Abstract
Fast inhibitory synaptic transmission is mediated by γ-aminobutyric acid type A receptors (GABAARs) that are enriched at functionally diverse synapses via mechanisms that remain unclear. Using isothermal titration calorimetry and complementary methods we demonstrate an exclusive low micromolar binding of collybistin to the α2-subunit of GABAARs. To explore the biological relevance of collybistin-α2-subunit selectivity, we generate mice with a mutation in the α2-subunit-collybistin binding region (Gabra2-1). The mutation results in loss of a distinct subset of inhibitory synapses and decreased amplitude of inhibitory synaptic currents. Gabra2–1 mice have a striking phenotype characterized by increased susceptibility to seizures and early mortality. Surviving Gabra2-1 mice show anxiety and elevations in electroencephalogram δ power, which are ameliorated by treatment with the α2/α3-selective positive modulator, AZD7325. Taken together, our results demonstrate an α2-subunit selective binding of collybistin, which plays a key role in patterned brain activity, particularly during development.
The inhibitory synaptic protein collybistin (CB) and GABAAR-α subunits are thought to interact, but strength and specificity are unclear. Here the authors study the CB–α2 interaction and show that a mouse mutated in the CB-binding region of α2 displays a loss of specific synapses and seizure.
Introduction
The brain functions via a dynamic partnership between excitatory principal cells and inhibitory interneurons, ensuring regulation of global cell firing rates, yet allowing for local control of excitability. Fast inhibitory synaptic transmission is mediated by γ-aminobutyric acid type A receptors (GABAARs), which are composed of combinations of subunit families α(1–6), β(1–3), γ(1–3), δ, ε, θ, and π1. Despite GABAAR-α-subunits being highly homologous, they are known to be specifically localized, with GABAARs containing α1 enriched at dendritic and somatic synapses, and α2 localized to synapses on the axon initial segment (AIS)2–4. It has been long speculated that α-subunits are prime mediators in selective targeting5; however, the precise mechanisms regulating this targeting remain unclear. Comparison of α1 and α2 structures reveals that the large intracellular loop between transmembrane domains 3 and 4 is the most divergent, but its necessity for targeting is unclear. Given the important role of AIS synapses in regulating excitatory cell output6,7, and that α-subunit isoforms determine the decay of inhibitory postsynaptic currents8, enrichment of α2 to AIS synapses may profoundly affect neuronal excitability. Therefore, it is of fundamental importance to understand how neurons regulate the formation of synapse subtypes and control selective targeting of α2-containing GABAARs.
A well-known inhibitory synaptic organizer, gephyrin, has been shown to interact with multiple α-subunits of GABAARs9–12. Structural and mutational studies have identified a universal binding site within gephyrin that allows for the alternative recruitment of glycine and GABAAR subunits9. Mutations that weaken the receptor–gephyrin interaction have been shown to alter receptor accumulation, diffusion, clustering, and transport, and can be related to severe phenotypes, including epilepsy13–15. Early studies demonstrated a link between the GABAAR-α2-subunit and gephyrin11, however, quantification of the interaction later revealed a very low interaction strength16. Collybistin (CB; ARHGEF9) has also been shown to interact with gephyrin, as well as GABAARs17–20, but quantification of the interaction strength has not been possible. CB and its human homolog hPEM-2 (human homolog of posterior end mark-2) are guanine nucleotide exchange factors that activate the small GTPase CDC-42, regulating the actin cytoskeleton21. CB is generally composed of a catalytic Rho guanine nucleotide exchange factor domain, a pleckstrin homology (PH) phosphoinositide-binding domain, as well as an Src homology 3 (SH3) domain. Yeast-two-hybrid (Y2H) assays suggested the GABAAR-α2-subunit19 and neuroligin-222 as possible activators for CB by directly interacting with the SH3 domain, resulting in its release from the PH and DH domains. Mapping studies proposed an overlapping binding site for gephyrin and CB on the large intracellular loop of the GABAAR-α2-subunit, and it has been suggested that synergistic binding could produce a tripartite complex19.
The study of both mouse models and human neurodevelopmental syndromes characterized by CB loss supports a functional link to GABAARs. Deletion of Arhgef9 in mice leads to selective reductions in the number of inhibitory synapses within the hippocampus21,23. A naturally occurring missense mutation of ARHGEF9 (G55A) is associated with hyperekplexia, epilepsy, and mental retardation24,25, and has been shown to disrupt the clustering of α2-containing GABAARs and gephyrin19. Similarly, a translocation mutation within the ARHGEF9 gene has been shown to result in a neurodevelopmental syndrome that includes epilepsy, anxiety, aggressive behavior, sleep–wake disruptions, and mental retardation, but not hyperekplexia24,26,27, suggesting a broad disruption of GABAergic but not glycinergic signaling in this variant of the disorder. Despite these findings, the respective affinity of CB for specific GABAAR subunits remains unknown24,28. Further, the precise molecular and cellular mechanisms mediating the severe symptoms seen following mutations in ARHGEF9 remain unknown.
Using isothermal titration calorimetry and complementary methods, we identify an exclusive binding of CB to the GABAAR-α2-subunit. Notably, this is the first report of a high-affinity protein interaction for both CB and α2. To reveal the biological relevance, we create a novel knock-in mouse where amino acids 358–375 from the α1 loop are substituted into α2 (Gabra2-1), a substitution that reduces CB binding. This mutation alters the expression of α2 and CB, inhibitory current amplitude and decay time, and results in the loss of AIS synapses. Gabra2-1 mice display a remarkable phenotype characterized by spontaneous seizures and early mortality, reminiscent of the human syndrome linked to ARHGEF9 mutation. Surviving Gabra2-1 mice do not show behavioral seizures, but are more susceptible to seizure induction by kainate. Gabra2-1 mice also display anxiety-like behavior, and an enhancement of power in the δ-band using electroencephalogram (EEG) recording, both of which are ameliorated by treatment with the α2/α3-selective GABAAR positive modulator AZD7325.
Results
Collybistin binds the GABAAR-α2-subunit with high affinity
Quantitative binding studies have shown that gephyrin displays a subunit binding profile that dictates receptor recruitment9,16. Prompted by Y2H studies reporting GABAAR-CB interactions19, we speculated that CB might display subunit specificity. We used purified proteins, including the GABAAR-α1, -α2, and -α3 loops, the SH3 domain of CB (CB-SH3), and the E domain of gephyrin (GephE; Fig. 1a). We applied mixtures of purified CB-SH3 and the intracellular loops of GABAAR-α1–3 to native agarose gel electrophoresis (NAGE) for identification of low affinity interactions by altered migration of the native proteins upon complex formation. While the SH3-GABAAR-α1L and -α2L complexes are retained in the pocket, the GABAAR-α3-subunit allows SH3 to enter the gel (Fig. 1b), suggesting that -α1L and -α2L (but not -α3L) are capable of forming a complex with CB-SH3.
Fig. 1.
Analyzing the interaction of GABAAR α subunits with collybistin. a Cartoon showing the purified proteins used for biochemical studies of α-subunit interaction with gephyrin and CB. b Receptor subunits were subject to NAGE (pockets mark sites of protein application) followed by coomassie staining, revealing that α-subunit loops slowly migrate to the cathode. CB-SH3 alone or mixed with a threefold excess of GABAAR intracellular domains reveals that α2L markedly alters the migration of SH3 (red arrow). c SEC analysis was used to explore the behavior of CB-SH3 alone or when mixed with equimolar amounts of α1L, α2L, or α3L. Fusion proteins were detected in eluates using absorbance at 280 nM and coomassie staining after SDS-PAGE (insets), verifying the presence of the SH3 domain at lower elution volumes in the fraction containing α2L. d CB-SH3 was titrated against α1L and α2L. The measured binding enthalpies are plotted as a function of the molar ratio of the SH3 domain to the GABAAR α1 and α2 loops. e GephE and GephE + CB-SH3 were subject to NAGE. The migration of the respective proteins is indicated. In presence of the SH3 domain the migration of gephyrin is not altered indicating that the proteins do not interact on NAGE (left panel). GephE in the presence of a fivefold molar excess of α2L is partly retained in the pocket and partly migrates towards the anode (lane 1 right panel). Addition of increasing amounts of the SH3 domain to the GephE-α2L complex allows more gephyrin to enter the gel and migrate towards the anode. At the same time SH3 binding to α2L retains SH3 in the pocket. This indicates that SH3 and gephyrin compete for α2 binding. f CB-SH3 was titrated against α2L alone or in combination with GephE, and the measured binding enthalpies are plotted
To estimate the stability of the GABAAR-SH3 complexes we further characterized them by analytical size exclusion chromatography (SEC). Either single proteins (Fig. 1c top) or equimolar mixtures of the SH3 domain combined with either -α1L, -α2L, or -α3L were applied to a column and the resulting fractions were analyzed by SDS-PAGE. While the NAGE-assay revealed an SH3-α1L complex, SEC analysis demonstrated its low stability, as the SH3 domain elution profile was unaltered by -α1L (Fig. 1c). In line with the NAGE experiments, -α3L did not alter SH3 elution (Fig. 1c). SEC demonstrated a tight SH3-α2L complex, resulting in a significant 10 ml shift of the SH3 domain toward higher molecular weights and correspondingly lower elution volumes (Fig. 1c). Taken together, the SEC and NAGE results define a weak SH3-α1L complex and a tight SH3-α2L complex.
Encouraged by the NAGE and SEC, we next quantified the interactions using isothermal titration calorimetry (ITC; Fig. 1d). ITC revealed that the -α2L forms a tight complex (Kd = 1 μM), while -α1L forms a rather weak complex (Kd ~500 μM; Table 1). To narrow down the GABAAR-α2 binding site, we determined the SH3 affinity of different truncated constructs. The first half of the receptor large intracellular loop displayed an affinity comparable to the full-length receptor loop (Supplementary Table 1). Further N-terminal truncations of this construct significantly decreased the SH3 affinity (Supplementary Table 2), suggesting an important role of secondary structure and/or cooperative effects for this interaction.
