Abstract
A morphological and morphometric study of the skin of a variety of newborn marsupials (Dasyurus viverrinus, Monodelphis domestica, Trichosurus vulpecula, Isoodon obesulus, Perameles nasuta, Phascolarctos cinereus, Potorous tridactylus, Petrogale penicillata, Thylogale thetidi, Macropus dorsalis) and of a monotreme hatchling (Ornithorhynchus anatinus) was undertaken to assess the possibility of cutaneous gas exchange. Additionally, the lungs of some of these species were investigated to assess its structural degree at birth. The skin in the different newborn marsupials and the monotreme hatchling had a similar structure (no hair follicles and no sebaceous or perspiratory glands) and was in all cases less developed than the skin of altricial eutherians. The thickness of the entire skin (36–186 μm) and its different layers, epidermis (6–29 μm) and dermis (29–171 μm) varied among the marsupial species and reflected the differences in size and developmental degree of the neonates. In the skin of all marsupial neonates and the monotreme hatchling, numerous superficial cutaneous capillaries were encountered, some closely associated with the epidermis, indicating the possibility that the skin participated in gaseous exchange. The skin of the newborn D. viverrinus had the highest capillary volume density and shortest skin diffusion barrier of all marsupial neonates, suggesting that skin gas exchange in the dasyurid neonate might be the most pronounced. A graduation of the skin capillary density among the marsupial neonates inversely followed the respective lung structure and general developmental degree of the neonates.
Keywords: gas exchange, lung, morphology, neonate, skin
Introduction
Newborn marsupials and newly hatched monotremes are distinguished from eutherian mammals by their extremely small size and high immaturity at birth (bodyweight ranging from ~ 6 mg in the smallest newborn, the honey possum, to ~ 830 mg in the largest newborn, the grey kangaroo; see Tyndale‐Biscoe & Renfree, 1987). Most of their growth and development occurs postnatally, supported by a prolonged lactation cycle (Renfree, 2006; Ferner & Mess, 2011). Despite the extremely small size, primary systems of the marsupial neonate have been described as basically functioning (e.g. digestive, neuronal, immune, respiratory; see Hughes & Hall, 1988). Increasing evidence, however, suggests that the immaturity of the respiratory system in marsupial neonates necessitates recruitment of an alternative organ system such as the skin for gas exchange (Frappell & MacFarlane, 2006).
The lungs of newborn marsupials and newly hatched monotremes are considerably less developed when compared with those of newborn eutherian mammals (Ferner et al. 2009, 2017). The lung structure is highly immature at birth and the majority of lung development takes place during early postnatal life (Szdzuy et al. 2008). A graduation of lung development from canalicular stage to saccular stage can be observed among newborn marsupials (Frappell & MacFarlane, 2006; Mess & Ferner, 2010). It follows the size variation in the sequence G1 to G3 as proposed by Hughes & Hall (1988), which is based on the developmental complexity of the organ systems and the external form of the newborns. The most underdeveloped of all marsupial neonates, the low birthweight Dasyuridae (G1), such as the eastern and northern native cat (Dasyurus viverrinus, 12.5 mg, Hill & Hill, 1955; Dasyurus hallucatus, 18 mg, Gemmell & Nelson, 1988), the stripe‐faced and fat‐tailed dunnart (Sminthopsis macroura,16 mg, Gemmell & Selwood, 1994; Sminthopsis crassicaudata, 10 mg, Simpson et al. 2011) and the Tasmanian devil (Sarcophilus harrisii; 18 mg, Hughes & Hall, 1988) have simple airways that end in tubular air spaces with vascularized partitions.
Neonates of the Peramelidae (bandicoot, Isoodon macrourus, 180 mg, Gemmell, 1986), Phalangeridae (brushtail possum, Trichosurus vulpecula, 200 mg, Gemmell & Nelson, 1988), and Didelphidae (North American opossum, Didelphis virginiana, 130 mg, Krause & Leeson, 1975; grey short‐tailed opossum, Monodelphis domestica, 100 mg, Ferner et al. 2017) represent the intermediate developmental type G2, with lungs consisting of a number of large terminal air spaces with capillaries located on either side of the thickened primary septa. The most developed lungs of all marsupial neonates (G3) can be found among the Macropodidae (tammar wallaby, Macropus eugenii, 370 mg, Runciman et al. 1996; Szdzuy et al. 2008). They already have a primitive bronchial tree terminating in several smaller saccules (saccules still larger than in eutherian neonates).
Despite the immaturity of the respiratory system in marsupial neonates, respiration and gas exchange via the lung is possible in most of the newborn marsupials investigated so far (M. eugenii: MacFarlane & Frappell, 2001; MacFarlane et al. 2002; Frappell & MacFarlane, 2006; M. domestica: Szdzuy et al. 2008). Exceptions are the smallest marsupial neonates, the dasyurids. In the fat‐tailed dunnart, S. crassicaudata (Frappell & MacFarlane, 2006) and Julia creek dunnart, Sminthopsis douglasi (Frappell & Mortola, 2000) no pulmonary ventilation was observed and gas exchange was conducted solely via cutaneous respiration. The onset of pulmonary ventilation is delayed for a number of days in these species (Mortola et al. 1999).
But even in comparatively well developed marsupial neonates, such as the tammar wallaby, the high resistance of the respiratory system and the distortion of the chest wall greatly reduce the mechanical efficiency of breathing (MacFarlane et al. 2002). Therefore, gas exchange through the skin is an important complement to pulmonary ventilation in the newborn tammar wallaby and accounts for 33% of the total oxygen uptake (MacFarlane & Frappell, 2001).
Considering the large surface area to volume ratio inherent in small body size, it is not surprising that cutaneous gas exchange occurs during the neonatal period in marsupials. Factors that likely contribute to cutaneous gas exchange in newborn marsupials may be the low oxygen requirements of the pouch young (Frappell & MacFarlane, 2006; Szdzuy et al. 2008; Simpson et al. 2011), neuro‐mechanical constraints on ventilation (Frappell & MacFarlane, 2006) and the presence of cardiac shunts (Runciman et al. 1995).
Given the similar relative immaturity at birth, the possibility exists that cutaneous respiration may be present to a varying extent in nearly all marsupial neonates and in monotreme hatchlings as well.
Earlier morphological studies of the newborn tammar wallaby largely dismissed the possibility that the skin may be an accessory or a major site of gas exchange in marsupial pouch young (Baudinette et al. 1988). The comparison of vascularity (sparse vs. rich) and blood–air diffusion distances (> 100 vs. < 1 μm) of skin and lung led to the assumption that cutaneous respiration might be negligible (Randall et al. 1984). However, these studies used young that were not newborn (youngest animals were ~ 3 days old) and a marsupial species, where the neonate is relatively large. A more recent study on the newborn quokka wallaby (Setonix brachyurus, 350 mg) reported a skin diffusion barrier between 30 and 35 μm, suggesting that the skin also participates in gaseous exchange (Makanya et al. 2007). Besides these studies on macropodids, there is no information about skin morphology of marsupial neonates with respect to diffusion distances or capillary density available. In view of the assumed importance of cutaneous respiration for newborn marsupials emerging from physiological studies, a structural investigation of the skin was needed.
In the current study, the structural characteristics of the skin in newborns of a wide range of marsupial species (Dasyurus viverrinus, Monodelphis domestica, Trichosurus vulpecula, Isoodon obesulus, Perameles nasuta, Phascolarctos cinereus, Potorous tridactylus, Petrogale penicillata, Thylogale thetidi, Macropus dorsalis) and one monotreme hatchling (platypus; Ornithorhynchus anatinus) were compared in order to examine the morphological basis for cutaneous gas exchange.
Materials and methods
Tissues
The taxa selected for this study represent the different developmental stages that exist in marsupial neonates (see Fig. 1). Neonates of eastern quoll (D. viverrinus), grey short‐tailed opossum (M. domestica), southern brown bandicoot (I. obesulus), long‐nosed bandicoot (P. nasuta), brush‐tail possum (T. vulpecula), koala (P. cinereus), long‐nosed potoroo (P. tridactylus), brush‐tailed rock wallaby (P. penicillata), red‐necked pademelon (T. thetidi) and black‐striped wallaby (M. dorsalis) were investigated. For comparison, a neonate of an altricial eutherian, the golden hamster (Mesocricetus auratus) and a newly hatched monotreme, the platypus (O. anatinus), were included in this study. To compare and understand the mechanisms of cutaneous respiration, the skin of two amphibian species with cutaneous respiration, the fire salamander (Salamandra salamandra) and Northern leopard frog (Lithobates pipiens), were investigated as well.
Figure 1.
