Abstract
Background
We compared balloon dacryocystorhinostomy with conventional endoscopic dacryocystorhinostomy for the management of acquired distal nasolacrimal obstruction and the quality of life post procedure.
Methods
98 patients, aged 10–73 years, were recruited and randomized into 2 groups of 49 each who underwent conventional endoscopic dacryocystorhinostomy (group 1) and 9 mm balloon assisted endoscopic dacryocystorhinostomy (group 2). Follow-up sessions were conducted at 3, 6 and 12 months post-op.
Results
Group 2 showed significantly shorter mean operative time (25.10 min versus 29.82; p < 0.001), lesser pain in the post-op evening (mean 2.12 versus 2.9 on NRS-11 pain scale; p < 0.001) as well as on first post-op day (mean 1.08 versus 1.73; p < 0.001). Success was achieved in 89.79% in group 1 and 93.87% in group 2 at 3 months (p = 0.46) which declined due to recurrences to 85.71% and 87.75% respectively at 12 months (p = 0.76). Complications occurred in 14 cases in group 1 and in 10 cases in group 2 (p = 0.34). All were minor. Mean GBI scores (for quality of life assessment) at 12 months follow-up were 27.20 and 28.38 respectively (p = 0.08).
Conclusion
The efficacy, safety and quality of life of balloon dacryocystorhinostomy and conventional endoscopic dacryocystorhinostomy were comparable. In addition, balloon dacryocystorhinostomy had significantly shorter operative time and lesser post-op pain.
Keywords: Balloon catheter, Endoscopic, Dacryocystorhinostomy, Lacrimal, Randomized
Introduction
Epiphora (excessive tearing) is a distressing symptom which causes social embarrassment to the patient and adversely affects vision related quality of life (QOL).1 This symptom can be relieved by dacryocystorhinostomy (DCR) which is an effective procedure for bypassing the obstruction in the distal nasolacrimal apparatus. The external approach of DCR (Ext DCR) described by Toti in 1904 had been the gold standard in the past, but with improvements in the endoscopes, now endoscopic endonasal approaches are becoming popular; especially among ENT surgeons due to shorter operative time, low complications, an absence of visible scar and high success rate which has been quoted as 95–100% in previous studies.2, 3
The conventional technique of endoscopic endonasal DCR (End DCR)4, 5 has undergone many modifications. These include the use of stents, newer flaps, and mucosal preservation methods, local application of mitomycin C (MMC), use of powered instruments like drills and micro-debriders, use of lasers, radiofrequency, composite technique and balloons. There are published reports for as well as against all of these modifications.6, 7, 8, 9, 10, 11, 12, 13, 26, 27
Balloon-assisted endoscopic endonasal DCR (Balloon DCR) is comparatively a newer modification, which was initially introduced by Becker et al. in 1996 as a dilatation technique for congenital nasolacrimal obstruction.14 The technique has been claimed to have high success, shorter operative time and lower complications. However, studies on Balloon DCR are limited in number and are mostly retrospective case records analyses only.15 Prospective, controlled trials employing Balloon DCR are even scarcer. One such study conducted by Ragab et al. compared 5 mm balloon assisted DCR with End DCR but did not find any difference in the success rate of the two procedures.16 Further, we did not find any study on QOL following Balloon DCR in the literature despite an extensive search. QOL studies are available only for End DCR.17, 18
Lack of enough prospective studies and complete absence of reports on QOL following Balloon DCR prompted us to undertake this prospective, randomized, interventional, controlled study comparing End DCR with Balloon DCR for their success rate, operative time, morbidity and post-surgery QOL.
Material and methods
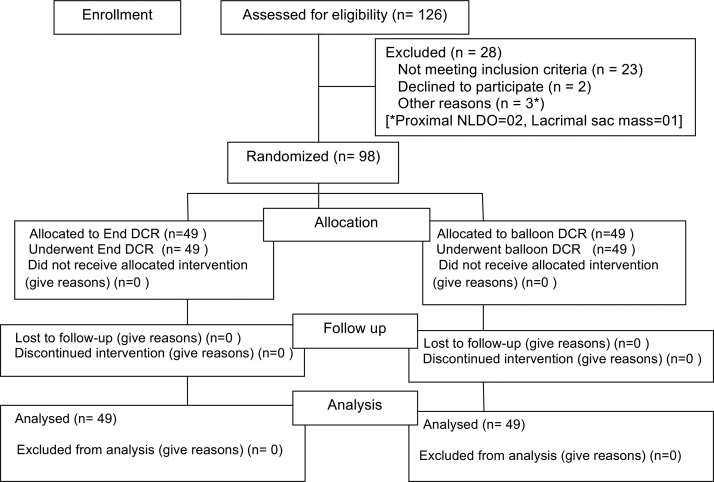
The study was performed at a tertiary care hospital from August 2014 to March 2017. Ethical clearance was obtained from the Institutional Ethics Committee and the study was approved by the Scientific Review Committee of our institute. Informed written consent was obtained from all participants. A total of 126 patients were assessed. Finally, 98 patients were selected as elaborated in the flow diagram of the study (Fig. 1).
Fig. 1.
Flow diagram of the study as per CONSORT statement.
Inclusion criteria
All patients presenting with features of acquired, complete, distal nasolacrimal drainage obstruction (NLDO) like epiphora, mucopurulent eye discharge, chronic dacryocystitis of more than one year duration were included in the study.
