/1000 in western countries [14, 15] to 69/1000 in Indian patients [16, 17].
The use of hypomethylating agents in the management of AML, the use of pediatric like induction protocols for treating adolescent and adult ALL and the use of non-chemotherapy based ATO–ATRA induction protocols in the management of APML are among the major advances in leukemia therapy in the last decade, however the behaviour of TB with the use of above treatment modalities has not been described previously. We have reviewed our recent experience which is comparable to the data previously published a decade earlier from another tertiary care hospital from TB endemic area and catering to a similar population background [17]. Both the studies demonstrate that the complications of TB in patients with acute leukemia are higher in patients with AML as compared to ALL.
In our study 76.9% patients definitively responded to ATT which is comparable to the response rate of 88.8% previously reported [15, 17]. A definitive diagnosis of TB (demonstration of AFB) could be established in 55.5% cases in the previous study [17]. In the current study 76.9% cases had definitive evidence of tuberculosis while the diagnosis was clinico-radiologic in 23.1% patients.
The presence of active TB often presents as pyrexia of unknown origin (PUO) in patients of acute leukemia and leads to delay in institution of high dose chemotherapy. A significant delay in the institution of chemotherapy in patients with acute leukemia has adverse impact on overall survival [18]. The role of HMA as a bridge to high dose therapy and transplant is well established in patients with Myelodysplastic syndrome [19]. In young patients with AML who present with a poor performance status due to an underlying reversible disorder such as an infection, the use of hypomethylating agents as bridge therapy to high dose therapy and transplant has not been systemically studied and may prove to be a useful strategy. We used hypomethylating agents as a bridge to high dose chemotherapy in 3 patients of AML with TB. Two of these 3 patients achieved complete remission with subsequent chemotherapy.
The use of ATO–ATRA as an induction regime for patients of APML is well established. Although the use of this chemotherapy free regime has decreased the rates of bacterial and fungal infections however the incidence of Tuberculosis in patients treated on ATO/ATRA regime remains undetermined [20]. All our APML patients received corticosteroids at the dose of 1 mg/kg as part of prophylaxis for differentiation syndrome during the induction phase. Whether the routine use of corticosteroid contributes to a higher incidence of tubercular reactivation in APML patients on ATO–ATRA requires further prospective studies. Most of the TB cases were diagnosed during the induction phase of APML except one of the patients who succumbed to disseminated tuberculosis in the maintenance phase. Although both ATO and ATRA are known to be hepatotoxic [21, 22]; the incidence of hepatitis in this group of patients who received concomitant ATT was 14.3% (n = 1). All the patients of APML who were diagnosed with TB antemortem are currently in a remission and have responded to ATT. Thus we conclude that ATT is well tolerated in patients of APML who are on ATO–ATRA combination therapy.
Apart from profound immunoparesis due to acute leukemia, treatment related immunosuppression itself is an additional risk factor for development of tuberculosis [23, 24]. In our study 80.8% patients developed TB during the neutropenic phase of chemotherapy. This is consistent with the data published by Chen et al. [25]; in which neutropenia was a significant risk factor for the development of TB. Among the three cohorts of leukemia patients, disseminated TB was found significantly more frequent in patients of AML (p value =0.016). AML patients had more atypical presentations of TB such as breast abscess, hepato-splenic involvement and monoarticular knee arthritis. This is in concordance to the previous studies, in which the incidence of extra-pulmonary tuberculosis was more common in patients with AML [17, 25]. On the contrary none of the patients with ALL and only 1 patient of APML had disseminated TB.
The clinico-radiologic syndrome of fever with reticulonodular shadows and lymphadenopathy may have a range of differential diagnosis in the setting of acute leukemia patients and accurate localization of involved sites is necessary to guide FNACs [26]. PET CT scan was able to guide the site of biopsy and pick up occult sites of TB in two of our patients of AML who developed PUO after AML induction therapy. All attempts should be made to attain a definitive microbiologic diagnosis for the etiology of fever in patients of acute leukemia rather than embarking on empiric antifungal or antibacterial therapy and PET CT scans may be used more frequently in this setting.
