Abstract
The panniculus carnosus is a thin striated muscular layer intimately attached to the skin and fascia of most mammals, where it provides skin twitching and contraction functions. In humans, the panniculus carnosus is conserved at sparse anatomical locations with high interindividual variability, and it is considered of no functional significance (most possibly being a remnant of evolution). Diverse research fields (such as anatomy, dermatology, myology, neuroscience, surgery, veterinary science) use this unique muscle as a model, but several unknowns and misconceptions remain in the literature. In this article, we review what is currently known about panniculus carnosus structure, development, anatomical location, response to environmental stimuli and potential function(s), with the aim of putting together the evidence arising from the different research communities and raising interest in this unique muscle, which we postulate as an ideal model for both vascular and muscular research.
Keywords: cutaneous maximus muscle, cutaneous muscle, cutaneous trunci, musculus cutaneous, subcutaneous muscle, superficial fascia system
Rudiments of various muscles have been observed in many parts of the human body; and not a few muscles, which are regularly present in some of the lower animals can occasionally be detected in man in a greatly reduced condition. Every one must have noticed the power which many animals, especially horses, possess of moving or twitching their skin; this is effected by the panniculus carnosus. Remnants of this muscle in an efficient state are found in various parts of our bodies; for instance, the muscle on the forehead, by which the eyebrows are raised… Some few persons have the power of contracting the superficial muscles on their scalps; and these muscles are in a variable and partly rudimentary condition. M. A. de Candolle has communicated to me a curious instance of the long‐continued persistence or inheritance of this power, as well as of its unusual development. He knows a family in which one member, the present head of the family, could, when a youth, pitch several heavy books from his head by the movement of the scalp alone, and he won wagers by performing this feat. His father, uncle, grandfather, and his three children possess the same power to the same unusual degree. This family became divided eight generations ago into two branches; so that the head of the above‐mentioned branch is cousin in the seventh degree to the head of the other branch. This distant cousin resides in another part of France; and on being asked whether he possessed the same faculty, immediately exhibited his power. This case offers a good illustration how persistent may be the transmission of an absolutely useless faculty… Charles Darwin, ‘The Descent of Man’ (1875).
Introduction
As described in Darwin's beautiful words, the cutaneous panniculus carnosus (PC) muscle has long been considered a vestigial (Turner, 1870; Perrin, 1871) and ‘absolutely useless’ organ in humans; a remnant of evolution (Darwin, 1879). This, together with interindividual variability may be the reason why it remains relatively ill‐studied and underappreciated, in stark comparison with other muscles of the body. However, research from various fields (such as anatomy, dermatology, myology, neuroscience, surgery, veterinary science) impacts on distinct aspects of PC muscle, and denominate it under differing names that may reflect either PC per se or developmentally related muscles. Alternative names found in the literature include cutaneous trunci (van Iwaarden et al. 2012), musculus cutaneous (or cutaneous muscle) (Matsumoto et al. 2010), cutaneous maximus muscle (Pan et al. 2012), subcutaneous muscle (Brunius et al. 1968), and superficial fascia system (Fodor, 1993). Additionally, anatomically restricted subsets of PC may include the sternalis, platysma colli (of the neck), orbicularis (lip) and axillary arch (axilla) muscles (see below) (Turner, 1867). Although some authors include smooth muscles such as subareolar muscle, dartos (scrotum) and M. corrugator cutis ani (anus) as PC, we believe it is more correct to reserve the term panniculus carnosus for striated muscles only, even if those smooth muscles are also localized immediately beneath the skin and may fulfil similar functions. We thus advocate reaching a consensus that the panniculus carnosus denomination be used as an umbrella term that includes superficially localized striated muscles in human. This term (as mentioned above) may be found in the scientific literature from the 1800s, and possibly earlier (Hallett, 1848). Panniculus carnosus relates to the immediately adjacent panniculus adiposus layer, which is nowadays designated dermal white adipose tissue (dWAT) (Driskell et al. 2014; Schneider, 2014; Alexander et al. 2015). We are aware that our definition may include striated muscles that traditionally have not been considered PC remnants in the human, such as the cremaster muscle of the scrotum. Conversely, muscles that traditionally are considered PC but are not superficially located (such as psoas minor) would be excluded from this definition (Table 1).
Table 1.
Muscles described as PC or PC‐related in the literature, and their re‐assignment as per our proposed definition
| Muscle | Reference(s) | Conforms to the novel definition |
|---|---|---|
| Striated muscles | ||
| Craneo‐facial muscles | Abdullahi et al. 2014; | Yes |
| Greenwood, 2010; | ||
| Hall, 2015; | ||
| Kulkarni, 2012; | ||
| Sánchez‐Yus & Simón, 2000; | ||
| Singh, 2015; | ||
| Platysma | Greenwood, 2010; | Yes |
| Hall, 2015; | ||
| Kulkarni, 2012; | ||
| Singh, 2015; | ||
| Turner, 1867; | ||
| Langer`s axillary arch | Bergman et al. 2017; | Yes |
| Besana‐Ciani & Greenall, 2005; | ||
| Hall, 2015; | ||
| Musculus esternalis | Bergman et al. 2017; | Yes |
| Hall, 2015; | ||
| Turner, 1867; | ||
| Musculus palmaris brevis | Greenwood, 2010; | Yes |
| Hall, 2015; | ||
| Kulkarni, 2012; | ||
| Singh, 2015; | ||
| Turner, 1867; | ||
| Musculus palmaris longus | Hall, 2015; | Yes |
| Trapezius muscle | Bergman et al. 2017; | Yes |
| Hall, 2015; | ||
| Turner, 1870; | ||
| Pectoral muscles | Bergman et al. 2017; | Yes |
| Hall, 2015; | ||
| Turner, 1867; | ||
| Serratus muscle | Bergman et al. 2017; | Yes |
| Hall, 2015; | ||
| Musculus psoas minor | Hall, 2015; | No |
| Cremaster | This work | Yes |
| Smooth muscles | ||
| Musculus corrugator cutis ani | Greenwood, 2010; | No |
| Jana, 2018; | ||
| Kulkarni, 2012; | ||
| McMinn, 1998; | ||
| Singh, 2015; | ||
| Dartos | Drake, 1727; | No |
| Greenwood, 2010; | ||
| Jana, 2018; | ||
| Kulkarni, 2012; | ||
| McMinn, 1998; | ||
| Singh, 2015; | ||
| Subareolar muscle | Greenwood, 2010; | No |
| Jana, 2018; | ||
| McMinn, 1998; | ||
| Singh, 2015 | ||
In this article, our aim is to review the available literature on the PC muscle, which is found only in mammals, with the primary objective of knitting together the evidence from separate areas of knowledge. Further, the presence of a skeletal muscle within the skin functional unit (albeit in distinct and variable parts of the human body) raises a certain curiosity about the roles it could play in cutaneous biology, not only in skin homeostasis but also in response to wounding. Recent evidence points to a continuous interaction (both in health and disease) among skin layers and appendages through direct intercellular contacts, secretion of extracellular matrix components and paracrine signals. This includes relatively ‘unexpected’ phenomena such as nerve‐, arrector pili muscle‐ and dWAT‐associated cells interacting with hair follicle stem cells, or wound myofibroblasts converting into adipocytes (Brownell et al. 2011; Festa et al. 2011; Fujiwara et al. 2011; Jahoda & Christiano, 2011; Plikus et al. 2017). Where present, it seems likely that the PC muscle may also be actively participating in relevant aspects of skin physiology and possibly in the aforementioned interlayer/inter‐appendage crosstalk. Moreover, in this review we address the relative importance of PC in mammals, and the conservation of its functions. Finally, its unique contractile reflex (Jones, 2012) and purportedly high regenerative capacity (Brazelton et al. 2003; Bahri et al. 2016; Naldaiz‐Gastesi et al. 2016) make the PC a generally attractive target for researchers in the skeletal muscle field, while its high vascularization and accessibility have long attracted angiogenesis‐related studies.
Panniculus carnosus: definition, fine structure and innervation
The PC consists of a thin layer of striated muscle that is intimately attached to the skin and fascia of most mammals (Fig. 1). This sheet, which we here postulate should be considered a functional part of the mammalian skin, is considerably more conserved in some species, e.g. rodents, cats, dogs, horses and whales. In these animals, the PC is widely spread in the proximal part of posterior limbs, the back, abdomen and chest (Langworthy, 1924, 1925; Ridgway & Carder, 1990; Inzunza et al. 2008; Greenwood, 2010).
Figure 1.