Exclusion criteria
Patients with symptoms due to any other cause except distal NLDO were excluded. This included proximal NLDO like common canalicular block, ocular pump failure, dry eye syndrome or those having post-traumatic bony deformity, bone diseases, Down's syndrome, suspicion of malignancy, radiation therapy, large dacryoliths, Sarcoidosis, Wegener's granuloma, chronic inflammatory disease of nose and sinuses, age less than 10 years, systemic disease likely to jeopardize safety in surgery like bleeding dyscrasia and non-consenting patients.
Sample size calculation
The sample size was calculated based on a projected difference of 20% in the main outcome measure, i.e. success rate of the two procedures. The success rate for sample size calculation was taken as 75% for End DCR and 95% for Balloon DCR based on results of a pilot study on 24 patients conducted earlier. Based on this, we calculated a sample size of minimum 49 patients per group, which would permit a type 1 error (alpha) of 0.05 with a type II error (beta) of 0.5 and power of 0.8 permitting a two tail analysis.
Patients’ evaluation
Patients were registered and demographic data recorded. History of various symptoms of NLDO was elicited. Complete Ophthalmologic and ENT evaluation were done.
Lacrimal sac syringing was used to determine the degree and site of obstruction. Reflux of fluid through the opposite punctum indicated distal NLDO, while the reflux from the same punctum indicated proximal NLDO. If the fluid passed into the nose freely with no reflux into the eye, the lacrimal system was labelled as patent not requiring surgery.
Probing was performed by inserting a lacrimal probe through the punctum and led into the canaliculus. If it stopped ‘hard’ against the bone, it ruled out the canalicular block. If it stopped ‘soft’, a blockage in the canaliculus was likely.
Dye disappearance test was used for assessing functional patency.
Diagnostic nasal endoscopy was performed with a 4 mm, 0 and 30-degree rigid endoscope after decongesting the nose. Intranasal pathology like DNS/polyps/rhinosinusitis was noted.
Pre-op Munk's score19 was recorded for each patient enrolled in the study.
CT scans/MRI were obtained only in cases of a history of prior trauma, surgery, suspected neoplasm or significant sinus disease.
Group allocation
Allocation of the patients to a particular group was done using table of random numbers, but stratification for age group and gender was made to keep the two groups demographically similar. The random number lists were generated by a staff member and neither the patient nor the investigators were aware of the treatment group allotted till the day of actual surgery.
Intervention
End DCR was performed in group 1 and Balloon DCR in group 2.
Anaesthesia
Surgery was carried out under LA (2% lignocaine with 1 in 200,000 adrenalin by local infiltration) or GA depending on patients’ acceptance and consent. The patient was cleaned and draped after induction of GA/infiltration of LA. Nasal endoscopy was performed with 0-degree/30-degree rigid nasal endoscope. Associated nasal pathology like DNS/polyps was corrected before performing DCR.
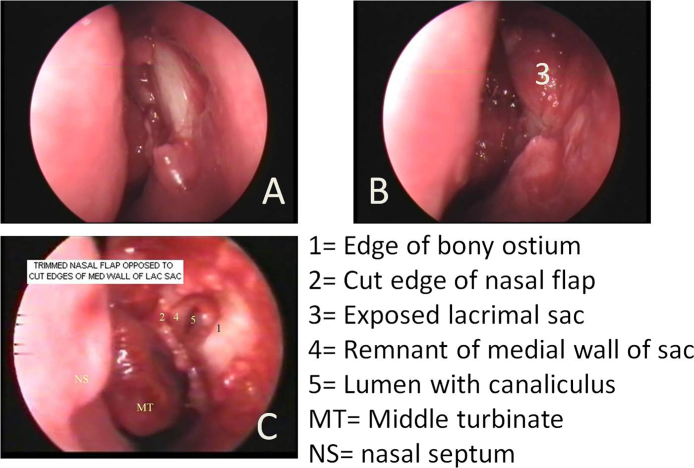
Details of operative procedure of End DCR (Fig. 2)
Fig. 2.
Main steps of conventional End DCR. (A) Bone overlying lacrimal sac has been removed; (B) well exposed lacrimal sac is ready for incision; (C) medial wall of lacrimal sac removed exposing the lumen of the sac and opening of the common canaliculus. The remnant of the medial wall of the sac has been placed opposite the trimmed nasal mucoperiosteal flap.
A curvilinear incision was made in the mucosa overlying lacrimal sac using a sickle knife and mucoperiosteal flap raised in a posterior direction up to the suture line between lacrimal bone and uncinate process, exposing the bone of lacrimal fossa completely. Bone was removed using a Kerrison DCR punch forceps starting at the suture between uncinate and lacrimal bone and advancing anteriorly to expose lacrimal sac which was confirmed by movement of the sac on pressing the medial canthus. The stoma thus created was enlarged to completely expose the lacrimal sac. The lacrimal punctum was cannulated and the lacrimal sac filled with saline. A vertical incision in the lacrimal sac was made with a lacrimal knife. The medial wall of the sac was removed completely and remaining edge of the medial wall was placed in opposition with the trimmed mucoperiosteal flap. Sac syringing was carried out. The free flow of saline indicated the successful creation of a nasolacrimal fistula. A silicon tube with wide outer diameter and thin central segment (STENTube® marketed by Quest Medical, Inc., Allen, TX75002, USA) was inserted bicanalicularly, the two ends of the tube were tied together within the nasal cavity. The extra length of the tube was trimmed. Light anterior nasal packing using non-absorbable material was done.