Allo-HSCT is frequently indicated in the management of high risk leukemia. Three of our patients who had Tuberculosis during chemotherapy could successfully undergo Allo-HSCT without any tubercular reactivation in the post transplant period. The median interval between the start of ATT and Allo-HSCT was 16 weeks. Tubercular reactivation following Allo-HSCT has been previously described [27, 28] and chronic GVHD, immunosuppressive therapy and total body irradiation are known risk factors for development of TB [27, 29]. Few studies have described the feasibility of Allo-HSCT and risk of re-activation in patients of acute leukemia who had a TB in recent past. In the study by Eom et al. [30], 13 patients with a recent history of TB underwent allo-HSCT, out of which, 9 patients had a diagnosis of acute leukemia. Post transplant, 12 of the 13 patients did not have reactivation of Tuberculosis post transplant with a follow up ranging from 0.7 to 87.5 months.
This study has the following limitations. Firstly, the diagnosis of tuberculosis was clinic-radiological in some of the patients. Secondly, the diagnosis was established by detection of AFB in the majority of the patients and was not proven by culture or molecular techniques and data for antibiotic susceptibility was not available. Finally, only hepatitis was studied as a side effect of anti-tubercular therapy and other side effects could not be analyzed due to retrospective analysis.
Conclusion
Tuberculosis is an under-diagnosed condition in patients with acute leukemia and may be a cause of febrile neutropenia in these patients. Anti-tubercular therapy is feasible in patients with acute leukemia undergoing high dose therapy and stem cell transplant. Hypomethylating agents may be used successfully in patients with acute myeloid leukemia with Tuberculosis as a bridge to definitive therapy. TB responds readily to therapy in most of these patients, however modifications of chemotherapy regime are associated with increased mortality. Therefore, in carefully selected cases, antileukemia therapy should continue after starting ATT as early as possible.
Funding
It was analysis of patient data base and did not involve any funding.
Appendix 1
See Table 6.
Table 6.
Individual patient characteristics of patients with ALL with TB
| Leukemia characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Age/sex | Hematological diagnosis | Phase of therapy when TB detected | Neutropenia at time of diagnosis of TB | Therapy | Interval (between leukemiadiagnosis and TB) | Outcome of leukemia | Impact of TB on therapy |
| P1 | 43/F | Ph + B ALL | Maintenance | No | BFM 90 | 28 weeks | CNS Relapse-week 19 Medullary Relapse- M6 | Rescheduling of Maintenance3rd |
| P2 | 29/M | Ph + B ALL | Salvage induction | Yes | Initial-BFM 90 Relapse-FLAG f/b HaploHSCT |
77 weeks | Remission | Rescheduling of HaploHSCT by 12 weeks |
| P3 | 20/F | B ALL | Induction | Yes | BFM 90 | Simultaneous | Remission | Alteration/omissionof anthracycline and cyclophosphamide for initial 3 weeks) |
| P4 | 20/M | B ALL | Induction | Yes | BFM 90 | 3 weeks | Remission | None |
| P5 | 37/M | Ph + MPAL | Maintenance | No | Vincristine + Steroids + TKIs | 24 weeks | Isolated CNS Relapse at 28 weeks | Rescheduling of maintenance by 4 weeks |
| P6 | 16/M | B ALL | Maintenance | No | BFM 90 | 66 weeks | Remission | Rescheduling of maintenance by 2 weeks |
| P7 | 28/M | MPAL | Induction | Yes | BFM 90 | Simultaneous | Relapse at week 8 | None |
| Tuberculosis characteristics | ||||||
|---|---|---|---|---|---|---|
| No. | Organs involved | Site of isolation | Evidence of TB | Outcome of TB | Side effects of ATT | Follow up after diagnosis of TB |
| P1 | Cervical lymph nodes | Cervical lymph node | AFB positive on FNA | Inadequate therapy due to adverse effects | Hepatitis | Died at 6 months |
| P2 | Lung | BAL fluid | Gene Xpert positive | Improved | Hepatitis | In remission at 38 weeks |
| P3 | Lung and mediastinal lymph nodes | TBNA and BAL Fluid | AFB and gene Xpert positive | Improved | Hepatitis | In remission at 40 weeks |
| P4 | Mediastinal lymph nodes, pleura | – | Clinico-radiologic | Improved | – | In remission at 84 weeks |
| P5 | Pleura, mediastinal lymph nodes | Pleura | Gene Xpert positive | Improved | – | In remission at 8 weeks |
| P6 | Lung, mediastinal lymph nodes | BAL fluid | AFB positive | Currently undergoing therapy | 1 week | |
| P7 | Lung, pleura | BAL fluid | Gene Xpert positive | Currently undergoing therapy | – | 2 weeks |
ALL acute lymphoblastic leukemia, TB tuberculosis, M male, f female, TB tuberculosis, ATT anti tubercular therapy, BFM Berlin Frankfurt Munster, Ph+ Philadelphia +, CNS central nervous system, BAL broncho alveolar lavage, TBNA trans bronchial node aspiration, FNA fine needle aspiration, Haplo haploidentical, HSCT hematopoietic stem cell transplant, FLAG fludarabine-arabinoside-C-GCSF, CR complete remission, TKI tyrosine kinase inhibitor, f/b followed by
Appendix 2
See Table 7.
