Abstract
Myocardial infarction (MI), occurs when the coronary artery is occluded resulting in the hypoxia of areas in heart tissue, is increasing in recent years because of the population ageing and lifestyle changes. Currently, there is no ideal therapeutic scheme because of the limitation of MI therapeutic strategies due to the lack of regenerative ability of the heart cells in adult humans. Recent advances in tissue engineering and regenerative medicine brings hope to the MI therapy and current studies are focusing on restoring the function and structure of damaged tissue by delivering exogenous cells or stimulating endogenous heart cells. However, attempts to directly inject stem cells or cardiomyocytes to the infract zone often lead to rapid cell death and abundant cell loss. To address this challenge, various soft repair cells and porous scaffold materials have been integrated to improve cell retention and engraftment and preventing left ventricle (LV) dilatation. In this article, we will review the current method for heart regeneration based on soft cell-porous scaffold interfacial tissue engineering including common stem cell types, biomaterials, and cardiac patch and will discuss potential future directions in this area.
Keywords: Regenerative medicine, cardiac repair, myocardial infarction (MI), tissue engineering, biomaterial
Introduction
Myocardial infarction (MI), the main cause of the damage to the heart muscle, is increasing in recent years because of the population ageing and lifestyle changes. MI is commonly known as a heart attack when blood flow to part of heart decreases or stops and is accompanied by symptoms such as chest pain, sweating, nausea and fainting. It occurs when the coronary artery is occluded resulting in the hypoxia of areas in heart tissue. As the hypoxia status sustains, in the final stage, MI may cause the cardiac fibrosis and heart failure affecting nearly 23 million worldwide (1).
At present, even with abundant advanced pharmacological and medical device treatment methods, the morbidity of MI still stays high. The initial treatment for MI is to restore the blood flow using pharmacologic, surgical or mechanical methods. With increasing duration and severity of ischemia, the cardiac tissue damage develops with reperfusion-associated pathologies which can be lethal. The pharmacological interventions to reduce the reperfusion injury are not ideal (2). The normal human left ventricle (LV) is approximately 1 cm thick and as the disease intensifies, the left ventricular size, shape and structure begin to change (3,4). Left ventricular reconstruction have been used to restore ventricular shape and reduce its volume to improve heart function. However, these procedures have not found general acceptance in the medical community (5). For heart failure, there is no effective and curative treatment except for the heart transplant (6), however, the shortage of donors and the difficulty of immunosuppression limit its application. The left ventricular assist devices have also improved the myocardial contractility, but it is plagued by the complications including bleeding, right ventricular failure, thromboembolism, and infection (7). Besides, some surgical approaches have been developed such as angioplasty, left ventricular reconstruction and cellular cardiomyoplasty.
Current limitation of MI therapeutic strategies is attributed to the lack of regenerative ability of the heart cells in adult humans. Moreover, the congenital malformations of the heart and stubbornly high morbidity and mortality require new modes of therapy. The emergence of tissue engineering and regenerative medicine brings hope to the therapy of MI. Regenerative medicine is a multidisciplinary field aims to revolutionize the way of improving the health and quality of life by restoring, maintaining or enhancing tissue and functions of organs (8). It has the potential to replace tissues or even organs damaged by diseases by building the cell regeneration system or mobilizing the body’s innate healing response to promote regeneration. The strategies of heart regenerative medicine combine multiple fields such as tissue engineering, biology, medicine and materials. Using the materials and de novo generated cells, the damaged tissue could be repaired and the structure and the function are recovered.
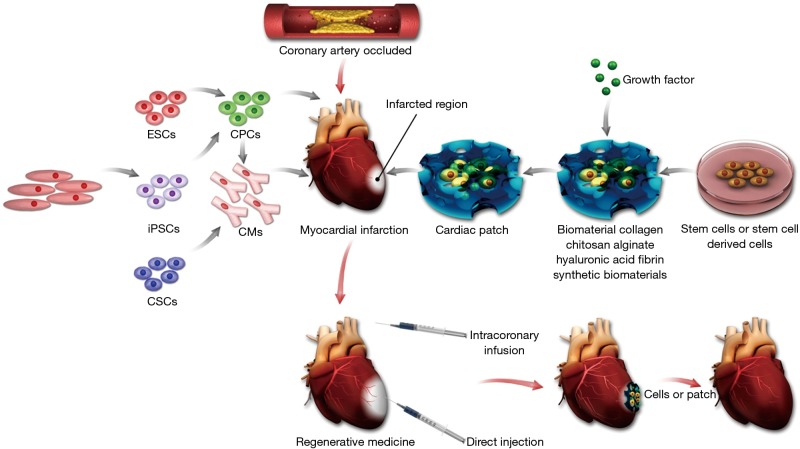
Regenerative medicine includes several aspects that we must take into account (Figure 1). First, the type of reparative cells to form a functional tissue must be carefully chosen. Second, if necessary, appropriate porous scaffolds for hosting the cells for transplantation can be selected. Third, some bioactive molecules such as cytokines and growth factors can be used to support the formation of the desired tissue. In this review, we will summarize the current method for heart regeneration based on soft cell-porous scaffold and discuss the potential future directions in this area.
Figure 1.
Summary of approaches for cardiac tissue regeneration. The left of this scheme shows the potential cell sources for heart regeneration. The right shows the diagrammatic representation of cardiac patch strategies using biomaterials. In the middle, the diagrammatic representation of the myocardial infarction and the injection methods used in heart regeneration is illustrated. ESCs, embryonic stem cells; CPCs, cardiac progenitor cells; SCs, somatic cells; iPSCs, induced pluripotent stem cells; CMs, cardiomyocytes; CSCs, cardiac stem cells.
Stem cells and heart regeneration
As the death and disability are mainly attributed to the limitation of heart’s regenerative capacity, current researchers are focusing on restoring the function and structure of damaged heart tissue by delivering exogenous cells or stimulating endogenous heart cells (9). Cells that have been used for this purpose include embryonic stem cells (ESCs) (10), induced pluripotent stem cells (iPSCs), cardiac stem cells (CSCs) (11), bone marrow mononuclear cells (12), skeletal myoblasts (13), and endothelial progenitor cells (14).