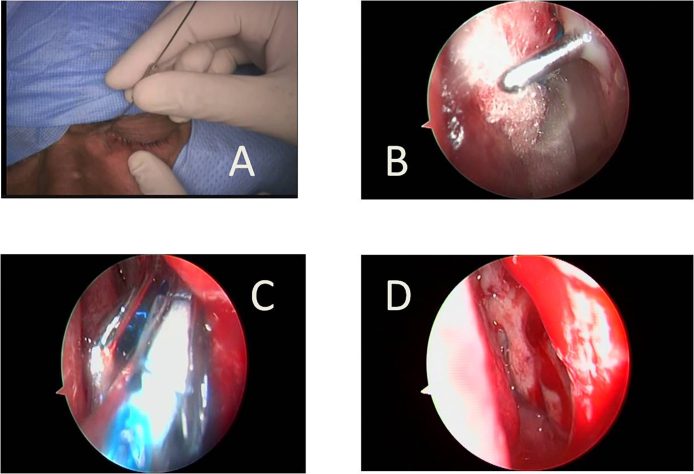
Details of operative procedure of Balloon DCR (Fig. 3)
Fig. 3.
Main steps of Balloon DCR. (A) A Bowman's 3–4 size reinforced probe is passed through the lacrimal punctum into the nose, piercing the medial wall of the sac, lacrimal bone and nasal mucosa creating a small rhinostoma; (B) probe is seen in the nose through the rhinostoma with some mucopus around it. The probe is moved up and down to enlarge the rhinostoma; (C) the opening is enlarged by dilatation using a 09 mm high-pressure balloon catheter; (D) adequate size (7–9 mm) of rhinostoma achieved.
-
(a)
Creation of rhinostoma:
After dilating the upper punctum, a reinforced stainless steel 3–4 size Bowman probe was passed into the lacrimal sac through the superior canaliculus. The probe was pushed in a postero-inferior direction through the lacrimal sac, bone of lacrimal fossa and the nasal mucoperiosteum. The appearance of the probe in the nose was seen by nasal endoscope. The probe had to be passed 3–4 times in this manner to make a slit-like opening which created a fistula passing through the medial wall of lacrimal sac, thin posterior lacrimal bone, and nasal mucoperiosteum and was represented by a small rhinostoma on the nasal side.
-
(b)
Enlargement of rhinostoma and fistula:
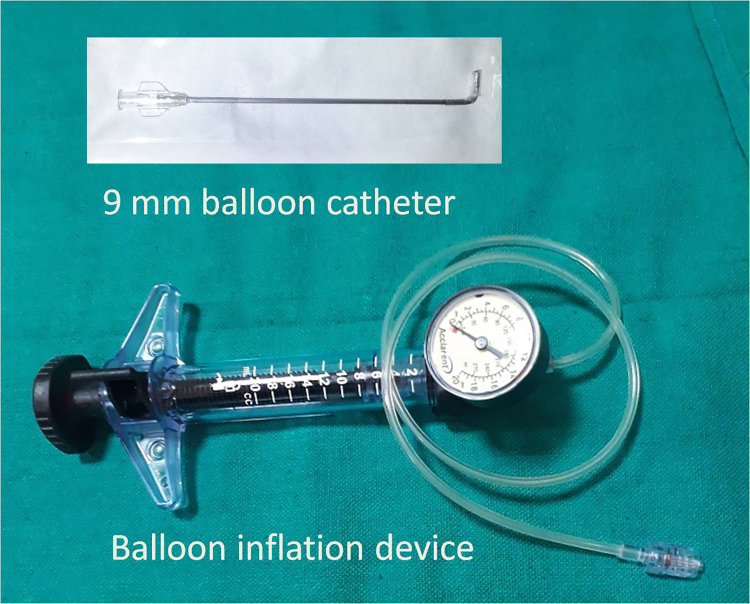
Any loose bone chips or mucosal tags were removed using microscissors and Blakesley forceps. A 09 mm Balloon catheter (LacriCATH® marketed by Quest Medical, Inc, Allen, TX75002, USA) (Fig. 4) was introduced through the nose. The lacrimal probe was used to guide the balloon catheter into rhinostoma. Then the catheter was connected to a saline filled inflation device and the balloon was inflated to 8 atm for 90 s. The balloon was then deflated and re-inflated to 8 atm for 60 s. This was repeated until the size of rhinostoma became satisfactory, usually 7–9 mm. Finally, the balloon was deflated fully and taken out of the nose.
-
(c)
Refining the stoma:
The rhinostoma was enlarged in an anterior direction using back biting forceps if required. Any loose mucosal tags were removed.
-
(d)
Confirmation of patency:
Sac syringing was carried out. Free flow of saline indicated the successful creation of a nasolacrimal fistula.
-
(e)
Stenting and nasal packing:
A silicon tube having same specifications as in End DCR group was inserted through both canaliculi, the two ends of the tube were tied together within the nasal cavity. The extra length of the tube was trimmed. Light anterior nasal packing using non-absorbable material was done.