Table 7.
Individual patient characteristics of patients with AML with TB
| Leukemia characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | Age/sex | Hematological diagnosis | Therapy | Phase of therapy at diagnosis of TB | Neutropenia at diagnosis of TB | Interval (between leukemia and TB) | Outcome of leukemia | Impact of TB on therapy |
| P1 | 51/M | AML-FABM1 | HMA 1 # f/b Induction(7 + 3) f/b AlloHSCT | Induction | Yes | Simultaneous | Relapse at 108 weeks | Received 1 #HMAas bridge to( 7 + 3) |
| P2 | 16/F | AML-FAB M2 | Induction f/b reinduction f/b 2#HIDAC | Consolidation | Yes | 20 weeks | Remission | Rescheduling in HIDAC#2 by 2 weeks |
| P3 | 44/F | AML-FAB M6 | 7# HMA f/b (7 + 3) f/b HMA f/b (5 + 2) | Induction | Yes | 62 weeks | Refractory Leukemia | Rescheduling of 5 # of HMA by2 months |
| P4 | 50/M | AML-FAB M2 | Induction f/b 4# HMA f/b 4# consolidation | Induction | Yes | 9 weeks | Remission | HIDAC consolidation altered to HMA |
| P5 | 14/F | AML-FAB M4 | Induction f/b 4# consolidation | Induction | Yes | 2 weeks | Remission | Rescheduling in starting induction by 2 weeks |
| P6 | 38/F | AML-FAB M1 | Induction f/b HMA | Induction | Yes | 28 weeks | Relapse at 60 weeks | HIDAC consolidation altered to HMA |
| P7 | 23/M | AML-FAB M4 | Palliation | Induction | Yes | Simultaneous | Not treated | Definitive therapy couldn’t be given |
| P8 | 45/M | AML FAB M2 | Induction f/b 3# consolidation | Post Therapy | No | 8 years | Relapse at 8.5 years | None |
| P9 | 66/M | AML FAB M2 | 1# HMA f/b induction f/b 3# consolidation | Post therapy | No | 64 weeks | Relapse after 128 weeks | None |
| P10 | 60/M | AML FAB M2 | 2# HMA | Induction | Yes | 8 weeks | Persistent leukemia | Interupption of HMA |
| P11 | 27/M | Myeloid sarcoma | Induction f/b 3# consolidation | Consolidation | Yes | 17 weeks | Relapse after 64 weeks | Rescheduling of 3# consolidation by 4 weeks |
| P12 | 46/M | AML FAB M1 | 16# of HMA | Induction | Yes | Simultaneous | Refractory Leukemia | 7 + 3 altered to HMA |
| Tuberculosis characteristics | ||||||
|---|---|---|---|---|---|---|
| No. | Organs involved | Site of isolation | Evidence of TB | Outcome of TB | Side effects of ATT | Follow up after diagnosis of TB |
| P1 | Lung, mediastinal lymph nodes | Sputum | AFB positive | Improved | Hepatitis | Died at 108 weeks due to AML relapse |
| P2 | Lungs, pleura, mediastinal lymph nodes, liver | Pleural nodule | AFB positive | Improved | Hepatitis | Death at 4 weeks with Pseudomonas sepsis |
| P3 | Breast, pleura, mediastinal and abdominal lymph nodes, liver, spleen, vertebra | Breast | AFB positive on FNAC | Improved | Hepatitis | Died with active disease 61 weeks |
| P4 | Cervical and Mediastinal lymph nodes, lung | Cervical lymph node | AFB positive | Improved | Transaminitis | In remission at 100 weeks |
| P5 | Lungs, pleura, pericardium, mediastinal nodes, intestine | – | Clinico-radiologic | Improved | – | Died of relapse at 72 weeks |
| P6 | Lung, mediastinal lymph nodes | – | Clinico-radiologic | Improved | – | Died at 32 weeks with relapse |
| P7 | Lung, mediastinal and cervical lymph nodes | – | Clinico-radiologic | Inadequate therapy | – | Died at 4 weeks with disease |