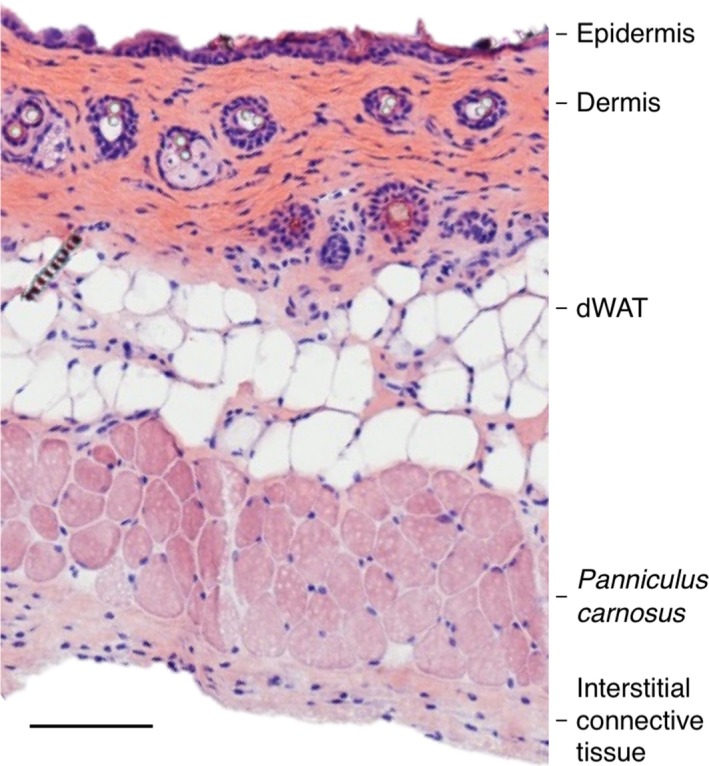
Histological section of adult mouse skin. A full thickness skin section taken from the back skin of a female 8‐week‐old C57/BL6 mouse was stained with hematoxylin/eosin. The panniculus carnosus muscle sits in between the dermal white adipose tissue (dWAT) and the interstitial connective tissue that overlies the fascia. Scale bar: 100 μm.
The PC is innervated by α‐motor neurons (not spindle organs; Holstege & Blok, 1989; Theriault & Diamond, 1988), to recruit muscle fibres in enabling the animal to move or twitch its skin independently of the underlying fixed mass muscle. Transversely, the PC muscle layer in rodents is composed of about three to four fibres in thickness running laterally to the skin (Langworthy, 1925; Menger et al. 2006). It is reported that the PC consists predominantly of fast type IIB muscle fibres (type IIB in rodents being equivalent to type IIX in humans) (Brazelton et al. 2003) with some fast type IIA fibres present (Bahri et al. 2016). Therefore, they are a fast twitch muscle fibre with associated properties of capacity to produce significant force over a short period of time. This fibre type arrangement would be consistent with purported functions of the PC in shaking off unwarranted foreign bodies or stimuli, perhaps even a potential role in contractile‐mediated shivering thermogenesis (Rowland et al. 2015).
Classical anatomists such as Langworthy indicated that PC of rodents is innervated by nn. thoracalis ant. terminal nerve (Langworthy, 1925). However, Theriault & Diamond (1988) proposed that PC of the rat is innervated by motor neurons that run rostrocaudally. These motor neurons rise from three nerve territories (ventral, lateral and dorsal) all of which are derived from the brachial plexus (Theriault & Diamond, 1988). By counting the somata of retrogradely labelled motor neurons, they estimated the average motor neuron population to be 1183 ± 33 motor neuron per side, extending originally from C6 to T1 nerves. More recently, it was confirmed that the lateral thoracic nerve (LTN) innervates the PC in the mouse (Petruska et al. 2014). Immediately after exiting the brachial plexus, LTN divides into three branches (dorsal, ventral and lateral), each innervating a different region of PC. These major branches divide further to give five to eight smaller branches that divide again into smaller ramifications to cover the whole PC. In contrast, the dorsal cutaneous nerve, originating from T4–L2 spinal nerves, is responsible for the sensation activity of the PC. Indeed, neuromuscular junctions are seen scattered along the PC myofibres (Petruska et al. 2014). Overall, these findings propose that distinct motor/sensory nerves supply the PC muscle fibres, enabling them to move or twitch independently of the underlying fixed mass muscle.
A similar situation applies to guinea pig, canines and felines (Langworthy, 1924, 1925), in which the thoracodorsal nerve (n. thoracodorsalis) supports innervation of the whole‐body PC. However, the human/primate neck platysma is supplied by the cervical division of the facial nerve (McMinn, 1998). The superficial part of the ulnar nerve supports the human PC, like the palmar brevis muscle in the hand. The scrotum PC is innervated by the genital portion of genitofemoral nerve (Standring, 2008). It is reported that the lateral thoracic nerve (LTN) innervates the cutaneous maximus (or PC) muscle in mouse and consists of some of the shortest (innervating the rostral PC area) and longest nerves (innervating the caudal PC) in the mouse (Pan et al. 2012).
Developmental origin of PC muscle
The origin of PC has been studied in mouse development. At the E10.5 dermomyotome, cells demarcated by the expression of homeobox‐containing protein Engrailed‐1 (EN1) originate muscle, fat and dermis (Atit et al. 2006). Follow‐up data with two different constructs that trace PAX7+ cells (Pax7CE‐βGal+ cells traced at E9.5 of development, or constitutive tracing of Pax7‐expressing cells at all stages of development by Pax7‐IRES‐Cre) confirmed labelling of the PC (Amini‐Nik et al. 2011; Lepper et al. 2011). However, the specific contribution of early embryonic muscle precursors to the adult PC satellite cell pool was poorly understood. Through lineage tracing studies in the mouse dorsal skin, we recently described that the adult PC derives from dermomyotomal precursors, more specifically MYF5‐, PAX3‐ and PAX7‐expressing precursor cells (Naldaiz‐Gastesi et al. 2016). In this sense, the PC is similar to other trunk muscles, although it is certainly unique in that it is particularly receptive in attracting the fusion of bone‐marrow derived cells (see below) (Brazelton et al. 2003; Naldaiz‐Gastesi et al. 2016). The functional implications of this supply of bone marrow‐derived material to the PC muscle, as well as its timing (whether it occurs in development, throughout adult life, or both) are currently unknown.
Anatomical location of PC muscle sheath and interindividual variation in human skin
Although this muscle is mostly absent in humans, different muscles at distinct anatomical locations are phylogenetically accepted as vestigial rudiments of the PC (Fig. 2, Table 1). Descriptions in the literature of this group of striated muscles includes occipitofrontalis muscles of the scalp, muscles of the auricle, the platysma in the ventral part of the neck, palmaris brevis in the hand, and Langer's axillary arch in the axilla region, which was last deemed to be of PC origin (Jung et al. 2016).
Figure 2.
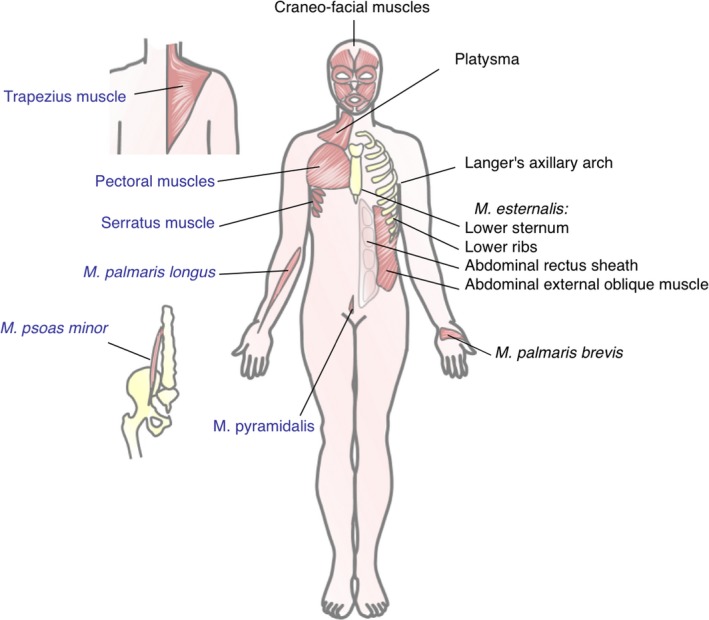
Localization of PC remnants in the human body. To the right of the human figure, black lettering shows the location and denomination of striated muscles that have been described as remnants of the PC by different authors. On the left, muscles that may also be part of PC but whose assignment as PC is not as clear, are indicated in blue lettering. Only striated muscles are included. Smooth muscles postulated by some authors as PC remnants have been purposely excluded from this figure.
As evolutionary remnants of low functional importance, PC muscles may vary among individuals and ethnic groups (Bergman et al. 2017). For instance, the M. sternalis exists in 3–5% of individuals and has no apparent functional significance (Hall, 2015). In contrast, pyramidalis muscle was found to be absent in 16–17%, the psoas minor muscle in 50% and the palmaris longus muscle in 11% of dissected human bodies (Hall, 2015).
Among humans, the thickness and expansion of the platysma muscle is remarkably variable (De la Cuadra‐Blanco et al. 2013). Ageing also has an effect on the platysma, which becomes thinner and less defined in older individuals (De Castro, 1980).
Conservation among mammals
In contrast to humans, the PC skeletal muscle layer is well developed and sits immediately beneath the skin of most mammalian animal species including mice, rats, guinea pig and rabbits (Lorenz & Longaker, 2008), cats and dogs (Pavletic, 2018), pigs and chattels (Wilder, 1923; Reese et al. 2004), horses (Higgins & Martin, 2012), dolphins (Yoshida, 1971) and skin areas of some higher primates (Turnquist & Minugh‐Purvis, 2012). In most of these, the PC covers the dorsal, ventral and lateral surfaces of the body trunk (musculi cutanei trunci, also termed cutaneous trunci or cutaneous maximus) and extends down to the upper part of the limbs.