Fig. 4.
A 09 mm balloon catheter and inflation device.
Post-op care
Patients were discharged on the next day, after removing the nasal pack. They were advised not to blow nose for 10 days post-op. Systemic antibiotics were not prescribed as a routine. But, antibiotic eye drops, steroid eye drops, and nasal decongestant drops were given for 7 days. Saline nasal spray twice daily was also used for 15 days to reduce crusting. Patients were reviewed on 7th day when endoscopic nasal toilet and sac syringing were done. Stents were removed at 3 months in all cases except when excessive granulations/infection warranted their early removal.
Follow-up
The patients were called for follow-up at 3, 6 and 12 months and more where possible. The follow-up visit comprised inquiry into symptoms, nasal endoscopy, sac syringing, dye disappearance test, documenting complications, Munk's score recording and filling Glasgow Benefit Inventory (GBI) questionnaire.
Main outcome measures
Success
A case was declared ‘successful’ if it fulfilled all the three of the following criteria:
-
1.
Anatomic success – Free flow of saline into the nose on sac syringing. Obstructed or partially obstructed flow was defined as failure.
-
2.
Functional success – Appearance of 2% fluorescein dye at the rhinostomy site within 10 s of instillation of few drops of it in the lower fornix of eye. Appearance of dye in nose later than 10 s or non-appearance was read as failure.
-
3.
Subjective success – Grade 0 or 1 on Munk's score was successful. Grade 2, 3, 4 were taken as failure.
Recurrence
Any patient who met all the 3 criteria of success at 3 months follow-up, but failed to meet these criteria in subsequent reviews (i.e. at 6 months or 12 months) was termed as recurrence.
QOL assessment
QOL after DCR surgery was assessed by Glasgow Benefit Inventory (GBI) score developed by Robinson et al.20 The GBI is a validated scale which comprises 18 questions and the response to each question is based on a five-point Likert scale. This response ranges from a large worsening of health status through ‘no change’ to a large betterment. Therefore, the scores range from −100 to +100 through 0. If the score is positive it means that the patient is satisfied with the procedure.
Operative time
Operative time was recorded from the start of the incision (in the case of End DCR) or starting punctal dilatation (in the case of Balloon DCR) till the insertion of the nasal pack. Time taken to handle associated nasal pathology like DNS was not included in the operative time.
Post-op pain
Post-op pain was assessed on Numeric Rating Scale (NRS-11)28 by asking the patient to record three pain ratings, corresponding to current, best and worst pain experienced over the past 24 h on a scale of 0 (no pain) to 10 (worst pain imaginable), on post-op evening and everyday for 7 days post-op. The average of the 3 worst ratings in a day was used to represent the patient's level of pain for that day.
Complications
Complications and adverse events during the surgery and post-operatively were looked for and recorded.
Statistical analysis
Data are presented as mean and standard deviation (SD) or percent of patients. Parametric tests were applied for data that followed a normal distribution. Non-parametric tests were applied for data that did not follow a normal distribution. Statistically significant difference was defined as p < 0.05. Statistical analyses were performed using IBM© SPSS© Statistics for Windows, Version 22 (Released 2013. IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.).
Results
There were 98 patients (49 in each group), aged 10–73 years. Both groups were well balanced for age and gender of the participants. Females outnumbered males (Table 1a, Table 1b).
Table 1a.
Patients’ demographics and descriptive statistics.
| Variable | All patients (n = 98) |
End DCR (n = 49) |
Balloon DCR (n = 49) |
Statistical tool (statistic, p value) |
|---|---|---|---|---|
| Age (years) | ||||
| Range | 10–73 | 13–73 | 10–67 | |
| Mean | 41.92 | 42.61 | 41.24 | Student's t test |
| SD | 15.72 | 16.16 | 15.40 | (0.43, 0.67) |
| Gender – number (%) | ||||
| Male | 35 (35.71) | 17 (34.69) | 18 (36.73) | Chi-square test |
| Female | 63 (64.28) | 32 (65.30) | 31 (63.26) | (0.4, 0.83) |
| Eye affected – number (%) | ||||
| Right | 29 (29.59) | 14 (53.13) | 15 (30.61) | Chi square test |
| Left | 31 (31.63) | 13 (26.53) | 18 (36.73) | (1.78, 0.40) |
| Both | 38 (38.77) | 22 (44.89) | 16 (32.65) | |
| Side operated – number (%) | ||||
| Right | 50 (51.02) | 26 (53.06) | 24 (48.97) | Chi-square test |
| Left | 48 (48.97) | 23 (46.93) | 25 (51.02) | (0.16, 0.69) |
| Fresh cases – number (%) | 87 (88.77) | 44 (89.79) | 43 (87.75) | Chi-square test |
| Revision cases – number (%) | 11 (11.22) | 05 (10.2) | 06 (12.24) | (0.10,0.75) |
| Prev End DCR | 10 | 05 | 05 | |
| Prev Balloon DCR | 01 | 00 | 01 | |
| Additional nasal pathology – number (%) | ||||
| Total | 12 (12.24) | 06 (12.24) | 06 (12.24) | Chi-square test |
| DNS | 04 | 03 | 01 | (2, 0.57) |
| Concha bullosa | 04 | 01 | 03 | |
| Hypertrophic IT | 02 | 01 | 01 | |
| Polyps | 02 | 01 | 01 | |
| Anaesthesia used – number (%) | ||||
| GA | 67 (68.36) | 34 (69.38) | 33 (67.34) | Chi square test |
| LA | 31 (31.63) | 15 (30.61) | 16 (32.65) | (0.04, 0.83) |
| Duration of follow-up (months) | ||||
| Range | 12–18 | 12–18 | 12–18 | Student's t test |
| Mean | 14.57 | 14.20 | 14.94 | (−1.22, 0.22) |
| SD | 2.98 | 2.92 | 3.03 | |
Table 1b.