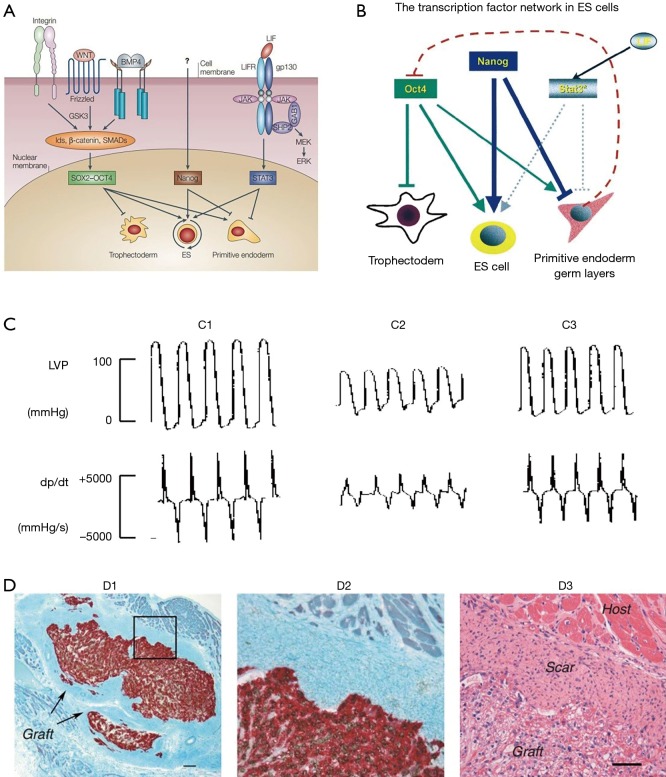
ESCs have the ability to differentiate into all types of specialized body cells under specific conditions, which gives them the potential in the therapy of heart diseases. ESCs were firstly isolated from mouse in 1981 (15) and was subsequently isolated from rat (16), rabbit (17), monkey (18) and human (19). In human, transcription factors like octamer-binding transcription factor 3/4 (Oct3/4), SRY-related high-mobility group-box protein-2 (Sox2) and Nanog mediate the pluripotency of ESCs (20-22), and the fibroblast growth factor maintains its activity (23) (Figure 2A). The transcription factor network in ESCs was discussed in Chambers’s article (21) (Figure 2B): Nanog and Oct4 play important role in ESCs identity and Stat3 has an accessory function. Oct4 blocks the differentiation into trophectoderm and promote differentiation into primitive endoderm and germ layers. On the contrary, Nanog blocks the differentiation into endoderm and germ layers. In establishing the cardiovascular system, the transforming growth factor β, Wnt, fibroblast growth factor and bone morphogenic protein pathways play important roles (25-28). The Wnt/β-catenin signaling pathway was summarized in Kwon’s article (26). Studies have suggested that the ESC-induced cardiomyocytes’ characteristics were similar to the adult cardiomyocytes and proved the function in the infarcted hearts (10,24,29) (Figure 2C,D). Although improved cardiac function was observed, several obstacles remain, such as the immature characteristics after engraftment, safety concerns, and ethical issues from human (30,31). More seriously, the tumor formation and immune rejection were observed, which emphasized the hurdles that need to be overcome before therapy with ESCs (32). This indicated huge obstacles in the clinical applications and the impendency to develop efficient methods to guide cardiac differentiation and prevent allogeneic graft immune rejection.
Figure 2.
Stem cells and heart regeneration. (A) Signal pathways involved in maintaining mouse ESC pluripotency. (B) Model of the transcription factor network in embryonic stem (ES) cells. The arrow indicates the promoting effect and the horizontal line means the blocking effect (21). (C) Embryonic stem cell transplantation improved left ventricular (LV) function (24). (C1) Sham-operated rats; (C2) postinfarcted rats injected with cell-free medium; (C3) postinfarcted rats transplanted with embryonic stem cells. (D) Human embryonic stem cells enhance function of infracted rat hearts (10). The bright field microscopic images were acquired from recipient hearts 4 weeks post-transplantation. (D1) The human pan-centromeric in situ hybridization (brown chromagen) and β-myosin-positive cardiomyocytes (red) indicated the formation of a large cell graft within infarct scar tissue. Scale bar =100 µm. (D2) High magnification of boxed part in D1. (D3) Hematoxylin and eosin stain showed the graft cells have a vacuolated appearance because of the glycogen. Scale bar =50 µm. [(A) reprinted with permission of reference (20), (B) reprinted with permission of reference (21), (C) reprinted with permission of reference (24) and (D) reprinted with permission of reference (10)]. LVP, left ventricular potential; dP/dt, peak LV systolic pressure.
To overcome this problem, Yamanaka and colleagues (33) reported a solution using the iPSCs which can be generated from patient’s own somatic cells. Similar to ESCs, the iPSCs also have the ability to differentiate into body cells, which was studied in mice (34), pigs (35) and humans (36). These studies also showed the molecular and functional similarity between ESCs and iPSCs, and the potential of iPSCs in cardiac repair was demonstrated. However, the gene expression patterns between these two kinds of cells are significantly different (37,38). Clinically, iPSC has not been recognized because of the potential immunogenic problems (39), which may cause gene mutations during the therapy.
CSCs population in the adult heart leads to 1% cardiomyocytes renew per year (40). The CSCs can self-renew and differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells (41,42). Therefore, CSCs were injected into the ischemic heart and used in regenerative repair to reduce the infarct size. Besides the three main stem cell types above, the stem cell population in skeletal muscle was also used for transplantation to improve cardiac function because of its functional and histological similarities with cardiac muscle (13). In addition, the cell population from bone marrow was also demonstrated to have broad potential in heart regenerative medicine (43).
In the stem cell therapy of heart diseases, it is important to deliver cells to the site of injury precisely and efficiently. Intramyocardial, intravenous and intracoronary injections are main methods to deliver cells (44). Unfortunately, the direct injection into infarcted regions can result in the mechanical leakage and massive loss of cells occur (45). Intracoronary infusion is the method to deliver cells that can minimize cell leakage, but the acute myocardial ischemia may be caused by this process (46). Currently, maintaining cell viability for a long time is challenging, irrespective of the different delivery methods aforementioned. The low cell retention/engraftment, cell delivery efficiency, electromechanical integration, and long-term safety are still the obstacles in stem cell therapy in MI. In addition, it was found that some engrafted stem cells could not differentiate into cardiomyocytes and could not contact with the host cells normally (13).