Although mammals share the same essential distribution of PC (Fig. 3), there are anatomical differences in the PC among species due to their development, evolution and the functional importance of the PC to a particular species. For instance, the platysma of horses is restricted to the lower part of the jaw, whereas in dogs it is wider (Langworthy, 1925). The cutaneous trunci muscle of hamsters is remarkably thin, being composed of a single layer of PC fibres, in contrast to the multilayers of fibres in mice and rats (Menger et al. 2006). The trunk portion of the PC layer extends as far as the knees in horses, whereas it extends down to the shin of the lower legs in the ox (Goldfinger, 2004).
Figure 3.
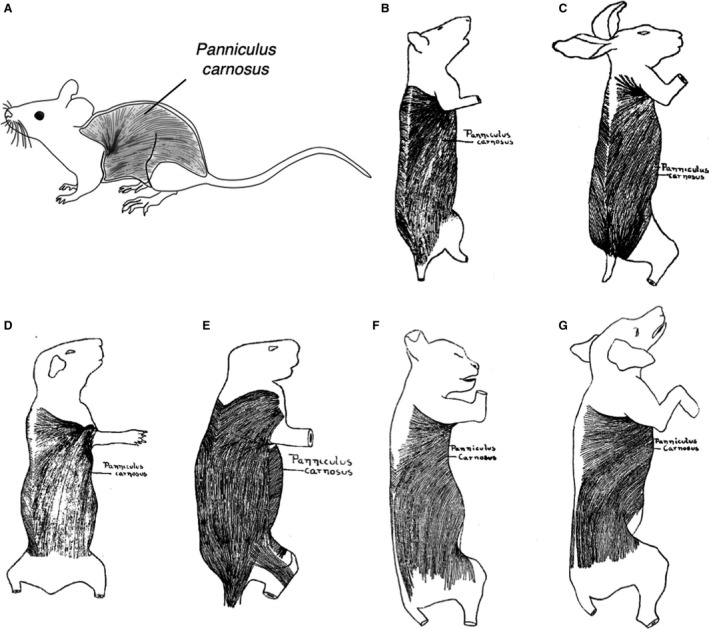
Localization of the panniculus carnosus (PC) in laboratory animals. Schemes show the anatomical location of PC in several species relevant for research. (A) Mouse. (B) Rat. (C) Rabbit. (D) Guinea pig. (E) Porcupine. (F) Cat. (G) Dog. (B‐E) Adapted with permission from Langworthy (1925). (F,G) Adapted with permission from Langworthy (1924).
It is not entirely clear why the PC muscle sheath varies among many mammals and why it is vestigial in humans. Throughout the literature, two reasonable hypotheses emerge. Greenwood hypothesized that such an element was essential for wound closure and healing, especially in the wild (Greenwood, 2010). Otherwise, animals would be weaker, slower to recover and more prone to further attacks. He argued that the persistence of PC tissue in humans, i.e. the platysma of the neck, is most likely due to its location at the most frequent site for attacks by large mammalian predators (Greenwood, 2010). Other authors attributed the regression of PC in humans to a twitching function to remove noxious irritants such as insects, but considered that is no longer necessary in humans due to the evolved wider mobility of the upper limbs (Hall, 2015; Jung et al. 2016; Bergman et al. 2017).
Known and postulated functions of PC muscle
The anatomical location of the PC muscle layer, in between the dWAT and the interstitial connective tissue (ICT) (Machado et al. 2011), has given it the flexibility for voluntary and independent movement from the underlying muscles (Pavletic, 2018). This has benefited animals with a low resistance loose skin, in contrast to skin areas lacking PC, where it is more firmly attached to the fascia (Lorenz & Longaker, 2008; Pavletic, 2018). These unique features of the PC sheath have facilitated the adaptation of many mammals to their surrounding environment. For instance, the presence of the PC muscle sheath allows canines to shake their wet skins, cattle and horses to twitch their skin as a defence mechanism against irritating stimuli (Wilder, 1923; Higgins & Martin, 2012) and naked‐mole rat to resist the abrasive forces and reduce the potential damage due to underground burrowing (Daly & Buffenstein, 1998). In higher primates and humans, the functional significance of PC as a defence from irritation is diminished, as previously mentioned.
Other purported functions of the PC muscle are in the generation of heat by contraction (shivering thermogenesis), which plays a major role in thermoregulation of endothermic vertebrates (‘warm‐blooded’ mammals) (Wilder, 1923; Pavletic, 2018). In marsupials, facial PC muscle sheaths are involved in external ear movement and PC muscle layers associated with the marsupium (pouch) may support its function and, to some extent, help in its closure. The body of the spiny anteater Echidna is almost completely covered by PC skeletal muscle (Griffiths, 1989). For the purpose of defence and protection it can elevate its spines (Ashwell & Musser, 2013) or roll itself into a spikey ball by PC fibre contraction (Griffiths, 1989). In the sirenians (sea cows), the PC muscle assists downstrokes of the tail, and in cetaceans it overlies the mammary gland to help in breastfeeding (Reidenberg, 2018). In humans, the rudiment PC facial muscles and platysma muscle of the neck may help facial expression formation (Sánchez‐Yus & Simón, 2000; Pavletic, 2018).
Facilitating wound contraction
With the major exceptions of humans and pigs, wound healing and thermal injury repair processes in most loose‐skinned mammals occur primarily via a phenomenon called wound contraction (Gottrup et al. 2000; Dahiya, 2009; Abdullahi et al. 2014), which is an alternative to forming new tissue to close the wound gap (through re‐epithelialization and granulation tissue formation) (Wong et al. 2011). The PC has traditionally been considered particularly important for this phenomenon due to its mobility characteristics (Kennedy & Cliff, 1979). PC role in wound contraction may be important during the first 2–3 days in the rat, but the PC may also contribute by secreting collagen (Cohen et al. 1979) and promoting the viability of the cells (Bakheit, 1996). Therefore, it is widely accepted that wound healing via wound contraction occurs much more rapidly than via re‐epithelialization alone (Lorenz & Longaker, 2008; Pavletic, 2018). In our opinion, it is difficult to assign a role to the PC in wound contraction other than providing a plane of low resistance between two fascial layers (Lorenz & Longaker, 2008). Wound contraction in loose skinned mammals is mainly mediated by myofibroblasts, cells that arise transiently in the wound bed (in response to injury; Cuthbertson, 1959; Watts et al. 1958), which are unrelated to the PC muscle per se (Naldaiz‐Gastesi et al. 2016). The main factor determining the direction of wound contraction seems to be mechanical tension (Watts, 1960). The classic works on wound contraction showed that the skin surrounding the wound pulled outwards, tending to produce wound distraction instead of contraction until the wound margins are fixed (Billingham & Russell, 1956). In fact, the wound tensile strength might be at least partially attributed to retraction of the severed PC (Billingham & Russell, 1956; Brunius et al. 1968).
Consequently, why do some authors believe that the PC has an active role in the contraction of wounds? This has come mostly through experiments that eliminated the PC and studied its effect on wound closure. In guinea pigs, Aksoy et al. showed that panniculectomy followed by two types of wounding (circular skin excision and burn injury) caused a delay in the healing of wounds and larger, more irregular scars, although it failed to produce hypertrophic scars (Aksoy et al. 2002). In a similar study performed in rabbits, these authors showed that third‐degree burn injuries inflicted in the presence or absence of PC had distinct outcomes: wound healing was slower and scars were broader on the panniculectomy side than on the control side (Aksoy et al. 2009). However, as the authors acknowledge, a reduction of blood supply to the skin flaps secondary to excision of panniculus carnosus layer, in addition to a putative role in fixing wound margins to promote contraction, may underlie these phenomena.
Revascularization and capillary remodelling
The PC is ideally situated to study revascularization and capillary remodelling in response to wounding (Machado et al. 2011), both of which are involved in full thickness excisional wound healing. The PC layer contributes to the skin microcirculation via the direct cutaneous arteries (Fig. 4). The vascular plexuses on and underneath the PC start to extend, irrigating the wound bed by incorporating blood from the evolving capillary structure already existing within the musculature sheath. This forms a new vascular bed that gradually expands from the severed edges of the PC and infiltrates in between the regenerating muscle fibres (Hughes & Dann, 1941; Billingham & Russell, 1956; Machado et al. 2011; Rittie, 2016).
Figure 4.
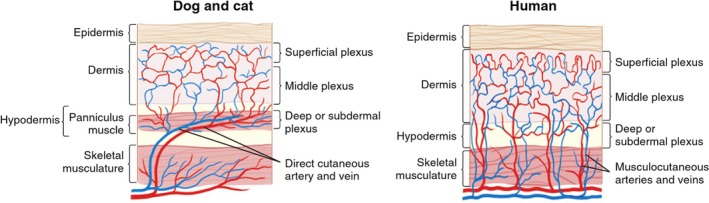
Cutaneous circulation in dogs, cats and humans. The subdermal plexus is formed and supplied by terminal branches of direct cutaneous vessels at the level of the panniculus carnosus muscle in dogs and cats. Note the parallel relation of the direct cutaneous vessels to the overlying skin. This is unlike the perpendicular orientation of musculocutaneous vessels in the human (modified from Pavletic, 2003).
