Abstract
Global population aging is one of the major social and economic challenges of contemporary society. During aging the progressive decline in physiological functions has serious consequences for all organs including brain. The age-related incidence of neurodegenerative diseases coincides with the sharp decline of the amount and functionality of adult neural stem cells. Recently, we identified a short list of brain age-regulated genes by means of next-generation sequencing. Among them znf367 codes for a transcription factor that represents a central node in gene co-regulation networks during aging, but whose function in the central nervous system (CNS), is completely unknown. As proof of concept, we analysed the role of znf367 during Xenopus laevis neurogenesis. By means of a gene loss of function approach limited to the CNS, we suggested that znf367 might act as a key controller of the neuroblast cell cycle, particularly in the progression of mitosis and spindle checkpoint. A candidate gene approach based on a weighted-gene co-expression network analysis, revealed fancd2 and ska3 as possible targets of znf367. The age-related decline of znf367 correlated well with its role during embryonic neurogenesis, opening new lines of investigation also in adult neurogenesis to improved maintenance and even repair of neuronal function.
Introduction
The age-related incidence of many brain diseases coincides with a reduced adult neurogenic potential. The regenerative capability and the amount of adult neural stem cells (aNSCs) decline with age, contributing to the reduced functionality of the aged brain1. Despite the great interest in age related diseases, the molecular factors responsible for age-dependent decay of aNSCs function and the transition between stemness and differentiating properties of these precursors are almost unknown. Recently, we identified a set of evolutionarily-conserved genes expressed in aNSCs and age-regulated by RNA-seq analysis in the short-lived fish Nothobranchius furzeri, a well-established animal model in aging studies2. Among them, zinc finger protein 367 (znf367) was suggested to occupy a central position in a regulatory network controlling cell cycle progression and DNA replication. We found that znf367 is expressed in the adult brain of N. furzeri, where its RNA level decreases with age, and in neuroblast and retinoblast of developing Zebrafish embryos2. Znf367 belongs to the Zinc finger (ZNF) transcription factors family that represents a large class of proteins that are encoded by 2% of human genes3,4. Their functions include DNA recognition, RNA packaging, transcriptional activation, regulation of apoptosis, protein folding and assembly, and lipid binding5. Zinc finger proteins have an evolutionarily conserved structure and the ones containing the Cys2-His2 motif, constitute the largest family6. The function of the majority of ZNF genes is largely unknown, but some of them play a critical role in the development and differentiation of the nervous system. For instance, the Kruppel-like zinc finger transcription factor Zic has multiple roles in the regulation of proliferation and differentiation of neural progenitors in the medial forebrain and cerebellum7. The Ikaros family of transcription factors is characterized by two sets of highly conserved Cys2His2-type zinc finger motif and is involved in the maturation and differentiation of striatal medium spiny neurons8. Znf367 gene (also known as ZFF29 or ZFP367) has been initially isolated in human fetal liver erythroid cells9. In the human genome, this gene is on chr 9q and two alterative mRNA splicing products were identified and designated ZFF29a and ZFF29b. They both code for nuclear proteins, but only ZFF29b seems to act as an activator factor of erythroid gene promoters9. In Human SW13 adrenocortical carcinoma cell line, znf367 is overexpressed and in this cell line Znf367 downregulation caused an increase of cellular proliferation, invasion and migration10. Furthermore, znf367 was also identified as a potential tissue-specific biomarker correlated with breast cancer where its expression level is dysregulated influencing cell proliferation, differentiation and metastatic processes11. To our knowledge, there are no data available regarding the putative role of znf367 in the Central Nervous System (CNS) during embryonic and adult neurogenesis. A very recent research paper analyzed the transcriptome of different pools of aNSC that comprise quiescent and activated neural stem cells in the mouse sub-ventricular zone12. Interestingly in the Supplementary materials (Table S7), the authors compared the trascriptome of young (3–4 month-old) quiescent neural stem cells to the one obtained from old (19–22 month-old) quiescent neural stem cells and znf367 (zfp367) emerged among the genes significantly down regulated in the old mice12. Despite this data confirmed that, even in mammals, znf367 is an age-regulated gene in the adult brain, its function in the CNS remained unknown. To shed light on the znf367 role in vertebrates CNS, we analyzed its function during Xenopus laevis neurogenesis. The clawed frog Xenopus is the favorite animal model to perform functional screening of genes. In Xenopus, it is possible to microinject mRNAs or morpholino oligos in just one side of the early cleaving embryo and compare, in each embryo, the manipulated side of the embryo with its wild-type counterpart that represents a perfect internal control. This unique vertebrate model also provide the possibility, to rapidly perform gene loss of function experiments in a tissue specific manner thanks to the well-defined fate map of each blastomere of the early cleaving embryo. This allowed us to target specific znf367 morpholinos to the central nervous system without interfering with the normal development of the other tissues. In this paper, we show that znf367 is expressed in the developing CNS in Xenopus and it could have a key role in primary neurogenesis, regulating the neuroblast progression of mitosis. These finding, together with the znf367 gene expression decline observed during CNS aging, lay the groundwork for future studies aimed to unveil znf367 role in adult neurogenesis and CNS aging.
Results
Embryonic expression analysis of znf367
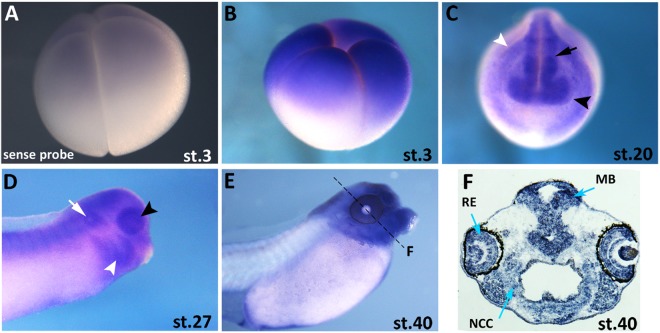
To verify the evolutionary conservation of znf367 sequence in vertebrates we performed an in silico analysis of the amino acid sequences of ZNF367 in Homo sapiens (both splicing variants: ZFF29a and ZFF29b), Nothobranchius furzeri and Xenopus laevis (both splicing variants: znf367a and znf367b). This approach revealed a high conservation of znf367 with a 66% of identity between the human and Xenopus amino acid sequence that reached 98% at the level of the zinc finger domains (Fig. 1) suggesting a conserved putative znf367 function in vertebrates, from fish to tetrapod and primates. To analyze the spatio-temporal gene expression pattern of znf367, whole mount in situ hybridization (WISH) was performed on Xenopus embryos at different stages. Znf367 is expressed maternally in the animal pole in Xenopus embryos at blastula stage (Fig. 2A,B) when compared to sense control probe treated siblings (Fig. 2A). At neurula stage znf367 is expressed in the neural tube, in the eye fields, in the pre-placodal territory and in the neural crest cells (NCC) (Fig. 2C). At the tadpole stage, znf367 is widely expressed in the central nervous system, in the eye, in the otic vesicle and in the NCC migrated in the branchial pouches (Fig. 2D). At larval stages of development, znf367 is widely expressed in the CNS as shown in transverse sections (Fig. 2E,F).