| P8 | Lung, liver, spleen, cervical and mediastinal lymph nodes | BAL fluid | AFB positive | Improved | – | Hematological relapse at 6 months |
| P9 | Right knee | Knee aspirate | TB PCR positive | Improved | Hepatitis | Hematological relapse at 64 weeks |
| P10 | Lungs, mediastinal lymph nodes | BAL fluid | TB PCR positive | Inadequate therapy | – | Died of disease at 4 weeks |
| P11 | Cervical lymph nodes, lung, pleura | Cervical lymph nodes | AFB Positive | Improved | None | Died of disease at 59 weeks |
| P12 | Lung, pleura | – | Clinico radiologic | Improved | None | With active disease at 70 weeks |
AML acute myeloid leukemia, TB tuberculosis, M male, F female, AFB acid fast bacilli, ATT anti tubercular therapy, HMA hypo methylating agent, # cycle, HIDAC high dose arabinoside C, f/b followed by, CNS central nervous system, BAL broncho alveolar lavage, FNA fine needle aspiration, Allo allogeneic, HSCT hematopoietic stem cell transplant
Appendix 3
See Table 8.
Table 8.
Individual patient characteristics of patients with APML with TB
| Leukemia characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Serial number | Age/sex | Hematological diagnosis | Therapy | Phase of therapy | Neutropenia at time of diagnosis of TB | Interval (between leukemia and TB) | Outcome of leukemia | Impact of TB on therapy |
| P1 | 28/M | APML | ATO + ATRA | Induction | Yes | 5 weeks | Remission | None |
| P2 | 28/F | APML | ATO + ATRA | Induction | Yes | 5 weeks | Remission | None |
| P3 | 30/M | APML | ATO + ATRA | Induction | Yes | 8 weeks | Remission | None |
| P4 | 55/F | APML | ATO + ATRA | Induction | Yes | 3 weeks | Remission | None |
| P5 | 29/F | APML | ATO + ATRA f/b maintenance with 6Mercatopurine and Methotrexate | Maintenance | Yes | 68 weeks | Remission | None |
| P6 | 23/M | APML | ATO + ATRA | Induction | Yes | Simultaneous | Remission | None |
| P7 | 55/M | APML | ATO + ATRA | Induction | Yes | 5 weeks | Remission | None |
| Tuberculosis characteristics | ||||||
|---|---|---|---|---|---|---|
| Serial number | Organs involved | Site of isolation | Evidence of TB | Outcome of TB | Side effects of ATT | Follow up after diagnosis of TB |
| P1 | Lung, mediastinal lymph nodes | Mediastinal node | FNA AFB positive | Improved | None | In remission at 74 weeks |
| P2 | Lungs, mediastinal nodes | Mediastinal node | FNA AFB positive | Improved | None | In remission at 64 weeks |
| P3 | Lungs, mediastinal node, pleura | Mediastinal node | FNA AFB positive | Improved | Hepatitis | In remission at 113 weeks |
| P4 | Lungs, pleura | Pleural fluid | Clinicoradiologic | Improved | None | In remission at 64 weeks |
| P5 | Lungs, pleura, pericardium, mediastinal lymph nodes, Intestine | Pleural, lung, intestine | AFB positive | Expired | – | TB diagnosed post mortem |
| P6 | Lung | BAL fluid | AFB positive | Improved | None | 54 weeks |
| P7 | Lung, mediastinal lymph Node | Mediastinal Node | Gene Xpert positive | Improved | None | 62 weeks |
APML acute promyelocytic leukemia, TB tuberculosis, M male, F female, AFB acid fast bacilli, ATO arsenic tri oxide, ATRA all trans retinoic acid, ATT anti tubercular therapy, BAL broncho alveolar lavage, FNA fine needle aspiration
Compliance with Ethical Standards
Conflict of interest
There are no potential conflict of interest of authors writing this article.