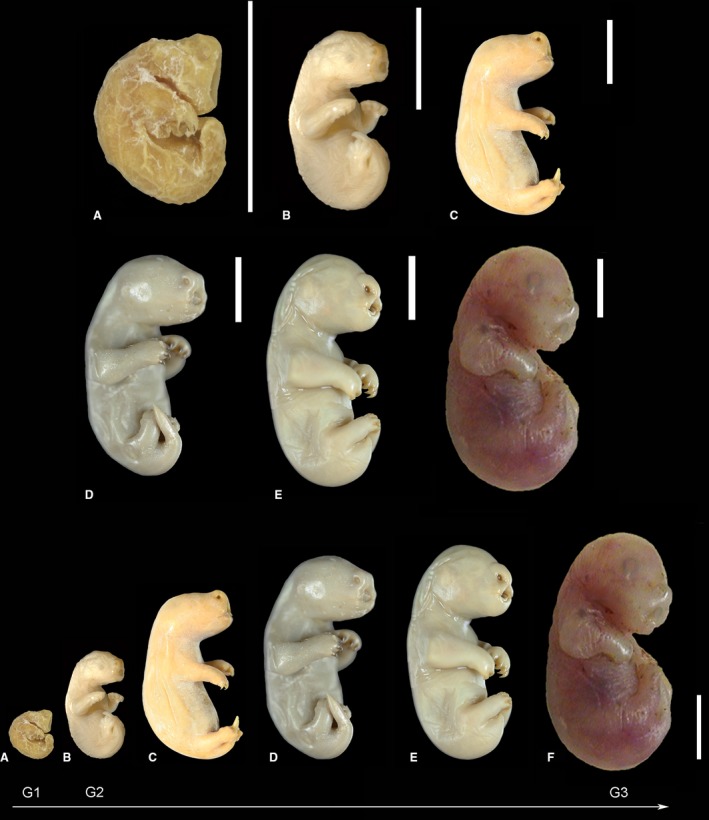
Macrographs of neonate marsupials reflecting the varying developmental degrees (G1–G3) among marsupial species: Dasyurus viverrinus (A), Monodelphis domestica (B), Isoodon obesulus (C), Trichosurus vulpecula (D), Phascolarctos cincereus (E) and Macropus eugenii (F, 5 days old). All neonate marsupials had stronger developed forelimbs than hindlimbs and laterally fused lips. The most immature newborn, D. viverrinus (G1), showed a massive oral shield and large prominent nostrils, eye and ear primordia were barely visible and a definitive neck was missing. M. domestica, I. obesulus and T. vulpecula (G2) had a definitive neck, eye and ear primordia were visible, the nasal swelling was moderate and an simple oral shield was present. Phascolarctos cincereus and M. eugenii (G3) showed a definitive neck, a slight nasal swelling, a vestigial oral shield and visible ear and eye primordia (prominent ring of retinal pigmentation in M. eugenii). Scale bar: 0.5 cm.
The samples of the newborn marsupials and of the newly hatched monotreme were available from the Hubrecht & Hill collection, which is a part of the embryological collection of the Museum für Naturkunde, Leibniz‐Institut für Evolutions‐ und Biodiversitätsforschung, Berlin.
The sections of the neonate grey short‐tailed opossum (M. domestica) and of the neonate Golden hamster (M. auratus) were obtained during the author's PhD work in 2006 (Supervisor: U. Zeller) at the Humboldt‐Universität zu Berlin. The skin sections of the two amphibian species were kindly provided by Collin van Buren (Museum für Naturkunde, Leibniz‐Institut für Evolutions‐ und Biodiversitätsforschung, Berlin).
All samples had been prepared for light microscope studies before (e.g. serially sectioned, transferred to glass slides, stained) and were already available for the investigation. The specimens from the Hubrecht & Hill collection were collected around 1900 in Australia. The exact time of sampling and process of preservation is not documented. However, it is most probable that the material was fixed with Bouin's solution and preserved in 70% ethanol as was state of the art at that time. The neonates of M. domestica and M. auratus were fixed with Bouin's solution and preserved in 70% ethanol as well (for detail see Szdzuy et al. 2008). The fixative Bouin's solution (picric acid, acetic acid and formaldehyde in an aqueous solution) and the entire embedding process in paraffin can cause some shrinkage of the material (~ 30%, see Ross, 1953). Considering the unavoidable shrinking during the processing of the material, the obtained values and morphometrical measurement of skin and lung sections might be underestimated. However, the comparability of the data among the species investigated in this study is a given, as the processing of the material was similar.
Animals labelled as ‘newborn’, ‘recently born’, ‘about to be born’ or ‘newly hatched’ in the collection were defined as neonate/hatchling (day 0 postpartum, first 24 h at the day of birth) in this study. The number of animals investigated per species varied depending on the availability in the Hubrecht & Hill collection. The specifics and numbers of the specimens used for light microscopy in this study are summarized in Table 1.
Table 1.
List of specimens examined in this study
| Species common name | Species scientific name | No. | CRL (mm) | Age/remark | Section | Staining |
|---|---|---|---|---|---|---|
| Eastern quoll | Dasyurus viverrinus | MS 138 | 5.45 | Newborn | 10 μm, longitudinal | H & E |
| MS 138(B) | 5.45 | Newborn | 5 μm, transversal | H & E | ||
| MS134 | 5.5 | Newborn | 10 μm, coronal | H & E | ||
| MS134(B) | 5.5 | Newborn | 5 μm, transversal | H & E | ||
| Grey short‐tailed opossum | Monodelphis domestica | 337* | 10 | Newborn | 10 μm, transversal | Azan |
| Brushtail possum | Trichosurus vulpecula | MS288 | 14.5 | Recently born | 15 μm, coronal | H & E |
| MS289 | 15 | Newborn | H & E | |||
| MS290 | 15.5 | Newborn | H & E | |||
| Southern brown bandicoot | Isoodon obesulus | MS219 | 15 | Newborn | 10 μm, coronal | Azan? |
| Long‐nosed bandicoot | Perameles nasuta | MS482 (A) | 11 | Late‐stage embryo | 7 μm, transversal | H & E |
| MS216 | 14 | Newborn | Sagittal | H & E | ||
| MS218 | 15 | Newborn | 10 μm, coronal | H & E? | ||
| Koala | Phascolarctos cinereus | MS351 | 17 | Newborn | Coronal | H & E |
| MS352 | 18 | Newborn | Coronal | Azan? | ||
| MS353 | 16.5 | Newborn | Coronal | H & E | ||
| Long‐nosed potoroo | Potorous tridactylus | MS492a | Newborn | 6 μm, transversal | H & E | |
| Brush‐tailed rock wallaby | Petrogale penicillata | MS484 | 19‐20 | Newborn | 7‐8 μm, transversal | H & E |
| MS448 | 20 | Newborn | 7 μm, transversal | H & E | ||
| Red‐necked pademelon | Thylogale thetidi | MS408 | 17 | About to be born | Transversal | H & E |
| Black‐striped wallaby | Macropus dorsalis | MS407 | 16.5 | Late‐stage embryo | Sagittal | H & E |
| Platypus | Ornithorhynchus anatinus | M44 | 16.75 | Newly hatched | 10 μm, coronal | Azan |
| Golden hamster | Mesocricetus auratus | 14* | 30 | Newborn | 10 μm, transversal | H & E |
All other specimens were available from the Hubrecht & Hill collection.
*Specimens of M. domestica and Mesocricetus auratus are available from the author's PhD.
Measurements and skin morphometry
Serial histological sections were investigated by light microscopy using a stereomicroscope (Leica MZ 12; Wildt, Switzerland). Subcutaneous capillaries were found in the skin of the ventral and dorsolateral sides of the trunk and in the skin of extremities (see Fig. 4). A preceding survey of the whole trunk suggested that the highest volume density of capillaries (V vc) was found in the skin of the dorsolateral side. For comparison, the volume density of capillaries was measured at five arbitrary positions in the skin of the ventral side of the trunk and in the skin of the extremities of one neonate of D. viverrinus (MS138B). The results confirmed a lower volume density of capillaries in the skin of extremities (V vc = 0.10 ± 0.00) and ventral side of the trunk (V vc = 0.14 ± 0.02) than in the skin on the dorsolateral side (V vc = 0.33 ± 0.05). Considering the curled up position of the neonates and the proximity of littermates fixed to the maternal teat, only the dorsolateral side of the trunk is freely exposed to the air. Therefore it represents probably the most important body region for cutaneous gas exchange and was selected for the comparative analysis. The measurements of the skin were conducted at arbitrary positions on the dorsolateral side of the trunk. Five selected sections of each specimen were captured in a light microscope (Zeiss Axiokop, Carl Zeiss Microscopy GmbH, Germany) equipped with a digital camera (Leica DFC490, Leica Microsystems, Switzerland), connected to a computer (software LAS V4.2, Leica Microsystems, Switzerland). Assessment of total skin thickness and of its different components (dermis, epidermis, stratum corneum) as well as the distance between the external surface and the capillaries closest to the epidermis (in marsupial and monotreme species) were performed by 50 measurements per specimen. Measurements were made directly on the computer screen using a digital ruler (imagej software, National Institutes of Health, USA). All measurements were averaged for each animal and the means and SEM are presented, and additional group means were obtained (presented in Table 2).
Figure 4.