Figure 1.
Multiple sequence alignments of znf367 amino acid sequences. The gray boxes highlighted the conserved zinc-fingers binding domains of znf367 from X. laevis (both splicing variants znf367a and znf367b), human (both splicing variants ZFF29a and ZFF29b) and N. furzeri (n) obtained using Clustal Omega.
Figure 2.
Znf367 gene expression pattern during Xenopus laevis development. (A,B) Znf367 expression at blastula stage (stage 3) using sense control probe (A) and antisense probe (B). (C,D) At neurula (C) and at tadpole stages (D) znf367 is expressed in the neural tube (black arrow), the developing eye (black arrowhead), the neural crest cells (white arrowhead) in the otic vesicle (white arrow) and in the most anterior regions of the nervous system. (E,F) Stage 40 embryo is shown in lateral view (E) and in a transversal section at the level of the hindbrain (F). MB, midbrain; NCC, skeletogenic neural crest cells; RE, retina.
Znf367 Knockdown inhibits neuronal differentiation in Xenopus laevis embryos
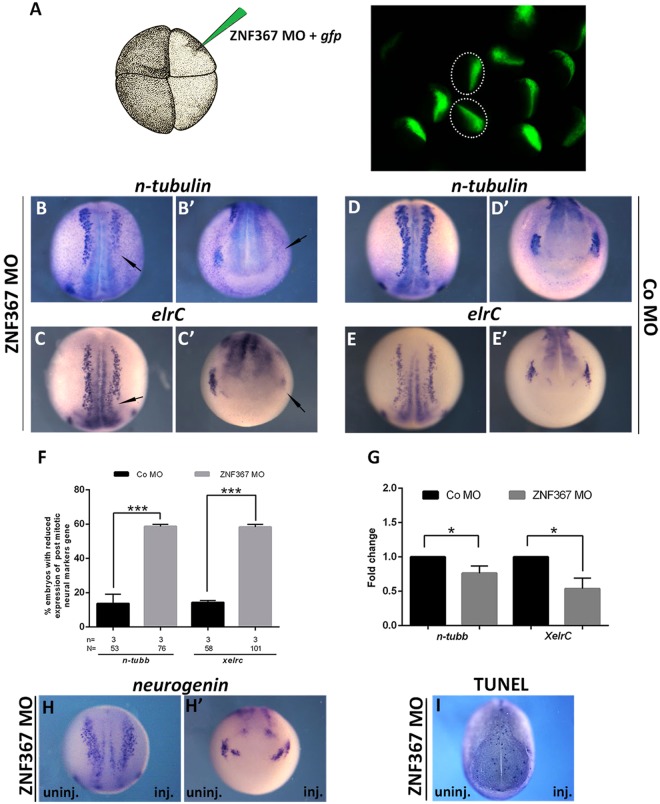
To investigate the znf367 function during neurogenesis in Xenopus, we performed knockdown experiments using a specific antisense oligonucleotide morpholino designed to block the translation of the endogenous mRNA (ZNF367-MO). For all the experiments described here, injections were performed unilaterally into one dorso-animal blastomere at the four-cell stage to target the neural tissue. The un-injected side served as an internal control and the co-injection with gfp (250 pg) RNA was used to screen for correctly injected embryos (Fig. 3A). The standard Gene Tools Control-morpholino (co-MO) was used to control for non-specific embryo responses. At neurula stage (st.18), znf367 morphants showed a strong reduction of cells expressing the markers of post-mitotic neurons N-tubulin and elrC (also known as HuC) in the injected side of the embryos compared to the control side and the co-MO injected embryos (Fig. 3B–E’). These data were also confirmed by qRT-PCR analysis that showed a significant reduction of both neuronal markers in znf367 morphants (Fig. 3F,G). Interestingly, the injection of ZNF367-MO did not affect the expression of ngnr1, a proneural marker necessary for the specification of primary neurons13 (N = 53) (Fig. 3H–H’), suggesting a role for znf367 during neuronal differentiation, but not in neuronal specification. The lack of differentiated primary neurons in znf367 morphants could be due to an increase in cell apoptosis during the differentiation process. To evaluate this aspect, we performed a TUNEL assay in znf367 morphants at the neurula stage and did not detect a significant increase in TUNEL positive cells between the znf367 injected side and the un-injected control side of each analyzed embryo (Fig. 3I).
Figure 3.
Loss of znf367 function interferes with the expression of neuronal differentiation markers. (A) Embryos injected with gfp (250 pg) and either ZNF367-MO or Control morpholino (Co-MO) (9 ng) at one dorsal blastomere at the four-cells stage showed fluorescence only in the neural plate at neurula stage (st. 18). (B–E’) mRNA distribution of N-tubulin and elrC in znf367 morphants and controls. (B,C) dorsal view and (B’,C’) frontal view of neurula morphants showing a clear loss of differentiation markers expression in primary neurons (arrows). (D,E) dorsal view and (D’,E’) frontal view of neurula embryos injected with Co-MO. (F) Quantification of the data in A and B. (G) qRT-PCR analysis. The levels of expression for the indicated mRNAs were evaluated for Co-MO or ZNF367-MO populations by qRT-PCR, and normalized to that of the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (gapdh) expression. The mean of the Control-MO was set to 1. For each gene, three independent RNA samples from morphants and controls were analysed. (H–H’) mRNA distribution of neurogenin in znf367 morphants. (I) TUNEL staining. ZNF367-MO injection did not lead to an increase of TUNEL positive cells compared to the un-injected side. Abbreviations: n, number of independent experiments; N, number of evaluate embryos in total; Error bars indicate standard error of the means (s.e.m); *p ≤ 0,05; ***p ≤ 0,001. P-value were calculated by Student’s t-test.