In summary, stem cells have enormous potential for MI treatment. With the advantages, people are committed to improve the current strategies and challenges for heart regeneration. To address these challenges, tissue engineering and biomaterials have been the choices to help improve the delivery efficiency and biomaterial-based porous scaffolds have proven their effectiveness in transplantation.
Biomaterials and heart regeneration
The high porosity, microenvironment adaptation, biocompatibility and biodegradability make the natural and synthetic biomaterials being the optimal choices to fabricate porous scaffolds. Moreover, acellular porous scaffolds can be immediately implanted and the immune reaction is limited. Some natural and synthetic polymers have been investigated as scaffolds for heart regeneration, such as collagen, chitosan, alginate, hyaluronic acid (HA), fibrin, and some synthetic biomaterials (Table 1).
Table 1. Biomaterials have been investigated for heart regeneration.
| Biomaterials | Advantages | Limitation |
|---|---|---|
| Collagen | Excellent biocompatibility and biodegradability | Low elastic modulus |
| Chitosan | Porosity; high elastic modulus | Non-cell adherent |
| Alginate | Gelation capacity; non-thrombogenic property | Lack of integration with cardiac cells |
| Synthetic materials | Improved mechanical properties; excellent strength and durability; better uniformity; lower risk of infection | Toxicity; low biocompatibility |
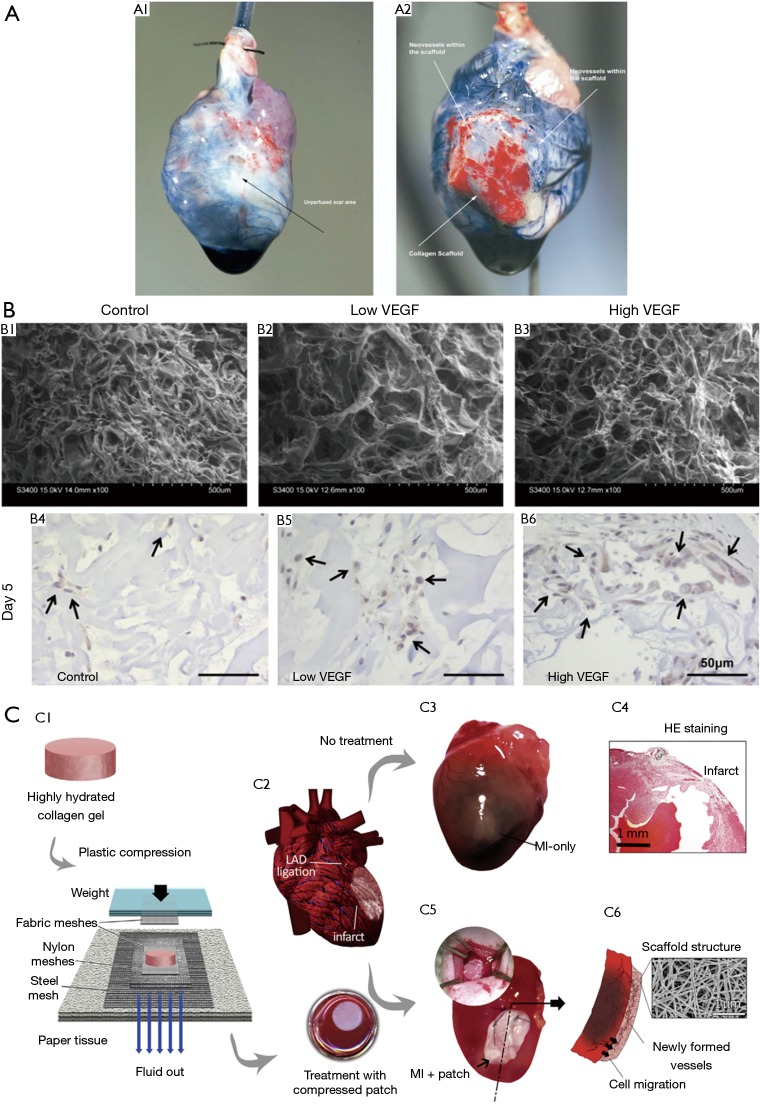
Collagen porous scaffold is one of the most popular natural materials used in heart repair due to its abundant distribution in extracellular matrix, which provides excellent biocompatibility and biodegradability for cell integration (47). Studies have showed that vessel formation and cardiac repair effect of collagen porous scaffolds in rat models (48,49) (Figure 3A). In Miyagi et al.’s study (49), a collagen patch with the vascular endothelial growth factor (VEGF) was proved to promote vascularization and ventricular wall thickness in right ventricle defect, which indicated the cardiac repair effect of VEGF-collagen patch (Figure 3B). The collagen plays the repair role through diminishing infarct region fibrosis, supporting blood vessel formation and attracting native cells (50) (Figure 3C). However, the lower elastic modulus of collagen limits its mechanical integration and stabilization, which can be improved by combination with other biomaterials such as chitosan and angiogenic factors (51,52).
Figure 3.
The collagen patch in cardiac regeneration. (A) Coronary artery perfusion of rat hearts (48). (A1) Infarcted heart without collagen patch. (A2) Infarcted heart with collagen patch. The Evans blue was used in the experiment to show the neo-vasculature. (B) The in vitro properties (B1–3) and in vivo effect on cell mobilization (B4–6) of porous collagen cardiac patch (49). (C) Schematic representation of the plastic compression of collagen gels (C1) and inducing MI via left anterior descending (LAD) artery ligation (C2) which was either treated with collagen patch (C3,4) or untreated (C5,6) (50). [Figure (A) reprinted with permission of reference (48), (B) reprinted with permission of reference (49), and (C) reprinted with permission of reference (50)].