Interestingly, a number of independent studies support a key role of the PC in revascularization of the wound bed. In a skin graft transplantation setting in rats, the presence of PC muscle eliminated the pro‐graft survival effect of VEGF treatment, indicating that the muscle was instrumental in avoiding graft necrosis (Richter et al. 2006). Revascularization of musculocutaneous and muscle flaps after division of the major vascular supply arises from the wound periphery and is independent of flap type (Gundeslioglu et al. 2013). Adeno‐associated viral (AAV) vectors encoding VEGF with exclusive targeting to PC fibres enhance healing of full thickness excisional wounds in rats, by inducing new vessel formation (Deodato et al. 2002). Finally, beads that enhanced angiogenesis within myocutaneous skin grafts, significantly increasing microvessel density, also thickened the transplanted PC layer (Eckhaus et al. 2008). This is significant because grafted muscle does not normally survive due to its low tolerance to ischemia. All of these studies may have a translational edge to them, as the presence of PC might improve prevascularization of the wound bed and thus enable improved skin grafting (Eckhaus et al. 2008).
Response of the PC to wounding and other insults
Unlike the extremely efficient and rapid wound healing process of lower mammals (by contraction), human wound healing requires more time to close the wound because of the need to create new epidermal tissue (Abdullahi et al. 2014). Generally, human wound margins remain separated, contraction being only provided by myofibroblasts, though it may lead to constriction, distortion and immobilization due to the loss of the anatomical prerequisites for efficient reparation of the skin wound through this process (Billingham & Medawar, 1955; Davidson et al. 2013). The same is true in some mammals during healing of the skin parts that are closely knit to the subcutaneous tissues (e.g. distal parts of the ears) or in those cases where the PC is damaged (Davidson et al. 2013). In these situations, the residual gap that cannot be closed by contracture is filled by a fibrous scar (Billingham & Medawar, 1955).
Some confusion has arisen from transplantation experiments. For instance, PC sheets transplanted into rabbit ear wounds do not seem to influence the wound contraction pattern or the rate of closure (Bakheit, 1996). However, it was unknown whether the transplanted PC sheets were functional or how they engaged with the recipient tissue. Subcutaneous injections of tumours (Argyris & Argyris, 1962) or even transplanted myoblasts forming ectopic muscle masses (Irintchev et al. 1998; Fukushima et al. 2005) are not able to cross the PC unless it has previously been damaged. This raises questions about the transplantation approach, because it may not be the optimal setting to understand function of these cells upon grafting. Similarly, Ibrahim et al. (2014) described a mouse burn injury model where the role of the PC in contraction was analysed in the dorsum after grafting of a skin piece from the ear. In this setting, the PC did not contribute to the contraction of the wound, although the graft itself contracted. One possibility is that the PC was not able to contract because the graft physically impedes this.
In response to wounding, a muscle‐specific variant of the alpha subunit of nascent polypeptide associated complex (skNAC) – a factor regulating postnatal muscle regeneration (Park et al. 2010) – is upregulated in the adjacent PC fibres (Munz et al. 1999), and TAp63+ cells appear both in the PC and in newly formed granulation tissue (Bamberger et al. 2005). Integrin α9+ cells were detected both on blood vessels at the granulation tissue and at the PC ends proximal to injury (Singh et al. 2004). These results somehow suggested that the PC might contribute cells to the wound bed in response to wounding.
As adult satellite stem cells are responsible for muscle cell turnover in response to wounding and other stimuli, it would be expected that these resident stem cells would provide any PC‐derived cells to the wounds. However, we have recently shown that there is no detectable contribution of adult PC satellite‐derived cells to the wound bed upon injury (Naldaiz‐Gastesi et al. 2016). Consequently, it might appear that the PC does not have a clear role stimulating full‐thickness wound contraction, at least at the cellular level. Intriguingly, Amini‐Nik et al. (2011) reported that in full thickness punch wounds in 10‐week‐old mice, cells constitutively traced by Pax7‐Cre expression (putative PC satellite cells) constitute up to 23% of the cells populating the wound bed 1 week post‐wounding. However, at the somite maturation stages, Pax7 is not a muscle‐specific gene, as it is expressed in the central domain of the dermomyotome. Cells leaving the dermomyotome to enter non‐myogenic lineages, downregulate Pax7 expression (Buckingham & Relaix, 2007). However, they would still be marked by the Pax7‐Cre reporter strain. Additionally, there seems to be some bidirectional plasticity between satellite cells and pericytes, at least during fetal muscle development (Cappellari et al. 2013; Stallcup, 2013). Those pericytes transiently induce Myf5 and are likely to maintain myogenic features and express Pax7 (Biressi et al. 2013). For all of these reasons, we believe it more likely that the Pax7+ cell progeny traced by Amini‐Nik et al. were activated dermal precursors populating the wound bed, and not postnatal PC satellite cells.
Response of the PC to microgravity, increased pressure and irradiation
In one astronaut, a long stay in space induced loss of skin elasticity associated to a rarefication of the underlying fibre structure (Tronnier et al. 2008). In rats, 10 days of microgravity exposure induced a significant delay in wound healing, associated to lower collagen content of the wounds (Davidson et al. 1999). In general terms, the long‐term effects of microgravity on skeletal muscle are the upregulation of muscle atrophy and stress‐related genes (Sandonà et al. 2012). In a mouse study, 3 months aboard the International Space Station were sufficient to induce dermal atrophy accompanied by a decreased collagen content, deregulation of the hair follicle cycle and upregulation of muscle homeostatic genes in the PC, via the MEF2C–myogenin pathway (Neutelings et al. 2015). Indeed, gravitational stress (changes in body tilt) induces microvascular responses (variations in arteriole and venule diameter) in the PC of rats (Puri & Segal, 1994). These results suggest that the PC somehow ‘senses’ differences in environmental pressure and reacts to them.
Interestingly, an increase in pressure also seems to have a profound impact on PC biology. In the field of pressure ulcers, the pressure‐induced injuries to the deep tissues of the body (i.e. muscle) that occur during long surgical procedures have long been known as ‘deep tissue injury (DTI)’ (Agam, 2007; Stewart & Salcido, 2012) or, more recently, as ‘myosubcutaneous infarcts’ (Salcido, 2007). In a rat model of DTI, the lesions most often associated with pressure loading and unloading (ischemia/reperfusion insults) were characterized by necrosis of the PC and damage to the associated adipose tissue (Salcido et al. 1995). These data should also be taken into account when evaluating the subcutaneous use of soft tissue fillers in preclinical models (Hillel et al. 2012) because the injected filler might increase loading pressure on the PC.
The body surface is exposed to insults from the environment, and some of these noxa (such as solar irradiation) may reach deep into the skin tissue. Continuous near‐infrared irradiation of the skin has been reported to induce thinning of PC muscle and skin ptosis (Tanaka et al. 2013). Degradation of collagen and elastin in sun‐exposed areas is associated to wrinkling, but at least in the human face the PC muscles contraction may also be involved in wrinkle formation, as demonstrated by the generalized use of ‘Botox’ (botulinum toxin) in this area (Matsumoto et al. 2010). Accordingly, UV irradiation of hairless mice following removal of the PC induced a decrease in wrinkle depth, as compared to controls (Matsumoto et al. 2010).
Unusual engraftment rate of bone marrow‐derived cells
After bone marrow transplantation, donor hematopoietic stem cells (HSC) may occasionally be detected as becoming part of skeletal muscle fibres (Ferrari et al. 1998). More specifically, it is believed that HSCs fuse to multinucleated MyoD‐expressing myofibrils and thus their nuclei become myonuclei (Dellavalle et al. 2011). We and others have seen that, in the absence of injury, the rate of bone marrow‐derived cell (possibly HSCs) incorporation into PC is highly significant when compared with the physiologically irrelevant rates achieved by other muscle groups (Brazelton et al. 2003; Corbel et al. 2003; Sherwood et al. 2004; Naldaiz‐Gastesi et al. 2016). The reasons for the increased incorporation of non‐resident cells into the PC remain unclear but may be related to increased cellular turnover in the PC. Our results indicated that cells of donor origin are capable of repopulating the muscle stem cell niche but may not fully acquire the relevant myogenic commitment, perhaps because they failed to reach full conversion to the satellite cell fate (Naldaiz‐Gastesi et al. 2016). The relevance of these phenomena remains unclear. We propose that the PC may be a key experimental model that can be used to understand mobilized cell engraftment in skeletal muscle, a thus far neglected research area due to the extreme rarity of the fusion events in the best studied muscle groups.
Does the PC form part of the functional skin unit? Role in disease
The disappearance of PC in humans, cetacea and pigs, and filling of the ‘empty’ PC space by dWAT is correlated with the absence of terminal hair follicles (HF) in most body areas (Greenwood, 2010). It is well known that the thickness of the adipose tissue layer varies concomitantly with the hair cycle (Kruglikov & Scherer, 2016). Similar to adipose tissue, it would be tempting to speculate that the PC reacts to HF cycling with oscillations in sheath thickness; however, to our knowledge no study has addressed this directly. Early studies observed that, in rats, the inactive HFs lie high in the dermis whereas in areas of active growth, the roots of the growing HFs extend to the PC and are sometimes deflected into a ‘hockey‐stick’ appearance (Butcher, 1934; Durward & Rudall, 1949). Interestingly, increased vascularity is also seen around the lower portion and bulb of the growing HFs due to the generation of new capillary networks (Durward & Rudall, 1949). In our view, it is certain that the closely positioned and highly vascularized PC may have a key structural role in promoting these angiogenic phenomena. PC sheath thickness is also related to contractile force. In a study in horses, it was observed that the maximal contractile response of the PC to cutaneous stimuli was detected in areas where the cutaneous trunci muscle was thicker and the skin was more mobile (Essig et al. 2013).