Znf367 knockdown increases proliferation markers in Xenopus laevis embryos
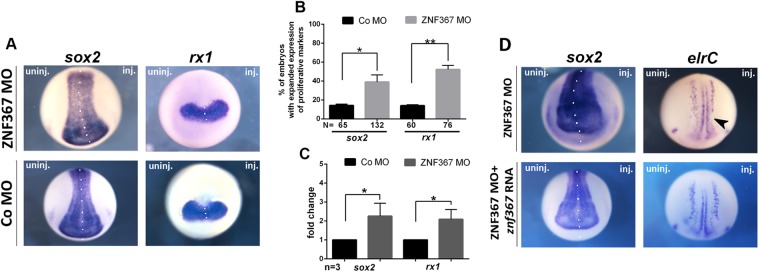
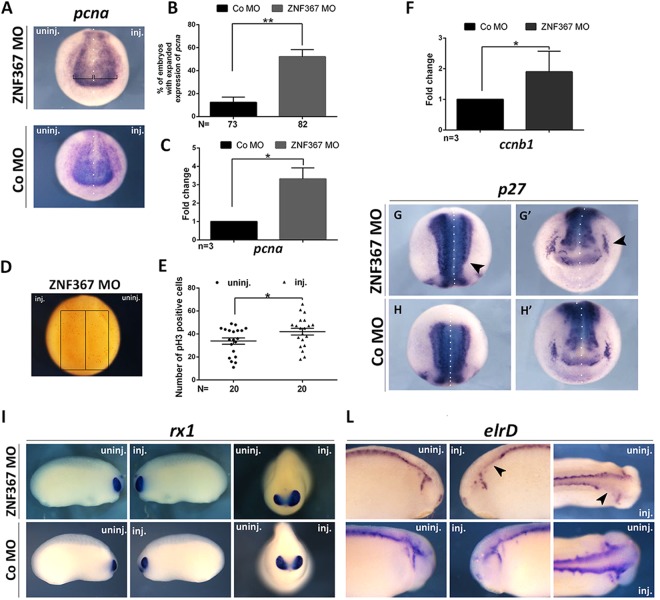
To determine whether the observed loss of post-mitotic neurons in znf367 morphants was the consequence of impairment in the maintenance of the neuronal progenitors pool, we visualized cells expressing the stemness genes sox2 and rx1, in the injected embryos. Sox2 and rx1 are involved in maintaining neuroblast and retinoblast as cycling precursors in the neural plate14,15. The expression domains of sox2 and rx1 were expanded on the ZNF367-MO injected side of the embryo as compared to either the un-injected and Co-MO injected sides (Fig. 4A,B) indicating a larger population of progenitors. These data were confirmed by qRT-PCR analysis that showed a significant increase of both mRNAs in znf367 morphants (Fig. 4C). On the base of these results, we can suggest that the znf367 knockdown enhances self-renewal of progenitors at the expense of differentiation. For testing the specificity of the ZNF367-MO to induce this phenotype, we performed functional rescue experiments by co-injecting 9 ng ZNF367 MO together with 500 pg full-length Xenopus znf367 mRNA. We observed a restoration of the phenotype of the injected embryos visualized by the sox2 and elrC markers at neurula stage (30% of rescue for sox2 N = 114; 25% of rescue for elrC N = 100) (Fig. 4D). To further verify whether znf367 downregulation could alter the regulation of neuroblast proliferation, we also examined the mRNA expression of pcna (proliferating cell nuclear antigen) and we directly counted mitotically active cells marked by the anti-phosphorylated H3 (p-H3) antibody. Znf367 morphants showed an increased pcna mRNA expression both in WISH (Fig. 5A,B) and qRT-PCR experiments (Fig. 5C). The p-H3 staining showed a significant increase in mitotic cells number upon ZNF367-MO injection as compared to the control side (Fig. 5D,E). Given that a larger pool of neuroblasts did not correspond to an increased number of differentiated cells in the absence of apoptotic cell death, it is tempting to speculate that znf367 could be required to exit the M phase or control the mitotic checkpoint that precedes the anaphase. To test this hypothesis, we first evaluated the relative expression of cyclin B1 that is expressed predominantly during M phase of the cell cycle16, by qRT-PCR analysis of znf367 morphants. This experiment revealed a significant increase of cyclin B1 expression in znf367 morphants (Fig. 5F) indicating again that znf367 deficient neuroblasts could enter M phase, but they could not correctly exit mitosis and differentiate. The differentiation of neuronal progenitors requires the withdrawal from the cell cycle, driven by cell cycle inhibitors, such as pak3 (p21) and p2717,18. Concomitantly with the increase in mitotically active cells, a mild loss of p27 expression (phenotype 55%, N = 93) was observed in neurula morphants indicating that znf367 depleted neuroblasts are unable to exit the cell cycle (Fig. 5G–H’). These data led us to hypothesize that znf367 could be involved in the cell cycle exit and/or for the initiation of maintenance of a differentiated state. Finally, we examined morphants at the tailbud stage by performing WISH using rx1 and elrD (also known as HuD). ElrD labels post-mitotic neurons in the neural tube and the developing cranial ganglia19. As stated above, znf367 knockdown, but not control MO, caused an increase in rx1 gene expression (52%, N = 72) (Fig. 5I) while inhibiting neuronal differentiation, thus affecting the expression of elrD (54% N = 64) (Fig. 5L). These data showed that the effects of depletion znf367 are not recovered even in the late phases of primary neurogenesis.
Figure 4.
znf367 morphants analysis and control rescue experiments. (A) mRNA distribution of sox2 and rx1 in znf367 morphants and controls. (B) Statistical analysis of the data in A. (C) The levels of expression for the indicated mRNAs were evaluated for Co-MO or ZNF367-MO populations by qRT-PCR, and normalized to that of the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (gapdh) expression, and the mean of the Control-MO was set to 1. For each gene, three independent RNA samples from morphants and controls were analysed. (D) The morphants phenotype can be rescued by the co-injection of morpholino plus full length Xenopus znf367 mRNA as shown by the recovered expression of sox2 and elrC. Abbreviations: n, number of independent experiments; N, number of evaluated embryos in total; Error bars indicate standard error of the means (s.e.m); *p ≤ 0,05; **p ≤ 0,01. P-value were calculated by Student’s t-test.