Distribution of patients in various strata for age and gender.
| Stratum for age and gender | Total number in the stratum | Distributed to Balloon DCR group | Distributed to End DCR group |
|---|---|---|---|
| Male 10–29 years | 12 | 7 | 5 |
| Female 10–29 years | 17 | 8 | 9 |
| Male 30–49 years | 13 | 7 | 6 |
| Female 30–49 years | 24 | 11 | 13 |
| Male 50–69 years | 8 | 3 | 5 |
| Female 50–69 years | 21 | 12 | 9 |
| Male 70 years or more | 2 | 1 | 1 |
| Female 70 years or more | 1 | 0 | 1 |
| Total | 98 | 49 | 49 |
Epiphora was the main presenting feature in the majority of cases (76.53%), followed by purulent eye discharge (9.18%) and mucocele (8.16%).
There was a shorter mean operative time (25.10 ± 2.16 min, 95% CI = 24.48, 25.72) in Balloon DCR group compared to End DCR group (29.82 ± 3.01 min, 95% CI = 28.96, 30.68). The difference was highly significant (two-tailed t-test, t = 8.88, p < 0.001). Mean operative time to tackle DNS (endoscopic septoplasty), concha bullosa (excision), hypertrophic turbinates (turbinate reduction) and polyps (FESS) was 7.5 min, 3.75 min, 4 min and 23.5 min respectively.
Post-op pain was significantly lesser in Balloon DCR group (mean 2.12 ± 0.45, CI 1.99, 2.24 on NRS-11 scale) compared to End DCR group (mean 2.9 ± 0.43, CI 2.77, 3.02) on post-op evening (two tailed t test, t = 8.06, p < 0.001). On first post-op day the mean NRS-11 score in Balloon DCR was 1.08 ± 0.34 (CI 0.98, 1.17) and in End DCR group it was 1.73 ± 0.53 (CI 1.57, 1.88) (two tailed t test, t = 7.22, p < 0.001).
By third post-op day pain score reduced to below ‘1’ and was zero thereafter in both groups. The mean post-op pain in both groups was below ‘3’ which translates to ‘mild pain’ in the clinical parlance.
Success was achieved in 91.83% cases (overall), 89.79% in End DCR group and 93.87% in Balloon DCR group at 3 months which in subsequent follow-ups declined due to recurrences to 88.77%, 87.75%, and 89.79% respectively at 6 months and 86.73%, 85.71% and 87.75% respectively at 12 months. The difference in success rates between the two groups was statistically not significant at any follow-up stage (p = 0.46, 0.74 and 0.76 respectively). Success rates in revision cases were poor in both groups – only about two third of revision cases were found to be successful at 12 months follow-up (Table 2).
Table 2.
Success of the procedure at various stages of the study.
| Number of cases | All patients | End DCR | Balloon DCR | Z-test |
|---|---|---|---|---|
| Successful/total (%) | (n = 98) | (n = 49) | (n = 49) | (Z, p value) |
| At 3 months post-op | ||||
| Total | 90/98 (91.83) | 44/49 (89.79) | 46/49 (93.87) | (−0.74, 0.46) |
| 95% CI (%) | 84.72, 95.81 | 78.25, 95.56 | 83.48, 97.90 | |
| Fresh cases | 83/87 (95.4) | 41/44 (93.18) | 42/43 (97.67) | (−1.00, 0.31) |
| Revision cases | 07/11 (63.63) | 03/05 (60) | 04/06 (66.66) | (−0.23, 0.81) |
| At 06 months post-op | ||||
| Total | 87/98 (88.77) | 43/49 (87.75) | 44/49 (89.79) | (−0.32, 0.74) |
| 95% CI (%) | 81.02, 93.62 | 75.76, 94.27 | 78.25,95.56 | |
| Fresh cases | 80/87 (91.95) | 40/44 (90.9) | 40/43 (93.02) | (−0.36,0.71) |
| Revision cases | 07/11 (63.63) | 03/05 (60) | 04/06 (66.66) | (−0.22, 0.81) |
| At 12 months post-op | ||||
| Total | 85/98 (86.73) | 42/49 (85.71) | 43/49 (87.75) | (−0.29, 0.76) |
| 95% CI (%) | 78.61, 92.08 | 73.33,92.90 | 75.76, 94.27 | |
| Fresh cases | 78/87 (89.65) | 39/44 (88.63) | 39/43 (90.69) | (−0.32, 0.75) |
| Revision cases | 07/11 (63.63) | 03/05 (60) | 04/06 (66.66) | (−0.22, 0.81) |
CI = confidence interval.