Table 1.
Kd values for the α1–3 subunits and glycine receptors with gephyrin (GephE) or CB (CB-SH3) are compared
| Receptor | Binding motif | GephE Kd | CB-SH3 Kd |
|---|---|---|---|
| GABAAR-α1 | LIKKNNTYAPTATSYT | 17.0 ± 11.0 | 500 ± 400 |
| GABAAR-α2 | VMIQNNAYAVAVANYA | >500 | 1.3 ± 0.8 |
| GABAAR-α3 | AKKTSTTFNIVGTTYP | 5.0 ± 2.0 | >500 |
| GlyR | NDLRSNDFSIVGSLPR | 0.1/8.0 | >500 |
Values listed are mean ± standard error
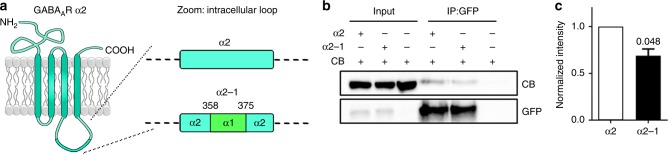
Based on the proposal of a tripartite complex19, and the overlapping binding site of GephE and CB-SH3 on -α2L, we probed these interactions for possible synergistic or competitive binding. This set of experiments revealed that GephE and CB-SH3 do not interact but instead compete for -α2L (Fig. 1e). After verification of an overlapping -α2L binding site for GephE and CB-SH3, we explored whether GephE can modulate the binding of SH3-α2L. Therefore, we titrated -α2L in the presence or absence of GephE with the SH3 domain (Fig. 1f). While the heat signatures were altered by differing concentrations of the -α2L, curve fitting revealed that the interaction strength remained unaltered in the presence of gephyrin (Fig. 1f). To compare the interaction of the full-length proteins, we performed GFP bead trap assays with pHlorin-tagged-α2, or a mutant form of pHlorin-tagged-α2, wherein amino acids 358–375 within the intracellular loop were replaced with those from GABAAR-α1 (α2–1; Fig. 2), which were expressed along with CB in human embryonic kidney (HEK) cells. We found that pHlorin-α2 could coimmunoprecipitate CB, while pHlorin-α2–1 coimmunoprecipitated significantly lower amounts of CB (Fig. 2b, c). These results validate that amino acids 358–375 within the intracellular loop of GABAAR-α2 are also important for interaction with CB in the context of the full-length proteins. Taken together, we establish a complementary subunit preference of CB compared to gephyrin, and competition between both proteins for α2-subunit binding.
Fig. 2.
Substitution of 13 amino acids from the GABAAR-α1 subunit loop into α2 reduces collybistin binding. a Cartoon of the GABAAR α2 subunit, showing the N- and C-termini and the large intracellular loop, expanded to show the site of the α2–1 mutation at amino acids 358–375. b Purified GFP-tagged α2 or α2–1 was combined with purified CB and subjected to coimmunoprecipitation. c Quantification of normalized intensities comparing GFP-tagged α2 or α2–1 (0.6895 ± 0.08221) interacting with CB. All plots shown and all values listed are mean ± standard error, t test
A mouse model with reduced collybistin-GABAAR-α2 binding
After having identified a tight complex between CB and the GABAAR-α2-subunit, we next wanted to investigate the functional and physiological relevance of this interaction, due to the implications of ARHGEF9 mutations in human disease24,26,27. To do so, we generated a knock-in mouse where amino acids 358–375 within the large intracellular loop of GABAAR-α2 were replaced by those of GABAAR-α1 (Gabra2–1; Fig. 3a).
Fig. 3.
Generation and basic characterization of α2–1 knock-in (Gabra2–1) mice. a Cartoon showing the targeting vector used to insert residues 358–375 from the α1 subunit into exon 10 of the α2 subunit, and the resulting allele. b Sequencing of PCR amplified genomic DNA from wildtype and Gabra2–1 homozygous mice, and predicted amino acid sequence. c Hippocampal extracts from wildtype and Gabra2-1 heterozygous and homozygous mice were immunoblotted with antibodies directed at the large intracellular loop (loop) and the c-terminus (c-term) of the GABAAR α2 subunit. d Immunoblotting with antibodies for gephyrin (Geph) and collybistin (CB) in wildtype and Gabra2-1 mice. See Supplementary Fig. 2 for uncropped blots and quantification of immunoblotting. e The 40 μm saggital sections immunostained with α2 c-term antibody and HRP-conjugated secondary antibody, and visualized using 3, 3′-diaminobenzidine staining. Scale bar = 1500 µm. See Supplementary Fig. 3 for quantification of α2 immunohistochemistry
To confirm the Gabra2–1 mutation we amplified this portion of genomic DNA from wildtype and homozygous Gabra2–1 mice and sequenced the polymerase chain reaction (PCR) product (Fig. 3b; Supplementary Fig. 1B, C). Sequencing results showed the site of mutation, and the predicted amino acid sequence alignments revealed insertion of the gephyrin-binding motif (GABAAR-α1 loop) into α210 (Fig. 3b), comparable to the alignment of α1 and α2 (Supplementary Fig. 1D). Further validation was obtained using an antibody that recognizes the α2-subunit loop (Fig. 3c, Supplementary Fig. 2), which showed a reduction in antibody recognition by a factor of ~2 in hippocampal lysates from heterozygous mice, and a complete loss in homozygotes. Using an antibody that recognizes the α2-subunit c-terminus we could demonstrate that α2 expression is maintained in Gabra2–1 mice (Fig. 3c). We also examined the expression of gephyrin and collybistin in hippocampal lysates and found that although total gephyrin was unchanged, collybistin decreased in expression in Gabra2–1 mice relative to control (Fig. 3d). To examine the regional expression of α2, we conducted DAB immunostaining using a GABAAR-α2-subunit c-terminal antibody comparing Gabra2–1 mice to wildtype controls (Fig. 3e, Supplementary Fig. 3A). GABAAR-α2-subunit c-terminal immunoreactivity was generally increased in Gabra2–1, with cortex, basal forebrain, and hippocampus showing increases (Supplementary Fig. 3B, C). Within the hippocampus, GABAAR-α2-subunit immunoreactivity was increased along the cell body layer of CA1, and in the molecular layer (Supplementary Fig. 3C). These studies confirmed effective Gabra2–1 loop mutation, and demonstrated that the mutated α2-subunit is stably expressed.
Mislocalization of GABAAR-α2 and collybistin in Gabra2-1
To investigate clustering of inhibitory synaptic proteins we conducted immunofluorescent staining for GABAAR-α2, -α1, CB, and the vesicular GABA transporter (VGAT; Fig. 4). Consistent with our immunoblotting and DAB immunohistochemistry (IHC) findings, we saw an increase in the total number of α2 clusters (Fig. 4a, c), although these clusters were reduced in size (Fig. 4b). Examination of α1-subunit immunofluorescence revealed no significant change in either the size or density of positive clusters (Fig. 4d, e; Supplementary Fig. 4A, B). The relationship between cluster size and intensity revealed a shift toward an increased number of smaller clusters of α2 in Gabra2–1, whereas the α1 relationship appears unaltered (Fig. 4e). Examination of CB immunostaining revealed a dramatic reduction in CB positive cluster size and number (Fig. 4f, h, i), while immunostaining for VGAT revealed no significant change in Gabra2–1 (Fig. 4g, j; Supplementary Fig. 4C). As a secondary measure of inhibitory presynaptic terminals we also stained for glutamate decarboxylase (GAD-65), which was also unaffected by the Gabra2–1 mutation (Supplementary Fig. 4F). To further evaluate inhibitory synapses we also assessed the colocalization of gephyrin and α2, as well as VGAT and α2. No change was observed in either the percentage of gephyrin colocalized with α2, or VGAT colocalized with α2 (Supplementary Figs. 5 and 6). A decrease in the percentage of α2 clusters colocalized with both gephyrin (Supplementary Fig. 5), and VGAT (Supplementary Fig. 6) declined upon Gabra2-1 mutation, which may reflect the increased expression and more diffuse localization of α2. These changes, however, do not reflect a loss of total numbers of inhibitory synapses, evidenced by unchanged VGAT and GAD-65 immunoreactivity, and maintenance of VGAT, and gephyrin, colocalization with α2. Taken together, the replacement of the CB binding site in α2 resulted in loss of CB and altered GABAAR-α2-subunit cluster distribution.
Fig. 4.

Expression of GABAAR α1 and α2 subunits, and related inhibitory synaptic proteins in Gabra2–1 mice. a Representative images of GABAAR α2-subunit staining in the CA1 region of the hippocampus. Quantification of the size: (b WT—0.365 ± 0.004; Het—0.279 ± 0.003; Homo—0.271 ± 0.003), and density (c WT—192.80 ± 7.851; Het—202.70 ± 17.490; Homo—347.20 ± 5.276) of α2 positive clusters in CA1. d Quantification of GABAAR α1 subunit cluster density (WT—145.00 ± 17.50; Het—184.10 ± 6.31; Homo—165.00 ± 28.33) in CA1. e Cluster size/intensity correlation plots for α2 and α1 staining, showing the shift toward smaller, less intense clusters of α2 in Gabra2–1 homozygous mice. f Representative images of CB immunostaining in CA1. g Representative images of VGAT immunostaining in CA1. Quantification of the size (h WT—0.344 ± 0.024; Het—0.232 ± 0.035; Homo—0.188 ± 0.020) and density i WT—3056.06 ± 338.30; Het—1913.26 ± 275.02; Homo—1087.94 ± 165.43) of CB positive clusters in CA1. j Quantification of VGAT positive cluster density (WT—3447.82 ± 250.00; Het—3165.59 ± 390.82; Homo—3346.53 ± 289.30) in CA1. Scale bar = 10 µm, applies to all images. All plots shown and all values listed are mean ± standard error, p values from KS test (cluster size), or ANOVA (cluster density)
Smaller sIPSC amplitude and faster decay time in Gabra2-1
Our immunoblotting and IHC experiments demonstrate modifications in the expression and clustering of α2. Based on these findings we assessed the impact of the Gabra2–1 mutation on inhibitory synaptic signaling. To address this, we recorded spontaneous inhibitory synaptic currents (sIPSCs) in CA1 principal neurons of hippocampal slices at postnatal day (PND) 2129,30 (Fig. 5). Quantification of sIPSC amplitudes revealed a significant decrease in Gabra2-1 heterozygous and homozygous slices compared to littermate controls (Fig. 5b, c). Analysis revealed a decrease of sIPSC decay times in CA1 neurons compared to littermate controls (Fig. 5d, e). Previous studies have indicated that the α-subunit in part determines the decay of GABAAR-mediated currents, thus this result may suggest a replacement of α2- with α1-subunits in CA18,31. Assessments of sIPSC frequency did not reveal a significant difference between Gabra2–1 slices and littermate controls (Supplementary Fig. 7). These results reveal that despite no loss of inhibitory synaptic contacts, Gabra2–1 mice have compromised inhibitory synaptic transmission that may be related to a decrease in α2 cluster size.