Figure 5.
znf367 morphants analysis for proliferating and differentiating precursors (A) mRNA distribution of pcna in znf367 morphant and control. (B) Statistical analysis of the data in A. (C) RT-PCR analysis revealed a significant increase of pcna. (D,E) Znf367 depletion leads to a significant reduction of proliferating cells compared to the un-injected side. pH3 positive cells were counted in the areas defined by the black rectangles. Statistical evaluation of the data is shown (E). (F) RT-PCR analysis of cyclinB1 (ccnb1). The levels of expression for the indicated mRNAs were evaluated for Co-MO or ZNF367-MO embryos by qRT-PCR in triplicate, and normalized to glyceraldehyde 3-phosphate dehydrogenase (gapdh) expression. (G–G’) p27 is down regulated at stage 18 in znf367 morphants (arrowheads). (G) dorsal view; (G’) frontal view. (H–H’). p27 expression in control embryo, (H) dorsal view; (H’) frontal view. (I–L) rx1 and elrD expression at tailbud stages confirmed the phenotype observed at neurula stages: increased expression of rx1 and downregulation of a neuronal differentiation marker (elrD) (arrowheads). Abbreviations: n, number of independent experiments; N, number of evaluated embryos in total; Error bars indicate standard error of the means (s.e.m); *p ≤ 0,05; **p ≤ 0,01. P-value were calculated by Student’s T-test.
Identification of putative Znf367 targets: a candidate gene approach
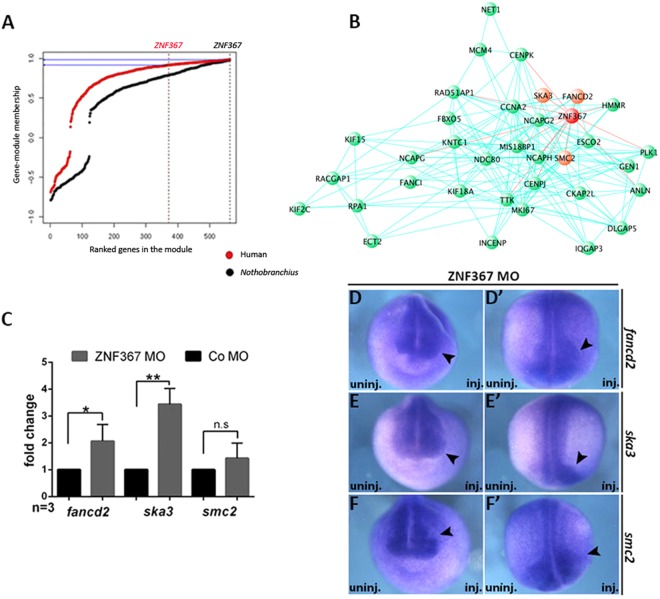
Our previous results suggested that znf367 represents a hub in the control of gene expression in the N. furzeri brain2. In order to test the conservation of this co-regulation across species, we analyzed CORTECON20 a public dataset of RNA-seq during cortical differentiation of human embryonic stem cells (hESCs) using weighted-gene co-expression network analysis (WGCNA)21. WGCNA constructs co-expression networks based on topological criteria. It was shown to be more robust than simple correlation and it has become the method of choice for gene expression studies in the nervous system22,23. This method identifies gene modules that are sets of tightly co-expressed genes, within the modules hub genes are identified as the genes with highest number of connections (highest connectivity) and are the putative drivers of the coherent expression of the genes within the module21. We therefore tested the conservation of gene co-expression networks between N. furzeri brain and human neuronal differentiation in vitro (Fig. 6A). We identified a conserved module that contains znf367 as a hub gene and then tested whether znf367 can be considered a hub in both species by computing its connectivity. Znf367 was among the top connected genes in the gene module in both species (98% percentile in N. furzeri and 92% percentile in human cells). Gene Ontology overrepresentation analysis revealed that cell-cycle related terms are highly overrepresented in this module. It should be also noted that all these genes have high expression in the hESCs, they are down-regulated during early differentiation, and show a peak of expression around 12 days of differentiation in vitro that correspond to the period of cortical specification20. Among the genes that showed the highest topological overlap, we noted enrichment in genes known to be involved in the progression of mitosis and in the mitotic spindle checkpoint (Fig. 6B). This corroborates the idea that znf367 has a role in the control of cell cycle and it could be preeminent in mitosis, when the dividing cell has the fundamental task of correctly arranging the genetic content of the two daughter cells. To verify our hypothesis, we decided to test by qRT-PCR and by whole mount in situ hybridization the expression of three of the genes closest to znf367 in the network (Fig. 6B). We analyzed the expression level of smc2, ska3 and fancd2 in znf367 morphants. The smc2 gene codes for one of the condensin components of the Structural Maintenance of Chromosomes (SMC) protein complexes, which play key roles in the regulation of higher-order chromosome organization and its role is crucial in the chromatin compaction in prophase24,25. Ska3 is one of the spindle and kinetochore-associated (Ska) proteins required for accurate chromosome segregation during mitosis26. During mitosis the cyclin-dependent kinase Cdk1 phosphorylates SKA3 to promote its direct binding to the Ndc80 complex, (also present in the Znf367 network). This event is required for the overcoming of spindle checkpoint and the beginning of anaphase26,27. FANCD2 encodes for a nuclear effector protein that is monoubiquitinated in response to DNA damage, targeting it to nuclear foci where it preserves chromosomal integrity28. Mutations in the FANC family are causative of the Fanconi Anemia in humans29. Greater than 60% of Fanconi anemia patients have developmental defects, such as growth retardation, short stature, microcephaly, and microphthalmia at birth, in addition to a highly elevated risk of bone marrow failure in the first decade of life30. This gene draws our attention as its knockdown in zebrafish embryos induced microcephaly, microphthalmia and pericardial edema28. It has been demonstrated that this factor is crucial for the S-phase rescue of damaged DNA, but also for the safeguarding of chromosome stability during mitosis31,32. The results obtained in three independent experiments, showed a significant increase in fancd2 and ska3 gene expression in znf367 morphants. The smc2 gene expression level followed the same trend without statistical significance (Fig. 6C). These results were also confirmed by WISH experiments: the expression domains of fancd2 (50%, N = 50), ska3 (53%, N = 50) and smc2 (48%, N = 50) were expanded on the ZNF367-MO injected side of the embryo (Fig. 6D–F’).
Figure 6.