Research Involving Human Participants and/or Animals
This is a retrospective data analysis and does not involve any human or animal intervention or experiment.
Informed Consent
Informed consent was obtained before data analysis from participating subjects.
References
- 1.Global Tuberculosis report (2016) WHO, Geneva. http://www.who.int/tb/publications/global_report/en/. Accessed 26 Jan 2017
- 2.Malone JL, Ijaz K, Lambert L, Rosencrans L, Phillips L, Tomlinson V, et al. Investigation of healthcare-associated transmission of Mycobacterium tuberculosis among patients with malignancies at three hospitals and at a residential facility. Cancer. 2004;101(12):2713–2721. doi: 10.1002/cncr.20698. [DOI] [PubMed] [Google Scholar]
- 3.Haidar C, Jeha S. Drug interactions in childhood cancer. Lancet Oncol. 2011;12(1):92–99. doi: 10.1016/S1470-2045(10)70105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EPOCRATES. Epocrates online: http://www.epocratess.com. Accessed 25 Jan 2017
- 5.Drug checker interaction. Medscape: http://www.medscape.com/druginfo/druginterchecker. Accessed 25 Jan 2017
- 6.Iseman MD (2000) Extrapulmonary tuberculosis. A clinicians guide to tuberculosis. Lippincott Williams Wilkins, Philadelphia, 2001, pp 145–197
- 7.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 8.Sharma SK, Balamurugan A, Saha PK, Pandey RM, Mehra NK. Evaluation of clinical and immunogenetic risk factors for the development of hepatotoxicity during antituberculosis treatment. Am J Respir Crit Care Med. 2002;166(7):916–919. doi: 10.1164/rccm.2108091. [DOI] [PubMed] [Google Scholar]
- 9.Jones GR, Konsler GK, Dunaway RP, Pusek SN. Infection risk factors in febrile, neutropenic children and adolescents. Pediatr Hematol Oncol. 1996;13(3):217–229. doi: 10.3109/08880019609030820. [DOI] [PubMed] [Google Scholar]
- 10.Narita M. Polymerase chain reaction for diagnosis of infectious diseases. Acta Paediatrica Japonica Overseas Ed. 1993;35(2):89–97. doi: 10.1111/j.1442-200X.1993.tb03015.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen CY, Tang JL, Hsueh PR, Yao M, Huang SY, Chen YC, et al. Trends and antimicrobial resistance of pathogens causing bloodstream infections among febrile neutropenic adults with hematological malignancy. J Formosan Med Assoc Taiwan yi zhi. 2004;103(7):526–532. [PubMed] [Google Scholar]
- 12.Casper C, Singh SP, Rave S, Daley CL, Schecter GS, Riley LW, et al. The transcontinental transmission of tuberculosis: a molecular epidemiological assessment. Am J Public Health. 1996;86(4):551–553. doi: 10.2105/AJPH.86.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamboj M, Sepkowitz KA. The risk of tuberculosis in patients with cancer. Clin Infect Dis. 2006;42(11):1592–1595. doi: 10.1086/503917. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan MH, Armstrong D, Rosen P. Tuberculosis complicating neoplastic disease. A review of 201 cases. Cancer. 1974;33(3):850–858. doi: 10.1002/1097-0142(197403)33:3<850::AID-CNCR2820330334>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 15.Feld R, Bodey GP, Groschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976;136(1):67–70. doi: 10.1001/archinte.1976.03630010051009. [DOI] [PubMed] [Google Scholar]
- 16.Choudhry VP. Pulmonary tuberculosis in children with acute lymphatic leukemia. Indian J Pediatr. 1981;48(390):117–119. doi: 10.1007/BF02895208. [DOI] [PubMed] [Google Scholar]