A number of older biochemical studies described skin proteins and metabolites associated to the PC, although in most cases they were purely descriptive and contributed little to understanding function. In rhesus macaques, high levels of glycolytic enzymes were detected in the dorsal PC than in arrector pili muscles (Im & Adachi, 1968). The rat PC expresses large quantities of calcium‐binding protein parvalbumin (Hawley‐Nelson et al. 1986), which is consistent with its fast fibre type profile (Celio & Heizmann, 1982). In mice, co‐expression of four creatine kinase isoenzymes has been associated to the PC (Schlattner et al. 2002).
Perhaps more direct evidence of a functional role of PC might be derived from mouse models of disease. For instance, in the SOD1 mouse model of amyotrophic lateral sclerosis (ALS), axonal degeneration is detectable earlier at the distal LTN innervating the PC than at the sciatic nerve, possibly because the axons are longer (Tallon et al. 2016). Therefore, the PC may emerge as a good model for studies of early neuromuscular junction degeneration in ALS. Additionally, several human muscular dystrophies do not affect all muscles equally (Emery, 2002). One characteristic example of focal myopathy is facioscapulohumeral dystrophy (FSHD), which affects shoulder and facial muscles. Interestingly, Fat1‐deficient mice reproduce the clinical picture of early phases of human FSHD in that constitutive ablation of Fat1 causes developmental abnormalities of subcutaneous muscles in the face and a severe reduction in thickness of the cutaneous maximus muscle (Caruso et al. 2013). In humans, FAT1 levels may reflect early and late disease onset within specific muscle groups (Mariot et al. 2015).
Thus PC muscle fibres can be employed as a dynamic tissue regeneration system to explore muscle cell turnover in normal health and disease states. Currently, we have characterized the PC muscle fibres in the mdx mouse model of Duchenne muscular dystrophy (DMD) (Bahri et al. 2016; and unpublished data). In the mdx mouse, PC muscle fibres appear to exemplify the dystrophic morphology and augmented levels of regeneration comparable to the more commonly studied limb muscles in this disease, such as the gastrocnemius (Dimitrijevic & Gracanin, 1968; Allamand & Campbell, 2000; Dowling et al. 2002). In addition, a pronounced level of muscle fibre hypertrophy was associated with aged dystrophic PC muscle fibres compared with wild‐type fibres, a known phenotypic response in muscular dystrophy (Duddy et al. 2015). Interestingly, we observed some gender dimorphisms for PC in the mdx mouse, with male mdx PC muscle cells showing higher regenerative turnover compared with females in vivo. This gender difference was also retained in vitro, with satellite cells derived from the PC muscle layer of male mdx mice giving rise to myotubes with a higher myogenic activity when compared with their female counterparts. Gender‐related differences in the population and behaviour of skeletal muscle satellite cells in normal and diseased states are inconsistently reported in the literature (La Colla et al. 2015; Williams et al. 2017). Exploration of gender differences in the dystrophic PC may reveal new therapeutic targets for ameliorating muscle degenerative disorders and other muscle deficiencies such as ageing‐associated sarcopenia.
PC – a unique model for vascular and muscular research
The PC muscle has many unique advantages as a model for use in vascular and musculoskeletal research (Laschke & Menger, 2016). The dorsal skinfold window chamber model originally developed for studying tumour growth in mice (Algire, 1943) has been widely used to study an array of cellular and physiological mechanisms around the PC tissue (Laschke & Menger, 2016). In brief, the glass chamber system consists of two titanium frames inserted on the dorsal skinfold of the mouse (Laschke & Menger, 2016). On one frame, a layer of cutis, subcutis and PC muscle is carefully removed in a circular area at the front of the chamber. This front circular chamber serves as the observational window which looks into the contralateral layer of PC muscle, subcutis and skin. A cover glass is fixed on to the exposed tissue in the chamber frame, allowing in vivo analyses of the PC. The dorsal skinfold chamber has the advantage that it can be horizontally positioned under a microscope for repeated non‐invasive intravital microscopic analyses. Fluorescent dyes can be intravenously delivered to highlight specific cells or cellular structures, which can be imaged through the dorsal skinfold window for use in quantifying an array of microvascular, cellular and molecular mechanisms.
Taking advantage of the dorsal skinfold window chamber model, the contralateral layer of the PC muscle may serve as a significant tool to understand a diverse range of physiological phenomena as well as preclinical disorders, including muscle wound healing (Sorg et al. 2007), vascular growth, angiogenesis (Fukumura & Jain, 2008; Machado et al. 2011), tumour growth and therapeutics (Huang et al. 1999; Jain et al. 2002), the effect of diabetes on angiogenesis in muscle regeneration (Langer et al. 2016), and nerve degeneration/regeneration (Pan et al. 2012). Moreover, the direct and non‐invasive accessibility to the PC muscle, via the glass chamber system, may also be beneficial to an array of translational research including muscle drug, gene and cell delivery, and biomaterial tissue engineering research (Laschke et al. 2011; Laschke & Menger, 2016; Langer et al. 2016).
Engineering PC
Contractile skeletal myotubes were successfully bioengineered in vitro using satellite cells of the PC muscle layer (Garcia‐Parra et al. 2014; Naldaiz‐Gastesi et al. 2016). Moreover, in another on‐going study in our labs, we were able to show that mdx satellite cells derived from the PC muscle layer gave rise to myotubes with a higher myogenic activity compared with their wild type counterparts. If this approach could be successfully applied to human PC tissue, it would be a promising cell transplantation‐based therapy for muscle regeneration.
Interestingly, other groups have exploited the panniculus carnosus muscle with the dorsal skinfold window chamber to study the engraftment of bioengineered muscles as a basis for human muscle therapies (Juhas et al. 2014). Briefly, Juhas et al. cultured and differentiated neonatal rat muscle cells in a 3D arrangement, such that they retain their stem cells and regenerative capacity similar to native muscle. Muscle cells were further engineered by anchoring the fibres end‐to‐end with tendon‐mimetic Velcro tabs pinned inside a polydimethylsiloxane well. The biomimetic muscles were implanted on the PC muscle in a skinfold window chamber on the back of immunodeficient mice. The rat muscle bundles were seen to thrive on the mouse PC muscle layer over a 2‐week period. Dramatically, the engineered muscles became increasingly vascularized and integrated with the host PC muscle. Muscle fibres hypertrophied and also responded to a toxin‐induced injury challenge at the expense of a reduction in the muscle stem cell pool. Increases in detected calcium spikes paralleled a progressive increase in maximal contractile force output such that muscles could contract as strongly as native neonatal skeletal muscle by 2 weeks post‐implantation (Juhas et al. 2014). In fact the biomimetic muscles produced 10–100 fold higher contractile‐force amplitudes compared with other previously in vitro engineered muscles (Hinds et al. 2011; Koffler et al. 2011). Others have shown that engineered muscles transplanted into mice can partially fulfil the functional properties of an excised muscle (Carosio et al. 2013).
More recently, the use of the dorsal skinfold window chamber has been extended for use in assessing the engraftment and functionality of human induced pluripotent stem cell (hPSC)‐derived skeletal muscle (Rao et al. 2018). Muscle progenitor cells were derived by transient overexpression of Pax7 in mesodermal cells differentiated from the hPSCs. The human muscle progenitor cells were grown in vitro into 3D bundles of muscle tissue and were subsequently implanted into the dorsal skin fold of immunocompromised mice. The hPSC‐derived muscle tissue survived and became progressively vascularized, exemplifying calcium firing patterns typical of contractile adult myofibres (Rao et al. 2018). The aforementioned studies reflect how the dorsal skinfold window chamber technique is increasingly being used and developed as an invaluable tool to examine and functionally test stem cell engineered tissues in the field of regenerative medicine (Laschke & Menger, 2016; Schreiter et al. 2017).
Conclusions
In this article, we have advocated a redefinition of PC muscle and a re‐assessment of its functional importance in the light of numerous studies and diverse aspects of its physiology. We hope that this review will improve knowledge of this unique anatomical structure and induce other researchers to add information on the knowledge gaps that still persist.
Disclosure of interests
No competing financial interests exist.
Author contributions
A.I. and K.M. wrote the manuscript with the support of N.N.‐G., O.B. and A.L.M.
Acknowledgements
Writing of this review was made possible by grants from Ministerio de Economía y Competitividad (RTC‐2015‐3750‐1) and Instituto de Salud Carlos III (PI13/02172, PI16/01430) to A.I., co‐funded by the European Union (ERDF/ESF, ‘Investing in your future’). N.N.‐G. received a studentship from the Department of Education, University and Research of the Basque Government (PRE2013‐1‐1168). A.L.M. was funded by grants from FIS (PI17/01841 and PI14/00436) and The Basque Government (2015/11038, RIS3 2017222021 and BIO16/ER/022). O.B. and K.M.C.'s laboratory were funded by the Ministry of Higher Education, Saudi Arabia. K.M.C. was funded by the Millennium Research fund, NUIG.
Contributor Information
Karl J. A. McCullagh, Email: karl.mccullagh@nuigalway.ie
Ander Izeta, Email: ander.izeta@biodonostia.org.