Network and molecular analysis of znf367 neighbors. (A) Distribution of membership values for the analyzed module in human cells and N. furzeri brain. The vertical line indicates the rank of znf367 and the horizontal line its membership value (black for N. furzeri and red for H. sapiens). Please note due to the high convexity of the human distribution ZNF367 has very high membership despite its rank. (B) Central part of the gene module containing ZNF367. Only genes showing topological overlap >0.3 are shown. ZNF367 is in red, while genes used for further analysis are in orange (SMC2, SKA3 and FANCD2). (C) qRT-PCR analysis of fancd2, ska3 and smc2. The levels of expression for the indicated mRNAs were evaluated for Co-MO or ZNF367-MO populations by qRT-PCR in triplicate and normalized to, glyceraldehyde 3-phosphate dehydrogenase (gapdh) expression. (D) mRNA distribution of fancd2, ska3 and smc2 in znf367 morphants. Neurula stage embryos are shown in frontal (D,E,F) and dorsal view (D’,E’,F’). Arrowheads indicated the expanded domain of the mRNA distribution of each gene in the injected side of the embryo. Abbreviations: n, number of independent experiments; Error bars indicate standard error of the means (s.e.m); n.s., not significant. *p ≤ 0,05; **p ≤ 0,01. P-value were calculated by Student’s T-test.
These suggest that znf367 could have a major role in the control of chromosome stability and the functionality of the spindle check point.
Discussion
We chose Xenopus laevis as a model system to directly modulate the znf367 expression in the CNS without affecting other tissues and to unveil its role in tetrapods. Znf367 is expressed in the neural tissue of the early Xenopus laevis embryo including the eye field and in the neural crest cells. The spatial expression pattern suggested a role in the context of primary neurogenesis. This was further supported by the marked loss of post mitotic neurons upon knockdown of znf367, suggesting that znf367 could be essential for neuronal differentiation. In Xenopus znf367 morphants, we did not observe an increase in apoptosis rate suggesting that the loss of post-mitotic neurons was not due to unspecific morpholino toxicity or to a specific triggering of apoptotic pathways. Indeed, we found that the loss of znf367 function led to an increased expression of genes involved in the maintenance of neuroblast and retinoblast as proliferating precursors. Accordingly, we observed a significant increase in the number mitotic cells in znf367 morphants. The co-ordinate regulation of cell proliferation and differentiation is of fundamental importance in the development of the central nervous system33. At early developmental stages, a period of extensive proliferation is needed to generate the required number of progenitor cells for correct tissue and organ formation, accompanied or closely followed by differentiation34. After the closure of the neural tube, the epithelial lining of the ventricles becomes specialized, consisting of a single sheet of progenitor cells (neuroepithelial cells). These cells undergo symmetrical cell divisions during the proliferative period to self-renew and expand the pool of progenitors34,35. The subsequent transition from a proliferative neural precursor cell to a post-mitotic neurons is a highly regulated step, which, in many instances, has been shown to involve a cascade of transcription factors that is triggered by pro-neural genes36. Differentiation of neural progenitor cells requires withdrawal from the cell cycle, which is regulated by the expression of cell cycle inhibitors, such as p27 in Xenopus18. Consistent with the increase in mitotically active cells, a reduced p27 expression was observed in znf367 morphants, thus raising the possibility that the neural progenitors are prevented from undergoing differentiation because they are not able to exit the cell cycle, remaining in an undifferentiated state. Znf367 morphants also expressed high levels of cyclin B1, which is required to drive cells into mitotic division, but that must be degraded to allow anaphase. Again, this datum corroborates our hypothesis that znf367 deficient neuroblasts could enter M phase, but they could not correctly exit mitosis and differentiate. Given the requirement of znf367 for both proliferation and neuronal differentiation of neuroectodermal cells, it is plausible that znf367 could be required to exit M phase or in the control of the spindle checkpoint that precedes anaphase. To have a wider view on the molecular mechanisms potentially regulated by znf367, we performed a correlation-based network analysis testing the conservation of gene co-expression networks between N. furzeri brain and human neuronal differentiation in vitro, identifying a conserved module that contains znf367. To this purpose we analysed the public RNA-seq database CORTECON. CORTECON reports transcript expression levels for all stages of in vitro differentiation from hESCs to cortical neurons, therefore capturing even the earliest stages of neurogenesis comparable to the stages analysed here in Xenopus.
We noted enrichment in genes involved in the regulation of the cell cycle and specifically in the progression of mitosis and mitotic spindle check point. The involvement of znf367 in the control of cell cycle is supported by functional studies that demonstrated its implication in regulating different aspects of cancer progression10. Some of the genes, correlated to znf367 in our correlation analysis are both implicated in CNS development as well as in cancer initiation and/or progression. Among these, we decided to closely analyze the relation between znf367 and smc2, ska3 and fancd2. SMC2 is part of the condensing complex required for the structural and functional organization of chromosomes37. Its role is crucial in the chromatin compaction in the prophase24. In our functional study the loss of znf367 seemed to interfere with smc2 mRNA level, but even if smc2 mRNA seemed to be more abundant in znf367 morphants than in controls, the results are suggestive of a trend, but not statistically significant. Fancd2 is essential during zebrafish CNS development to prevent neural cell apoptosis during neuroblast proliferative expansion28. FANCD2, when mutated, is one of the causative genes of Fanconi anemia, an inherited disorder characterized by developmental defects, progressive bone marrow failure, and predisposition to cancer30. In particular, FANCD2 in postnatal and adult life is required for the functional maintenance of the hematopoietic stem cell pool38. The link between znf367 and fancd2 seems therefore particularly intriguing since the znf367 function seemed to be required to repress fancd2 expression and allow cells to inactivate the spindle checkpoint and proceed towards differentiation. The level of fancd2 mRNA is significantly up regulated in znf367 morphants. It is tempting to speculate that during primary neurogenesis in Xenopus znf367 could regulate fancd2 expression levels in order to define the pool of neuroblast and coordinate the cell cycle exit necessary for post-mitotic differentiation. SKA3 is one of the spindle assembly checkpoint proteins. SKA3 is strongly associated with kinetochores during prometaphase and metaphase, while being diminished during anaphase and lost in telophase27. Its major role is to contribute to the silencing of spindle checkpoint during metaphase and to the maintenance of chromosome cohesion in mitosis27,39. In our znf367 morphants, ska3 is upregulated, supporting the idea that znf367 could play a key role in the control of mitosis and in particular during metaphase. As ska3 has to be downregulated to allow the progression towards anaphase and telophase. A possible hypothesis we can formulate is that the loss function of znf367 could maintain abnormally high levels of ska3, keeping cells blocked in mitosis (metaphase). This analysis provided us with a deeper view of the possible action of znf367 during neurogenesis but deeper analysis, necessary to shed light on the direct targets of znf367 and their function, should be addressed in the future.