Five cases (5.1%) recurred during follow-up; 03 cases between 3 months and 6 months follow-up and 2 cases between 6 months and 12 months follow-up. Overall 2 cases recurred in End DCR group and 3 in Balloon DCR group. There was no significant difference in the recurrence rates of the two groups (Chi = 0.21, p = 0.64). The commonest cause of initial failure (8 cases) and recurrence (5 cases) was gradual blocking of stoma due to granuloma or synechiae.
A total of 24 adverse events occurred – 14 in End DCR group and 10 in Balloon DCR group. The difference was not significant (p = 0.34). All were of minor nature. No major event like severe epistaxis, optic nerve injury, orbital muscle injury, CSF leak occurred (Table 3).
Table 3.
Complications.
| All patients (n = 98) |
End DCR (n = 49) |
Balloon DCR (n = 49) |
Z-test (Z, p value) |
|
|---|---|---|---|---|
| Total adverse eventsa | 24 | 14 | 10 | 0.93, 0.34 |
| Synechiae | 06 | 01 | 05 | −1.68, 0.09 |
| Stent loss | 2 | 2 | 00 | 1.4, 0.15 |
| Orbital fat exposure | 3 | 3 | 00 | 1.75, 0.07 |
| Orbital emphysema | 1 | 1 | 00 | 1.0, 0.31 |
| Eye lid oedema | 4 | 3 | 1 | 1,02, 0.30 |
| Cheesewiring of canaliculus | 1 | 00 | 1 | −1.0, 0.31 |
| Granuloma | 6 | 5 | 1 | 1.68, 0.09 |
| Keratoconjunctivitis | 1 | 00 | 1 | −1.0, 0.31 |
| Intolerance to nasal pack | 1 | 00 | 1 | −1.0, 0.31 |
All were minor; no major complications like severe epistaxis/optic nerve injury/medial rectus/sup oblique injury etc. occurred in our series.
For assessing QOL, patients were asked to fill GBI questionnaire at 3 months, 6 months and 12 months post-operatively. The scores are summarized in Table 4.
Table 4.
GBI scores during the course of follow-up.
| All patients (n = 98) |
End DCR (n = 49) |
Balloon DCR (n = 49) |
Mann–Whitney U test (U, Z, p value) |
|
|---|---|---|---|---|
| At 3 months | ||||
| Range | −16 to 43 | −16 to 35 | −21 to 43 | |
| Mean | 23 | 21.55 | 24.44 | 950, −1.77, 0.07 |
| SD | 11.85 | 12.09 | 11.54 | |
| 95% CI | 20.62, 25.38 | 18.08, 25.02 | 21.13, 27.75 | |
| At 6 months | ||||
| Range | −7 to 36 | −7 to 34 | −16 to 36 | |
| Mean | 27.46 | 27.34 | 27.84 | 811.5, 1.61, 0.10 |
| SD | 6.93 | 6.96 | 9.71 | |
| 95% CI | 26.07, 28.85 | 25.34, 29.34 | 25.05, 30.63 | |
| At 12 months | ||||
| Range | −13 to 36 | −13 to 34 | −19 to 36 | |
| Mean | 27.34 | 27.20 | 28.38 | 743, 1.71, 0.08 |
| SD | 7.65 | 7.69 | 8.71 | |
| 95% CI | 25.81, 28.87 | 24.99, 29.41 | 25.88, 30.88 | |
CI = confidence interval; SD = standard deviation.
Discussion
Patients’ demography and descriptive statistics in our study were in line with earlier published works.
Significantly shorter mean operative time (25.10 ± 2.16 min) in Balloon DCR group compared to End DCR group (29.82 ± 3.01 min) is in line with other studies.16 Thin lacrimal bone is a fragile bone and can be broken easily by reinforced Bowman probe – drills, debriders or punches are not really needed. This considerably reduced the operating time in Balloon DCR group. However, it should be appreciated that mean reduction in operative time in Balloon DCR as compared to End DCR was only 4.72 min (95% CI = 3.86, 5.58) which may not be relevant in clinical practice especially if we consider the high cost of Balloon DCR.
Though mean post-op pain in Balloon DCR group was significantly lesser on NRS-11 scale, it was less than ‘3’ (denoting mild pain only) in both groups. Hence, this difference is not likely to be appreciated by the patients clinically as the minimal value of clinically important change for NRS-11 is 2 points or 30% change.28
Although only a few studies are available on Balloon DCR in the literature, these have reported either comparable or better success rates of Balloon DCR with lesser morbidity compared to End DCR.15, 16 Since the initial puncture in Balloon DCR is made by a Bowman's probe passed through the upper punctum, the opening in the lacrimal sac, lacrimal bone, and nasal mucosa–are all in one line. This ensures better anatomical alignment of the channel and also prevents sump effect. During End DCR the openings are made via the nose and may not lie in one line. A drawback of Balloon DCR, at least in theory, may be that the final rhinostoma created in this technique is much smaller than the one created in End DCR. However, the researchers have found no correlation between the size of stoma and success of DCR.21, 22
Recurrences after End DCR are mainly due to the closure of the rhinostoma with synechia and fashioning of the small misplaced bony window. Both these disadvantageous factors are less likely to occur in the case of Balloon DCR as already discussed above. Avoiding undue manipulation of proximal lacrimal drainage system like punctum and canaliculus is very important in both the techniques as this can lead to proximal obstruction resulting in failure.