References
- Abdullahi A, Amini‐Nik S, Jeschke MG (2014) Animal models in burn research. Cell Mol Life Sci 71, 3241–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agam L (2007) Pressure ulcers and deep tissue injury: a bioengineering perspective. J Wound Care 16, 336–342. [DOI] [PubMed] [Google Scholar]
- Aksoy MH, Vargel I, Canter IH, et al. (2002) A new experimental hypertrophic scar model in guinea pigs. Aesthetic Plast Surg 26, 388–396. [DOI] [PubMed] [Google Scholar]
- Aksoy B, Aksoy HM, Civas E, et al. (2009) A new experimental delayed wound healing model in rabbits. Eur J Dermatol 19, 565–569. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Kasza I, Yen CL, et al. (2015) Dermal white adipose tissue: a new component of the thermogenic response. J Lipid Res 56, 2061–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algire GH (1943) An adaptation of the transparent‐chamber technique to the mouse. J Natl Cancer Inst 4, 1–11. [Google Scholar]
- Allamand V, Campbell KP (2000) Animal models for muscular dystrophy: valuable tools for the development of therapies. Hum Mol Genet 9, 2459–2467. [DOI] [PubMed] [Google Scholar]
- Amini‐Nik S, Glancy D, Boimer C, et al. (2011) Pax7 expressing cells contribute to dermal wound repair, regulating scar size through a beta‐catenin mediated process. Stem Cells 29, 1371–1379. [DOI] [PubMed] [Google Scholar]
- Argyris TS, Argyris BF (1962) Differential response of skin epithelium to growth‐promoting effects of subcutaneously transplanted tumor. Can Res 22, 73–77. [PubMed] [Google Scholar]
- Ashwell KWS, Musser AM (2013) Atlas and tables of peripheral nervous system anatomy In: Neurobiology of Monotremes: Brain Evolution in Our Distant Mammalian Cousins. (ed. Ashwell KWS.), p. 311, Collingwood, Victoria: CSIRO Publishing. [Google Scholar]
- Atit R, Sgaier SK, Mohamed OA, et al. (2006) Beta‐catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol 296, 164–176. [DOI] [PubMed] [Google Scholar]
- Bahri OA, Naldaiz‐Gastesi N, Wheatley AM, Izeta A, and McCullagh KJA (2016). Highly regenerative panniculus carnosus skeletal muscle in the pre‐clinical mdx model of DMD. Human Gene Therapy 27, A67–A67. [Google Scholar]
- Bakheit MA (1996) Effects of transplantation of the panniculus carnosus on wound contraction. Sudan Med J 34, 10–14. [Google Scholar]
- Bamberger C, Hafner A, Schmale H, et al. (2005) Expression of different p63 variants in healing skin wounds suggests a role of p63 in reepithelialization and muscle repair. Wound Repair Regen 13, 41–50. [DOI] [PubMed] [Google Scholar]
- Bergman RA, Afifi AK, Miyauchi R (2017) Illustrated encyclopedia of human anatomic variation: opus I: muscular system: alphabetical listing of muscles: P. Panniculus carnosus In: Anatomy Atlases. (eds D'Alessandro MP, Bergman RA.). Website available from http://www.anatomyatlases.org [Accessed 19 September 2017]. [Google Scholar]
- Besana‐Ciani I, Greenall MJ (2005) Langer's axillary arch: anatomy, embryological features and surgical implications. Surgeon 3, 325–327. [DOI] [PubMed] [Google Scholar]
- Billingham RE, Medawar PB (1955) Contracture and intussusceptive growth in the healing of extensive wounds in mammalian skin. J Anat 89, 114–123. [PMC free article] [PubMed] [Google Scholar]
- Billingham RE, Russell PS (1956) Studies on wound healing, with special reference to the phenomenon of contracture in experimental wounds in rabbits’ skin. Ann Surg 144, 961–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biressi S, Bjornson CR, Carlig PM, et al. (2013) Myf5 expression during fetal myogenesis defines the developmental progenitors of adult satellite cells. Dev Biol 379, 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton TR, Nystrom M, Blau HM (2003) Significant differences among skeletal muscles in the incorporation of bone marrow‐derived cells. Dev Biol 262, 64–74. [DOI] [PubMed] [Google Scholar]
- Brownell I, Guevara E, Bai CB, et al. (2011) Nerve‐derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8, 552–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunius U, Zederfeldt B, Ahren C (1968) Healing of skin incisions with intact subcutaneous muscle closed by non‐suture technique. A tensiometric and histologic study in the rat. Acta Chir Scand 134, 187–193. [PubMed] [Google Scholar]
- Buckingham M, Relaix F (2007) The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol 23, 645–673. [DOI] [PubMed] [Google Scholar]
- Butcher EO (1934) The hair cycles in the albino rat. Anat Rec 61, 5–19. [Google Scholar]
- Cappellari O, Benedetti S, Innocenzi A, et al. (2013) Dll4 and PDGF‐BB convert committed skeletal myoblasts to pericytes without erasing their myogenic memory. Dev Cell 24, 586–599. [DOI] [PubMed] [Google Scholar]
- Carosio S, Barberi L, Rizzuto E, et al. (2013) Generation of eX vivo‐vascularized Muscle Engineered Tissue (X‐MET). Sci Rep 3, 1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso N, Herberth B, Bartoli M, et al. (2013) Deregulation of the protocadherin gene FAT1 alters muscle shapes: implications for the pathogenesis of facioscapulohumeral dystrophy. PLoS Genet 9, e1003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MR, Heizmann CW (1982) Calcium‐binding protein parvalbumin is associated with fast contracting muscle fibres. Nature 297, 504–506. [DOI] [PubMed] [Google Scholar]
- Cohen IK, Moore CD, Diegelmann RF (1979) Onset and localization of collagen synthesis during wound healing in open rat skin wounds. Proc Soc Exp Biol Med 160, 458–462. [DOI] [PubMed] [Google Scholar]
- Corbel SY, Lee A, Yi L, et al. (2003) Contribution of hematopoietic stem cells to skeletal muscle. Nat Med 9, 1528–1532. [DOI] [PubMed] [Google Scholar]
- Cuthbertson AM (1959) Contraction of full thickness skin wounds in the rat. Surg Gynecol Obstet 108, 421–432. [PubMed] [Google Scholar]
- Dahiya P (2009) Burns as a model of SIRS. Front Biosci 14, 4962–4967. [DOI] [PubMed] [Google Scholar]
- Daly TJ, Buffenstein R (1998) Skin morphology and its role in thermoregulation in mole‐rats, Heterocephalus glaber and Cryptomys hottentotus . J Anat 193, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C (1879) The evidence of the descent of man from some lower form In: The Descent of Man, and Selection in Relation to Sex. (ed. Murray J.), pp. 29–30, London: Penguin Books. [Google Scholar]
- Davidson JM, Aquino AM, Woodward SC, et al. (1999) Sustained microgravity reduces intrinsic wound healing and growth factor responses in the rat. FASEB J 13, 325–329. [DOI] [PubMed] [Google Scholar]
- Davidson JM, Yu F, Opalenik SR (2013) Splinting strategies to overcome confounding wound contraction in experimental animal models. Adv Wound Care 2, 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro CC (1980) The anatomy of the platysma muscle. Plast Reconstr Surg 66, 680–683. [DOI] [PubMed] [Google Scholar]
- De la Cuadra‐Blanco C, Peces‐Pena MD, Carvallo‐de Moraes LO, et al. (2013) Development of the platysma muscle and the superficial musculoaponeurotic system (human specimens at 8–17 weeks of development). Sci World J 2013, 716962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A, Maroli G, Covarello D, et al. (2011) Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2, 499. [DOI] [PubMed] [Google Scholar]
- Deodato B, Arsic N, Zentilin L, et al. (2002) Recombinant AAV vector encoding human VEGF165 enhances wound healing. Gene Ther 9, 777–785. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Gracanin F (1968) Differential involvement of tibialis anterior, gastrocnemius and soleus in muscular dystrophy. J Neurol Sci 6, 105–115. [DOI] [PubMed] [Google Scholar]
- Dowling P, Culligan K, Ohlendieck K (2002) Distal mdx muscle groups exhibiting up‐regulation of utrophin and rescue of dystrophin‐associated glycoproteins exemplify a protected phenotype in muscular dystrophy. Naturwissenschaften 89, 75–78. [DOI] [PubMed] [Google Scholar]
- Drake J (1727) Appendix In: Anthropologia Nova, or, A New System of Anatomy: Describing the Animal Oeconomy, and a Short Rationale of Many Distempers Incident to Human Bodies, p. 48, London: W. and J. Innys. [Google Scholar]
- Driskell RR, Jahoda CA, Chuong CM, et al. (2014) Defining dermal adipose tissue. Exp Dermatol 23, 629–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddy W, Duguez S, Johnston H, et al. (2015) Muscular dystrophy in the mdx mouse is a severe myopathy compounded by hypotrophy, hypertrophy and hyperplasia. Skelet Muscle 5, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durward A, Rudall KM (1949) Studies on hair growth in the rat. J Anat 83, 325–335. [PMC free article] [PubMed] [Google Scholar]
- Eckhaus AA, Fish JS, Skarja G, et al. (2008) A preliminary study of the effect of poly(methacrylic acid‐co‐methyl methacrylate) beads on angiogenesis in rodent skin grafts and the quality of the panniculus carnosus. Plast Reconstr Surg 122, 1361–1370. [DOI] [PubMed] [Google Scholar]