In conclusion, we unveiled a role for znf367 during neurogenesis in vertebrates. In particular, znf367 emerged as a key controller of the neuroblast cell cycle, and it seemed to act regulating the events that are strictly controlled during the metaphase to allow the progression of the cell cycle and the onset of anaphase. The observed age-related down-regulation of znf367 correlated with the age-related decline of quiescent aNSC to activate and give rise to new neural progenitors2,12. Our data shed light on the role of znf367 during neural progenitors development giving a proof of concept of the continuity of molecular control in developing and adult neurogenesis. It will be of interest for future studies to identify both the upstream regulators and the downstream effectors of znf367. This is important not only due to the requirement of znf367 during X. laevis neurogenesis, but more generally for the identification of the molecular factors involved in neuronal progenitors cell cycle exit and differentiation. If our findings will be validated also in adult neurogenesis they could represent the first step in defining new strategies to increase adult neurogenesis, leading to improved maintenance of functional aNSC.
Methods
Synteny analysis of znf367
Synteny analysis was performed using the NCBI GeneBank for the following organisms: Xenopus laevis znf367a (NP_001085362.1); Xenopus laevis znf367b (XP_018114684 PREDICTED); Homo sapiens ZFF29A (AY554164.1) and Homo sapiens ZFF29b (AY554165.1); Nothobranchius furzeri (HADY01011608.1).
Embryo preparation
Animal handling and care were performed in strict compliance with protocols approved by Italian Ministry of Public Health and of the local Ethical Committee of University of Pisa (authorization n.99/2012-A, 19.04.2012). Xenopus laevis embryos were obtained by hormone-induced laying and in vitro fertilization then reared in 0.1 X Marc’s Modified Ringer’s Solution (MMR 1×: 0.1 M NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES pH 7.5) till the desired stage according to Nieuwkoop and Faber40.
Morpholino oligonucleotides, cloning and microinjections
ZNF367 antisense Morpholino oligonucleotides (MO) and a standard Control MO were provided by Gene Tools, Philomath, OR, USA. ZNF367 MO sequence: 5′-CAGCCTATCTGACATTTGTTACTAC-3′. Co MO sequence: 5′-CCTCTTACCTCAGTTACAATTTATA-3′. Microinjections were performed as described previously41. Injected MO amounts were: 9 ng ZNF367 MO and 9 ng Control MO. Correct injections were verified by co-injected of 250 pg of GFP mRNA and using a fluorescence microscope. The un-injected side represents an internal control in each embryo. For functional rescue experiments, the open reading frame of X. laevis znf367 (XM_018259195.1 PREDICTED) was cloned into the pCS2+. For Rescue experiments, 9 ng ZNF367 MO and 500 pg full-length znf367 mRNA were co-injected. Higher doses of morpholino, 12 or 15 ng per embryos, were tested. Morphants injected with 12 or 15 ng of morpholino showed the described phenotype of altered neurogenis but also defects in the neural tube closure that were not efficiently rescued by the injection of znf367 mRNA (not shown). For this reason all the experiments showed in the results section were performed at the 9 ng ZNF367 MO dose. Capped znf367 mRNA was obtained using the MegaScript in vitro transcription kit (Ambion), according to manufacturer’s instructions.
In situ hybridization (ISH) experiments
Whole mount in situ hybridization (WISH) approaches was performed as described42. BM purple (Roche) was used as a substrate for the alkaline phosphatase; digoxigenin-11-UTP-labelled sense, and antisense RNA probes were generated via in vitro transcription. After color development, embryos were post-fixed and bleached over light to remove the pigment. For ISH on cryosections (12 µm), embryos were fixed in 4% paraformaldehyde (PFA) in PBS, cryoprotected with 30% sucrose in PBS and embedded in Tissue-Tek O.C.T. compound (Sakura, 4583). ISH on cryosections was performed as described42. Unpublished new plasmids for in situ hybridization were generated as follows: X. laevis znf367 EST clone image (ID_6637026) was cloned in pBKS-vector. X. laevis-Ska3 (NM_001127749), fancd2 (NM_AY633665) and smc2 (NM_001087904) were obtained by RT-PCR and cloned in pGEM-T vector. The following plasmids were used for preparation of antisense RNA probes, enzyme used for linearization and polymerases are indicated: X. laevis znf367 EST-pBKS- (XhoI, T7); pcna-pBSK (SalI, T7); sox2-pCS2+ (EcoR1, T7); n-tubulin-pBKS (NotI, T3); elrC-pBKS (NOTI, T7); elrD-pBSK (XhoI, T3); rx142. nrg1-pBKS (BamHI, T3); p27-Pbsk (BamHI, T7). X. laevis fancd2-pGEM-T (ClaI; SP6); X. laevis ska3-pGEM-T (NcoI; SP6); X. laevis smc2-pGEM-T (NcoI; SP6).
TUNEL and PH3 staining in Xenopus
TUNEL (TdT-mediated dUTP-dig nick end labeling) and PH3 (phosho histone 3) staining was performed at neurula stage according to established protocols41,43. TUNEL and PH3 positive cells were counted within defined areas in control and injected side of each manipulated embryo. P-values were calculated by paired Student’s T-test using GraphPad Prism 6 software (San Diego, CA, USA). Statistical significance was indicated as: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from 30 Xenopus morphants (at stage 18) using Nucleospin® RNA (Macherey-Nagel) according to the manufacturer’s instruction. cDNA was prepared by using iScript™ cDNA Synthesis Kit (Bio-Rad) and quantitative real-time PCR was performed using GoTaq®qPCR master mix (Promega) according to the manufacturer’s instruction. Relative expression levels of each gene were calculated using the 2−ΔΔCt method44 and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The following primers were used to perform qRT-PCR: pcna45 (Forward: 5′-CGTCGCGGTAATCCCTTA-3′; Reverse: 5′-TTGACCTCCTAGGGCAGAGA-3′); N-tubulin and sox2 (De Robertis’ lab, web site: http://www.hhmi.ucla.edu/derobertis/); elrC46 (Forward: 5′-GCTTTCTATCCTCCCCAGGT-3′; Reverse: 5′-TGCCACAGGACACTCTCATC-3′); cby1 (Forward: 5′-TGAAGCGGTTCCAGTTGTCG-3′; Reverse: 5′-TTGGTGGCAACAACCCTCTT-3′); ska3 (Forward: 5′-ACCGGAACTTTCCTACAGGC-3′; Reverse: 5′-ATTTCTGGGCGTGTTGGTGT-3′); fancd2 (Forward: 5′-CCCTACACTCACCAGGCAAAC-3′; Reverse: 5′-AGCGTTTCAGCTTTCTTGCTATT-3′); scm2 (Forward: 5′-GCTGAAAGAGAGAAGAAACGCAAA; Reverse: 5′-CTTGCAGAGAGCTCAGACCATC-3′); rx1 (Forward: 5′-GAGGAACCGGACAACATTCAC-3′; Reverse: 5′-TCATAGCCAGCTCTTZCTCTGC-3′); gapdh (Forward: 5′-CTTTGATGCTGATGCTGGA-3′; Reverse: 5′-GAAGAGGGGTTGACAGGTGA-3′).