Fig. 5.
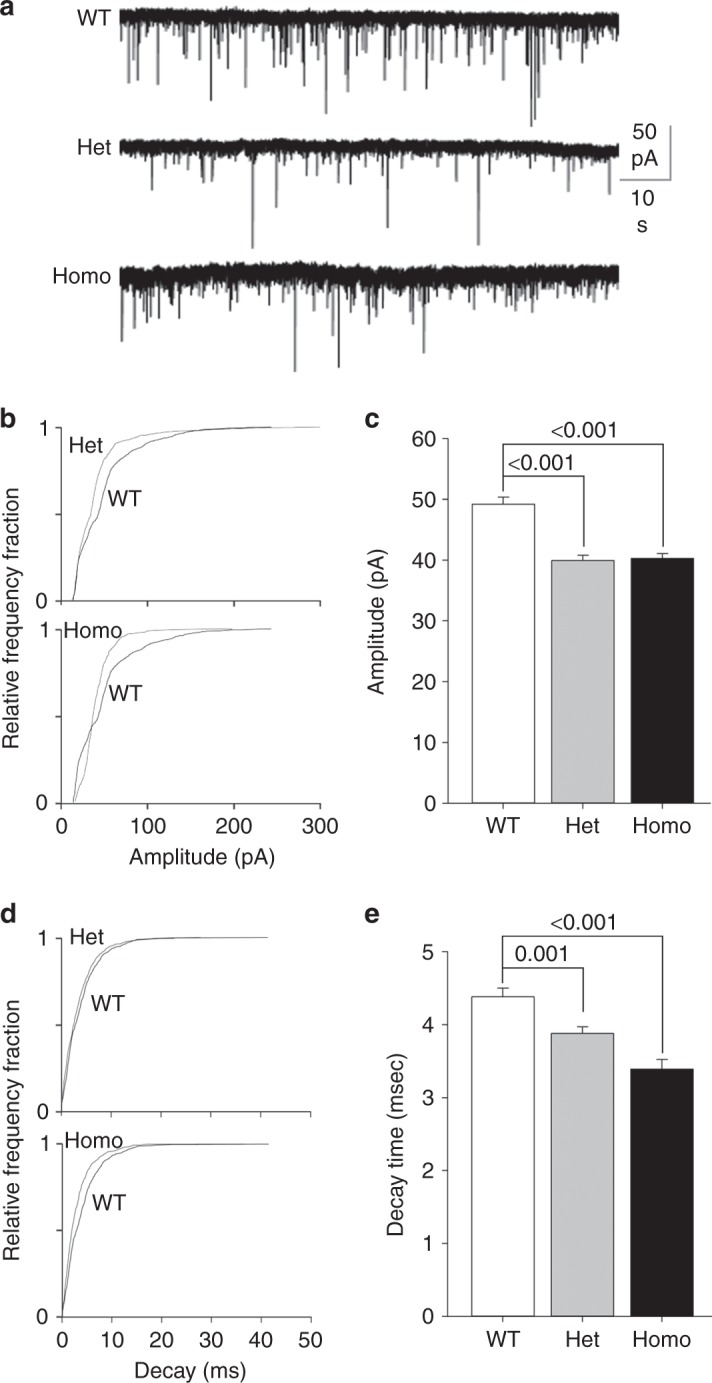
Gabra2–1 mice display alterations in hippocampal phasic current. a Representative recordings made from CA1 in hippocampal slices from p21 Gabra2–1 heterozygous and homozygous mice compared to wildtype littermate control mice. Gabra2–1 recordings displayed smaller sIPSC amplitudes and decreased decay times compared to wildtype littermate or age-matched controls as seen in the representative traces. Analysis of traces revealed a shift in the cumulative probability plots and bar graphs for sIPSC amplitude (b, c WT—49.2 ± 1.16; Het—39.9 ± 0.91; Homo—40.3 ± 0.74) and decay (d, e. WT—4.38 ± 0.12; Het—3.88 ± 0.09; Homo—3.39 ± 0.13). The frequency was comparable between the genotypes, for quantification see Supplementary Fig. 7. All plots shown and all values listed are mean ± standard error, p values from t test
Loss of GABAAR-α2 clusters at specific types of synapses
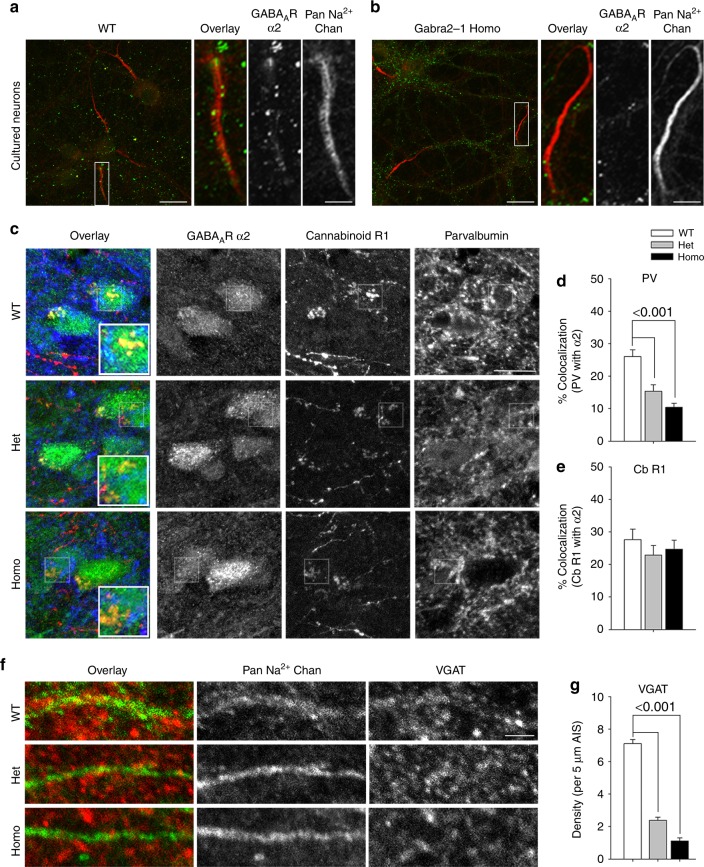
GABAARs containing α2 have been identified at a variety of different types of synaptic contacts, but are strongly enriched at synaptic contacts onto the AIS2–4, hence we examined the subcellular localization of α2. We began by culturing cortical cells to more readily identify the AIS (pan-Na+ channel antibody), and colabeled for the GABAAR-α2 subunit, which revealed a dramatic loss of α2-subunit clusters on the AIS in Gabra2–1 derived cultures (Fig. 6a, b). We followed with colocalization of α2 with PV (marker for interneurons including axo-axonic chandelier cells and terminals), and CB1R (marker for CCK positive basket cell terminals) in cortical sections. Overlay images reveal segregated populations of GABAAR-α2-subunit clusters (Fig. 6c) where α2 can be seen colocalized with either PV (teal–green + blue), or CB1R (yellow–green + red) in zoomed panels. Despite a total increase in GABAAR-α2 cluster density in Gabra2-1 mice, there was a significant decrease in the percent of α2 colocalized with PV (Fig. 6c, d). Percent colocalization with CB1R positive clusters was not altered (Fig. 6c, e). These findings suggest a specific loss of α2 positive clusters opposing contacts from PV positive cells, including chandelier cells. To follow up on this, we examined the number of inhibitory presynaptic contacts stained with VGAT, onto the AIS of cortical pyramidal cells (Fig. 6f). We found that both heterozygous and homozygous cells showed a significant reduction in the number of VGAT positive clusters per 5 µm AIS (Fig. 6f, g). The number of VGAT positive clusters per 100 µm2 of soma was unaffected (Supplementary Fig. 4D, E). These data indicate a selective loss of α2 opposed to PV positive contacts, paralleled by a reduction of VGAT positive clusters along the AIS.
Fig. 6.