Chitosan, derived from chitin in crustacean shells, has been widely used in biomedical area. The porosity of chitosan is important for the cell migration and integration (53). Generally, chitosan is mixed with other biomaterials for the cardiac regeneration to achieve optimal properties because of its high compressive modulus. Pok et al. (54) reported a multi-layer porous scaffold with gelatin-chitosan hydrogel and polycaprolactone (PCL) and used it in the cardiac repair. And the chitosan-hyaluronan/silk fibroin patch could reduce LV dilatation and improve heart function (55). It may attribute to the non-cell adherent characteristic of chitosan which need to be improved by combination with other lower compressive moduli and cell-adherent materials to increase the tissue integration and mechanical stability.
Alginate is an anionic polysaccharide derived from seaweed and the implantation in MI models that can reinforce scar thickness, attenuate ventricular dilatation and improve cardiac function (56,57). In Deng et al.’s research (58), it has been identified that early intramyocardial injection of alginate-chitosan in rat model of MI could prevent ventricular remodeling and cell apoptosis. Alginate has the gelation capacity and non-thrombogenic property, which makes it to be an attractive biomaterial in cardiac repair applications. The major limitation for alginate is the lack of integration with cardiac cells (5). The use of hyaluronic acid (HA) for cardiac function recovery in MI has been another effective measure since Yoon et al.’s research (59). Like other biomaterials used in cardiac repair, HA can decrease the infracted area and increase local vasculature (60). HA-mediated repair depends on the molecular weight and injection time of HA and a higher compression modulus is more suitable (61). In addition, fibrin (62) and extracellular matrix (63) have also been reported as natural biomaterials for heart regeneration.
Compared with natural biomaterials, synthetic materials have improved mechanical properties, excellent strength and durability, better uniformity and lower risk of infection, but the toxicity and biocompatibility are the main concerns for these materials. To meet specific properties of tissues, the properties of synthetic biomaterials can be modified, such as the degradation rates and porosity. Polymers are the most investigated synthetic biomaterials for cardiac repair, including polylactic acid, polyester, poly(propylene)and poly(caprolactone) (64-66). Among them, poly(ethylene glycol) is the most commonly explored synthetic polymer and it is already approved by FDA for certain applications. Beside these, conductive carbon nanofibers have also been investigated for cardiac engineering (67,68).
The cardiac patch
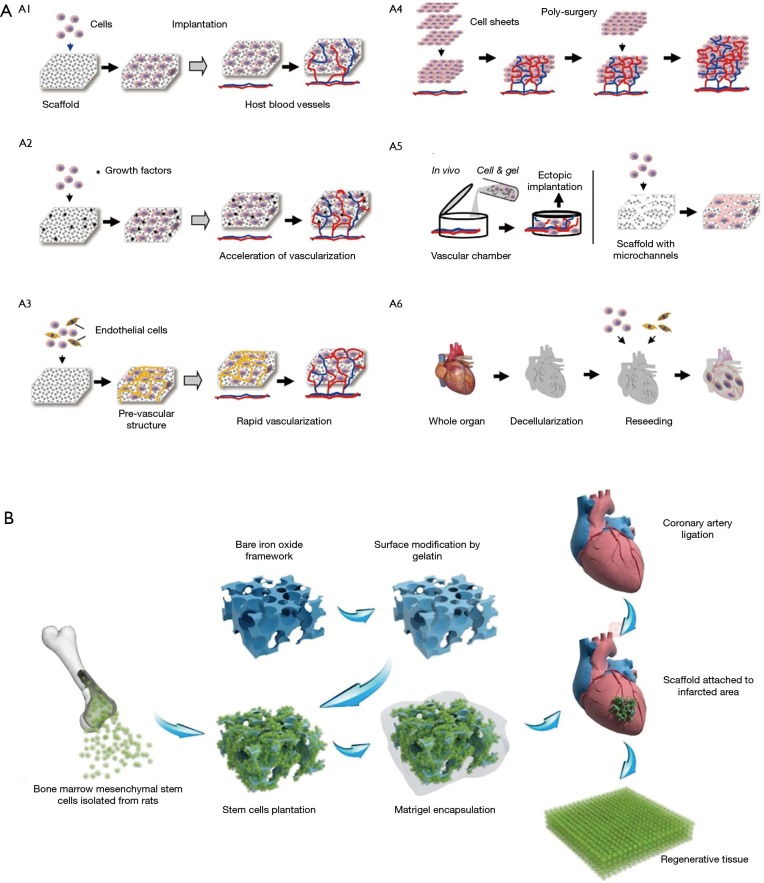
In the therapy of heart regeneration, the methods that attempt to directly inject stem cells or cardiomyocytes to the infract zone are followed by rapid cell death with abundant cell loss (69). Tissue engineering technology is one of the solutions for long-term engraftment upon transplantation. With the abundant natural and synthetic material above mentioned, integrating an implantable framework can improve the ability to mimic the architecture of the extracellular matrix, which will allow stem cells and cardiomyocytes to be adhered at the target area and maximize their chances of retention (70). The frameworks are fabricated in vitro with cells and implanted over the MI tissue. Scaffolds with optimal porous structure should have properties including high porosity that allows efficient diffusion of nutrients and metabolic wastes, natural microenvironment, biodegradability and biocompatibility. In addition, the implanted patch should have the balance between promoting cell migration and avoiding excessive internal space (71). With the goals of improving cell retention and engraftment and preventing LV dilatation, several kinds of porous scaffold materials and repair cells have been chosen and integrated. This may provide a method to replace the injured myocardium and activate the endogenous repairing mechanisms. The fabricated cellular framework can be a complex 3-D construct, or simple cell sheets (72) and various strategies for vascularization in tissue engineering was discussed in this article (Figure 4A).
Figure 4.
The cardiac patch and heart regeneration. (A) Diagrammatic representation of vascularization strategies in heart regeneration (72); (B) schematic of the macroporous iron oxide frameworks in the repair of infracted heart (73). [Figure (A) reprinted with permission of reference (72) and (B) reprinted with permission of reference (73)].