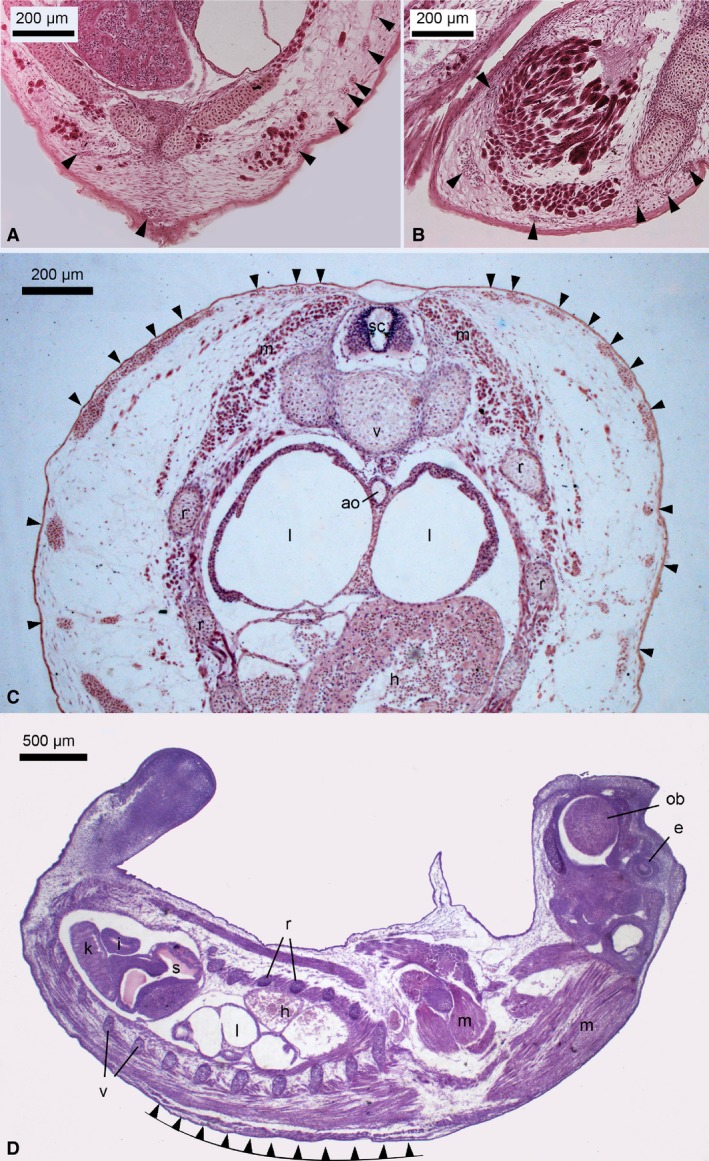
Light micrographs of transversal (A‐C) and a longitudinal (D) histological section through a newborn Dasyurus viverrinus. Numerous superficial capillaries (arrowheads) were closely associated with the epidermis, providing an extremely thin diffusion barrier. Capillaries were present in the skin of the ventral side of the trunk (A) and in the skin of the extremities (B) as well. However, capillary density was highest in the skin of the dorsolateral side of the trunk (C,D). The general developmental degree of D. viverrinus was highly immature at birth, showing cartilaginous skeletal elements, lungs with tubular air spaces, a simple mesonephros as kidney, a small and undifferentiated stomach and a short intestine. Remarkable was the pronounced para‐axial musculature, which enables crawling movement toward the pouch immediately after birth. ao, aorta; e, eye primordium; h, heart; i, intestine; k, kidney; l, lung; m, musculature; ob, olfactory bulbus; r, rib; s, stomach; sc, spinal cord; v, vertebrae. Magnification is indicated by the scale bar.
Table 2.
Cutaneous and pulmonary morphometrical data of neonates from 10 marsupial species, of the newly hatched platypus and of the neonate golden hamster
| Specimen No. | Entire skin thickness, μm | Dermal thickness, μm | Epidermal thickness, μm | Stratum corneum thickness, μm | Diffusion distance, μm | Volume density of capillaries in the skin, Vvc | Area of the gas exchange surface per unitary lung volume, Sv (g.e.s., lung), cm−1 | |
|---|---|---|---|---|---|---|---|---|
| Dasyururs viverrinus | MS 138 | 33 ± 1 | 26 ± 1 | 6 ± 0 | 2 ± 0 | 9 ± 0 | 0.26 ± 0.03 | 108.79 ± 4.03 |
| MS 138(B) | 31 ± 1 | 24 ± 1 | 7 ± 0 | 2 ± 0 | 10 ± 1 | 0.33 ± 0.05 | 112.82 ± 14.10 | |
| MS134 | 44 ± 1 | 38 ± 1 | 5 ± 0 | 2 ± 0 | 7 ± 0 | 0.44 ± 0.04 | 60.44 ± 4.03 | |
| MS134(B) | 36 ± 1 | 27 ± 1 | 6 ± 0 | 2 ± 0 | 10 ± 0 | 0.29 ± 0.02 | 84.62 ± 9.40 | |
| Mean | 36 ± 3 | 29 ± 3 | 6 ± 0 | 2 ± 0 | 9 ± 1 | 0.33 ± 0.04 | 91.67 ± 12.13 | |
| Monodelphis domestica | 337 | 63 ± 2 | 39 ± 2 | 21 ± 1 | 5 ± 0 | 38 ± 1 | 0.12 ± 0.00 | 130.26 ± 11.96 |
| Trichosurus vulpecula | MS288 | 107 ± 6 | 90 ± 5 | 14 ± 0 | 6 ± 0 | 33 ± 1 | 0.11 ± 0.01 | 137.50 ± 24.68 |
| MS289 | 84 ± 1 | 58 ± 1 | 23 ± 1 | 11 ± 0 | 39 ± 1 | 0.16 ± 0.02 | 148.28 ± 15.31 | |
| MS290 | 76 ± 2 | 55 ± 2 | 17 ± 0 | 6 ± 0 | 40 ± 1 | 0.13 ± 0.02 | 148.33 ± 3.27 | |
| Mean | 89 ± 9 | 68 ± 11 | 18 ± 3 | 8 ± 2 | 37 ± 2 | 0.13 ± 0.01 | 144.70 ± 3.60 | |
| Isoodon obesulus | MS219 | 75 ± 2 | 54 ± 1 | 15 ± 0.4 | 4 ± 0 | 41 ± 2 | 0.11 ± 0.03 | n.a. |
| Perameles nasuta | MS482(A) | 76 ± 1 | 59 ± 1 | 14 ± 0 | 4 ± 0 | 50 ± 1 | 0.11 ± 0.01 | 178.88 ± 6.40 |
| MS216 | 118 ± 3 | 89 ± 3 | 24 ± 0 | 4 ± 0 | 84 ± 2 | 0.06 ± 0.01 | 166.12 ± 14.00 | |
| MS218 | 68 ± 2 | 49 ± 2 | 15 ± 0 | 4 ± 0 | 47 ± 1 | 0.13 ± 0.02 | 162.18 ± 7.05 | |
| Mean | 87 ± 16 | 66 ± 12 | 18 ± 3 | 4 ± 0 | 60 ± 12 | 0.10 ± 0.02 | 169.06 ± 5.04 | |
| Phascolarctos cinereus | MS351 | 126 ± 2 | 107 ± 2 | 15 ± 1 | 5 ± 0 | 50 ± 3 | 0.11 ± 0.02 | 231.64 ± 15.95 |
| MS352 | 110 ± 4 | 84 ± 3 | 16 ± 1 | 6 ± 0 | 38 ± 2 | 0.12 ± 0.03 | 170.34 ± 18.03 | |
| MS353 | 110 ± 3 | 84 ± 3 | 19 ± 1 | 4 ± 0 | 48 ± 3 | 0.09 ± 0.02 | 172.88 ± 19.58 | |
| Mean | 115 ± 5 | 92 ± 8 | 17 ± 1 | 5 ± 1 | 45 ± 4 | 0.11 ± 0.01 | 191.62 ± 20.02 | |
| Potorous tridactylus | MS492a | 83 ± 2 | 66 ± 2 | 14 ± 0 | 5 ± 0 | 35 ± 2 | 0.12 ± 0.02 | n.a. |
| Petrogale penicillata | MS484 | 110 ± 4 | 88 ± 3 | 19 ± 1 | 6 ± 0 | 36 ± 1 | 0.05 ± 0.01 | 236.22 ± 44.10 |
| MS448 | 119 ± 2 | 99 ± 2 | 17 ± 1 | 8 ± 0 | 40 ± 2 | 0.04 ± 0.01 | 201.26 ± 22.62 | |
| Mean | 115 ± 5 | 94 ± 6 | 18 ± 1 | 7 ± 1 | 38 ± 2 | 0.05 ± 0.01 | 218.74 ± 14.27 | |
| Thylogale thetidi | MS408 | 159 ± 3 | 127 ± 3 | 29 ± 1 | 4 ± 0 | 65 ± 2 | 0.07 ± 0.01 | n.a. |
| Macropus dorsalis | MS407 | 186 ± 6 | 171 ± 6 | 17 ± 1 | 6 ± 0 | 23 ± 1 | 0.07 ± 0.01 | 235.13 ± 24.45 |
| Ornithorhynchus anatinus | M44 | 75 ± 5 | 53 ± 4 | 19 ± 1 | 7 ± 0 | 47 ± 2 | 0.09 ± 0.02 | 135.09 ± 13.50 |
| Mesocricetus auratus | 14 | 243 ± 8 | 226 ± 9 | 21 ± 0 | 7 ± 0 | ‐ | ‐ | 321 ± 39.36 |
All values are means ± SEM.