Examination of the subcellular localization of GABAAR α2-subunit clusters in Gabra2-1 mice. Cultured cortical neurons from wildtype (a) and Gabra2-1 homozygous (b) pups stained with antibodies directed against GABAAR α2 (green) and sodium channels (red), showing zooms of representative AIS segments. c Colocalization of GABAAR α2 (green) with CB1R (red) and parvalbumin (blue) clusters in the CA1 of wildtype, heterozygous and homozygous Gabra2–1 mice. Quantification of the colocalization of GABAAR α2 with parvalbumin (d WT—26.05 ± 2.12; Het—15.34 ± 2.06; Homo—10.41 ± 1.24) and CB1R (e WT—27.64 ± 3.30; Het—22.90 ± 2.99; Homo—24.73 ± 2.74). f Staining for VGAT (red) positive clusters to mark inhibitory presynaptic compartments, along with a Pan-sodium channel antibody (green) to label the axon initial segment. g Quantification of the number of VGAT positive clusters per 5 µm of AIS (WT—7.11 ± 0.26; Het—2.39 ± 0.19; Homo—1.11 ± 0.18). Scale bar = 10 µm (A low mag, C), scale bar = 5 µm (A zoom, F). All plots shown and all values listed are mean ± standard error, p values from ANOVA
Developmental seizure susceptibility and mortality in Gabra2–1
Gabra2–1 mice are viable and fertile, but a subset of heterozygous and homozygous Gabra2–1 pups die prior to weaning, with the peak of mortality falling on PND 20 (Fig. 7a). At PND 5, wildtype × heterozygous matings produce normal Mendelian ratios (1:1 wt:het), whereas pups genotyped following weaning are approximately 63% wildtype and 37% heterozygous, further demonstrating mortality in Gabra2–1 prior to weaning, but after PND 5. We genotyped the offspring found dead from heterozygous × heterozygous mating pairs and found that when corrected for predicted offspring ratio (2:1 het:homo), heterozygous and homozygous offspring have similar likelihood of mortality, but homozygous mice appear to die earlier in development (mode: het = PND 20, homo = PND 17; Fig. 7b). A representative survival plot from a heterozygous × homozygous mating, again reflects the peak of mortality around PND 20 (Fig. 7c). We also compared the percentage of litters that had one or more dead pups, and the percentage of pups weaned comparing all mating schemes (shown on the x-axis; Fig. 7d, e). Dead pups were rarely found in wildtype × wildtype mating pairs and approximately 98% of wildtype pups born survive through weaning. In contrast, Gabra2–1 mating schemes had at least 69% of litters with one or more pups found dead after PND 5, and only about 36% of pups born survive through weaning in Gabra2–1 homozygous mating pairs.
Fig. 7.
Early postnatal mortality and spontaneous seizures in Gabra2–1 heterozygous and homozygous mice. a Plot showing the proportion of mice found dead (out of the total found dead) across a 120 day lifespan. b Cumulative proportion of heterozygous and homozygous mice found dead in the early postnatal period, corrected for predicted Mendelian ratio of 2:1 het:homo. c Survival plot of litters from a typical heterozygous × homozygous mating pair. Quantification of the percentage of litters from different mating schemes that had one or more dead pups (d WT × WT—3.20 ± 3.20; WT × Het—69.05 ± 8.29; Het × Het 77.38 ± 4.49; Het × Homo 90.91 ± 9.09; Homo × Homo—100.00 ± 0.00) and the percentage of pups that were weaned (e WT × WT—97.78 ± 2.22; WT × Het—60.63 ± 4.98; Het × Het 63.57 ± 4.42; Het × Homo 46.41 ± 14.13; Homo × Homo—36.50 ± 2.21) according to mating scheme. f. Quantification of spontaneous seizure severity according to the Racine scale in pups observed seizing. g. Quantification of kainate seizure severity according to the Racine scale in WT (LS Mean 0.74), heterozygous (LS Mean 3.40), and homozygous (LS Mean 4.19) littermates (SE of LS Mean = 0.401; Het vs Homo p = 0.197). h Representative traces of EEG activity in wildtype, and Gabra2-1 heterozygous and homozygous mice following kainate injection. Quantification of the latency to SE (i WT—58.16 ± 3.31; Het—33.39 ± 5.52; Homo—31.09 ± 4.43; Het vs. Homo p = 0.732), latency to first seizure event (j WT—9.44 ± 0.973; Het—9.95 ± 2.523; Homo—12.39 ± 2.836), and the average event duration (k WT—143.08 ± 13.05; Het—390.71 ± 88.21; Homo—501.92 ± 57.63; Het vs. Homo p = 0.214) in wildtype, heterozygous, and homozygous Gabra2–1 mice treated with kainate. All plots shown and all values listed are mean ± standard error, p values from ANOVA, or repeated measures ANOVA (Kainate Racine over time)
We evaluated failure to thrive by assessing size, weight, and gross physical development of Gabra2–1 pups, finding no difference from wildtype (Supplementary Fig. 8A, B, Supplementary Table 4). Extensive monitoring did reveal the occurrence of rare spontaneous seizures in Gabra2–1 offspring, with clear behavioral characteristics that we classified according to the Racine scale (Fig. 7f; Supplementary Movie 1). To confirm this apparent predisposition to seizure activity, we also assessed the susceptibility of adult Gabra2–1 mice to kainate (20 mg kg−1 i.p.) induced seizures (Fig. 7g). Using the Racine scale we found that Gabra2–1 mice consistently showed more severe seizures and increased mortality resulting from kainate injection. EEG recordings also revealed increased sensitivity to kainate, with all Gabra2–1 mice developing status epilepticus (SE—less than a 2-min interval between events), while only half of wildtype mice developed SE. The latency to the development of SE was reduced in Gabra2–1 mice (Fig. 7h, i), while latency to the first seizure event did not differ (Fig. 7h, j). Gabra2–1 mice also have seizure events of increased duration (Fig. 7h, k). Taken together, we identified a susceptibility of Gabra2–1 mice to spontaneous and chemically induced seizures that can result in early mortality.
Gabra2–1 anxiety and EEG corrected by α2-selective compound
To explore the phenotype caused by Gabra2–1 mutation, we examined surviving adult Gabra2–1 mice using behavior tests and EEG recordings. To rule out the possibility of gross behavioral impairment we conducted a modified SHIRPA screen32 (Supplementary Table 4; Supplementary Fig. 8B). Gabra2–1 mice were shown to have normal physical health, weight, body condition, as well as sensory and motor function (Supplementary Table 4; Supplementary Fig. 8). Assessment of the startle response to evaluate hyperekplexia, which is linked to glycine receptor function33 and human mutations in ARHGEF924, revealed no significant difference between Gabra2–1 and littermate controls (Supplementary Fig. 8E, F). We also evaluated the possibility of a deficit in prepulse inhibition due to reports of mislocalization of the GABAAR-α2-subunit on the AIS in post mortem tissue from schizophrenic patients34, but no difference was detected between Gabra2–1 mice and littermate controls (Supplementary Fig. 8G,H). Next, we assessed anxiety behavior, due to the role of the GABAAR-α2-subunit in mediating the anxiolytic effects of benzodiazepines35. Using both the light–dark boxes and elevated plus maze, we detected increased anxiety behavior in Gabra2–1 mice shown by increases in total time spent in the dark chamber, and percent time spent in the closed arms respectively (Fig. 8a, b). Due to the low numbers of viable homozygotes we examined the responsiveness of Gabra2–1 heterozygotes to the nonselective benzodiazepine, diazepam (2 mg kg−1), and the α2/α3-selective GABAAR positive modulator AZD7325 (3 mg kg−1). As expected, wildtype mice showed a reduction in anxiety behavior following treatment with either diazepam or AZD7325 as evidenced by an increased percent time spent in the open arms (Fig. 8c), and increased open arm entries (Supplementary Fig. 9A). In contrast, Gabra2–1 mice did not show a reduction in anxiety behavior following diazepam treatment, but were responsive to treatment with AZD7325 (Fig. 8c, d; Supplementary Fig. 9A). Analysis of total distance traveled did not reveal any differences in exploration of the maze between genotypes, or treatment compared to vehicle control (Supplementary Fig. 9B). It is possible that Gabra2–1 mice require an increased dose of diazepam to exert anxiolytic effects; however, higher doses of diazepam cause sedation. As such, we also examined the responsiveness of Gabra2–1 mice to sedative doses of diazepam (10 mg kg−1) to rule out any difference in drug metabolism. We found no difference in the sedative effects of diazepam comparing wildtype and Gabra2–1 mice (Supplementary Fig. 9C, D). These results suggest that Gabra2–1 mice display an anxiety phenotype possibly related to impaired clustering and function of α2-containing GABAARs.
Fig. 8.
Anxiety and EEG in Gabra2–1 mice are corrected by treatment with an α2-selective compound. a Quantification of the percent time spent in the light chamber of the light-dark box, comparing wildtype (104.52 ± 11.11), heterozygous (71.77 ± 6.89) and homozygous (73.50 ± 10.34) Gabra2-1 mice. b Time spent in the arms expressed as percent of total in the elevated plus maze comparing wildtype (open—25.22 ± 4.87; closed—74.78 ± 4.87), heterozygous (open—10.95 ± 1.90; closed—89.05 ± 1.89) and homozygous (open—8.23 ± 2.95; closed—91.78 ± 2.95) Gabra2–1 mice. c Assessment of the percent time spent in the open arms of the elevated plus maze comparing wildtype (LS Mean 31.58) and Gabra2–1 heterozygous (LS mean 20.10; SE of LS mean for genotype—1.51) mice treated with vehicle (beta cyclodextran; LS mean 15.29), 2 mg kg−1 diazepam (LS mean 22.67), or 3 mg kg−1 AZD7325 (LS mean 39.55; SE of LS Mean for treatment—1.85). d Analysis of percent closed arm time in Gabra2–1 heterozygous mice. e Spectrograms of representative EEG recordings from Gabra2–1 heterozygotes and wildtype controls during baseline and after treatment with AZD7325. f FFT of EEG recordings comparing wildtype (LS mean 82.02 ± 8.23) and Gabra2–1 heterozygous (112.60 ± 7.13) mice. g Comparison of average δ power between wildtype and Gabra2–1 mice, and after treatment with AZD7325. All plots shown and all values listed are mean ± standard error, p values from ANOVA, or repeated measures ANOVA (EEG FFT)
We next assessed adult Gabra2–1 mice for baseline abnormalities in EEG recordings. Gabra2–1 mice and controls were recorded during 1 h of wakefulness at least one week following implantation. Representative spectrograms and fast-Fourier analysis revealed increased power of specific frequency bands when Gabra2–1 mice and littermate controls were compared (Fig. 8e, f). To examine this more closely, we parsed the EEG into frequency bands as follows: δ (0.25-4 Hz), θ (4–12 Hz), α (12–18 Hz), β (18–30 Hz), and γ (>30 Hz), revealing an elevation particularly in δ and θ ranges in Gabra2–1 mice (Fig. 8g; Supplementary Fig. 10). To follow up on the findings with anxiety behavior, we investigated the beneficial effects of AZD7325 (3 mg kg−1) in correcting the EEG abnormalities in Gabra2–1 (Fig. 8e, g). We found that AZD7325 was successful in reducing EEG δ and θ power in Gabra2–1 mice (Fig. 8e, g; Supplementary Fig. 10A–E). We also found that AZD7325 resulted in increases in the power of high frequency bands in both wildtype and Gabra2–1 heterozygotes (Supplementary Fig. 10F, G) similar to previous findings36. Diazepam is also known to suppress δ-power, so we conducted assessment of the response to diazepam following 1 h of baseline EEG, comparing wildtype to Gabra2–1. Diazepam suppressed δ in wildtype mice, but not in Gabra2–1 mice (Supplementary Fig. 10H), which is in keeping with the lack of behavioral response to diazepam (Fig. 8c, d). Collectively, the EEG analysis revealed abnormally elevated δ power in Gabra2–1 mice compared to wildtype controls, which could be alleviated by treatment with AZD7325 but not diazepam, mirroring the behavioral results.
