Abstract
Recent reports have shown that excellent survival outcomes can be achieved in adult Burkitt’s lymphoma with the use of DA-EPOCH-R regimen. When compared to earlier intense pediatric-type protocols, this regimen is less toxic. There are limited reports available on the use of this regimen outside the context of clinical trials. We analyzed the outcomes of patients who were treated with the DA-EPOCH-R regimen [Burkitt’s lymphoma (BL), primary mediastinal B cell lymphoma (PMBCL) and HIV-positive patients with diffuse large B cell lymphoma (DLBCL)] at our center over a 3 year period. Baseline characters, responses, and toxicity data was captured from records. Event-free survival (EFS—from therapy initiation till occurrence of event (non-achievement of complete response or relapse) and overall survival (OS—from therapy initiation till death due to any cause) were estimated using Kaplan–Meier method. Among 41 patients [median age 40 years (18–76)], the following diagnoses were included-HIV negative patients (N = 29): BL (N = 24), PMBCL (N = 5); HIV positive patients (N = 12): BL (N = 8), and DLBCL (N = 4). Among those with BL, majority had stage III/IV disease (N = 21/32, 65%). At the completion of planned therapy, 33 had achieved CR (81%). One patient died due to toxicity. The actuarial EFS and OS at 2 years were 80 and 77% respectively for all patients. The OS at 2 years was 100% for PMBCL, 80% for BL and 50% for HIV-positive DLBCL. Majority of the failures in BL were in patients with advanced disease. DA-EPOCH-R can be used in real-world setting and allows treatment of older patients with BL.
Keywords: Burkitt’s lymphoma, DA-EPOCH-R, Primary mediastinal B cell lymphoma, HIV-positive lymphoma
Introduction
Excellent outcomes have been demonstrated in adult Burkitt’s lymphoma (BL) and primary mediastinal B cell lymphoma (PMBCL) with the dose adjusted DA-EPOCH-R (rituximab, etoposide, prednisolone, vincristine, doxorubicin, and cyclophosphamide) regimen [1–3]. Burkitt’s lymphoma was conventionally treated with intense regimens modeled upon pediatric protocols. These regimens (Hyper CVAD, CODOX-M-IVAC) were very toxic and had high treatment related morbidity and mortality [4, 5]. The advantage of the infusional DA-EPOCH-R regimen over the latter was improved tolerability while preserving the high curability [1]. Even among patients who were infected with human immunodeficiency virus (HIV), high survival was achieved with infusional regimens [1, 6, 7]. Reduced toxicity of the DA-EPOCH-R regimen (compared to more intense protocols) increases the feasibility of treating BL in older patients and in those with co-morbidities outside the context of clinical trials even in the absence of a highly-specialized cancer center. Primary mediastinal B cell lymphoma is another malignancy where this regimen has shown exceptional results [3].
Since 2013, we have used the DA-EPOCH-R chemotherapy in all adult patients with BL, PMBCL and HIV positive B cell NHL. This reflects the field experience in the use of this regimen outside the context of a trial.
Methods
The outcomes of patients treated with DA-EPOCH-R chemotherapy at our center between October 2013 and December 2016 were analyzed. All patients had completed planned treatment and final response was known at the time of analysis. Diagnosis of Burkitt’s lymphoma was based on histopathology and immunohistochemistry and only few patients had demonstration of MYC-translocation.
Chemotherapy Protocol
All patients were treated with DA-EPOCH-R chemotherapy with doses similar to those described by Dunleavy et al. [1]. Rituximab was used at 375 mg/m2 on day 1 of chemotherapy cycles. EPOCH was delivered in a step-wise dose escalation method. A “pre-phase” of either cyclophosphamide (500 mg) alone or with vincristine and steroids (COP) was administered to patients with poor performance status, age > 60 years, or HIV positive status or extensive disease load ± features of tumor lysis at presentation. In these patients, if there was no improvement in the parameters, the first cycle was delivered with a 25–50% dose reduction and escalated subsequently based on tolerance. Intrathecal methotrexate (12.5 mg) was administered prophylactically on day 4 of each cycle (total of 6 doses). In those who were CSF positive, biweekly doses of methotrexate were administered until CSF cleared and thereafter with each cycle. All patients were given granulocyte colony stimulating factor (GCSF) starting 24 h after completion of EPOCH chemotherapy and this was continued through the nadir until ANC ≥ 5000/cmm for at least 2 days. Similar scheme was used for the HIV-positive patients except that escalation beyond dose level 2 was not practiced for these patients.