For species in which more than one specimen was investigated, additional group means are presented. For better inter‐species comparison the values for the species (obtained from single specimen or group means) are highlighted bold.
Using LM morphometry the volume densities of capillaries (V vc) in the selected skin sections were obtained by point‐counting methods (Howard & Reed, 2005), following the equation:
where P c and P tot are point counts on capillaries and total number of points on the skin tissue.
Lung morphology and estimation of surface areas
As a measure of the developmental degree of the neonates in general and of the respiratory system in particular, the lung structures of neonate marsupials, a recently hatched monotreme and an altricial eutherian neonate were included in this study. Serial sections of the lungs of newborn eastern quoll, grey short‐tailed opossum, long‐nosed bandicoot, brush‐tail possum, koala, brush‐tailed rock wallaby, black‐striped wallaby, golden hamster and newly hatched platypus were available from the same specimens examined for skin morphology (Table 1). Five arbitrary sections of the entire lung were captured using a stereomicroscope equipped with a digital camera (specifics above). The airspace surface areas of the lungs were obtained by the intersection counting method (Howard & Reed, 2005). The area of the gas exchange surface (g.e.s.) per unitary lung volume Sv, expressed as cm−1 can be estimated using following equation:
where I is the number of intersection counts between the test lines and the surface of interest (airspaces), P is the number of points hitting the reference space (parenchymal tissue) and l/p is the length of cycloid associated with each point for the given magnification.
All data of individuals and of the group are presented as means ± 1 SEM.
Results
Skin structure and morphometry
The skin in all newborn marsupials investigated in this study was less developed than that of the altricial eutherian neonate. The skin thickness varied among the marsupial species and reflected the differences in size and developmental degree of the neonates (G1–G3, see Fig. 1). Structural details of the skin of newborn marsupials are given in Figs 2 and 3, and the morphometric measurements are presented in Table 2. The thickness of the entire skin ranged from 36 ± 3 μm in D. viverrinus to 186 ± 6 μm in M. dorsalis. Hairs and hair follicles were conspicuously absent in the skin of all marsupial newborns and sebaceous and perspiratory glands were missing as well. The skin of the newly hatched O. anatinus resembled in structure and thickness (75 ± 5 μm) the skin of newborn marsupials. For comparison, the skin of the altricial eutherian newborn M. auratus measured 243 ± 8 μm in thickness. Hair follicles were already visible in the dermis, yet not breaking through the epidermis, as in this species newborn are hairless.
Figure 2.
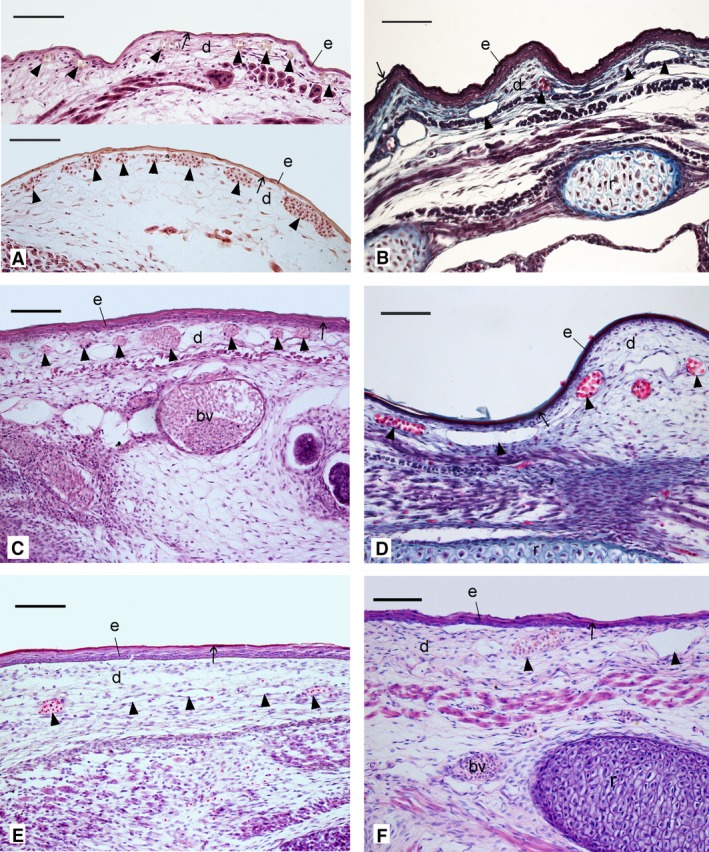
Histological sections comparing the structural characteristics of the skin in the neonate Dasyurus viverrinus (A), Monodelphis domestica (B), Trichosurus vulpecula (C), Isoodon obesulus (D), Perameles nasuta (E) and Phascolarctos cincereus (F). The skin layers varied in thickness between the marsupial neonates. Capillaries (arrowheads) were encountered in all marsupial neonates at various levels of the dermis, some closely associated with the epidermis. The highest capillary density was present in the newborn D. viverrinus. As part of the epidermis, a thin layer of keratin (stratum corneum, arrow) formed the outer covering of the skin. Hairs were absent. bv, blood vessel; d, dermis; e, epidermis; r, rib. Scale bar: 100 μm.
Figure 3.
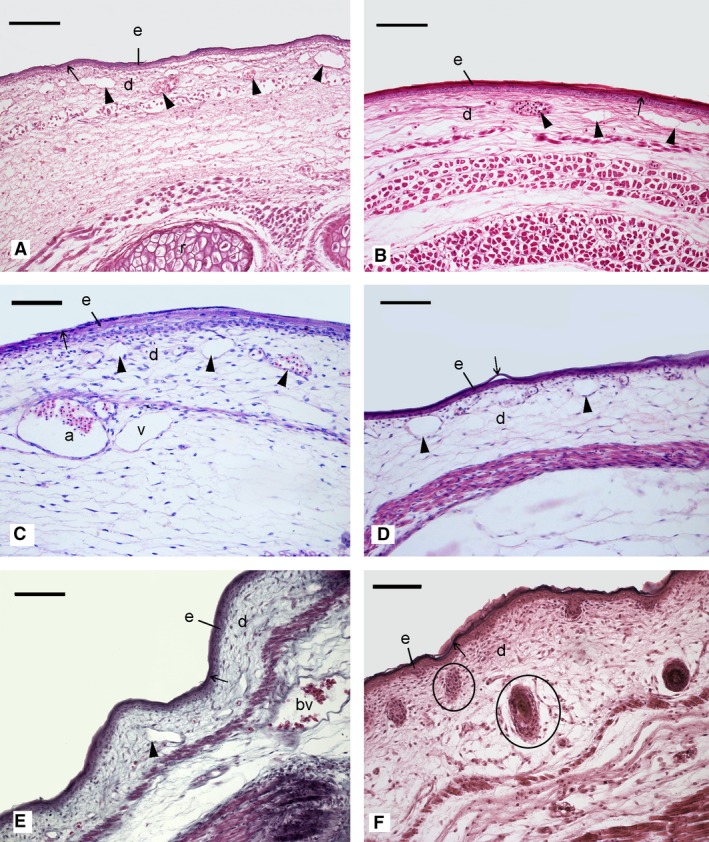
Histological sections comparing the structural characteristics of the skin in the neonate Potorous tridactylus (A), Petrogale penicillata (B), Thylogale thetidi (C), Macropus dorsalis (D), Ornithorhynchus anatinus (E) and Mesocricetus auratus (F). Capillaries (arrowheads) were present in the dermis of all marsupial neonates and the monotreme hatchling, but were absent in the altricial neonate. A thin layer of keratin (stratum corneum, arrow) formed the outer rim of the epidermis, providing cover and protection of the skin. Hairs were absent in all marsupial neonates and the monotreme hatchling, but hair follicles (encircled) were prominent in the dermis of the altricial neonate. a, artery; bv, blood vessel; d, dermis; e, epidermis; r, rib; v, vein. Scale bar: 100 μm.
The skin of the newborn marsupials consisted basically of two layers: the dermis and the epidermis. The dermis accounted for the largest part of the skin and consisted mainly of connective tissue and several capillaries. The thickness of the dermal layer varied between 29 ± 3 μm in D. viverrinus and 171 ± 6 μm in M. dorsalis.
The epidermis of the marsupial neonates lacked the typical stratification characteristic of the mammalian integument and consisted mainly of a stratum basale with one layer of amorphous rounded cells and a thin flattened layer of keratinized cells, the stratum corneum. Only in the more developed macropodid neonates (G3: M. dorsalis, P. penicillata) did a thin stratum spinosum, visible as attenuated cells, adjoin the stratum basale. The entire epidermis of the most immature neonate of D. viverrinus (G1) was extremely thin and measured 6 ± 0 μm in thickness, and the stratum corneum was only 2 ± 0 μm thick. Similar thicknesses were found in all other marsupial neonates (G2–G3), in the newly hatched O. anatinus and in the altricial eutherian neonate. The epidermal thickness measured between 14 ± 0 μm in P. tridactylus and 29 ± 1 μm in T. thetidi. The stratum corneum varied between 4 ± 0 μm in P. nasuta and 8 ± 2 μm in T. vulpecula.