Discussion
We show that the CB-SH3 domain specifically binds the GABAAR-α2-subunit loop with low micromolar affinity, which is the strongest interaction identified for both proteins to date. Despite its high affinity, the interacting α2-subunit fragment is devoid of canonical PxxP motifs, indicating an atypical SH3 interaction. Earlier Y2H studies demonstrated that full-length CB and gephyrin together exhibit a potentiated GABAAR-α2 affinity19. Our results exclude a possible gephyrin-SH3 domain interaction, as well as synergistic binding to the GABAAR-α2-subunit in the presence of gephyrin. Instead we propose that Y2H-demonstration of gephyrin’s ability to amplify CB’s binding uncovered gephyrin-mediated un-masking of CB-SH337. Isolated CB-SH3 therefore might exhibit the maximal affinity displayed by CB after full activation by gephyrin37. Comparison with gephyrin reveals a striking reciprocal relationship in CB-affinities with different α-subunits. A possible gephyrin–CB complex would, therefore, have the potential to interact with all three synaptic α-subunits, thus explaining reports of both CB and gephyrin colocalizing with GABAARs containing α1–3-subunits. Therefore, our results indicate that the molecular basis of GABAAR and, possibly, also GlyR accumulation at synaptic sites lies in the so far unknown architecture and modulation of the CB–gephyrin complex, as well as in its exact interplay with other binding partners (PI3P, neuroligin-2, and CDC 42).
Both GABAAR-subunits38–40 and CB24–27 have been implicated in human disease. We have previously shown that GABAAR signaling can be modulated by interfering with the underlying protein–protein interactions41,42. Here, we used a novel mouse expressing a chimeric α2–α1 loop to investigate the disease relevance of the CB-GABAAR-α2 interaction. The Gabra2–1 mutation reduces GABAAR-α2 interaction with CB in vitro, and results in an increase in α2, but a decrease in CB expression in vivo. The increase in α2 density may reflect an increased stability due to interaction with gephyrin or other partners in place of CB, or may reflect poor clustering of α2-containing receptors, which is suggested by decreased cluster size and reduced mIPSC amplitude. Although the Gabra2–1 mutation appears to reduce clustering of α2-containing receptors, we do not see a detectable reduction in the size or density of VGAT (or GAD-65) positive clusters, or when measured specifically on the soma, or in the proportion of VGAT clusters that have α2, suggesting no widespread loss of inhibitory synaptic contacts. These results suggest that the Gabra2–1 mutation is impairing the interaction of α2 with CB, leading to reduced clustering of α2-containing receptors and increased turnover of CB. CB-KO mice show a loss of α2-containing GABAARs in CA1 pyramidal cells, accompanied by a loss of gephyrin and α1-containing GABAARs23. In contrast, PV interneurons retain gephyrin and α1 expression upon CB KO23. This may suggest that CA1 pyramidal cells express heteromeric α1–α2 receptors, which rely upon CB–α2 for expression and retention at synapses, while other cells expressing GABAARs with two α1-subunits are not impacted by the loss of CB–α2 interaction43. We also found that the Gabra2–1 mutation has a significant effect on inhibitory synaptic currents recorded from CA1, reducing their amplitude and accelerating decay time. A decrease in decay time in Gabra2–1 slices likely reflects the loss of effective activation of α2-containing GABAARs, which have been shown to display a 2–6 times slower deactivation8,31.
As we could not detect a reduction in total GABAergic synapses (VGAT, GAD-65 clusters; colocalization of VGAT-α2) we next evaluated the effect of the Gabra2–1 mutation on specific subsets of synapses. We observed fewer α2 positive clusters along the AIS in Gabra2–1 cells compared to wildtype cells. We compared the colocalization of α2 with CB1R and PV in tissue sections, to differentiate between axo-somatic contacts and AIS contacts from chandelier cells. We found that the Gabra2–1 mutation caused a substantial loss of α2 at PV + sites, and no significant difference in α2 at CB1R + sites, suggesting an impact on AIS synapses. Gabra2–1 mice indeed displayed a significant reduction in the number of VGAT clusters opposed to the AIS, suggesting that other subunits do not compensate for the loss of α2. A recent study has questioned the selective segregation of α2, suggesting that α1-subunits are also enriched on the AIS44. The approach relied upon different polyclonal antibodies that have distinct affinities, hence an absolute quantification of subunit levels is not possible. Moreover, data interpretation is complicated by estimates that in excess of 20% of GABAARs containing α2-subunits also contain α145,46. Another recent study identified some region-specific expression of α1 and α3 on the AIS, by comparing AIS expression to non-AIS expression to overcome antibody affinity differences47. While α1 and α3 are detected on the AIS, α2 shows the greatest enrichment, and further gephyrin is not preferentially enriched and may not contribute to AIS recruitment of GABAAR-α-subunits47. Our new findings suggest that α2 interaction with CB is the primary mediator of localization of GABAARs to AIS synapses, and that other subunits and gephyrin may be localized there in small amounts when complexed with α2 and CB. The specific impact of Gabra2–1 mutation on AIS inhibitory synapses may be related to interaction of the α2–CB complex with other proteins that have been implicated in the stabilization of AIS functional components such as ankyrinG48, neurofascin49, NrCAM50, and βIV-spectrin51.
Stemming from these alterations to GABAergic synapses and inhibitory synaptic function, Gabra2–1 mice display a remarkable phenotype characterized by spontaneous seizures, resulting in mortality in a subset of both heterozygous and homozygous mice. This is particularly striking since a relatively small number of GABAAR-subunit mutations result in spontaneous seizures38–40,52–54. A role for AIS abnormalities in epilepsy has been suggested in other studies55–57, implicating trafficking and localization of voltage-gated channels to the AIS. Other analyses have found alterations to AIS inhibitory synapses resulting from or contributing to epilepsy; however, both decreases and increases in AIS synapse number have been found, leaving the contribution of this synaptic subtype unclear58–60. Our results directly link a mutation in a specific GABAAR-subunit, accompanied by an alteration in AIS GABAergic synapses, to an epileptic phenotype. An additional feature of the Gabra2–1 model is that it reduces the possibility of gene compensation relative to a subunit knock-out. This is supported by the fact that spontaneous seizures and early mortality are not reported in the α261 or CB21 knock-out models where a compensatory feedback enhancing the expression of other proteins may occur.
Surviving Gabra2–1 mice show anxiety-like behavior and alterations in EEG, including an enhancement of δ-power. Gabra2–1 anxiety may be related to prior seizures, as it is well documented that anxiety is a common comorbidity in human epilepsy and in animal models62. The alterations in δ-power also corroborate the anxiety findings. δ-frequency oscillations have been most closely tied to salience detection63,64, and anxiety as a heightened salience state is correspondingly linked to elevations in the δ-range65. Further, nonsedating doses of diazepam and other benzodiazepines are known to decrease δ-power66,67. While AZD7325 reduced anxiety-like behavior in both Gabra2–1 mice and wildtype controls, we found that the classical benzodiazepine diazepam was not effective at reducing anxiety-like behavior in Gabra2–1 mice. Further, when we analyzed the impact of diazepam on the EEG, we also found that Gabra2–1 mice do not show the typical suppression of δ. Previous studies have suggested that binding of benzodiazepines to α2-containing receptors is required for the suppression of EEG δ-power68, while α1 and α3 binding has a limited role in δ-suppression69,70. Although it is not immediately apparent why diazepam is ineffective in Gabra2–1 mice, this difference may be related to the predominance of α1-/α3-mediated effects of diazepam when α2-containing receptors are compromised. In contrast, since AZD7325 acts preferentially on α2-containing receptors, it remains effective at suppressing δ and reducing anxiety-like behavior.
The Gabra2–1 mutation demonstrates a striking parallel with the effects of ARHGEF9 mutation in human syndromes, whose symptoms include epilepsy, anxiety, mental retardation, aggressive behavior, sleep–wake cycle disruptions, early mortality, and hyperekplexia24–27. It is likely that the hyperekplexia is linked to CB interaction with glycine receptors15, however, the remaining symptoms may be linked to dysfunctions at the AIS due to mislocalization of α2-containing GABAARs. Gabra2–1 mice do not display hyperekplexia, but have increased seizure susceptibility, early mortality, and anxiety, drawing several parallels. The responsiveness of the anxiety and EEG phenotype to the α2/α3-selective GABAA positive modulator AZD7325 suggests a possible novel therapeutic strategy to relieve symptoms in this set of human developmental syndromes linked to CB dysfunction.
Taken together, our results reveal a clear and selective interaction between the α2-subunit of GABAARs and CB, an interaction that has been long speculated about but has not previously been characterized. Furthermore, using the novel Gabra2–1 mouse model, we demonstrate that this interaction is critical for the targeting of α2-containing GABAARs, specifically to AIS sites needed for the stabilization of VGAT positive contacts onto the AIS. Gabra2–1 mice display spontaneous seizures during development, accompanied by early mortality in a subset of pups. Adult Gabra2–1 mice that survive have increased anxiety-like behavior and EEG abnormalities that are responsive to α2-selective benzodiazepines, revealing a novel therapeutic potential for these compounds.