Recently, significant researches reported several kinds of cardiac patch with biomaterials and appropriate cells. Simpson et al. (74) embedded human mesenchymal stem cells into a collagen matrix to form the cardiac patch, and the pluripotent cells could be efficiently delivered to a site of MI and resulted in improved myocardial remodeling. Moreover, vitronectin/collagen porous scaffold seeded with endothelial progenitor cells has been shown to have the ability of inducing vasculogenesis and preserving ventricular function (75). As a hydrogel that contains adhesion molecules, cardiac patch consisted of fibrin porous scaffolds seeded with neonatal rat cardiac cells could reduce the infarct size and eliminate ventricular wall thinning (76). Tang et al. (77) demonstrated the safety and efficacy of nanogel-encapsulated human CSCs in mouse and pig models of MI. Some synthetic materials such as polyester and polycaprolactone are also considered as appropriate biomaterials to deliver differentiated cardiomyocytes from stem cells (78,79). In the past few years, hawse has focused on the development of macroporous materials and the potential of application in the field of tissue engineering (80-85). Recently, we reported a multilayered iron oxide-based macroporous composite framework in MI therapy (73), which has excellent biocompatibility, improved mechanical strength, controlled biodegradability and enormous potential in cardiac repair (Figure 4B).
Except for the numerous studies of the cell-based cardiac patches, the drug/ligand-based patches are also the research hotspot with promoting neo-vascularization and restoring blood flow in MI (86). The basic-fibroblast growth factor (bFGF) (87) and VEGF (88) are the common exogenous pro-angiogenic factors used in cardiac patch. And a collagen-based cardiac patch containing follistatin-like 1 (Fstl1) was also reported that can attenuate MI-induced heart injuries (89).
For heart tissue engineering applications, a porous scaffold with high porosity, appropriate pore sizes and mechanical strength is essential. To this regard, many fabrication techniques were developed to meet the complex heterogeneous nature of endogenous tissue. The traditional techniques include solvent casting, particle leaching, freeze drying, and gas foaming. With these methods, the pore size of the scaffold can be well controlled but the cell encapsulation process cannot be performed in situ as cytotoxic solvents were involved during the scaffold fabrication processes (90-92). Bioprinting with a top-down approach has been a popular method in complex architecture fabrication. With this, 3-D constructs analogous to tissues or organs are built in a layer-by-layer process, and prepolymer solutions and cell aggregates can be rapidly deposited onto a substrate over relatively large areas (93). Bioprinting has the capacity to construct complex architectures automatically. Photolithography techniques, used widely in the semiconductor industry, can be used to create a 2-D porous scaffold for cell growth or a 3-D network for cell encapsulation (94,95). The photolithography system has the ability to uniformly encapsulate cells and has good spatial and temporal control. However, although the photolithography techniques have been successfully employed for tissue engineering, the reactive species created by the absorbed incident light can be cytotoxic to cells (96). Electrospinning has been a popular method for cardiac repair application as a technique that uses an electrical charge to produce nanofibers from polymer solutions. This technique has the ability to produce a network of interconnected nanofibers which is similar to the architecture in natural extracellular matrix. Several polymers have been used to develop nanofiber porous scaffolds using this technique to construct a biocompatible and biodegradable network in cardiac tissue regeneration application (97-99).
Future direction in the perspective of heart regeneration
The achievement in material sciences, tissue engineering and nanotechnology has led to an explosion in the variety of frameworks available for tissue grafting in heart tissue regeneration. Stem cell therapy, biomaterials and several kinds of cardiac patch have shown promise in the past but it is still elusive to restore the myocardial function fully. As there are still many obstacles in this field, the future of biomaterials and cardiac regenerative medicine is certainly moving towards finding the ideal biomaterial-cell combination types. It is critical to develop elastic biomaterials to meet native myocardium, minimize the immune response and inflammatory, and properly encapsulate cells (100). Since the electrophysiological characteristic is essential in heart as the largest bioelectrical source, synthesizing conductive materials may facilitate the beating of cardiomyocytes (101). And it could enhance the communication between transplanted patch and host myocardium.
In addition, the minimal kinds of cell and biomaterial composition are necessary to increase biological activity. And the exchange of gases, nutrients and metabolic products between the repair patch with the vascular network is also an urgent and important issue. In the future, the development of whole heart organ regeneration in vitro can eliminate the quest for organ donators completely, which may revolutionize the health care medicine and improve quality of life.
Acknowledgements
Funding: This work was supported by the National Key R&D Program of China (2017YFA0206901, 2017YFA0206900), the NSF of China (21705027), the Major Scientific and Technological Innovation Project of Shandong (2017CXGC0501), Natural Science Foundation of Shandong Province (ZR2016HQ11, ZR2017LEM010), the Recruitment Program of Global Experts of China and the Recruitment Program of Global Experts of Shanghai.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011;8:30-41. 10.1038/nrcardio.2010.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibáñez B, Heusch G, Ovize M, et al. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 2015;65:1454-71. 10.1016/j.jacc.2015.02.032 [DOI] [PubMed] [Google Scholar]
- 3.Artenie R, Artenie A, Ungureanu D, et al. Myocardial remodeling. Rev Med Chir Soc Med Nat Iasi 2003;107:35-9. [PubMed] [Google Scholar]