A marked difference between the marsupial neonates/monotreme hatchling and the altricial eutherian neonate was the presence of numerous cutaneous capillaries in the dermis of the marsupial neonates and monotreme hatchling. These were missing in the eutherian neonate. Several superficial capillaries were encountered, some closely apposed to the epidermis (Figs 2 and 3A‐E). The skin of the newborn D. viverrinus had the highest capillary volume density (0.33 ± 0.02). It was three‐fold higher than that of marsupial neonates at the G2 developmental stage (0.10 ± 0.02 to 0.13 ± 0.01) and four‐fold higher than that of monotreme hatchling (0.09 ± 0.02) and macropodid neonates that referred to developmental stage G3 (0.05 ± 0.01 to 0.07 ± 0.01) (Table 2). A survey of the whole trunk of newborn marsupials revealed that subcutaneous capillaries were not evenly distributed over the entire body (for detail see ‘Measurements and skin morphometry’ in Methods). Although capillaries were present in the skin of the extremities and the ventral side of the trunk as well, the capillary density was highest on the dorsolateral side of the trunk (Fig. 4).
The skin diffusion barrier (distance between the external surface of the skin and the most superficial capillaries) in the skin of the newborn D. viverrinus was very thin and measured only 9 ± 1 μm. The skin diffusion barriers of the other marsupial neonates and the monotreme hatchling ranged from 23 ± 1 μm in M. dorsalis to 65 ± 2 μm in T. thetidi (Table 2).
The pathways of cutaneous gas exchange must also include the blood supply to the skin. Beside superficial capillaries, which were located close to the epidermis (Fig. 5A), deeper lying capillaries were running through the dermis (Fig. 5B), indicating a transport of blood to and from the subcutaneous capillaries. Various large blood vessels were drawing from peripheral regions towards the heart, providing the blood supply for the cutaneous gas exchange (Fig. 5C,D). Structural details of the heart of the newborn D. viverrinus indicated a large blood vessel (possibly a pulmocutaneous vein) communicating with the left atrium (Fig. 5E), and the lack of an inter‐ventricular septum (Fig. 5F).
Figure 5.
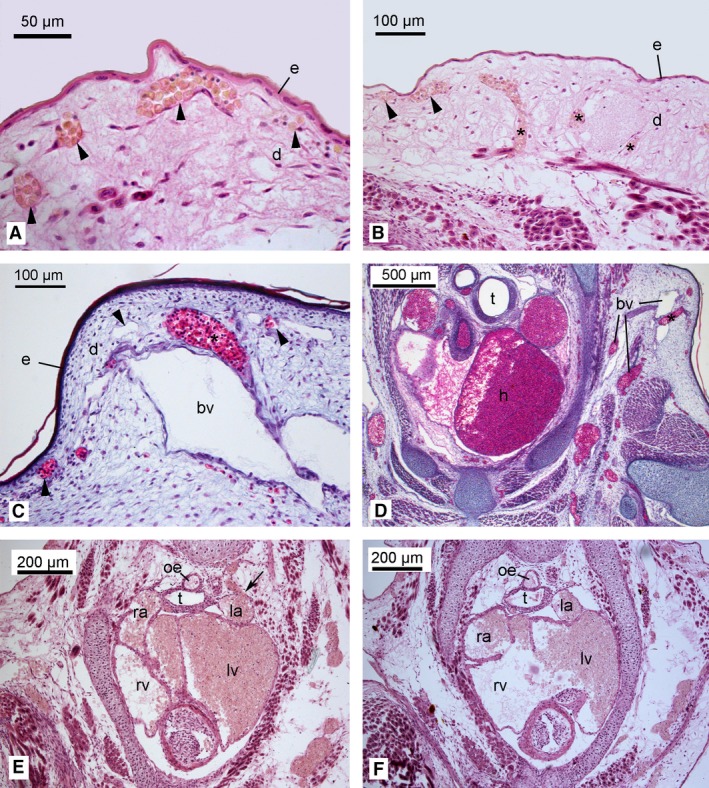
Light micrographs of the skin and heart of the neonates of Dasyurus viverrinus (A,B,E,F) and Trichosurus vulpecula (C,D). Superficial capillaries (indicated by arrowheads) were located close to the epidermis (4A), allowing for transcutaneous gas exchange. Capillaries (indicated by asterisk) were running through the dermis (Fig. 4B‐D), indicating a transport of blood to and from the subcutaneous capillaries. Various large blood vessels were running from peripheral regions towards the heart (C,D). Structural details of the heart of the newborn D. viverrinus indicated a large blood vessel (possibly a pulmocutaneous vein) communicating with the left atrium (E, arrow) and the lack of an inter‐ventricular septum (F). bv, blood vessel; d, dermis; e, epidermis; h, heart; la, left atrium; lv, left ventricle; oe, oesophagus; ra, right atrium; right ventricle; t, trachea. Magnification is indicated by the scale bar.
For comparison the skin of the fire salamander (S. salamandra) and of the Northern leopard frog (L. pipiens), was investigated (Fig. 6). The thickness of the entire skin of S. salamandra and L. pipiens measured 248 ± 3 μm and 178 ± 1, respectively. The skin consisted of the epidermis, the dermis and the subcutis (Tela subcutanea). The epidermis was composed of a stratum germinativum with stratified epithelium and a stratum corneum with flattened keratinized cells. The epidermal thickness measured 20 ± 0 μm (stratum corneum thickness: 10 ± 0 μm) in S. salamandra and 25 ± 0 μm (stratum corneum thickness: 3 ± 0 μm) in L. pipiens. The dermis also consisted of two layers. The outer stratum spongiosum was made up of areolar connective tissue with interlacing fibres, capillaries and various types of cells, including pigment‐bearing chromatophores. Mucous glands of epidermal origin were imbedded in the stratum spongiosum. The underlying stratum compactum was composed of compactly arranged collagenous fibres, nerve fibres and smooth musculature.
Figure 6.
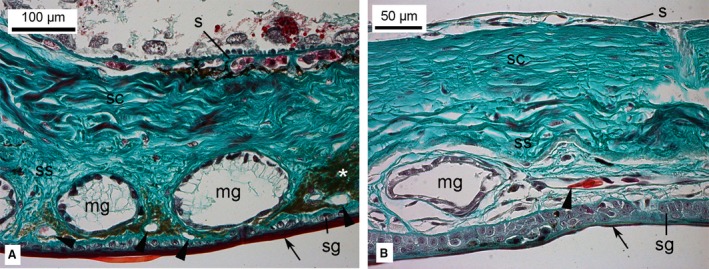
Histological sections of the skin in two amphibian species, Salamandra salamandra (A) and Lithobates pipiens (B). The epidermis was composed of a stratum germinativum with stratified epithelium and a stratum corneum (arrow) with flattened keratinized cells. The dermis consisted of an outer stratum spongiosum and the underlying stratum compactum. Mucous glands and chromatophores (asterisk) were embedded in the stratum spongiosum. A capillary network of numerous small capillaries (indicated by arrowheads) was located in a plane directly beneath the epidermis. A network of larger arteries and veins was located in the subcutis. e, epidermis; d, dermis; mg, mucous gland; s, subcutis; sc, stratum compactum; sg, straum germinativum; ss, stratum spongiosum. Magnification is indicated by the scale bar.
A capillary network of numerous small capillaries were located directly beneath the epidermis. Associated arterioles and venules ran perpendicular to the skin surface, communicating with a network of larger arteries and veins located in the subcutis. The skin diffusion barrier measured 31 ± 1 μm in S. salamandra and 41 ± 1 μm in L. pipiens.
Lung morphology
Lung development in newborn marsupials followed the general developmental degree of the neonates (G1–G3). The most immature lungs were present in the newborn D. viverrinus (G1; Fig. 7A,B). They were at the canalicular stage of lung development, characterized by primitive airways consisting of tubules, partially subdivided by septal crests. Only a few capillaries were located at the inner surface of the lungs, predominantly type II pneumocytes formed the inner lining within the airspaces. The area of the gas exchange surface per unitary lung volume in the neonate D. viverrinus was at 91.67 ± 12.13 cm−1 the lowest of all marsupial lungs examined (Table 2).
Figure 7.