- Emery AE (2002) The muscular dystrophies. Lancet 359, 687–695. [DOI] [PubMed] [Google Scholar]
- Essig CM, Merritt JS, Stubbs NC, et al. (2013) Localization of the cutaneus trunci muscle reflex in horses. Am J Vet Res 74, 1428–1432. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella‐De Angelis G, Coletta M, et al. (1998) Muscle regeneration by bone marrow‐derived myogenic progenitors. Science 279, 1528–1530. [DOI] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, et al. (2011) Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146, 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor PB (1993) Editorial: from the Panniculus Carnosus (PC) to the Superficial Fascia System (SFS). Aesthetic Plast Surg 17, 179–181. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Ferreira M, Donati G, et al. (2011) The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144, 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura D, Jain RK (2008) Imaging angiogenesis and the microenvironment. APMIS 116, 695–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima MG, Furlan I, Chiavegatti T, et al. (2005) Ectopic development of skeletal muscle induced by subcutaneous transplant of rat satellite cells. Braz J Med Biol Res 38, 367–374. [DOI] [PubMed] [Google Scholar]
- Garcia‐Parra P, Naldaiz‐Gastesi N, Maroto M, et al. (2014) Murine muscle engineered from dermal precursors: an in vitro model for skeletal muscle generation, degeneration, and fatty infiltration. Tissue Eng 20, 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger E (2004) Individual muscles: attachments, action and structure In: Animal Anatomy for Artists: The Elements of Form. (ed Goldfinger E.), p. 65, New York: Oxford University Press. [Google Scholar]
- Gottrup F, Agren MS, Karlsmark T (2000) Models for use in wound healing research: a survey focusing on in vitro and in vivo adult soft tissue. Wound Repair Regen 8, 83–96. [DOI] [PubMed] [Google Scholar]
- Greenwood JE (2010) Function of the panniculus carnosus – a hypothesis. Vet Rec 167, 760. [DOI] [PubMed] [Google Scholar]
- Griffiths M (1989) Tachyglossidae In: Fauna of Australia ‐ Volume 1B Mammalia. (eds Walton DW, Richardson BJ.), pp. 410, 456, Canberra: AGPS. [Google Scholar]
- Gundeslioglu AO, Selimoglu N, Toy H, et al. (2013) Neo‐vascularisation of musculocutaneous and muscle flaps after division of the major vascular supply: an experimental study. J Plast Reconstr Aesthet Surg 66, 978–986. [DOI] [PubMed] [Google Scholar]
- Hall JG (2015) Muscle In: Human Malformations and Related Anomalies (eds Stevenson RE, Hall JG, Everman DB, et al. 327–328, Oxford: Oxford University Press. [Google Scholar]
- Hallett CH (1848) An account of the anomalies of the muscular system, met with in the dissecting‐room of the University during the years 1846–1847; with general remarks. Edinb Med Surg J 69, 1–32. [PMC free article] [PubMed] [Google Scholar]
- Hawley‐Nelson P, Berchtold MW, Huitfeldt H, et al. (1986) Skin calcium‐binding protein is a parvalbumin of the Panniculus Carnosus. J Invest Dermatol 86, 157–162. [DOI] [PubMed] [Google Scholar]
- Higgins G, Martin S (2012) The integumentary system In: Horse Anatomy for Performance: A Practical Guide to Training, Riding and Horse Care. (ed Higgins G, Martin S.), p. 8, Newton Abbot: David & Charles. [Google Scholar]
- Hillel AT, Nahas Z, Unterman S, et al. (2012) Validation of a small animal model for soft tissue filler characterization. Dermatol Surg 38, 471–478. [DOI] [PubMed] [Google Scholar]
- Hinds S, Bian W, Dennis RG, et al. (2011) The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials 32, 3575–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G, Blok BF (1989) Descending pathways to the cutaneous trunci muscle motoneuronal cell group in the cat. J Neurophysiol 62, 1260–1269. [DOI] [PubMed] [Google Scholar]
- Huang Q, Shan S, Braun RD, et al. (1999) Noninvasive visualization of tumors in rodent dorsal skin window chambers. Nat Biotechnol 17, 1033–1035. [DOI] [PubMed] [Google Scholar]
- Hughes AFW, Dann L (1941) Vascular regeneration in experimental wounds and burns. Br J Exp Pathol 22, 9–14. [Google Scholar]
- Ibrahim MM, Bond J, Bergeron A, et al. (2014) A novel immune competent murine hypertrophic scar contracture model: a tool to elucidate disease mechanism and develop new therapies. Wound Repair Regen 22, 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im MJC, Adachi K (1968) Glycolytic enzyme profiles in the arrector pili muscle and the subcutaneous muscle. J Invest Dermatol 50, 429–434. [DOI] [PubMed] [Google Scholar]
- Inzunza O, Pino F, Navarrete C, et al. (2008) Panniculus carnosus, vestigial remnants in the axillary region. Int J Morphol 26, 841–844. [Google Scholar]
- Irintchev A, Rosenblatt JD, Cullen MJ, et al. (1998) Ectopic skeletal muscles derived from myoblasts implanted under the skin. J Cell Sci 111, 3287–3297. [DOI] [PubMed] [Google Scholar]
- van Iwaarden A, Stubbs NC, Clayton HM (2012) Topographical anatomy of the equine M. Cutaneus Trunci in relation to the position of the saddle and girth. J Equine Vet Sci 32, 519–524. [Google Scholar]
- Jahoda CA, Christiano AM (2011) Niche crosstalk: intercellular signals at the hair follicle. Cell 146, 678–681. [DOI] [PubMed] [Google Scholar]
- Jain RK, Munn LL, Fukumura D (2002) Dissecting tumour pathophysiology using intravital microscopy. Nat Rev Cancer 2, 266–276. [DOI] [PubMed] [Google Scholar]
- Jana TK (2018) Window dissections: introduction In: Exam‐Oriented Practical Anatomy: A Student's Manual, p. 10, New Delhi: Jaypee Brothers Medical Publishers. [Google Scholar]
- Jones BR (2012) Twitch or no twitch? The cutaneous trunci reflex. J Small Anim Pract 53, 431. [DOI] [PubMed] [Google Scholar]
- Juhas M, Engelmayr GC Jr, Fontanella AN, et al. (2014) Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo . Proc Natl Acad Sci U S A 111, 5508–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S‐J, Lee H, Choi I‐J, et al. (2016) Muscular axillary arch accompanying variation of the musculocutaneous nerve: axillary arch. Anat Cell Biol 49, 160–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DF, Cliff WJ (1979) A systematic study of wound contraction in mammalian skin. Pathology 11, 207–222. [DOI] [PubMed] [Google Scholar]
- Koffler J, Kaufman‐Francis K, Shandalov Y, et al. (2011) Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc Natl Acad Sci U S A 108, 14789–14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov IL, Scherer PE (2016) Dermal adipocytes and hair cycling: is spatial heterogeneity a characteristic feature of the dermal adipose tissue depot? Exp Dermatol 25, 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni NV (2012) Skin, hypodermis and deep fascia In: Clinical Anatomy (A Problem Solving Approach), 2nd edn (ed. Kulkarni NV.), p. 33, New Delhi: Jaypee Brothers, Medical Publishers Pvt. Limited. [Google Scholar]
- La Colla A, Pronsato L, Milanesi L, et al. (2015) 17β‐Estradiol and testosterone in sarcopenia: role of satellite cells. Ageing Res Rev 24, 166–177. [DOI] [PubMed] [Google Scholar]
- Langer S, Beescho C, Ring A, et al. (2016) A new in vivo model using a dorsal skinfold chamber to investigate microcirculation and angiogenesis in diabetic wounds. GMS Interdiscip Plast Reconstr Surg DGPW, 5, Doc09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langworthy OR (1924) The Panniculus Carnosus in cat and dog and its genetical relation to the pectoral musculature. J Mammal 5, 49–63. [Google Scholar]
- Langworthy OR (1925) A morphological study of the Panniculus Carnosus and its genetical relationship to the pectoral musculature in rodents. Am J Anat 35, 283–302. [Google Scholar]
- Laschke MW, Menger MD (2016) The dorsal skinfold chamber: a versatile tool for preclinical research in tissue engineering and regenerative medicine. Eur Cell Mater, 32, 202–215. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Vollmar B, Menger MD (2011) The dorsal skinfold chamber: window into the dynamic interaction of biomaterials with their surrounding host tissue. Eur Cell Mater, 22, 147–164. [DOI] [PubMed] [Google Scholar]
- Lepper C, Partridge TA, Fan CM (2011) An absolute requirement for Pax7‐positive satellite cells in acute injury‐induced skeletal muscle regeneration. Development 138, 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz HP, Longaker MT (2008) Wounds: biology, pathology, and management In: Surgery: Basic Science and Clinical Evidence. (eds Norton J, Barie PS, Bollinger RR, et al. ), p. 195, Berlin: Springer. [Google Scholar]
- Machado MJC, Watson MG, Devlin AH, et al. (2011) Dynamics of angiogenesis during wound healing: a coupled in vivo and in silico study. Microcirculation 18, 183–197. [DOI] [PubMed] [Google Scholar]