Statistics
Statistical analysis for qRT-PCR experiments were performed by Student’s T-test using GraphPad Prism 6 software (San Diego, CA, USA). The levels of mRNA expression for individual genes were evaluated for Control-Morpholino or ZNF367-Morpholino injected embryos by qRT-PCR. The results obtained in three independent experiments were normalized to the expression of the hosekeeping gene, gapdh. The mean of the Control-Morpholino was set 1. The relative mRNA levels were calculated using the comparative Ct method (2−ΔΔCt)44,47. Statistical significance was indicated as: *p ≤ 0.05.
Statistical analysis for the phenotypes observed after the injection of the Control-Morpholino or the injection of ZNF367-Morpholino, were performed by Student’s T-test using GraphPad Prism 6 software (San Diego, CA, USA). We compared the percentage of the embryos with altered markers gene expression between the Control-Morpholino injected embryos and the ZNF367-Morpholino injected embryos. Statistical significance was indicated as: **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
WGCNA (Weighted Gene Co-expression Network Analysis)
Network analysis was performed using WGCNA method21. Samples used for the workflow were derived from two independent datasets, one from Nothobranchius furzeri’s brain, comprehensive of two strains (MZM-04010 and GRZ), six different time points and five replicates per time point2, and the other one from human embryonic stem cells. In particular the latter was obtained from a cerebro-cortical developmental experiment performed on hESC with 9 different time points20.
Network analysis was performed through different steps:
- Setting of the soft threshold, coefficient necessary for the adjacency matrix construction, as shown in the formula:
- Adjacency matrix and TOM (Topological Overlap Matrix), defined as:
Hierarchical clustering and modules detection after measuring the module eigengenes; every module is characterized by a color (as the module which has been studied for the analysis, defined by the turquoise color)
Module-trait relationship table construction, as correlation between single gene expression and external trait (in this case aging/development)
Module membership plot (as correlation between single gene expression and module eigengene): this was done for both the N. furzeri and the H. sapiens datasets, as described in Fig. 6A
Visualization with Cytoscape software.
Network construction was done in two independent analyses: the first one only on Nothobranchius furzeri dataset, while the latter using a consensus network obtained matching the two datasets. As soft threshold, we chose β = 6 for both the analyses to obtain the correspondent adiacency matrix and TOM, and significant modules negatively correlated with N. furzeri brain aging were selected. The genes contained in the selected modules were then tested for GO analysis using WebGestalt software, and then visualized using Cytoscape. Finally, the overall module membership of the genes contained in the “turquoise” module (as specified above, and only for the second analysis) was plotted on the ranked genes for both the killifish and the human data.
Data availability
All data generated or analysed during this study are included in the manuscript.
Acknowledgements
We thank Guglielma De Matienzo and Dr. Elena Landi for technical support. We would like to thank Dr. Joanne Spataro, EAP Senior Instructor, Language Center, University of Pisa for manuscript revision and Prof. Alessandro Massolo, University of Pisa for suggestions and feedbacks on data statistical analysis. This work was supported by funding from University of Pisa (Michela Ori).
Author Contributions
V.N., S.M., D.C. performed all the Xenopus experiments. M.T.M. and A.C. performed WGCNA (Weighted Gene Co-expression Network Analysis), contributed in the manuscript discussion and writing. M.O. contributed to conceptualization, provided necessary financial resources, experimental supervision, data analysis and discussion, writing.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Encinas JM, et al. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgart M, et al. RNA-seq of the aging brain in the short-lived fish N. furzeri - conserved pathways and novel genes associated with neurogenesis. Aging Cell. 2014;13:965–974. doi: 10.1111/acel.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lander ES, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 4.Tupler R, Perini G, Green MR. Expressing the human genome. Nature. 2001;409:832–833. doi: 10.1038/35057011. [DOI] [PubMed] [Google Scholar]
- 5.Laity JH, Lee BM, Wright PE. Zinc finger proteins: new insights into structural and functional diversity. Curr Opin Struct Biol. 2001;11:39–46. doi: 10.1016/S0959-440X(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 6.Cassandri M, et al. Zinc-finger proteins in health and disease. Cell death discovery. 2017;3:17071. doi: 10.1038/cddiscovery.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue T, Ogawa M, Mikoshiba K, Aruga J. Zic deficiency in the cortical marginal zone and meninges results in cortical lamination defects resembling those in type II lissencephaly. J Neurosci. 2008;28:4712–4725. doi: 10.1523/JNEUROSCI.5735-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiehl TR, Fischer SE, Ezzat S, Asa SL. Mice lacking the transcription factor Ikaros display behavioral alterations of an anti-depressive phenotype. Exp Neurol. 2008;211:107–114. doi: 10.1016/j.expneurol.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Asano H, Murate T, Naoe T, Saito H, Stamatoyannopoulos G. Molecular cloning and characterization of ZFF29: a protein containing a unique Cys2His2 zinc-finger motif. Biochem J. 2004;384:647–653. doi: 10.1042/BJ20040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain M, et al. ZNF367 inhibits cancer progression and is targeted by miR-195. PLoS One. 2014;9:e101423. doi: 10.1371/journal.pone.0101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li WX, et al. Comprehensive tissue-specific gene set enrichment analysis and transcription factor analysis of breast cancer by integrating 14 gene expression datasets. Oncotarget. 2017;8:6775–6786. doi: 10.18632/oncotarget.14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leeman DS, et al. Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during aging. Science. 2018;359:1277–1283. doi: 10.1126/science.aag3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/S0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- 14.Andreazzoli M, et al. Xrx1 controls proliferation and neurogenesis in Xenopus anterior neural plate. Development. 2003;130:5143–5154. doi: 10.1242/dev.00665. [DOI] [PubMed] [Google Scholar]