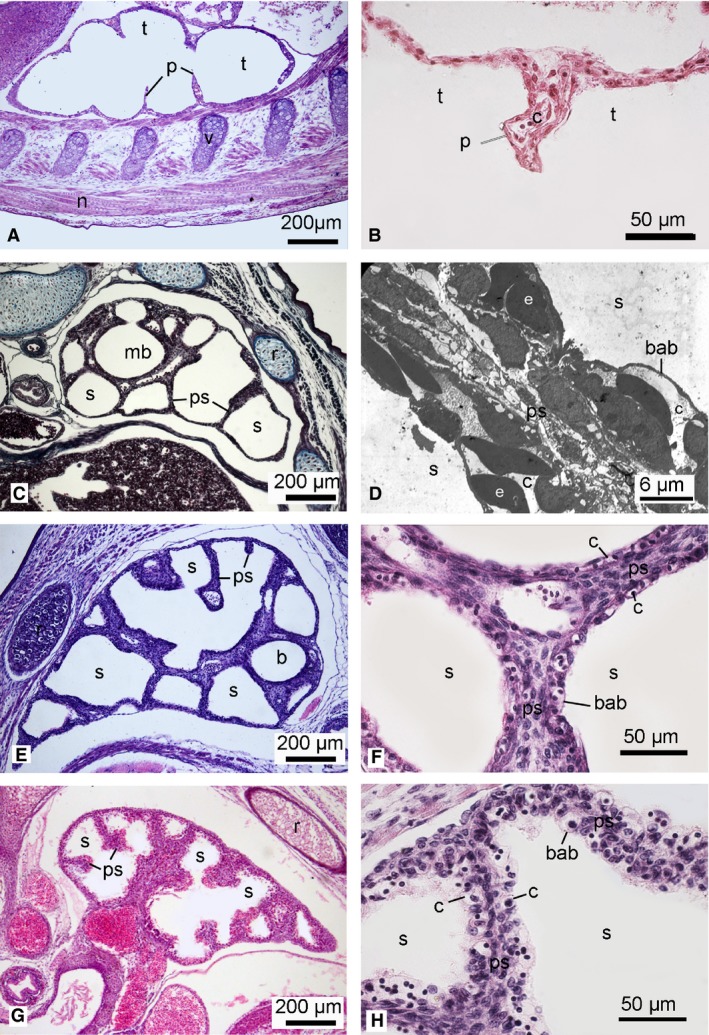
Histological sections of the lung in the neonate Dasyurus viverrinus (A,B), Monodelphis domestica (C), Trichosurus vulpecula (E,F) and Perameles nasuta (G,H). Higher magnification micrographs show the septa between airspaces: (B,F,H) histological sections; (D) TEM micrograph of M. domestica (from the author's PhD work). The lung of the newborn D. viverrinus consisted of poorly vascularized airspaces (tubules). No double capillary septa and onlys a few portions of the blood–air barrier were present, thus showing characteristics of the canalicular stage (G1). The lungs of M. domestica, T. vulpecula and P. nasuta were at the early saccular stage. The large smooth airspaces were separated by thick primary septa with a double capillary network and a blood–air barrier was present (G2). b, bronchus; bab, blood‐air‐barrier; c, capillary; e, erythrocyte; mb, main bronchus; n, spinal cord; p, partitions; ps, primary septum; r, rib; s, sacculus; t, tubulus; v, cartilage of vertebra. Magnification is indicated by the scale bar.
The neonates of M. domestica (Fig. 7C,D), T. vulpecula (Fig. 7E,F) and P. nasuta (Fig. 7G,H) represented the intermediate developmental degree G2, with lungs at the early saccular stage of lung development. Their lungs consisted of primitive airways, which opened in large terminal saccules separated by thick tissue septa with a double capillary network. The lining of the airspace surface was formed by squamous type I pneumocytes and interspersed cuboidal type II pneumocytes. The area of the gas exchange surface per unitary lung volume was 130.26 ± 11.96 cm−1 in M. domestica, 144.70 ± 3.60 cm−1 in T. vulpecula and 169.06 ± 5.04 cm−1 in P. nasuta. The lung structure of the monotreme hatchling (Fig. 8E,F) resembled that of M. domestica, T. vulpecula and P. nasuta neonates. The area of the gas exchange surface per unitary lung volume was with 135.09 ± 13.50 cm−1, similar to the G2 neonates.
Figure 8.
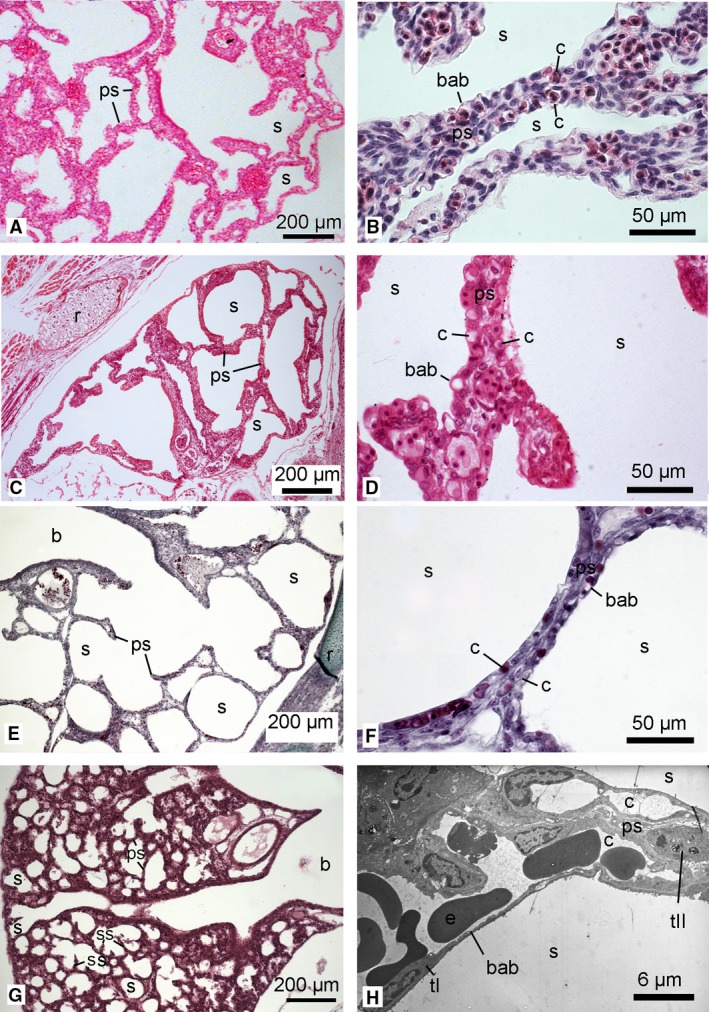
Histological sections of the lung in the neonate Phascolarctos cinereus (A,B), Petrogale penicillata (C,D), O. anatinus (E,F) and Mesocricetus auratus (G). Higher magnification micrographs show the septa between airspaces: (B,D,F) histological sections; (H) TEM micrograph of M. auratus (from the author's PhD work). The lungs of P. cinereus, P. penicillata and O. anatinus were at the saccular stage, characterized by large terminal saccules separated by thick primary septa with a double capillary network. In the lungs of neonate P. cinereus and P. penicillata, numerous septal crests indicated a further proliferation of the lung parenchyma, resulting in more irregular shapes of the terminal saccules (G3). In the altricial placental neonate M. auratus, the bronchial tubes led to the periphery of the lung and terminated in numerous small saccules which were separated by a thin primary double capillary septum. A well developed blood–air barrier was present (8H). b, bronchus; bab, blood‐air‐barrier; c, capillary; e, erythrocyte; ps, primary septum; r, rib; s, sacculus; tI, type I pneumocytes; tII, type II pneumocytes. Magnification is indicated by the scale bar.
Phascolarctos cincereus (Fig. 8A,B), P. penicillata (Fig. 8C,D) and M. dorsalis (not shown) had the most developed lungs of all the marsupial neonates. The lungs of these G3 neonates were at the saccular stage of lung development. Prominent airways extended to the pleural surface and branched out into wide channels that opened directly into terminal saccules. The terminal saccules were large, with a few low septal ridges protruding from the thick double capillary septa, indicating an increasing subdivision of the lung parenchyma. Compared with the other marsupial neonates (G1 and G2) the saccules were smaller and more numerous. The area of the gas exchange surface per unitary lung volume was 191.62 ± 20.02 cm−1 in P. cincereus, 218.74 ± 14.27 cm−1 in P. penicillata and 235.13 ± 24.45 cm−1 in M. dorsalis.
The lung of the newborn M. auratus, representing altricial eutherians (Fig. 8G,H), showed characteristics typical for the late saccular stage of lung development. The ramified bronchial tree extended out to the periphery of the lung. The numerous small saccules were separated by thin double capillary septa and a few secondary septa (tissue crests) and a well developed blood–gas barrier were present. The area of the gas exchange surface per unitary lung volume was 321.49 ± 39.36 cm−1.
Discussion
Lung morphology
Newborn marsupials and monotreme hatchlings are born very small and with a low requirement for oxygen (Szdzuy & Zeller, 2009). Generally, marsupials species with a very low birthweight have lungs at birth consisting of a few tubular airspaces (G1; e.g. D. viverrinus), neonates with intermediate birthweights have lungs consisting of large terminal saccules separated by thick primary septa (G2; e.g. M. domestica, T. vulpecula, P. nasuta) and the neonates with the highest birthweights have further divided lungs (G3; e.g. P. cincereus, P. penicillata, M. dorsalis). A summary diagram defines the consecutive developmental stages of lung development and shows the degree of the lung development at birth/hatching for the species investigated in this study (Fig. 9).
Figure 9.