- Mariot V, Roche S, Hourde C, et al. (2015) Correlation between low FAT1 expression and early affected muscle in facioscapulohumeral muscular dystrophy. Ann Neurol 78, 387–400. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Ikuta N, Mori M, et al. (2010) Mechanics of wrinkle formation: micromechanical analysis of skin deformation during wrinkle formation in ultraviolet‐irradiated mice. Skin Res Technol 16, 179–189. [DOI] [PubMed] [Google Scholar]
- McMinn RMH (1998) Head and neck and spine In: Last's Anatomy: Regional and Applied. (ed. McMinn RMH.), p. 435, Chatswood: Churchill Livingstone, Elsevier Australia. [Google Scholar]
- Menger MD, Laschke MW, Vollmar B (2006) Chamber assays In: Angiogenesis Assays: A Critical Appraisal of Current Techniques. (eds Staton CA, Lewis C, Bicknell R.), p. 244, Hoboken: John Wiley and Sons, Ltd. [Google Scholar]
- Munz B, Wiedmann M, Lochmu H, et al. (1999) Cloning of novel injury‐regulated genes. J Biol Chem 274, 13305–13310. [DOI] [PubMed] [Google Scholar]
- Naldaiz‐Gastesi N, Goicoechea M, Alonso‐Martin S, et al. (2016) Identification and characterization of the dermal Panniculus Carnosus muscle stem cells. Stem Cell Rep 7, 411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutelings T, Nusgens BV, Liu Y, et al. (2015) Skin physiology in microgravity: a 3‐month stay aboard ISS induces dermal atrophy and affects cutaneous muscle and hair follicles cycling in mice. NPJ Microgravity, 1, 15002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Grunewald B, Nguyen T, et al. (2012) The lateral thoracic nerve and the cutaneous maximus muscle – a novel in vivo model system for nerve degeneration and regeneration studies. Exp Neurol 236, 6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Pierce SA, von Drehle M, et al. (2010) skNAC, a Smyd1‐interacting transcription factor, is involved in cardiac development and skeletal muscle growth and regeneration. Proc Natl Acad Sci U S A 107, 20750–20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavletic MM (2018) The skin In: Atlas of Small Animal Wound Management and Reconstructive Surgery, (ed. Pavletic MM.), pp. 8–9, Hoboken: Wiley‐Blackwell. [Google Scholar]
- Pavletic MM (2003) The integument In: Textbook of Small Animal Surgery. (ed. Slatter DH.), pp. 253–255, Philadelphia: Saunders (W.B.) Co. Ltd. [Google Scholar]
- Perrin JB (1871) On a rudiment of the dorsal portion of the panniculus carnosus, superficial to the trapezius. J Anat Physiol 5, 241. [PMC free article] [PubMed] [Google Scholar]
- Petruska JC, Barker DF, Garraway SM, et al. (2014) Organization of sensory input to the nociceptive‐specific cutaneous trunk muscle reflex in rat, an effective experimental system for examining nociception and plasticity. J Comp Neurol 522, 1048–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Guerrero‐Juarez CF, Ito M, et al. (2017) Regeneration of fat cells from myofibroblasts during wound healing. Science 355, 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri RK, Segal SS (1994) Microvascular responses to body tilt in cutaneous maximus muscle of conscious rats. J Appl Physiol 77, 2426–2433. [DOI] [PubMed] [Google Scholar]
- Rao L, Qian Y, Khodabukus A, et al. (2018) Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat Commun 9, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese S, Budras K‐D, Mülling C, et al. (2004) Common integument (integumentum commune) In: Veterinary Anatomy of Domestic Mammals: Textbook and Colour Atlas. (eds König HE, Liebich H‐G.), p. 610, Stuttgart: Schattauer. [Google Scholar]
- Reidenberg JS (2018) Musculature In: Encyclopedia of Marine Mammals, 3rd edn (eds Würsig B, Thewissen JGM, Kovacs KM.), p. 623, London: Academic Press. [Google Scholar]
- Richter GT, Fan CY, Ozgursoy O, et al. (2006) Effect of vascular endothelial growth factor on skin graft survival in Sprague‐Dawley rats. Arch Otolaryngol Head Neck Surg 132, 637–641. [DOI] [PubMed] [Google Scholar]
- Ridgway SH, Carder DA (1990) Tactile sensitivity, somatosensory responses, skin vibrations, and the skin surface ridges of the bottle‐nose dolphin, Tursiops truncatus In: Sensory Abilities of Cetaceans: Laboratory and Field Evidence. (eds Thomas JA, Kastelein RA.), p. 165 New York: Plenum Press. [Google Scholar]
- Rittie L (2016) Cellular mechanisms of skin repair in humans and other mammals. J Cell Commun Signal 10, 103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LA, Bal NC, Periasamy M (2015) The role of skeletal‐muscle‐based thermogenic mechanisms in vertebrate endothermy. Biol Rev 90, 1279–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcido RS (2007) Myosubcutaneous infarct: deep tissue injury. Adv Skin Wound Care 20, 248–250. [DOI] [PubMed] [Google Scholar]
- Salcido R, Donofrio JC, Fisher SB, et al. (1995) Evaluation of ibuprofen for pressure ulcer prevention: application of a rat pressure ulcer model. Adv Wound Care 8, 30–32. [PubMed] [Google Scholar]
- Sánchez‐Yus E, Simón P (2000) Striated muscle: a normal component of the dermis and subcutis in many areas of the face. Am J Dermatopathol 22, 503–509. [DOI] [PubMed] [Google Scholar]
- Sandonà D, Desaphy J‐F, Camerino GM, et al. (2012) Adaptation of mouse skeletal muscle to long‐term microgravity in the MDS mission. PLoS ONE 7, e33232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlattner U, Mo N, Speer O, et al. (2002) Creatine kinase and creatine transporter in normal, wounded, and diseased skin. J Invest Dermatol 118, 416–423. [DOI] [PubMed] [Google Scholar]
- Schneider MR (2014) Coming home at last: dermal white adipose tissue. Exp Dermatol 23, 634–635. [DOI] [PubMed] [Google Scholar]
- Schreiter J, Meyer S, Schmidt C, et al. (2017) Dorsal skinfold chamber models in mice. GMS Interdiscip Plast Reconstr Surg DGPW, 6, Doc10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RI, Christensen JL, Weissman IL, et al. (2004) Determinants of skeletal muscle contributions from circulating cells, bone marrow cells, and hematopoietic stem cells. Stem Cells 22, 1292–1304. [DOI] [PubMed] [Google Scholar]
- Singh V (2015) Skin, superficial fascia and deep fascia In: General Anatomy, 2nd edn, p. 61 New Delhi: Reed Elsevier India. [Google Scholar]
- Singh P, Reimer CL, Peters JH, et al. (2004) The spatial and temporal expression patterns of integrin α9β1 and one of its ligands, the EIIIA segment of fibronectin, in cutaneous wound healing. J Invest Dermatol 123, 1176–1181. [DOI] [PubMed] [Google Scholar]
- Sorg H, Krueger C, Vollmar B (2007) Intravital insights in skin wound healing using the mouse dorsal skin fold chamber. J Anat 211, 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB (2013) Bidirectional myoblast‐pericyte plasticity. Dev Cell 24, 563–564. [DOI] [PubMed] [Google Scholar]
- Standring S (2008) Male reproductive system In: Gray's Anatomy, 40th edn (ed. Standring S.), pp. 1938–2312, Philadelphia: Elsevier/Churchill Livingstone. [Google Scholar]
- Stewart T, Salcido S (2012) Deep tissue injury : 25 years of learning. Adv Skin Wound Care 25, 59–60. [DOI] [PubMed] [Google Scholar]
- Tallon C, Russell KA, Sakhalkar S, et al. (2016) Length‐dependent axo‐terminal degeneration at the neuromuscular synapses of type II muscle in SOD1 mice. Neuroscience 312, 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Tsunemi Y, Kawashima M, et al. (2013) The impact of near‐infrared in plastic surgery. Plast Surg 2013, 1–13. [Google Scholar]
- Theriault E, Diamond J (1988) Nociceptive cutaneous stimuli evoke localized contractions in a skeletal muscle. J Neurophysiol 60, 446–462. [DOI] [PubMed] [Google Scholar]
- Tronnier H, Wiebusch M, Heinrich U (2008) Change in skin physiological parameters in space – report on and results of the first study on man. Skin Pharmacol Physiol 21, 283–292. [DOI] [PubMed] [Google Scholar]
- Turner W (1867) On the musculus sternalis. J Anat Physiol 1, 246–253. [PMC free article] [PubMed] [Google Scholar]
- Turner W (1870) On a rudiment of the panniculus carnosus superficial to the trapezius. J Anat Physiol 5, 116–117. [PMC free article] [PubMed] [Google Scholar]
- Turnquist JE, Minugh‐Purvis N (2012) Functional morphology In: Nonhuman Primates in Biomedical Research, 2nd edn (eds Abee CR, Mansfield K, Tardif S, Morris T.), p. 116, Boston: Academic Press. [Google Scholar]
- Watts GT (1960) Wound shape and tissue tension in healing. Br J Surg 47, 555–561. [DOI] [PubMed] [Google Scholar]
- Watts GT, Grillo HC, Gross J (1958) Studies in wound healing: II. The role of granulation tissue in contraction. Ann Surg 148, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder HH (1923) The History of the Human Body. New York City: Henry Holt and Company. [Google Scholar]
- Williams JK, Mariya S, Suparto I (2017) Gender, age and differences in stem cell expression and efficacy. J Stem Cell Res Ther 3(2), 00097. [Google Scholar]
- Wong VW, Sorkin M, Glotzbach JP, et al. (2011) Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol 2011, 969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K (1971) On the panniculus carnosus of the bridled dolphin (Stenella frontalis). Acta Med Nagasaki 15, 13–25. [PubMed] [Google Scholar]