Methods
Protein expression and purification
GephE (AA 318–736) as well as the large cytoplasmic loop of the GlyR β (AA 378–425), GABAAR α1 (AA334–420), α2 (AA 335–419) and α3 (AA 392–406) subunits and the corresponding truncation variants GABAAR α2 (AA 307–334), and (AA 307–350) were expressed and purified. The pGEX Vector SH3 construct provided by T. Soykan from the Brose Lab corresponds to residues 4–72 of murine CB1 and encompasses the complete SH3 domain (residues 8–67) of this protein which is identical to the SH3 domain of the human CB isoform, hPEM-2. The protein was expressed in Escherichia coli and purified via its N terminal GST-tag. After in-situ thrombin cleavage the GST–SH3 mixture was applied to a 26/60 Superdex 200 size exclusion column (Amersham Biosciences) equilibrated with 10 mM Tris-HCl pH 8.0, 250 mM NaCl, 1 mM β-mercaptoethanol. Two separate peaks could be resolved, one containing both the cleaved and uncleaved GST and GST–SH3, respectively, and the other containing pure SH3 domain.
Peptide synthesis
Peptides were purchased as lyophilized powder from Genscript (USA) at a minimal purity of 90%.
GFP bead trap and western blotting
HEK293T cells were transfected by electroporation with 2 µg DNA of each construct, and used for experiments 48 h later. HEK cells were transfected with full-length CB and either phluorin-tagged full-length α2 or phluorin-tagged full-length α2–1 constructs. Cultures were chilled on ice and lysed in 20 mM Tris-HCl (pH 8), 150 mM NaCl, 1% triton, 5 mM EDTA, 10 mM NaF, 2 mM Na3VO4, 10 mM Na4P2O7 with protease inhibitors. Lysates were cleared by centrifugation and incubated with GFP-trap overnight at 4 °C. After four washes in lysis buffer, bound proteins were detected by western blotting.
A table of antibodies, their sources and concentrations used can be found in Supplementary Table 3. For western blotting, HEK cells or brain tissue samples (n = 6 mice per genotype in >2 independent experiments) were rapidly harvested and homogenized in TEEN buffer (50 mM Tris-HCL, 1 mM EGTA, 150 mM NaCl) supplemented with a protease inhibitor mixture (Roche Applied Science). Following homogenization the protein concentration was assessed using a BCA assay (Pierce) and heated to 65 °C in SDS-PAGE sample buffer with 10% β-mercaptoethanol for 15 min. After SDS-PAGE, western blot signals were detected using ECL. For quantification, specific protein levels were determined by densitometry and were normalized to actin loading densitometry results. Results were quantified using Image J and were analyzed using t test (bead trap) or analysis of variance (ANOVA) (western blotting) to compare mean values, graph plotted as mean ± standard error.
Mouse generation and maintenance
Animals were cared for according to the NIH Guide for the Care and Use of Laboratory Animals71 and protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Tufts University School of Medicine or the IACUC of the University of Nevada Las Vegas. Germ line transmission of the transgenes was detected using PCR with primers spanning the intronic region which contained the remaining loxP site (Supplementary Fig. 1). Nontransgenic littermates were used as controls in all experiments (wildtype). Following genotyping, animals of each genotype were randomly assigned to treatment and/or assessment groups. Animals were generated by Genoway and maintained at the vivarium for Tufts University’s Boston campus, as well as at the University of Nevada Las Vegas vivarium. Mice were group housed with a 12 h light–dark cycle with constant temperature. After founders were received, mice were back-crossed onto C57 Bl6J for more than ten generations (congenic) prior to behavioral and EEG assessments. For tissue IHC, behavior, pharmacology, and EEG experiments, only male mice, aged 6–10 weeks, were used. All other experiments included both males and females (western blotting, culture immuno (cultures made a P0), electrophysiology (slices cut at P18-22), and assessments of mortality).
Genotyping Primers:
Gabra2-1F: AAGGGGAATTGTTTGGAGCAAGTGAC
Gabra2-1R: TCCACTCGCCTTATGGTGAACTTATAAGG
Sequencing Primers:
α2se1: GCGCCGGTATTGTTCTCTG
α2se2: TGACCCCTAATACAGGCTCCC
α2se3: ATGCAGCCTAAACCCACATTG
α2se4: GCTTCTTGTTCGGTTCTGGC
Immunocytochemistry and IHC
A table of antibodies, their sources and concentrations used can be found in supplementary Table 3. For immunocytochemistry (ICC) and IHC, four preservation protocols were used (n = 4 coverslips per genotype ICC; n = 6 mice per genotype per tissue preparation method IHC). (1) For ICC, at day in vitro 14 (DIV 14) coverslips of cultured cortical cells were fixed in −20 °C methanol for 10 min. (2) For nonfluorescent IHC of GABAA receptor α2-subunits, animals were transcardially perfused with a periodate lysine paraformaldehyde fixative solution. (3) For fluorescent IHC of GABAAR-α1- and -2-subunits, animals were transcardially perfused with oxygenated artificial cerebrospinal fluid followed by post fixation in 4% paraformaldehyde in PB. (4) For fluorescent IHC of CB, VGAT, colocalization of α2 subunit with parvalbumin and cannabinoid receptor 1, and VGAT/sodium channel experiments, brains were harvested freshly and flash frozen in optimal cutting temperature embedding medium in liquid nitrogen cooled isopentane. Following one of the above preparation methods, sections were cut on a cryostat at thicknesses of 10 μm (fresh frozen—mounted directly on slides, fixed in −20 °C methanol) or 30 μm (paraformaldehyde fixed—free floating) and were incubated in blocking solution (2.5% Bovine Serum Albumin, 5% Normal Goat Serum, 0.1% triton-x, and 0.02% sodium azide in PBS) for 45 min, followed by primary antibody incubations diluted in modified blocking solution (2% Normal Goat Serum) overnight at 4 °C, and biotinylated (Vector) or Alexa conjugated (ThermoFisher) secondary antibody incubations diluted (1:2000) in modified blocking solution for 1 h at room temperature. Immunostaining intensity, density and colocalization was quantified using ImageJ, and results were analyzed using ANOVA to compare mean values, graphs were plotted as mean ± standard error.
Electrophysiology
Brain slices were prepared from 3-week-old male Gabra2–1 mice (n = 6–13 cells/genotype, slices prepared from 5–6 mice and assessed independently, one mouse sliced per day) in ice-cold cutting solution (mM: 126 NaCl, 2.5 KCl, 0.5 CaCl2, 2 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, 1.5 sodium pyruvate, and 3 kynurenic acid), and measurements of sIPSCs in CA1 principal neurons were performed. Slices were then transferred into incubation chamber filled with prewarmed (31–32 °C) oxygenated artificial cerebrospinal fluid of the following composition (mM: 126 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, 1.5 sodium pyruvate, 1 glutamine, 3 kynurenic acid, and 0.005 GABA bubbled with 95% O2–5% CO2).
Whole-cell currents were recorded from the hippocampal CA1 region using patch pipettes (5–7 MΩ) pulled from borosilicate glass (World Precision Instruments) and filled with intracellular solution (mM: 140 CsCl, 1 MgCl2, 0.1 EGTA, 10 HEPES, 2 Mg-ATP, 4 NaCl and 0.3 Na-GTP; pH = 7.2 with CsOH). A 5 min period for stabilization after obtaining the whole-cell recording conformation (holding potential of −60 mV) was allowed before currents were recorded using an Axopatch 200B amplifier (Molecular Devices), low-pass filtered at 2 kHz, digitized at 20 kHz (Digidata 1440A; Molecular Devices), and stored for off-line analysis. Spontaneous inhibitory postsynaptic currents (sIPSCs) were analyzed using Synaptosoft minianalysis software, and were analyzed using t test to compare mean values, graphs were plotted as mean ± standard error.
Electroencephalography
EEG and electromyography electrodes were implanted under ketamine and xylazine anesthesia (n = 6 mice per genotype per experimental agent—kainate and AZD7325). After a minimum of 5 d of postoperative recovery, EEG activity was measured using the Pinnacle system for mouse, during the dark phase of the cycle. For kainate experiments, mice were acclimatized to the recording chamber and preamplifier for one hour, then recorded for one hour of baseline before receiving 20 mg kg−1 kainate i.p. The animals were then recorded for an additional 2 h postinjection. For baseline and AZD7325 experiments, mice were acclimatized to the recording chamber and preamplifier for 1 h, then recorded for 1 h of baseline before receiving 3 mg kg−1 AZD7325 (or 40% (2-hydroxypropyl)-β-cyclodextrin vehicle control) i.p. For these experiments, vigilance was further promoted by providing animals with a fresh nestlet just prior to initiation of recordings. Thus, during the recording period mice were engaged with nest building, grooming, eating and drinking behaviors, and if mice became inactive a series of random tones was played. Results were quantified using SleepSign, and MatLab, and were analyzed using ANOVA (two way—Kainate and AZD7325 average experiments; repeated measures—FFT spectral analysis) to compare mean values, graphs were plotted as mean ± standard error.
Behavioral assessments
Genotype-blinded behavioral assessments were conducted on Gabra2–1 and wildtype mice during the light phase. For behavioral scoring of seizures, mice were assessed based on a Racine scale modified for mice, with 0 = normal behavior/no change; 1 = behavioral arrest, shivering; 2 = head nodding, Straub tail; 3 = forelimb clonus, champing; 4 = forelimb clonus with rearing; 5 = generalized tonic clonic, wild jumping; animals were assigned a 6 upon death. The preliminary screen was based on the modified SHIRPA protocol used by European Mouse Phenotyping Resource of Standardized Screens (EMPReSS) designed to evaluate the basic phenotype of transgenic mouse strains (n = 8 mice per genotype run in >2 independent cohorts). The open field apparatus was based on that used in the EMPReSS resource. Open field behavior of mice was assessed using the Noldus Ethovision Tracking system from video recordings taken from above. To assess the sedative effects of diazepam, naïve mice were first habituated to the open field for 30 min, and then removed and dosed with 10 mg kg−1 diazepam i.p. before being returned to the open field for an additional 60 min.