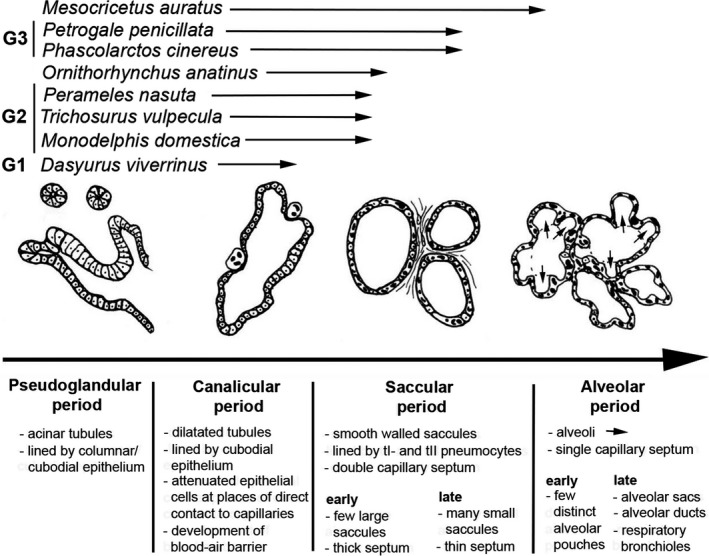
Summary diagram of lung development. The developmental degree of the lung at birth/hatching is indicated for six marsupials (Dasyurus viverrinus, M. domestica, Trichosurus vulpecula, Perameles nasuta, Phascolarctos cincereus, Petrogale penicillata, a monotreme (Ornithorhynchus anatinus) and an alticial eutherian (Mesocricetus auratus).
Although the lungs at birth are either at the canalicular or saccular stages of lung development, they show qualitative characteristics of a mature gas‐exchanging organ, such as the full complement of surfactant (Ribbons et al. 1989; Miller et al. 2001; Makanya et al. 2007) and a thin blood–gas barrier (Runciman et al. 1996; Szdzuy et al. 2008). Furthermore, the respiratory system is neuronally developed, a reflex control of breathing exists and the muscles required for breathing are innervated (Frappell & MacFarlane, 2006). Thus from the standpoint of passive mechanics there may be no major constraints to inspiration (Makanya et al. 2007). However, dynamic factors such as poor muscle co‐ordination and chest‐wall distortion impose severe constraints on ventilation (MacFarlane & Frappell, 2001). Increasing evidence from physiological studies suggests that the functional immaturity of the respiratory system at birth in marsupials necessitates recruitment of an alternative organ system such as the skin for gas exchange (Mortola et al. 1999; Frappell & Mortola, 2000; MacFarlane & Frappell, 2001; MacFarlane et al. 2002; Frappell & MacFarlane, 2006). Physiological studies found that 30–40% of gas exchange occurs across the skin in the newborn M. eugenii (MacFarlane & Frappell, 2001; MacFarlane et al. 2002). The extremely small neonates of the dasyurids rely for 95% of their air on gas exchange via the skin (Mortola et al., 1999; Frappell & Mortola, 2000). In the newborn Julia creek dunnart (Sminthopsis douglasi) gas exchange through the skin is a requirement because of the inefficient pulmonary ventilation (no breathing movements and volume changes) due to the immature neuro‐muscular development (Frappell & Mortola, 2000).
Gas exchange through the skin
The utility of the skin as a gas exchange organ depends on both morphological and physiological variables. Diffusion is proportional to the skin surface area, the skin permeability to gases and the difference in gas concentration (gas partial pressure) on either side of the skin, and is inversely proportional to skin thickness (Feder & Burggren, 1985). The physiological role of the cutaneous gas exchange area needs to be considered in relation to the surface area to mass ratio and the metabolic needs of the organism. Skin gas exchange is favoured in the newborn marsupial because of a relatively a large surface‐to‐mass ratio and a low metabolic rate (Frappell & MacFarlane, 2006). However, gas exchange via the skin would not be possible without the very thin skin, which provides an excellent opportunity for gas diffusion. Gas exchange through the body surface may be adequate even in organisms with a small surface‐to‐mass ratio, as long as oxygen needs are low, as found in many invertebrates and a few vertebrates such as amphibians (Mortola, 2015). Percutaneous gas exchange is known to be the primary mode of gas exchange in the lungless European salamander (Salamandra maculosa). The skin has an epidermis measuring 40–60 μm in thickness with a 5‐μm‐thick stratum corneum (Spearman, 1968). The thickness of the epidermis and stratum corneum is even smaller in the skin of the fire salamander and Northern leopard frog examined here (Table 3), and numerous small sub‐epidermal capillaries are present. In addition to the lung, amphibians use the skin of the body (surface area can be increased by skin folds, flaps, dermal papillae), the skin of the hind extremities and the inside surfaces of the mouth and pharynx for the exchange of respiratory gases (Whitford & Hutchinson, 1965; Feder & Burggren, 1985). In the majority of vertebrates, cutaneous gas exchange is limited by the poor gas diffusion properties of the skin tissues, the low surface‐to‐mass ratio resulting from large body size, and the high metabolic demands (Makanya et al. 2007; Mortola, 2015). In the shrew, one of the smallest mammals with a large body surface‐to‐mass ratio but a high metabolic rate, the skin contributes not more than 3% of total gaseous metabolism (Mover‐Lev et al. 1998). A special case of percutaneous gas exchange in mammals is reported from bats. The structural modifications of the wing web membrane of the epauletted fruit bat (Epomophorus wahlbergi) permit a substantial contribution (6–10%) to the total gas exchange (Makanya & Mortola, 2007). This value might have been underestimated, as it was obtained in anaesthetized bats with folded wings. Higher values would be expected with awake and active bats with wings spread out, as occurs during flight (Makanya & Mortola, 2007).
Table 3.
Comparison of thickness of selected skin layers in vertebrates that use percutaneous gas exchange. All values are in μm
| Species | Skin component | |||
|---|---|---|---|---|
| Epidermis | Stratum corneum | Dermis | Diffussion distance | |
| Amphibia | ||||
| European frog (Rana temporaria) * | 80–100 | 5 | NR | NR |
| African clawed frog (Xenopus laevis) * | 16–22 | NR | NR | NR |
| European salamander (Salamandra maculosa) * | 40–60 | 5 | NR | NR |
| Fire salamander (Salamandra salamandra) | 20 | 10 | 248 | 31 |
| Northern leopard frog (Lithobates pipiens) | 25 | 3 | 178 | 41 |
| Mammals | ||||
| Epauletted fruit bat (Epomorphorus wahlbergi) wing web skin** | 9.1–10.5 | 3.7–4.4 | 15.5 | 26.8 |
| Quokka wallaby (Setonix brachyurus) neonate*** | 29.97 | 4.87 | NR | 30–35 |
Compared with the considerable percutaneous gas exchange measured in newborn marsupials (33% in Macropus eugenii; MacFarlane et al. 2002, and 95% in Sminthopsis douglasi; Mortola et al. 1999) the reported rates are negligible. However, even if the skin might provide only a negligible contribution to O2 exchange, CO2 exchange via the skin might be more important. Due to a higher permeation coefficient of CO2, the skin figures generally more prominently in CO2 excretion than in O2 uptake (Feder & Burggren, 1985).
In all neonate marsupials investigated here, the epidermis was poorly developed with a thin stratum corneum and no hair follicles, providing a weak barrier to transcutaneous gas exchange. The presence of numerous capillaries in the dermal layer of all marsupial neonates and the monotreme hatchling supports the notion that cutaneous exchange in newborn marsupials and probably also newly hatched monotremes might be commonplace (see MacFarlane et al. 2002). The extremely short diffusion distance (blood–gas barrier) as well as the high capillary volume density in the skin of the newborn dasyurid D. viverrinus would provide excellent preconditions for effective cutaneous gas exchange. The highly immature lung structure of the neonate D. viverrinus with an area of the gas exchange surface per unitary lung volume of only 91.67 cm−1 might have an influence on the pulmonary function. It is possible that the neonate D. viverrinus is almost totally dependent on skin gas exchange, as has been reported for other newborn dasyurids (see Frappell & MacFarlane, 2006).
The newborn D. viverrinus had, with only 9 μm, the thinnest skin diffusion barrier of all marsupial neonates examined in this study. The barrier is thinner than the skin diffusion barrier reported from other species with cutaneous gas exchange as well. However, the value is close to the estimation of the epidermal thickness (9.8 μm) in the stretched wing web membrane of the fruit bat (Makanya & Mortola, 2007). The thickness of the skin diffusion barrier of the other marsupial neonates and of the monotreme hatchling (23–65 μm) were within a similar range reported for another newborn marsupial (Setonix brachyurus; 30–35 μm), amphibians (31.3–41.5 μm) and the folded wing web skin of a fruit bat (Epomorphorus wahlbergi; 26.8 μm) (see Table 3). These diffusion distances should be sufficient for gas exchange, as Stücker et al. (2002) have shown that cutaneous oxygen diffusion for the supply of underlying tissue can reach depths of 250–400 μm.