Chemotherapy was administered through central venous catheters. All patients were admitted during the chemotherapy and subsequently followed as outpatients. Daily GCSF was given as described above with hemograms done once in 2 days till recovery of counts. Patients were re-admitted in case of fever or other severe toxicity.
Initial staging was done mostly with PET CT scanning though occasional patients underwent only contrast CT of chest and abdomen. Bone marrow examination was performed prior to treatment in all patients and CSF evaluation at the time of the first intrathecal dose of methotrexate. Final evaluation of response was performed with PET CT in the majority of patients. Examination of BM was repeated if it was positive initially. Residual masses with PET uptake were considered as negative if the Deauville score was ≤ 3 [8]. If the Deauville score was more than 3 a biopsy of the residual lesion was performed or the scan was repeated after 3 months (if biopsy was not feasible). If there was increase in size of the mass after 3 months, biopsy was reconsidered. If there was no increase in size or PET uptake, it would be considered negative and patient would be followed.
Assessment of final response was based on recent criteria utilizing both CT and PET imaging [8, 9]. Briefly, complete response (CR) was considered when the residual disease was less than 1.5 cm in size and bone marrow was normal (if involved earlier). Partial response (PR) was defined as more than 50% reduction in size of the masses and progression as increase in size of involved sites of disease or occurrence of new lesions (PD). Patients who had residual masses that were PET negative (Deauville score ≤ 3) were also considered to be in CR.
Patients were followed up after therapy once in 3 months for the first 2 years and once in 6 months thereafter. Imaging studies were done during follow up only when clinically indicated or in those with indeterminate end therapy status as described above. Event free survival was calculated from the time of therapy initiation till occurrence of event (non-achievement of complete response or relapse at any point). Overall survival was calculated from time treatment was started till death due to any cause. Survival analysis was done as per the Kaplan–Meier method using SPSS version 13 (IBM Inc.). Data was censored on July 1st 2017. Toxicity data was computed from patient records.
Results
Between October 2013 and Dec 2016, 41 patients (Table 1) with the following CD20 positive B cell lymphomas received treatment with DA-EPOCH-R: HIV negative patients (N = 29): Burkitt’s lymphoma (N = 24), Primary mediastinal B cell lymphoma (N = 5); HIV positive patients (N = 12): Burkitt’s lymphoma (N = 8), and DLBCL (N = 4). There were 4 patients with HIV negative BL who were excluded because of previous therapy with LMB-89 prior to EPOCH (N = 1), revised diagnosis of DLBCL and not BL (N = 1), and untreated after initial cyclophosphamide (N = 2). Among HIV-positive patients with CD20 + NHL diagnosed during this period, 6 were excluded from this analysis because they received RCHOP (N = 2), received EPOCH without rituximab (N = 2) and did not receive any treatment other than steroids (N = 2). Twenty-three (37%) patients had stage III/IV disease including 7 (17%) with involvement of the bone marrow. The median age was 40 years (range 20–76 years) and there were 8 patients ≥ 50 years including 2 who were ≥ 60 years. Among patients with BL (N = 32), 12 patients (38%) had stage I/II disease and 20 (62%) had stage III/IV disease.
Table 1.
Baseline characteristics
| Parameter | All patients N = 41(%) |
|---|---|
| Age (median, range) | 40 (20–76) |
| Male sex | 22 (54) |
| HIV positive | 12 (29) |
| Diagnosis | |
| Burkitt’s lymphoma | 32 (78) |
| PMBCLa | 5 (12) |
| DLBCLb | 4 (10) |
| Performance status | |
| 1 | 26 (63) |
| 2 | 8(20) |
| 3,4 | 7 (17) |
| Stage | |
| 1 | 2 (5) |
| 2 | 16 (39) |
| 3 | 8 (20) |
| 4 | 15 (37) |
| aaIPI | |
| 0,1 | 17 (42) |
| 2 | 14 (34) |
| 3 | 10 (24) |
| Bone marrow positivec | 7 (17) |
| CSF positivec | 2 (5) |
| Bulky disease | 15 (37) |
aaIPI age-adjusted international prognostic index
aPMBCL, primary mediastinal B cell lymphomas, all patients were HIV negative
bDLBCL, diffuse large B cell lymphoma all patients were HIV positive
cBaseline
Feasibility of Delivering R DA-EPOCH-R
Twelve patients (29%) received “pre-phase” cyclophosphamide or COP 1 week prior to the start of DA-EPOCH-R. Additionally, 4 patients with poor general condition received the first cycle at 50–75% doses. The median number of cycles of DA-EPOCH-R delivered was 6 (range 1–7). Five patients did not complete the planned 6 cycles of therapy. Of these, 3 patients progressed after 1 (HIV positive, BL), 4 (HIV positive, BL) and 5 (HIV-negative, Burkitt’s leukemia) cycles of chemotherapy respectively. Another patient (55, male, Burkitt’s leukemia, with chronic lung disease) developed pulmonary infection and sepsis after the 5th course and died of these complications. Another 60 year old female with Burkitt’s leukemia developed serious infection after the 5th cycle and the last cycle was omitted. There were 2 patients with BL who had baseline CSF positive disease and they received additional weekly doses of triple intratheceal therapy. However both these patients systemic progression later.