Protocols for both the light/dark exploration test and the elevated plus maze (n = 8 mice per genotype per treatment run in >2 independent cohorts) were based on standard protocols. The light/dark arena is composed of a larger chamber that is transparent and brightly illuminated from above, and a smaller chamber that is black walled and dark. The two chambers are separated by a partition with a small doorway to allow the animal to freely pass from chamber to chamber while exploring for 10 min. The elevated plus maze was composed of two open and two closed arms extending from a central platform, elevated to a height of 40 cm above the floor. Test sessions were initiated by placing individual animals in the center square facing an open arm, and were terminated after 5 min of free exploration. For light-dark and elevated plus, video recordings (30 fps) of test sessions were digitized and both manually assessed by a genotype-blinded observer and analyzed using Noldus Ethovision, to quantify number of transitions between the light and dark compartments, and the total time spent in the light and dark compartments (light–dark), or the number of entries (all four paws into an arm) and time spent in open and closed arms (elevated plus). To test the anxiolytic effects of diazepam (2 mg kg−1, 15 min prior to testing) or AZD7325 (3 mg kg−1 in 40% (2-hydroxypropyl)-β-cyclodextrin, 45 min prior to testing) animals were given an ip injection prior to initiation of the elevated plus maze, and compared to 40% (2-hydroxypropyl)-β-cyclodextrin vehicle controls. Results from all behavioral assessments (n = 8 mice per genotype, per treatment, run in 2–3 independent cohorts) were analyzed using ANOVA to compare mean values.
Data collection and analyses
Power analyses run on pools of data from independent drug treatment or genetic modification experiments reveal a range of sample sizes between 5 and 12 animals (with n = 5 animals per group yielding a power of 80% on simple tests, and n = 10 yielding a power close to 100%). For the purposes of the behavioral assessments within this set of studies, we estimated that eight animals would be required per genetic manipulation, and per drug group, per behavioral experiment.
Biochemistry, EEG and confocal imaging experiments are subject to somewhat less variability, and thus we estimated that fewer animals (n = 6) would be needed to obtain statistical power for these experiments. For electrophysiology, power analysis of prior data sets provided an estimate of five to six animals needed per group to achieve a power close to 100%. All findings were reproduced in >2 independent experiments (biochemistry, confocal microscopy, electrophysiology) or matched cohorts (EEG and behavior). Data from independent experiments or cohorts were compared to ensure that findings were reproduced, and then the data was combined for statistical analysis. Data were normally distributed, and data being compared had similar variance. No data were excluded from this manuscript. Investigators were blinded to genotype and/or treatment group for all confocal, electrophysiology, behavioral and EEG experiments, and subsequent analyses.
Data availability
The data that support the findings of this study have been deposited in an institutional repository, and are available from the primary author upon reasonable request.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgments
This work was supported by NIH-NINDS Grants NS101888, NS081735, and NS087662; NIMH Grants MH097446 and MH106954 (S.J.M.) and NIH-DA Grant DA037170 to S.J.M. S.J.M. serves as a consultant for SAGE Therapeutics and AstraZeneca, relationships that are regulated by Tufts University. This work was also supported by the Deutsche Forschungsgemeinschaft grants FZ 82, SFB 487 C7, SCHI 425/8-1 awarded to H.S. R.M.H. was supported by a CIHR postdoctoral fellowship. H.M.M. was supported by a grant of the German Excellence Initiative to the Graduate School of Life Sciences, University of Würzburg.
Author contributions
R.M.H. generated and maintained the mice, performed genotyping, western blotting, perfusions, immunohistochemistry (cultured cells and fresh frozen tissue), confocal microscopy, assessments of mortality, seizure susceptibility, behavioral assessments, and performed data analysis on the aforementioned experiments, as well as assisted with EEG implants, recordings, and data analysis. H.M.M. expressed and purified recombinant proteins and carried out the native PAGE, analytical size exclusion experiments and isothermal titration calorimetry experiments. D.J.H. assisted with seizure susceptibility, and behavioral assessments, and conducted EEG implantation, recording, and analysis. P.P. and J.M.F. carried out the immunohistochemistry (perfusion fixed tissue) and analysis. A.M., T.D., and P.D. performed electrophysiological assessments. Y.N. conducted the GST-pull down experiment. A.J.N. assisted with genotyping and amplification of GABRA2 for sequencing. A.C. and N.B. contributed to the experiments examining diazepam and AZD7325. Figures for the manuscript were made by R.M.H. Experiments were designed by R.M.H., H.M.M., H.S., and S.J.M. The manuscript was written by R.M.H. with input from H.M.M., H.S., and S.J.M. S.J.M. and R.M.H. conceived the project.
Competing interests
S.J.M. serves as a consultant for SAGE Therapeutics and AstraZeneca, relationships that are regulated by Tufts University. The remaining authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/5/2018
This Article was originally published without the accompanying Peer Review File. This file is now available in the HTML version of the Article; the PDF was correct from the time of publication.
Contributor Information
Rochelle M. Hines, Email: Rochelle.Hines@unlv.edu
Stephen J. Moss, Email: Stephen.Moss@tufts.edu
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-05481-1.
References
- 1.Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr. Opin. Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Klausberger T, Roberts JDB, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc. Natl Acad. Sci. USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyíri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur. J. Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- 5.Fritschy JM, Johnson DK, Mohler H, Rudolph U. Independent assembly and subcellular targeting of GABA(A)-receptor subtypes demonstrated in mouse hippocampal and olfactory neurons in vivo. Neurosci. Lett. 1998;249:99–102. doi: 10.1016/S0304-3940(98)00397-8. [DOI] [PubMed] [Google Scholar]
- 6.Dugladze T, Schmitz D, Whittington MA, Vida I, Gloveli T. Segregation of axonal and somatic activity during fast network oscillations. Science. 2012;336:1458–1461. doi: 10.1126/science.1222017. [DOI] [PubMed] [Google Scholar]
- 7.Viney TJ, et al. Network state-dependent inhibition of identified hippocampal CA3 axo-axonic cells in vivo. Nat. Neurosci. 2013;16:1802–1811. doi: 10.1038/nn.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eyre MD, Renzi M, Farrant M, Nusser Z. Setting the time course of inhibitory synaptic currents by mixing multiple GABA(A) receptor α subunit isoforms. J. Neurosci. Off. J. Soc. Neurosci. 2012;32:5853–5867. doi: 10.1523/JNEUROSCI.6495-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maric HM, et al. Molecular basis of the alternative recruitment of GABA(A) versus glycine receptors through gephyrin. Nat. Commun. 2014;5:5767. doi: 10.1038/ncomms6767. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee J, et al. The residence time of GABA(A)Rs at inhibitory synapses is determined by direct binding of the receptor α1 subunit to gephyrin. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tretter V, et al. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J. Neurosci. Off. J. Soc. Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tretter V, et al. Molecular basis of the γ-aminobutyric acid A receptor α3-subunit interaction with the clustering protein gephyrin. J. Biol. Chem. 2011;286:37702–37711. doi: 10.1074/jbc.M111.291336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dejanovic B, Schwarz G. Neuronal nitric oxide synthase-dependent S-nitrosylation of gephyrin regulates gephyrin clustering at GABAergic synapses. J. Neurosci. Off. J. Soc. Neurosci. 2014;34:7763–7768. doi: 10.1523/JNEUROSCI.0531-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dejanovic B, et al. Simultaneous impairment of neuronal and metabolic function of mutated gephyrin in a patient with epileptic encephalopathy. EMBO Mol. Med. 2015;7:1580–1594. doi: 10.15252/emmm.201505323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey RJ, et al. A critical role for glycine transporters in hyperexcitability disorders. Front. Mol. Neurosci. 2008;1:1. doi: 10.3389/neuro.02.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maric HM, Mukherjee J, Tretter V, Moss SJ, Schindelin H. Gephyrin-mediated γ-aminobutyric acid type A and glycine receptor clustering relies on a common binding site. J. Biol. Chem. 2011;286:42105–42114. doi: 10.1074/jbc.M111.303412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiou TT, et al. Differential regulation of the postsynaptic clustering of γ-aminobutyric acid type A (GABAA) receptors by collybistin isoforms. J. Biol. Chem. 2011;286:22456–22468. doi: 10.1074/jbc.M111.236190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patrizi A, et al. Selective localization of collybistin at a subset of inhibitory synapses in brain circuits. J. Comp. Neurol. 2012;520:130–141. doi: 10.1002/cne.22702. [DOI] [PubMed] [Google Scholar]