- 15.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/S0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 16.Hochegger H, et al. New B-type cyclin synthesis is required between meiosis I and II during Xenopus oocyte maturation. Development. 2001;128:3795–3807. doi: 10.1242/dev.128.19.3795. [DOI] [PubMed] [Google Scholar]
- 17.Souopgui J, Solter M, Pieler T. XPak3 promotes cell cycle withdrawal during primary neurogenesis in Xenopus laevis. EMBO J. 2002;21:6429–6439. doi: 10.1093/emboj/cdf644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vernon AE, Devine C, Philpott A. The cdk inhibitor p27Xic1 is required for differentiation of primary neurones in Xenopus. Development. 2003;130:85–92. doi: 10.1242/dev.00193. [DOI] [PubMed] [Google Scholar]
- 19.Perron M, Furrer MP, Wegnez M, Theodore L. Xenopus elav-like genes are differentially expressed during neurogenesis. Mech Dev. 1999;84:139–142. doi: 10.1016/S0925-4773(99)00056-8. [DOI] [PubMed] [Google Scholar]
- 20.van de Leemput J, et al. CORTECON: a temporal transcriptome analysis of in vitro human cerebral cortex development from human embryonic stem cells. Neuron. 2014;83:51–68. doi: 10.1016/j.neuron.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4:Article17. doi: 10.2202/1544-6115.1128. [DOI] [PubMed] [Google Scholar]
- 22.Hawrylycz MJ, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldham MC, et al. Functional organization of the transcriptome in human brain. Nature neuroscience. 2008;11:1271–1282. doi: 10.1038/nn.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fazzio TG, Panning B. Control of embryonic stem cell identity by nucleosome remodeling enzymes. Curr Opin Genet Dev. 2010;20:500–504. doi: 10.1016/j.gde.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirano T. At the heart of the chromosome: SMC proteins in action. Nature reviews. Molecular cell biology. 2006;7:311–322. doi: 10.1038/nrm1909. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, et al. Ska3 Phosphorylated by Cdk1 Binds Ndc80 and Recruits Ska to Kinetochores to Promote Mitotic Progression. Curr Biol. 2017;27:1477–1484 e1474. doi: 10.1016/j.cub.2017.03.060. [DOI] [PubMed] [Google Scholar]
- 27.Daum JR, et al. Ska3 is required for spindle checkpoint silencing and the maintenance of chromosome cohesion in mitosis. Curr Biol. 2009;19:1467–1472. doi: 10.1016/j.cub.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu TX, et al. Knockdown of zebrafish Fancd2 causes developmental abnormalities via p53-dependent apoptosis. Developmental Cell. 2003;5:903–914. doi: 10.1016/S1534-5807(03)00339-3. [DOI] [PubMed] [Google Scholar]
- 29.Rogers KJ, Fu W, Akey JM, Monnat RJ., Jr. Global and disease-associated genetic variation in the human Fanconi anemia gene family. Human molecular genetics. 2014;23:6815–6825. doi: 10.1093/hmg/ddu400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong H, et al. Update of the human and mouse Fanconi anemia genes. Human genomics. 2015;9:32. doi: 10.1186/s40246-015-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naim V, Rosselli F. The FANC pathway and mitosis: a replication legacy. Cell Cycle. 2009;8:2907–2911. doi: 10.4161/cc.8.18.9538. [DOI] [PubMed] [Google Scholar]
- 32.Naim V, Rosselli F. The FANC pathway and BLM collaborate during mitosis to prevent micro-nucleation and chromosome abnormalities. Nat Cell Biol. 2009;11:761–768. doi: 10.1038/ncb1883. [DOI] [PubMed] [Google Scholar]
- 33.Politis PK, Thomaidou D, Matsas R. Coordination of cell cycle exit and differentiation of neuronal progenitors. Cell cycle. 2008;7:691–697. doi: 10.4161/cc.7.6.5550. [DOI] [PubMed] [Google Scholar]
- 34.Hardwick LJ, Ali FR, Azzarelli R, Philpott A. Cell cycle regulation of proliferation versus differentiation in the central nervous system. Cell Tissue Res. 2015;359:187–200. doi: 10.1007/s00441-014-1895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miguez DG. A Branching Process to Characterize the Dynamics of Stem Cell Differentiation. Scientific reports. 2015;5:13265. doi: 10.1038/srep13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diez del Corral R, Storey KG. Markers in vertebrate neurogenesis. Nat Rev Neurosci. 2001;2:835–839. doi: 10.1038/35097587. [DOI] [PubMed] [Google Scholar]
- 37.Romeo F, Falbo L, Costanzo V. Replication, checkpoint suppression and structure of centromeric DNA. Nucleus. 2016;7:540–546. doi: 10.1080/19491034.2016.1255836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li XL, et al. Fancd2 Is Required for Nuclear Retention of Foxo3a in Hematopoietic Stem Cell Maintenance. Journal of Biological Chemistry. 2015;290:2715–2727. doi: 10.1074/jbc.M114.619536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaitanos TN, et al. Stable kinetochore-microtubule interactions depend on the Ska complex and its new component Ska3/C13Orf3. The EMBO journal. 2009;28:1442–1452. doi: 10.1038/emboj.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nieuwkoop, P. D. & Faber, J. Normal table of Xenopus laevis (Daudin)-a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. Australian: North-Holland Publishing company (1967).
- 41.Casini P, Nardi I, Ori M. Hyaluronan is required for cranial neural crest cells migration and craniofacial development. Dev Dyn. 2012;241:294–302. doi: 10.1002/dvdy.23715. [DOI] [PubMed] [Google Scholar]
- 42.Reisoli E, De Lucchini S, Nardi I, Ori M. Serotonin 2B receptor signaling is required for craniofacial morphogenesis and jaw joint formation in Xenopus. Development. 2010;137:2927–2937. doi: 10.1242/dev.041079. [DOI] [PubMed] [Google Scholar]
- 43.Ori M, Nardini M, Casini P, Perris R, Nardi I. XHas2 activity is required during somitogenesis and precursor cell migration in Xenopus development. Development. 2006;133:631–640. doi: 10.1242/dev.02225. [DOI] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 45.Huyck RW, Nagarkar M, Olsen N, Clamons SE, Saha MS. Methylmercury exposure during early Xenopus laevis development affects cell proliferation and death but not neural progenitor specification. Neurotoxicol Teratol. 2015;47:102–113. doi: 10.1016/j.ntt.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seo S, Lim JW, Yellajoshyula D, Chang LW, Kroll KL. Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. EMBO J. 2007;26:5093–5108. doi: 10.1038/sj.emboj.7601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arango D, et al. c-Myc overexpression sensitises colon cancer cells to camptothecin-induced apoptosis. British journal of cancer. 2003;89:1757–1765. doi: 10.1038/sj.bjc.6601338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in the manuscript.