Dose Escalation and Toxicity
In HIV-positive patients (n = 12), dose escalation was not practised beyond step 1 or 2 and most received the step 1 dose level. Among HIV negative patients (N = 29), only 1 patient could escalate up to dose level 4. In the remaining patients, maximum dose levels attained were dose level 3 (N = 12), dose level 2 (N = 12) and dose level 1 (N = 4). In addition, 5 patients required reduction in doses of vincristine due to grade 2 + peripheral neuropathy/vincristine ileus beyond cycles 3–4.
Other Serious Toxicities
1 patient developed grade 4 thrombocytopenia and intracranial hemorrhage (sub arachnoid bleed) in the 6th cycle- and had complete recovery subsequently. Majority of the patients (32/41, 78%) patients developed at least 1 episode of febrile neutropenia during treatment. Five (12%) had life-threatening infections [fungal pneumonia (n = 1), gram negative (Pseudomonas sp.) sepsis (n = 1), septic shock with unidentified organism (n = 2), pneumonia with hypoxia requiring ventilator support (n = 1)]. One patient who developed respiratory failure due to pneumonia in a pre-existing background of obstructive pulmonary disease died.
Responses and Survival
At the completion of planned therapy, 33 had achieved CR (81%). Of these, 3 had residual disease > 1.5 cm, but were PET non-avid and considered as CR and continued on follow up (none have recurred). The CR rate was 50, 81 and 100% among those with DLBCL, BL and PMBCL respectively. Seven patients had progressive disease before completion of the planned 6 cycles of DA-R-EPOCH. All these 7 patients have subsequently died due to disease. Another patient with Burkitt’s leukemia died prior to completion of treatment due to pulmonary infection and the post-mortem bone marrow was normal. Of the 33 patients who had achieved CR, 1 patient had recurrence of disease 10 months after completion of treatment He had second-line chemotherapy without durable response and died 25 months after diagnosis. Another patient (76-year female with Burkitt’s lymphoma) who had achieved CR after 6 cycles of chemotherapy) died 14 months after diagnosis due to ischemic heart disease.
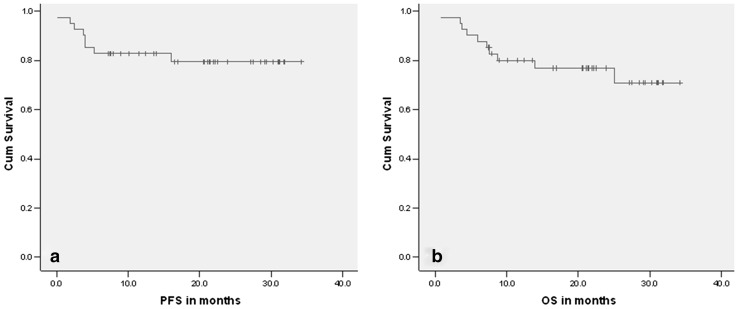
After a median follow up of 21 months (0.9–34), 8 patients had progressive disease (7 had primary progression and 1 patient recurred after achieving CR). The actuarial EFS at 24 months was 80% (± SE: 0.066) (Fig.1a). Ten patients have died (8 due to disease progression, 1 due to toxicity and 1 due to other causes). The actuarial OS at 24 months was 77% (± SE: 0.068) (Fig.1b).
Fig. 1.