- 17.Mishra P, Kumar R, Mahapatra M, Sharma S, Dixit A, Chaterjee T, et al. Tuberculosis in acute leukemia: a clinico-hematological profile. Hematology. 2006;11(5):335–340. doi: 10.1080/10245330600915818. [DOI] [PubMed] [Google Scholar]
- 18.Ostgard LS, Norgaard JM, Sengelov H, Holm MS, Jensen MK, Kallenbach M, et al. Impact of chemotherapy delay on short- and long-term survival in younger and older AML patients: a Danish population-based cohort study. Leukemia. 2014;28(9):1926–1929. doi: 10.1038/leu.2014.157. [DOI] [PubMed] [Google Scholar]
- 19.Yakoub-Agha I, Deeg J. Are hypomethylating agents replacing induction-type chemotherapy before allogeneic stem cell transplantation in patients with myelodysplastic syndrome? Biol Blood Marrow Transplant. 2014;20(12):1885–1890. doi: 10.1016/j.bbmt.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Lo-Cocco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Lacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 21.Mathews V, George B, Chendamarai E, Lakshmi KM, Desire S, Balasubramanian P, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J Clin Oncol. 2010;28(24):3866–3871. doi: 10.1200/JCO.2010.28.5031. [DOI] [PubMed] [Google Scholar]
- 22.de-Medeiros BC, Strapasson E, Pasquini R, de-Medeiros CR. Effect of all-trans retinoic acid on newly diagnosed acute promyelocytic leukemia patients: results of a Brazilian center. Braz J Med Biol Res Revista brasileira de pesquisas medicas e biologicas. 1998;31(12):1537–1543. doi: 10.1590/S0100-879X1998001200005. [DOI] [PubMed] [Google Scholar]
- 23.Coburn RJ, England JM, Samson DM, Walford DM, Blowers R, et al. Tuberculosis and blood disorders. Br J Haematol. 1973;25:793–799. doi: 10.1111/j.1365-2141.1973.tb01791.x. [DOI] [PubMed] [Google Scholar]
- 24.Fu LM. The potential of human neutrophil peptides in tuberculosis therapy. Int J Tuberc Lung Dis. 2003;7(11):1027–1032. [PubMed] [Google Scholar]
- 25.Chen CY, Sheng WH, Cheng A, Tsay W, Huang SY, Tang JL, et al. Clinical characteristics and outcomes of Mycobacterium tuberculosis disease in adult patients with hematological malignancies. BMC Infect Dis. 2011;11:324. doi: 10.1186/1471-2334-11-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nucci M, Nouer SA, Anaissie E. Distinguishing the causes of pulmonary infiltrates in patients with acute leukemia. Clin Lymphoma Myeloma Leuk. 2015;15(Suppl):S98–S103. doi: 10.1016/j.clml.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Roy V, Weisdorf D. Mycobacterial infections following bone marrow transplantation: a 20 year retrospective review. Bone Marrow Transplant. 1997;19(5):467–470. doi: 10.1038/sj.bmt.1700686. [DOI] [PubMed] [Google Scholar]
- 28.George B, Mathews V, Srivastava V, Srivastava A, Chandy M. Tuberculosis among allogeneic bone marrow transplant recipients in India. Bone Marrow Transplant. 2001;27(9):973–975. doi: 10.1038/sj.bmt.1702993. [DOI] [PubMed] [Google Scholar]
- 29.Ip MS, Yuen KY, Woo PC, Luk WK, Tsang KW, Lam WK, et al. Risk factors for pulmonary tuberculosis in bone marrow transplant recipients. Am J Respir Crit Care Med. 1998;158(4):1173–1177. doi: 10.1164/ajrccm.158.4.9712072. [DOI] [PubMed] [Google Scholar]
- 30.Eom KS, Lee DG, Lee HJ, Cho SY, Choi SM, Choi JK, et al. Tuberculosis before hematopoietic stem cell transplantation in patients with hematologic diseases: report of a single-center experience. Transplant Infect Dis. 2015;17(1):73–79. doi: 10.1111/tid.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]