- 4.Gaudron P, Eilles C, Ertl G, et al. Compensatory and noncompensatory left ventricular dilatation after myocardial infarction: Time course and hemodynamic consequences at rest and during exercise. AM Heart J 1992;123:377-85. 10.1016/0002-8703(92)90649-G [DOI] [PubMed] [Google Scholar]
- 5.Domenech M, Polo-Corrales L, Ramirez-Vick JE, et al. Tissue engineering strategies for myocardial regeneration: acellular versus cellular scaffolds? Tissue Eng Part B Rev 2016;22:438-58. 10.1089/ten.teb.2015.0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangini S, Alves BR, Silvestre OM, et al. Heart transplantation: review. Einstein 2015;13:310-8. 10.1590/S1679-45082015RW3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis 2006;6:426-37. 10.1016/S1473-3099(06)70522-9 [DOI] [PubMed] [Google Scholar]
- 8.Stoltz JF, de Isla N, Li YP, et al. Stem cells and regenerative medicine: myth or reality of the 21th century. Stem Cells Int 2015;2015:734731. 10.1155/2015/734731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho GS, Fernandez L, Kwon C. Regenerative medicine for the heart: perspectives on stem-cell therapy. Antioxid Redox Signal 2014;21:2018-31. 10.1089/ars.2014.6063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 2007;25:1015-24. 10.1038/nbt1327 [DOI] [PubMed] [Google Scholar]
- 11.Bolli R, Chugh AR, D'Amario D, et al. Effect of cardiac stem cells in patients with ischemic cardiomyopathy: initial results of the SCIPIO trial. Lancet 2011;378:1847-57. 10.1016/S0140-6736(11)61590-0 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 2004;364:141-8. 10.1016/S0140-6736(04)16626-9 [DOI] [PubMed] [Google Scholar]
- 13.Menasché P, Alfieri O, Janssens S, et al. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189-1200. 10.1161/CIRCULATIONAHA.107.734103 [DOI] [PubMed] [Google Scholar]
- 14.Katritsis DG, Sotiropoulou PA, Karvouni E, et al. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv 2005;65:321-9. 10.1002/ccd.20406 [DOI] [PubMed] [Google Scholar]
- 15.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A 1981;78:7634-8. 10.1073/pnas.78.12.7634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iannaccone PM, Taborn GU, Garton RL, et al. Pluripotent embryonic stem cells from the rat are capable of producing chimeras. Dev Biol 1994;163:288-92. 10.1006/dbio.1994.1146 [DOI] [PubMed] [Google Scholar]
- 17.Graves KH, Moreadith RW. Derivation and characterization of putative pluripotential embryonic stem cells from preimplantation rabbit embryos. Mol Reprod Dev 1993;36:424-33. 10.1002/mrd.1080360404 [DOI] [PubMed] [Google Scholar]
- 18.Thomson JA, Kalishman J, Golos TG, et al. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci U S A 1995;92:7844-48. 10.1073/pnas.92.17.7844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomson JA, Itskovitzeldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145-7. 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- 20.Boiani M, Schöler HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol 2005;6:872-84. 10.1038/nrm1744 [DOI] [PubMed] [Google Scholar]
- 21.Chambers I, Colby D, Robertson M, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003;113:643-55. 10.1016/S0092-8674(03)00392-1 [DOI] [PubMed] [Google Scholar]
- 22.Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998;95:379-91. 10.1016/S0092-8674(00)81769-9 [DOI] [PubMed] [Google Scholar]
- 23.Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol 2000;227:271-8. 10.1006/dbio.2000.9912 [DOI] [PubMed] [Google Scholar]
- 24.Min JY, Yang Y, Converso KL, et al. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol 2002;92:288-96. 10.1152/jappl.2002.92.1.288 [DOI] [PubMed] [Google Scholar]
- 25.Gadue P, Huber TL, Paddison PJ, et al. Wnt and TGF-β signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A 2006;103:16806-11. 10.1073/pnas.0603916103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon C, Cordes KR, Srivastava D. Wnt/beta-catenin signaling acts at multiple developmental stages to promote mammalian cardiogenesis. Cell Cycle 2008;7:3815-8. 10.4161/cc.7.24.7189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon C. Chapter309-Signaling pathways involved in cardiogenesis. In: Bradshaw RA, Dennis EA. editors. Handbook of Cell Signaling. 2nd edition. Amsterdam: Elsevier Inc., 2010:2601-9. [Google Scholar]
- 28.van Weerd JH, Koshibatakeuchi K, Kwon C, et al. Epigenetic factors and cardiac development. Cardiovasc Res 2011;91:203-11. 10.1093/cvr/cvr138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singla DK, Hacker TA, Ma L, et al. Transplantation of embryonic stem cells into the infarcted mouse heart: formation of multiple cell types. J Mol Cell Cardiol 2006;40:195-200. 10.1016/j.yjmcc.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 30.Kehat I, Gepstein L. Human embryonic stem cells for myocardial regeneration. Heart Fail Rev 2003;8:229-36. 10.1023/A:1024709332039 [DOI] [PubMed] [Google Scholar]
- 31.Nussbaum J, Minami E, Laflamme MA, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. Faseb J 2007;21:1345. 10.1096/fj.06-6769com [DOI] [PubMed] [Google Scholar]
- 32.Nussbaum J, Minami E, Laflamme MA, Vi, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J 2007;21:1345-57. 10.1096/fj.06-6769com [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 34.Mauritz C, Schwanke K, Reppel M, et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation 2008;118:507-17. 10.1161/CIRCULATIONAHA.108.778795 [DOI] [PubMed] [Google Scholar]
- 35.Montserrat N, Bahima EG, Batlle L, et al. Generation of pig iPS cells: a model for cell therapy. J Cardiovasc Transl Res 2011;4:121-30. 10.1007/s12265-010-9233-3 [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Wilson GF, Soerens AG, et al. Functional Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells. Circ Res 2009;104:e30-41. 10.1161/CIRCRESAHA.108.192237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chin MH, Mason MJ, Xie W, et al. Induced Pluripotent Stem Cells and Embryonic Stem Cells Are Distinguished by Gene Expression Signatures. Cell Stem Cell 2009;5:111-23. 10.1016/j.stem.2009.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson KD, Venkatasubrahmanyam S, Jia F, et al. MicroRNA profiling of human-induced pluripotent stem cells. Stem Cells Dev 2009;18:749-58. 10.1089/scd.2008.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gore A, Li Z, Fung HL, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature 2011;471:63-7. 10.1038/nature09805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science 2009;324:98-102. 10.1126/science.1164680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A 2007;104:14068-73. 10.1073/pnas.0706760104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003;114:763-76. 10.1016/S0092-8674(03)00687-1 [DOI] [PubMed] [Google Scholar]