- 19.Saiepour L, et al. Complex role of collybistin and gephyrin in GABAA receptor clustering. J. Biol. Chem. 2010;285:29623–29631. doi: 10.1074/jbc.M110.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyagarajan SK, Ghosh H, Harvey K, Fritschy JM. Collybistin splice variants differentially interact with gephyrin and Cdc42 to regulate gephyrin clustering at GABAergic synapses. J. Cell Sci. 2011;124:2786–2796. doi: 10.1242/jcs.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadopoulos T, et al. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. EMBO J. 2007;26:3888–3899. doi: 10.1038/sj.emboj.7601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poulopoulos A, et al. Neuroligin-2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Papadopoulos T, et al. Collybistin is required for both the formation and maintenance of GABAergic postsynapses in the hippocampus. Mol. Cell Neurosci. 2008;39:161–169. doi: 10.1016/j.mcn.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Harvey K, et al. The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. J. Neurosci. Off. J. Soc. Neurosci. 2004;24:5816–5826. doi: 10.1523/JNEUROSCI.1184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalscheuer VM, et al. A balanced chromosomal translocation disrupting ARHGEF9 is associated with epilepsy, anxiety, aggression, and mental retardation. Hum. Mutat. 2009;30:61–68. doi: 10.1002/humu.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lionel AC, et al. Rare exonic deletions implicate the synaptic organizer Gephyrin (GPHN) in risk for autism, schizophrenia and seizures. Hum. Mol. Genet. 2013;22:2055–2066. doi: 10.1093/hmg/ddt056. [DOI] [PubMed] [Google Scholar]
- 27.Shimojima K, et al. Loss-of-function mutation of collybistin is responsible for X-linked mental retardation associated with epilepsy. J. Hum. Genet. 2011;56:561–565. doi: 10.1038/jhg.2011.58. [DOI] [PubMed] [Google Scholar]
- 28.Kins S, Betz H, Kirsch J. Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat. Neurosci. 2000;3:22–29. doi: 10.1038/71096. [DOI] [PubMed] [Google Scholar]
- 29.Vien TN, et al. Compromising the phosphodependent regulation of the GABAAR β3-subunit reproduces the core phenotypes of autism spectrum disorders. Proc. Natl Acad. Sci. USA. 2015;112:14805–14810. doi: 10.1073/pnas.1514657112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modgil A, et al. Endogenous and synthetic neuroactive steroids evoke sustained increases in the efficacy of GABAergic inhibition via a protein kinase C-dependent mechanism. Neuropharmacology. 2017;113:314–322. doi: 10.1016/j.neuropharm.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein PA, et al. Prolongation of hippocampal miniature inhibitory postsynaptic currents in mice lacking the GABA(A) receptor alpha1 subunit. J. Neurophysiol. 2002;88:3208–3217. doi: 10.1152/jn.00885.2001. [DOI] [PubMed] [Google Scholar]
- 32.Brown SDM, Chambon P, de Angelis MH, Eumorphia Consortium. EMPReSS: standardized phenotype screens for functional annotation of the mouse genome. Nat. Genet. 2005;37:1155. doi: 10.1038/ng1105-1155. [DOI] [PubMed] [Google Scholar]
- 33.Rees MI, et al. Mutations in the gene encoding GlyT2 (SLC6A5) define a presynaptic component of human startle disease. Nat. Genet. 2006;38:801–806. doi: 10.1038/ng1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volk DW, et al. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb. Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- 35.Rudolph U, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 36.Christian EP, et al. EEG-β/γ spectral power elevation in rat: a translatable biomarker elicited by GABA(Aα2/3)-positive allosteric modulators at nonsedating anxiolytic doses. J. Neurophysiol. 2015;113:116–131. doi: 10.1152/jn.00539.2013. [DOI] [PubMed] [Google Scholar]
- 37.Soykan T, et al. A conformational switch in collybistin determines the differentiation of inhibitory postsynapses. EMBO J. 2014;33:2113–2133. doi: 10.15252/embj.201488143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baulac S, et al. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat. Genet. 2001;28:46–48. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- 39.Cossette P, et al. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat. Genet. 2002;31:184–189. doi: 10.1038/ng885. [DOI] [PubMed] [Google Scholar]
- 40.Wallace RH, et al. Mutant GABAA receptor γ2-subunit in childhood absence epilepsy and febrile seizures. Nat. Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 41.Maric HM, et al. Design and synthesis of high-affinity dimeric inhibitors targeting the interactions between gephyrin and inhibitory neurotransmitter receptors. Angew. Chem. Int. Ed. Engl. 2015;54:490–494. doi: 10.1002/anie.201409043. [DOI] [PubMed] [Google Scholar]
- 42.Maric HM, et al. Gephyrin-binding peptides visualize postsynaptic sites and modulate neurotransmission. Nat. Chem. Biol. 2017;13:153–160. doi: 10.1038/nchembio.2246. [DOI] [PubMed] [Google Scholar]
- 43.Fritschy JM, Panzanelli P, Tyagarajan SK. Molecular and functional heterogeneity of GABAergic synapses. Cell. Mol. Life Sci. CMLS. 2012;69:2485–2499. doi: 10.1007/s00018-012-0926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerti-Szigeti, K. & Nusser, Z. Similar GABAA receptor subunit composition in somatic and axon initial segment synapses of hippocampal pyramidal cells. eLife5, e18426 (2016). [DOI] [PMC free article] [PubMed]
- 45.Benke D, et al. Analysis of the presence and abundance of GABAA receptors containing two different types of alpha subunits in murine brain using point-mutated alpha subunits. J. Biol. Chem. 2004;279:43654–43660. doi: 10.1074/jbc.M407154200. [DOI] [PubMed] [Google Scholar]
- 46.Duggan MJ, Pollard S, Stephenson FA. Immunoaffinity purification of GABAA receptor alpha-subunit iso-oligomers. Demonstration of receptor populations containing alpha 1 alpha 2, alpha 1 alpha 3, and alpha 2 alpha 3-subunit pairs. J. Biol. Chem. 1991;266:24778–24784. [PubMed] [Google Scholar]
- 47.Gao, Y. & Heldt, S. A. Enrichment of GABAA receptor α-subunits on the axonal initial segment shows regional differences. Front. Cell Neurosci. 10, 39 (2016). [DOI] [PMC free article] [PubMed]
- 48.Kordeli E, Lambert S, Bennett V. Ankyrin A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J. Biol. Chem. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- 49.Kriebel M, et al. The cell adhesion molecule neurofascin stabilizes axo-axonic GABAergic terminals at the axon initial segment. J. Biol. Chem. 2011;286:24385–24393. doi: 10.1074/jbc.M110.212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J. Cell. Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berghs S, et al. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J. Cell Biol. 2000;151:985–1002. doi: 10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Audenaert D, et al. A novel GABRG2 mutation associated with febrile seizures. Neurology. 2006;67:687–690. doi: 10.1212/01.wnl.0000230145.73496.a2. [DOI] [PubMed] [Google Scholar]
- 53.Harkin LA, et al. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am. J. Hum. Genet. 2002;70:530–536. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kananura C, et al. A splice-site mutation in GABRG2 associated with childhood absence epilepsy and febrile convulsions. Arch. Neurol. 2002;59:1137–1141. doi: 10.1001/archneur.59.7.1137. [DOI] [PubMed] [Google Scholar]
- 55.Kole MHP, Stuart GJ. Signal processing in the axon initial segment. Neuron. 2012;73:235–247. doi: 10.1016/j.neuron.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Vacher H, Trimmer JS. Trafficking mechanisms underlying neuronal voltage-gated ion channel localization at the axon initial segment. Epilepsia. 2012;53(Suppl 9):S21–S31. doi: 10.1111/epi.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wimmer VC, Reid CA, So EYW, Berkovic SF, Petrou S. Axon initial segment dysfunction in epilepsy. J. Physiol. 2010;588:1829–1840. doi: 10.1113/jphysiol.2010.188417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeFelipe J. Chandelier cells and epilepsy. Brain J. Neurol. 1999;122(Pt 10):1807–1822. doi: 10.1093/brain/122.10.1807. [DOI] [PubMed] [Google Scholar]
- 59.Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 2005;28:310–316. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Zhang P, Wyskiel DR. Chandelier cells in functional and dysfunctional neural circuits. Front. Neural Circuits. 2016;10:33. doi: 10.3389/fncir.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dixon CI, Rosahl TW, Stephens DN. Targeted deletion of the GABRA2 gene encoding alpha2-subunits of GABA(A) receptors facilitates performance of a conditioned emotional response, and abolishes anxiolytic effects of benzodiazepines and barbiturates. Pharmacol. Biochem. Behav. 2008;90:1–8. doi: 10.1016/j.pbb.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 62.Farrell JS, et al. Postictal hypoperfusion/hypoxia provides the foundation for a unified theory of seizure-induced brain abnormalities and behavioral dysfunction. Epilepsia. 2017;58:1493–1501. doi: 10.1111/epi.13827. [DOI] [PubMed] [Google Scholar]
- 63.Knyazev GG. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007;31:377–395. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci. Biobehav. Rev. 2012;36:677–695. doi: 10.1016/j.neubiorev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 65.Gauthier AK, Chevrette T, Bouvier H, Godbout R. Evening vs. morning wake EEG activity in adolescents with anxiety disorders. J. Anxiety Disord. 2009;23:112–117. doi: 10.1016/j.janxdis.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Buchsbaum MS, et al. Topographic EEG changes with benzodiazepine administration in generalized anxiety disorder. Biol. Psychiatry. 1985;20:832–842. doi: 10.1016/0006-3223(85)90208-2. [DOI] [PubMed] [Google Scholar]
- 67.Siok CJ, et al. Comparative analysis of the neurophysiological profile of group II metabotropic glutamate receptor activators and diazepam: effects on hippocampal and cortical EEG patterns in rats. Neuropharmacology. 2012;62:226–236. doi: 10.1016/j.neuropharm.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 68.Kopp C, Rudolph U, Löw K, Tobler I. Modulation of rhythmic brain activity by diazepam: GABA(A) receptor subtype and state specificity. Proc. Natl Acad. Sci. USA. 2004;101:3674–3679. doi: 10.1073/pnas.0306975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kopp C, Rudolph U, Keist R, Tobler I. Diazepam-induced changes on sleep and the EEG spectrum in mice: role of the alpha3-GABA(A) receptor subtype. Eur. J. Neurosci. 2003;17:2226–2230. doi: 10.1046/j.1460-9568.2003.02651.x. [DOI] [PubMed] [Google Scholar]
- 70.Tobler I, Kopp C, Deboer T, Rudolph U. Diazepam-induced changes in sleep: role of the alpha 1 GABA(A) receptor subtype. Proc. Natl Acad. Sci. USA. 2001;98:6464–6469. doi: 10.1073/pnas.111055398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.NIH. Guide for the Care and Use of Laboratory Animals 3rd edn (National Academies Press, 2011). [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data that support the findings of this study have been deposited in an institutional repository, and are available from the primary author upon reasonable request.