PFS (1a) and OS (1b) in months of all patients
The 2-year EFS and OS among the patients with primary mediastinal B cell lymphoma was 100%. Those with DLBCL (N = 4), all of whom were HIV positive the EFS and OS was only 50%. Among those with Burkitt’s lymphoma/leukemia, the EFS and OS were 77 and 80% respectively at 2 years. No factor could be identified to be predictive of the outcomes by univariate analysis (Table 2, Fig. 2a and b).
Table 2.
Univariate analysis of outcome
| Parameter | N | EFS 2-yr % | P value | OS 2-yr % | P value |
|---|---|---|---|---|---|
| Age | |||||
| ≤ 40 years | 22 | 71 | 0.20 | 68 | 0.7 |
| > 40 years | 19 | 90 | 76 | ||
| Sex | |||||
| Male | 22 | 71 | 0.1 | 72 | 0.29 |
| Female | 19 | 90 | 83 | ||
| HIV status | |||||
| Positive | 12 | 67 | 0.1 | 67 | 0.21 |
| Negative | 29 | 85 | 74 | ||
| Stage | |||||
| 1,2 | 18 | 89 | 0.2 | 82 | 0.29 |
| 3,4 | 23 | 78 | 73 | ||
| Subtype of NHL | |||||
| BL | 32 | 80 | 0.138 | 77 | 0.124 |
| PMBCL | 5 | 100 | 100 | ||
| DLBCL | 4 | 50 | 50 | ||
| Risk group | |||||
| aaIPI code 0,1 | 17 | 85 | 0.2 | 72 | 0.32 |
| aaIPI score 2,3 | 24 | 75 | 70 | ||
EFS event-free survival, BL Burkitt’s lymphoma, PMBCL primary mediastinal B cell lymphoma, DLBCL diffuse large B cell lymphoma, aaIPI age adjusted International prognostic index
Fig. 2.
Overall survival by stage and aaIPI. 2a shows the difference in OS between stages 1,2 (black line) and stages 3,4 (grey line). 2b shows the differences in OS between patients with aaIPI 0,1 (black line) and aaIPI 3,4 (grey line)
Among patients with BL (N = 32), 1/11 patients with stage I/II disease had PD, while 6/20 patients with stage III/IV disease (N = 21) had PD. Six of the 7 failures happened before the completion of chemotherapy or within 3 months of completion of therapy and only 1 patient failed after 10 months of achieving CR.
Discussion
At our center, the earlier treatment protocol used for adult BL was a modified version of pediatric LMB-89 protocol. Only a handful of patients (2–3 per year) with adult BL who were very fit (usually below 30–40 years) used to receive therapy (data not shown). When we started using DA-EPOCH-R, it greatly enhanced the delivery of treatment for adult BL and we could treat 32 patients in 3 years with 2-year survival of 80%. Though the toxicity was significant with more than three-quarters developing febrile neutropenia, it was manageable. Notably, 7 patients over the age of 50 years were treated in this period.
The results in PMBCL were excellent (100% survival) as reported earlier [3]. The survival among patients with BL was 77% at 2 years. This is lower than the 90–100% survival reported in the original paper by Dunleavy et al. [1]. However, the latter study included patients with predominantly low risk disease from a selected population of patients enrolled for a clinical trial at a single dedicated center (NCI). In a report of 77 patients from a multi-center setting, with higher risk patients, the OS was lower (88%) [2, 10]. In our study, over half the patients had stage III/IV disease (7 had bone marrow involvement), 7 were in ECOG PS 3/4, and 12/41 were HIV positive. Most of the patients who failed had advanced disease. Whether DA-EPOCH-R alone is adequate therapy in higher risk BL or in those with leukemic presentation is not known at this point.
Earlier approaches using dose intense chemotherapy have reported improved survival with the addition of rituximab, though the toxicities were quite high [11–13]. The feasibility of reduction in number of DA-EPOCH-R cycles from 6 to 3 in very low risk disease has to be evaluated prospectively [10]. Whether intensification of DA-EPOCH-R is possible without significantly increasing its toxicity and if this would improve survival in high risk disease is not known. Toxicity can be reduced in HIV + patients with modified short course EPOCH with double dose rituximab [1]. This particular modification was not feasible for us due to the additional cost of rituximab and we chose to use the conventional DA-EPOCH-R in our HIV-positive patients though dose escalation was not done. Non-hematological toxicities were less commonly seen except for vincristine neuropathy/ileus because of which the dose needed modification in subsequent cycles. Other toxicities were all grade 1–2 though these were less stringently captured as this was a retrospective analysis.