QOL after the surgery in our study was assessed by GBI which is a validated tool and has been found useful for ENT procedures.23 There was no significant difference in GBI scores of the two procedures, signifying equal subjective satisfaction with the procedure in both groups. These scores rose during 6 months follow-up and were stable thereafter. This denotes a continued improvement in QOL up to 6 months after surgery which was thereafter stable and maintained without deterioration. A similar pattern was also observed by Smirnov et al.18 in their study of End DCR patients. There is no study available in the literature on QOL after Balloon DCR for comparison. Our study may be the first to report GBI scores after Balloon DCR.
Limitations of the study
GBI is generic for all ENT interventions and not specific to DCR surgery. Further, there is no scope for comparing pre-op and post-op scores in GBI. However, this limitation of GBI is well offset by its uniqueness of negative to positive scale imparting it ability to reveal not only the number of patients who benefitted by a procedure but also those not benefitted or who became worse.
Newer scores like LacQ,24 vision related QOL,1 nasolacrimal duct obstruction symptoms score (NLDO-SS),25 etc. which allow pre- and post-op comparison and are specific to lacrimation/eye related interventions were not available at the beginning of our study. Further, these are not fully validated as yet. Future studies utilizing these newer scores may elucidate their usefulness vis a vis GBI and are recommended.
In our study, the investigators were not aware of the procedure to be performed on a particular patient till the morning of surgery only, but were aware of it during operation and follow-ups. Unlike drugs, the surgical procedure cannot be concealed during operative and follow-up stage in trials involving surgical procedures. It is likely that some bias might have crept in on this account, although all attempts were made by the investigators to remain unbiased. The results of the study may be accepted with these limitations in mind.
We did not aim at achieving exactly equal groups in this study but equal groups occurred purely ‘by chance’ due to the process of randomization itself; as a random occurrence. No increase or decrease in the power/credibility of the study is being claimed by us on account of equal groups.
Conclusions
There was no significant difference in the proportion of successful outcomes, recurrence rate, post-op morbidity and the change in QOL between the two groups.
Both End DCR and Balloon DCR showed high success rate, low recurrence, minimal post-op morbidity and high level of patients’ satisfaction and improvement in QOL.
Balloon DCR scored over End DCR in having shorter operative time which may translate into lesser operative costs and faster clearance of waiting list. However, at present the cost of balloons is high (rupees eighteen to twenty thousand per balloon) which may offset any economic gain made due to lesser operative time.
Conflicts of interest
The authors have none to declare.
Acknowledgement
This paper is based on Armed Forces Medical Research Committee Project No. 4567/2014 granted and funded by the office of the Directorate General Armed Forces Medical Services and Defence Research Development Organisation, Government of India.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.mjafi.2017.08.010.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Shin J.H., Kim Y.D., Woo K.I. Impact of epiphora on vision-related quality of life. BMC Ophthalmol. 2015;15:6. doi: 10.1186/1471-2415-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jha K.N., Ramalingam W.V.B.S. External versus endoscopic dacryocystorhinostomy: a retrospective study. Med J Armed Forces India. 2009;65(1):23–25. doi: 10.1016/S0377-1237(09)80048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozer S., Ozer P.A. Endoscopic vs external dacryocystorhinostomy–comparison from the patients’ aspect. Int J Ophthalmol. 2014;7(4):689–696. doi: 10.3980/j.issn.2222-3959.2014.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh A.P., Narula V., Meher R. A new approach to endoscopic DCR. Braz J Otorhinolaryngol. 2012;78:7–11. doi: 10.5935/1808-8694.20120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farahani F. Endoscopic dacryocystorhinostomy. In: Gendeh B.S., editor. Otolaryngology. InTech; 2012. pp. 55–57. ISBN: 978-953-51-0624-1. Available from: http://www.intechopen.com/books/otolaryngology/endoscopicdacryocystorhinostomy. Accessed 27.05.17. [Google Scholar]
- 6.Codère F., Denton P., Corona J. Endonasal dacryocystorhinostomy: a modified technique with preservation of the nasal and lacrimal mucosa. Ophthal Plast Reconstr Surg. 2010;26(3):161–164. doi: 10.1097/IOP.0b013e3181b80af6. [DOI] [PubMed] [Google Scholar]
- 7.Sonkhya N., Mishra P. Endoscopic transnasal dacryocystorhinostomy with nasal mucosal and posterior lacrimal sac flap. J Laryngol Otol. 2009;123(3):320–326. doi: 10.1017/S0022215108003897. [DOI] [PubMed] [Google Scholar]