- 43.Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 2001;107:1395-402. 10.1172/JCI12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perin EC. Stem cell therapy for cardiovascular disease. Tex Heart Inst J 2006;33:204-8. [PMC free article] [PubMed] [Google Scholar]
- 45.Teng CJ, Luo J, Chiu RCJ, et al. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: Implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg 2006;132(3):628-32. 10.1016/j.jtcvs.2006.05.034 [DOI] [PubMed] [Google Scholar]
- 46.Bui QT, Gertz ZM, Wilensky RL. Intracoronary delivery of bone-marrow-derived stem cells. Stem Cell Res Ther 2010;1:29. 10.1186/scrt29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace DG, Rosenblatt J. Collagen gel systems for sustained delivery and tissue engineering. Adv Drug Deliv Rev 2003;55:1631-49. 10.1016/j.addr.2003.08.004 [DOI] [PubMed] [Google Scholar]
- 48.Gaballa MA, Sunkomat JN, Thai H, et al. Grafting an acellular 3-dimensional collagen scaffold onto a non-transmural infarcted myocardium induces neo-angiogenesis and reduces cardiac remodeling. J Heart Lung Transplant 2006;25:946-54. 10.1016/j.healun.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 49.Miyagi Y, Chiu LLY, Cimini M, et al. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials 2011;32:1280-90. 10.1016/j.biomaterials.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 50.Serpooshan V, Zhao M, Metzler SA, et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials 2013;34:9048-55. 10.1016/j.biomaterials.2013.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng C, Zhang P, Vulesevic B, et al. A collagen–chitosan hydrogel for endothelial differentiation and angiogenesis. Tissue Eng Part A 2010;16:3099-109. 10.1089/ten.tea.2009.0504 [DOI] [PubMed] [Google Scholar]
- 52.Chiu LL, Radisic M. Controlled release of thymosin β4 using collagen-chitosan composite hydrogels promotes epicardial cell migration and angiogenesis. J Control Release 2011;155:376-85. 10.1016/j.jconrel.2011.05.026 [DOI] [PubMed] [Google Scholar]
- 53.Song K, Qiao M, Liu T, et al. Preparation, fabrication and biocompatibility of novel injectable temperature-sensitive chitosan/glycerophosphate/collagen hydrogels. J Mater Sci Mater Med 2010;21:2835-42. 10.1007/s10856-010-4131-4 [DOI] [PubMed] [Google Scholar]
- 54.Pok S, Myers JD, Madihally SV, et al. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater 2013;9:5630-42. 10.1016/j.actbio.2012.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chi NH, Yang MC, Chung TW, et al. Cardiac repair using chitosan-hyaluronan/silk fibroin patches in a rat heart model with myocardial infarction. Carbohydr Polym 2013;92:591-7. 10.1016/j.carbpol.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 56.Lee KY. Alginate: properties and biomedical applications. Prog Polym Sci 2012;37:106-26. 10.1016/j.progpolymsci.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landa N, Miller L, Feinberg MS, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 2008;117:1388-96. 10.1161/CIRCULATIONAHA.107.727420 [DOI] [PubMed] [Google Scholar]
- 58.Deng B, Shen L, Wu Y, et al. Delivery of alginate-chitosan hydrogel promotes endogenous repair and preserves cardiac function in rats with myocardial infarction. J Biomed Mater Res A 2015;103:907-18. 10.1002/jbm.a.35232 [DOI] [PubMed] [Google Scholar]
- 59.Yoon SJ, Fang YH, Lim CH, et al. Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel. J Biomed Mater Res B Appl Biomater 2009;91:163-71. 10.1002/jbm.b.31386 [DOI] [PubMed] [Google Scholar]
- 60.Abdalla S, Makhoul G, Duong M, et al. Hyaluronic acid-based hydrogel induces neovascularization and improves cardiac function in a rat model of myocardial infarction. Interact Cardiovasc Thorac Surg 2013;17:767. 10.1093/icvts/ivt277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ifkovits JL, Tous E, Minakawa M, et al. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A 2010;107:11507-12. 10.1073/pnas.1004097107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y, Meng H, Liu Y, et al. Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. ScientificWorldJournal 2015;2015:685690. 10.1155/2015/685690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ravi S, Caves JM, Martinez AW, et al. Effect of bone marrow-derived extracellular matrix on cardiac function after ischemic injury. Biomaterials. 2012;33:7736. 10.1016/j.biomaterials.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukherjee S, Venugopal JR, Ravichandran R, et al. Evaluation of the biocompatibility of PLACL/collagen nanostructured matrices with cardiomyocytes as a model for the regeneration of infarcted myocardium. Adv Funct Mater 2011;21:2291-300. 10.1002/adfm.201002434 [DOI] [Google Scholar]
- 65.Fujimoto KL, Ma Z, Nelson DM, et al. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30:4357-68. 10.1016/j.biomaterials.2009.04.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang T, Wu DQ, Jiang XJ, et al. Novel thermosensitive hydrogel injection inhibits post-infarct ventricle remodelling. Eur J Heart Fail 2009;11:14-9. 10.1093/eurjhf/hfn009 [DOI] [PubMed] [Google Scholar]
- 67.Martins AM, Eng G, Caridade S, et al. Electrically conductive chitosan/carbon scaffoldsfor cardiac tissue engineering. Biomacromolecules 2014;15:635-43. 10.1021/bm401679q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou J, Chen J, Sun H, et al. Engineering the heart: Evaluation of conductive nanomaterials for improving implant integration and cardiac function. Sci Rep 2014;4:3733. 10.1038/srep03733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang M, Methot D, Poppa V, et al. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol 2001;33:907-21. 10.1006/jmcc.2001.1367 [DOI] [PubMed] [Google Scholar]
- 70.Sui R, Liao X, Zhou X, et al. The current status of engineering myocardial tissue. Stem Cell Rev 2011;7:172-80. 10.1007/s12015-010-9131-8 [DOI] [PubMed] [Google Scholar]
- 71.Davis ME, Hsieh PCH, Grodzinsky AJ, et al. Custom design of the cardiac microenvironment with biomaterials. Circ Res 2005;97:8-15. 10.1161/01.RES.0000173376.39447.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimizu T. Cell sheet-based tissue engineering for fabricating 3-dimensional heart tissues. Circ J 2014;78:2594-603. 10.1253/circj.CJ-14-0973 [DOI] [PubMed] [Google Scholar]