Although the overall toxicity of R-DA-EPOCH was manageable, a significant proportion of patients developed serious events including 1 death in a patient with pre-existing lung disease after the 5th cycle. Two-third of our patients hailed from poor economic conditions and delivering this protocol was a significant logistic challenge (despite the medicine cost being supported using various sources). The only other report on adult BL from India had 11 patients (age 15–25 years) who were treated with a pediatric type protocol and had a survival of 56% [14]. In this context, R-DA-EPOCH represents a major improvement which is particularly suited for older patients with BL and PMBCL. Future studies should focus on attempts to improve the outcomes of patients with more advanced stages of BL such as those with bone marrow involvement and leukemic presentations.
Acknowledgements
Vanitha N and Rekhadevi for help in collection of data.
Funding
The study was supported by Cancer Institute (WIA) funds. No grant number is applicable. There were no external sources of funding for this project.
Compliance with Ethical Standards
Conflict of interest
None of the authors have any relevant conflicts of interests to declare.
References
- 1.Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. 2013;369(20):1915–1925. doi: 10.1056/NEJMoa1308392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunleavy K, Fanale M, LaCasce A, Noy A, Caimi P, Parekh S, et al. Preliminary report of a multicenter prospective phase II study of DA-EPOCH-R in MYC-rearranged aggressive B-cell lymphoma. Blood. 2014;2014(124):395. [Google Scholar]
- 3.Dunleavy K, Pittaluga S, Maeda LS, Advani R, Chen CC, Hessler J, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368(15):1408–1416. doi: 10.1056/NEJMoa1214561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas DA, Faderl S, O’Brien S, Bueso-Ramos C, Cortes J, Garcia-Manero G, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106(7):1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 5.Evens AM, Carson KR, Kolesar J, Nabhan C, Helenowski I, Islam N, et al. A multicenter phase II study incorporating high-dose rituximab and liposomal doxorubicin into the CODOX-M/IVAC regimen for untreated Burkitt’s lymphoma. Ann Oncol. 2013;24(12):3076–3081. doi: 10.1093/annonc/mdt414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sparano JA, Lee JY, Kaplan LD, Levine AM, Ramos JC, Ambinder RF, et al. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood. 2010;115(15):3008–3016. doi: 10.1182/blood-2009-08-231613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barta SK, Lee JY, Kaplan LD, Noy A, Sparano JA. Pooled analysis of AIDS malignancy consortium trials evaluating rituximab plus CHOP or infusional EPOCH chemotherapy in HIV-associated non-Hodgkin lymphoma. Cancer. 2012;118(16):3977–3983. doi: 10.1002/cncr.26723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol. 2014;32(37):3048–3058. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 10.Dunleavy K, Noy A, Abramson JS, LaCasce AS, Link BK, Parekh S, et al. Risk-adapted therapy in adults with Burkitt lymphoma: preliminary report of a multicenter prospective phase II study of DA-EPOCH-R. Blood. 2015;126:342. [Google Scholar]
- 11.Ribera JM, García O, Grande C, Esteve J, Oriol A, Bergua J, et al. Dose-intensive chemotherapy including rituximab in Burkitt’s leukemia or lymphoma regardless of human immunodeficiency virus infection status: final results of a phase 2 study (Burkimab) Cancer. 2013;119(9):1660–1668. doi: 10.1002/cncr.27918. [DOI] [PubMed] [Google Scholar]
- 12.Xicoy B, Ribera JM, Müller M, García O, Hoffmann C, Oriol A, et al. Dose-intensive chemotherapy including rituximab is highly effective but toxic in human immunodeficiency virus-infected patients with Burkitt lymphoma/leukemia: parallel study of 81 patients. Leuk Lymphoma. 2014;55(10):2341–2348. doi: 10.3109/10428194.2013.878933. [DOI] [PubMed] [Google Scholar]
- 13.Hoelzer D, Walewski J, Döhner H, Viardot A, Hiddemann W, Spiekermann K, et al. Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial. Blood. 2014;124(26):3870–3879. doi: 10.1182/blood-2014-03-563627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sengar M, Akhade A, Nair R, Menon H, Shet T, Gujral S, et al. A retrospective audit of clinicopathological attributes and treatment outcomes of adolescent and young adult non-Hodgkin lymphomas from a tertiary care center. Indian J Med Paediatr Oncol. 2011;32(4):197–203. doi: 10.4103/0971-5851.95140. [DOI] [PMC free article] [PubMed] [Google Scholar]