- 8.Trimarchi M., Giordano Resti A., Bellini C., Forti M., Bussi M. Anastomosis of nasal mucosal and lacrimal sac flaps in endoscopic dacryocystorhinostomy. Eur Arch Otorhinolaryngol. 2009;266(11):1747–1752. doi: 10.1007/s00405-009-1002-z. [DOI] [PubMed] [Google Scholar]
- 9.Shah H., Sharma S., Suri N., Patel A. Comparison of surgical outcome in endoscopic dacryocystorhinostomy with and without silicon stent placement. Natl J Med Res. 2013;3(1):34–37. [Google Scholar]
- 10.Callejas C.A., Tewfik M.A., Wormald P.J. Powered endoscopic dacryocystorhinostomy with selective stenting. Laryngoscope. 2010;120(7):1449–1452. doi: 10.1002/lary.20916. [DOI] [PubMed] [Google Scholar]
- 11.Dolmetsch A.M. Nonlaser endoscopic endonasal dacryocystorhinostomy with adjunctive mitomycin C in nasolacrimal duct obstruction in adults. Ophthalmology. 2010;117(5):1037–1040. doi: 10.1016/j.ophtha.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Panwar S.S., Lal P., Sukthankar P.S. Comparative analysis of laser assisted endoscopic and conventional endoscopic dacryocystorhinostomy. Med J Armed Forces India. 2006;62:228–230. doi: 10.1016/S0377-1237(06)80006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javate R., Pamintuan F. Endoscopic radiofrequency-assisted dacryocystorhinostomy with double stent: a personal experience. Orbit. 2005;24(1):15–22. doi: 10.1080/01676830590890864. [DOI] [PubMed] [Google Scholar]
- 14.Becker B.B., Berry F.D., Koller H. Balloon catheter dilatation for treatment of congenital nasolacrimal duct obstruction. Am J Ophthalmol. 1996;121:304–309. doi: 10.1016/s0002-9394(14)70279-x. [DOI] [PubMed] [Google Scholar]
- 15.Silbert D.I., Matta N.S. Outcomes of 9 mm balloon-assisted endoscopic dacryo cystorhinostomy: retrospective review of 97 cases. Orbit. 2010;29(1):25–28. doi: 10.3109/01676830903229715. [DOI] [PubMed] [Google Scholar]
- 16.Ragab S.M., el-Koddousy M.S., Badr M. Endocanalicular, high-pressure balloon catheter, endoscopic dacryocystorhinostomy: a randomized controlled trial. Otolaryngol Head Neck Surg. 2011;145(4):683–688. doi: 10.1177/0194599811410534. [DOI] [PubMed] [Google Scholar]
- 17.Jutley G., Karim R., Joharatnam N., Latif S., Lynch T., Olver J.M. Patient satisfaction following endoscopic endonasal dacryocystorhinostomy: a quality of life study. Eye. 2013;27:1084–1089. doi: 10.1038/eye.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smirnov G., Tuomilehto H., Kokki H. Symptom score questionnaire for nasolacrimal duct obstruction in adults – a novel tool to assess the outcome after endoscopic dacryocystorhinostomy. Rhinology. 2010;48:446–451. doi: 10.4193/Rhino10.069. [DOI] [PubMed] [Google Scholar]
- 19.Munk P.L., Lin D.T., Morris D.C. Epiphora: treatment by means of dacryocystoplasty with balloon dilation of the nasolacrimal drainage apparatus. Radiology. 1990;177:687–690. doi: 10.1148/radiology.177.3.2243969. [DOI] [PubMed] [Google Scholar]
- 20.Robinson K., Gatehouse S., Browning G.G. Measuring patient benefit from otorhinolaryngological surgery and therapy. Ann Otol Rhinol Laryngol. 1996;105(6):415–422. doi: 10.1177/000348949610500601. [DOI] [PubMed] [Google Scholar]
- 21.Deka A., Bhattacharjee K., Bhuyan S.K. Effect of mitomycin C on ostium in dacryocystorhinostomy. Clin Exp Ophthalmol. 2006;34:557–561. doi: 10.1111/j.1442-9071.2006.01265.x. [DOI] [PubMed] [Google Scholar]
- 22.Yazici B., Yazici Z. Final nasolacrimal ostium after external dacryocystorhinostomy. Arch Ophthalmol. 2003;121:76–80. doi: 10.1001/archopht.121.1.76. [DOI] [PubMed] [Google Scholar]
- 23.Hendry J., Chin A., Swan I.R.C., Akeroyd M.A., Browning G.G. The Glasgow Benefit Inventory: a systematic review of the use and value of an otorhinolaryngological generic patient-recorded outcome measure. Clin Otolaryngol. 2016;41:259–275. doi: 10.1111/coa.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mistry N., Rockley T.J., Reynolds T., Hopkins C. Development and validation of a symptom questionnaire for recording outcomes in adult lacrimal surgery. Rhinology. 2011;49(5):538–545. doi: 10.4193/Rhino11.042. [DOI] [PubMed] [Google Scholar]
- 25.Penttila E., Smirnov G., Seppa J., Tuomilehto H., Kokki H. Validation of a symptom-score questionnaire and long-term results of endoscopic dacryocystorhinostomy. Rhinology. 2014;52(1):84–89. doi: 10.4193/Rhino13.041. [DOI] [PubMed] [Google Scholar]
- 26.Ali M.J., Psaltis A.J., Bassiouni A., Wormald P.J. Long-term outcomes in primary powered endoscopic dacryocystorhinostomy. Br J Ophthalmol. 2014;98(12):1678–1680. doi: 10.1136/bjophthalmol-2014-305510. [DOI] [PubMed] [Google Scholar]
- 27.Cannon P.S., Chan W., Selva D. Incidence of canalicular closure with endonasal dacryocystorhinostomy without intubation in primary nasolacrimal duct obstruction. J Ophthalmol. 2013;120(8):1688–1692. doi: 10.1016/j.ophtha.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Hawker G.A., Mian S., Kendzerska T., French M. Measures of adult pain. Arthritis Care Res. 2011;63(S11):240–252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.