- 73.Wang W, Tao H, Zhao Y, et al. Implantable and Biodegradable Macroporous Iron Oxide Frameworks for Efficient Regeneration and Repair of Infracted Heart. Theranostics 2017;7:1966-75. 10.7150/thno.16866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simpson D, Liu H, Fan TH, et al. A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells 2007;25:2350-7. 10.1634/stemcells.2007-0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frederick JR, Iii JRF, McCormick RC, et al. Stromal cell-derived factor-1α activation of tissue engineered endothelial progenitor cell matrix enhances ventricular function after myocardial infarction by inducing neovasculogenesis. Circulation 2010;122:S107-17. 10.1161/CIRCULATIONAHA.109.930404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wendel JS, Ye L, Zhang P, et al. Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue Eng Part A 2014;20:1325-35. 10.1089/ten.tea.2013.0312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang J, Cui X, Caranasos TG, et al. Heart repair using nanogel-encapsulated human cardiac stem cells in mice and pigs with myocardial infarction. ACS Nano 2017;11:9738-49. 10.1021/acsnano.7b01008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen QZ, Ishii H, Thouas GA, et al. An elastomeric patch derived from poly(glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials. 2010;31:3885-93. 10.1016/j.biomaterials.2010.01.108 [DOI] [PubMed] [Google Scholar]
- 79.Kai D, Wang QL, Wang HJ, et al. Stem cell-loaded nanofibrous patch promotes the regeneration of infarcted myocardium with functional improvement in rat model. Acta Biomater 2014;10:2727-38. 10.1016/j.actbio.2014.02.030 [DOI] [PubMed] [Google Scholar]
- 80.Kong B, Tang J, Wu Z, et al. Bio-inspired porous antenna-like nanocube/nanowire heterostructure as ultrasensitive cellular interfaces. Npg Asia Mater 2014;6:e117 10.1038/am.2014.56 [DOI] [Google Scholar]
- 81.Kong B, Tang J, Zhang Y, et al. Branched artificial nanofinger arrays by mesoporous interfacial atomic rearrangement. J Am Chem Soc 2015;137:4260-6. 10.1021/jacs.5b01747 [DOI] [PubMed] [Google Scholar]
- 82.Kong B, Tang J, Selomulya C, et al. Oriented mesoporousnanopyramids as versatile plasmon-enhanced interfaces. J Am Chem Soc 2014;136:6822-5. 10.1021/ja501517h [DOI] [PubMed] [Google Scholar]
- 83.Liang K, Richardson JJ, Cui J, et al. Metal-organic framework coatings as cytoprotective exoskeletons for living cells. Adv Mater 2016;28 7910-4. 10.1002/adma.201602335 [DOI] [PubMed] [Google Scholar]
- 84.Kang L, Joseph R, Christian D, et al. An enzyme-coated metal-organic framework shell for synthetically adaptive cell survival. Angew Chem 2017; 129: 8630-5. 10.1002/ange.201704120 [DOI] [PubMed] [Google Scholar]
- 85.Chu Y, Hou J, Boyer C, et al. Biomimetic synthesis of coordination network materials: recent advances in MOFs and MPNs. Appl Mater Today 2018; 10: 93-105. 10.1016/j.apmt.2017.12.009 [DOI] [Google Scholar]
- 86.Jiang F. Search for magic patches that heal the broken heart. Clin Exp Pharmacol Physiol 2016;43:290-3. 10.1111/1440-1681.12529 [DOI] [PubMed] [Google Scholar]
- 87.Fathi E, Nassiri SM, Atyabi N, et al. Induction of angiogenesis via topical delivery of basic-fibroblast growth factor from polyvinyl alcohol-dextran blend hydrogel in an ovine model of acute myocardial infarction. J Tissue Eng Regen Med 2013;7:697-707. 10.1002/term.1460 [DOI] [PubMed] [Google Scholar]
- 88.Gao J, Liu J, Gao Y, et al. A myocardial patch made of collagen membranes loaded with collagen-binding human vascular endothelial growth factor accelerates healing of the injured rabbit heart. Tissue Eng Part A 2011;17:2739-47. 10.1089/ten.tea.2011.0105 [DOI] [PubMed] [Google Scholar]
- 89.Wei K, Serpooshan V, Hurtado C, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 2015;525:479-85. 10.1038/nature15372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mehrabanian M, Mojtaba NE. HA/nylon 6,6 porous scaffolds fabricated by salt-leaching/solvent casting technique: effect of nano-sized filler content on scaffold properties. Int J Nanomedicine 2011;6:1651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quirk RA, France RM, Shakesheff KM, et al. Supercritical fluid technologies and tissue engineering scaffolds. Curr Opin Solid State Mater Sci 2004;8:313-21. 10.1016/j.cossms.2003.12.004 [DOI] [Google Scholar]
- 92.Zellander A, Gemeinhart R, Djalilian A, et al. Designing a gas foamed scaffold for keratoprosthesis. Mater Sci Eng C Mater Biol Appl 2013;33:3396-403. 10.1016/j.msec.2013.04.025 [DOI] [PubMed] [Google Scholar]
- 93.Mironov V, Visconti RP, Kasyanov V, et al. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009;30:2164-74. 10.1016/j.biomaterials.2008.12.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee SH, Moon JJ, West JL. Three-dimensional micropatterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration. Biomaterials 2008;29:2962-68. 10.1016/j.biomaterials.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Tsang V, Chen AA, Cho LM, et al. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. Faseb J 2007;21:790-801. 10.1096/fj.06-7117com [DOI] [PubMed] [Google Scholar]
- 96.Williams CG, Malik AN, Kim TK, et al. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005;26:1211-8. 10.1016/j.biomaterials.2004.04.024 [DOI] [PubMed] [Google Scholar]
- 97.Zong X, Bien H, Chung CY, et al. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials 2005;26:5330-8. 10.1016/j.biomaterials.2005.01.052 [DOI] [PubMed] [Google Scholar]
- 98.Rockwood DN, Akins RE, Parrag I, et al. Culture on electrospun polyurethane scaffolds decreases atrial natriuretic peptide expression by cardiomyocytes in vitro. Biomaterials 2008;29:4783-91. 10.1016/j.biomaterials.2008.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Şenel Ayaz HG, Perets A, Ayaz H, et al. Textile-templated electrospun anisotropic scaffolds for regenerative cardiac tissue engineering. Biomaterials 2014;35:8540-52. 10.1016/j.biomaterials.2014.06.029 [DOI] [PubMed] [Google Scholar]
- 100.Reis LA, Chiu LL, Feric N, et al. Biomaterials in myocardial tissue engineering. J Tissue Eng Regen Med 2016;10:11-28. 10.1002/term.1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tandon N, Cannizzaro C, Chao PH, et al. Electrical stimulation systems for cardiac tissue engineering. Nat Protoc 2009;4:155-73. 10.1038/nprot.2008.183 [DOI] [PMC free article] [PubMed] [Google Scholar]