The skin diffusion distances of the newborn marsupials offer an enormous diffusion barrier when compared with the blood–gas barrier of the lung in neonatal marsupials, which can be as thin as 0.3–0.6 μm (Runciman, 1994; Szdzuy et al. 2008). However, percutaneous gas exchange is advantageous in that the animal is exposed to an infinite pool of air with high partial pressures of oxygen. Contrary to the lung values with a partial oxygen pressure (PO2) of 100 mm Hg in the saccules and 46 mm Hg in venous blood, capillary blood of the skin of newborn marsupials is exposed to the environmental PO2 of about 150 mm Hg. Hence, the O2 pressure gradient for the skin capillaries of newborn marsupials would be twice that in the lung, which would be equivalent to halving the diffusion distance (Makanya & Mortola, 2007). A right‐shifted P50 curve (PO2 at which the blood is 50% saturated) in newborn marsupials ensures that O2 can be unloaded at high PO2, enabling diffusion over a greater distance. The high P50 may be an adaptation for the early neonatal period in marsupials when pulmonary circulation is limited and the immaturity of the lung function favours a reliance, to varying extents, on exchange of oxygen across the skin (Frappell & MacFarlane, 2006). Generally, the risk of excessive water loss via the skin is high in any terrestrial organism that uses the skin as blood gas barrier and therefore animals with percutaneous gas exchange need to live in a moist environment (Mortola, 2015). The humidity of the pouch in marsupials probably helps the neonate to circumvent this problem; even under pouch conditions, cutaneous blood would be exposed to a relatively high PO2 (Frappell & MacFarlane, 2006).
Increasing the number of blood vessels per unit area (i.e. skin capillary density) can also enhance the functional surface area of the skin (Feder & Burggren, 1985). The skin of amphibians, which almost always rely on cutaneous gas exchange, is heavily vascularized and between 20 and 95% of the total respiratory capillarization may occur in the skin, even in species that also breathe with lungs and gills (Czopek, 1965). In this study the highest capillary volume density was measured in the newborn D. viverrinus. The value was three‐ to four‐fold higher than that of the other marsupial neonates, indicating that skin gas exchange in the dasyurid neonate might be the most pronounced. It is not surprising that the capillary volume density in the skin seems to be diametrically opposed to the degree of lung development in marsupial neonates. The following hypothesis is possible: G1 neonates with the most immature and impaired lungs, such as dasyurids, rely up for to 90–100% (Mortola et al. 1999) of their air on cutaneous respiration. G2 and G3 neonates, depending on their respective structural degree and capacity of the lung, supplement pulmonary respiration with cutaneous respiration to a higher (G2 neonates possibly 50–70%) or lower (G3 neonates 30–40%; MacFarlane et al. 2002) degree. However, this hypothesis needs to be verified by physiological measurements of more marsupial species, in particular of neonates representing a developmental degree of G2.
Blood supply of the skin
The pathways of cutaneous gas exchange must include not only the superficial subepidermal capillary network but also the blood transport to and from the skin. In the skin of adult anurans, ramifications of the cutaneous arteries and veins join the subepidermal capillaries through segments running vertical to the skin surface, penetrating stratum compactum and stratum spongiosum of the dermis without branching in these layers (de Saint‐Aubain, 1982).
In the marsupial neonates as well, there were deeper‐lying capillaries running through the dermis, indicating a transport of blood to and from the subcutaneous capillaries. These capillaries joined larger blood vessels, probably ramifications of cutaneous arteries and veins, indicating a blood flow from peripheral regions towards the heart and vice versa. The exact structural details of the communication of the large blood vessels coming from the periphery with the cardiac system remain obscure. However, the gas exchange via the skin requires certain specializations, such as the presence of a skin artery and skin vein (as in amphibians) or a temporary pulmocutaneous artery and vein. Normally the skin is provided with oxygenated blood, as it is the case for all other body tissues. In amphibians a special skin artery (equivalent to the pulmonary artery) supplies the skin with deoxygenated blood, similar to the function of the lung artery. The skin vein leads oxygenated blood toward the heart and opens into the right atrium (in contrast to the pulmonary vein, which opens into the left atrium). As amphibians have a heart that consists of a single ventricle and two atria, it is possible that oxygenated blood from the skin mixes with that of the systemic circulation (Stephenson et al. 2017). In the heart of the newborn D. viverrinus as well, communication between the left and right sides of the heart is provided by incomplete inter‐atrial, inter‐ventricular and aortico‐pulmonary septa (Hill & Hill, 1955; Baudinette et al. 1988), enabling a solution similar to that present in amphibians. However, in more mature marsupial neonates such as Macropus eugenii, the inter‐ventricular septa and aortico‐pulmonary septa are complete; only the inter‐atrial connection is still wide open (Baudinette et al. 1988). The presence of the right‐to‐left shunt prevents discrete pulmonary and systemic circuits. The shunt permits blood to bypass the lungs and allows venous admixture of blood low in oxygen content (Frappell & MacFarlane, 2006). Considering the temporary role of cutaneous respiration in marsupial pouch young (see Mortola et al. 1999; MacFarlane & Frappell, 2001) and the fast structural changes of the pulmonary and systemic circulations (closing all shunts) in the early postnatal period, a temporary solution for the cardiovascular–cutaneous circulation (e.g. pulmocutaneous artery and vein) would be advantageous. Indeed, structural details of the heart of the newborn D. viverrinus indicated a large blood vessel (possibly the pulmocutaneous vein) communicating with the left atrium.
Conclusion
The structural basis for gas exchange via the skin is given in all newborn marsupial species and in the newly hatched monotreme investigated in this study. Especially in the most immature marsupial neonate, D. viverrinus, a pronounced cutaneous capillarization was evident. A graduation of the skin capillary density among the marsupial neonates inversely followed the respective lung structure and general developmental degree of the neonates. Skin gas exchange is achievable in newborn marsupials because of the relatively large surface‐to‐mass ratio, low metabolic rate and very thin skin. Unlike the pulmonary ventilation, cutaneous gas diffusion does not involve energy expenditure, which may be advantageous for the highly immature marsupial neonates.
The findings support the assumption that cutaneous gas exchange in newborn marsupials and probably newly hatched monotremes may be common and a required compensation for incomplete pulmonary architecture and impaired ventilation.
Acknowledgements
Thank you to Collin van Buren for letting me inspect the tissues of amphibians that he used for his PhD (Supervisor: Nadia Fröbisch, Museum für Naturkunde, Leibniz‐Institut für Evolutions‐ und Biodiversitätsforschung, Berlin). I thank Peter Giere for providing access to the Hubrecht & Hill collection and for his help in finding the appropriate specimens. I further would like to thank the referees for their inspiring comments.
References
- Baudinette RV, Runciman SIC, Frappell PF, et al. (1988) Development of the marsupial cardiorespiratory system In: The Developing Marsupial. Models for Biomedical Research. (eds Tyndale‐Biscoe CH, Janssens PA.), pp. 132–147. Berlin: Springer. [Google Scholar]
- Czopek J (1965) Quantitative studies on the morphology of respiratory surfaces in amphibians. Acta Anat 62, 296–323. [DOI] [PubMed] [Google Scholar]
- Feder ME, Burggren WW (1985) Cutaneous gas exchange in vertebrates: design, patterns, control and implications. Biol Rev Camb Philos Soc 60, 1–45. [DOI] [PubMed] [Google Scholar]
- Ferner K, Mess A (2011) Evolution and development of fetal membranes and placentation in amniote vertebrates. Respir Physiol Neurobiol 178, 39–50. [DOI] [PubMed] [Google Scholar]
- Ferner K, Zeller U, Renfree MB (2009) Lung development of monotremes: evidence for the mammalian morphotype. Anat Rec 292, 190–201. [DOI] [PubMed] [Google Scholar]
- Ferner K, Schultz JA, Zeller U (2017) Comparative anatomy of neonates of the three major mammalian groups (monotremes, marsupials, placentals) and implications for the ancestral mammalian neonate morphotype. J Anat 231, 798–822. 10.1111/joa.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frappell PB, MacFarlane PM (2006) Development of the respiratory system in marsupials. Respir Physiol Neurobiol 154, 252–267. [DOI] [PubMed] [Google Scholar]
- Frappell PB, Mortola JP (2000) Respiratory function in a newborn marsupial with skin gas exchange. Respir Physiol 120, 35–45. [DOI] [PubMed] [Google Scholar]
- Gemmell RT (1986) Lung development in the marsupial bandicoot, Isoodon macrourus . J Anat 148, 193–204. [PMC free article] [PubMed] [Google Scholar]
- Gemmell RT, Nelson J (1988) The ultrastructure of the lung of two newborn marsupial species, the northern native cat, Dasyurus hallucatus, and the brushtail possum, Trichosurus vulpecula . Cell Tissue Res 252, 683–685. [DOI] [PubMed] [Google Scholar]
- Gemmell RT, Selwood L (1994) Structural development in the newborn marsupial, the Stripe‐faced dunnart, Sminthopsis macroura . Acta Anat 149, 1–12. [DOI] [PubMed] [Google Scholar]
- Hill JP, Hill WCO (1955) The growth stages of the pouch young of the native cat (Dasyurus viverrinus) together with observations on the anatomy of the newborn young. Trans Zool Soc London 28, 350–427. [Google Scholar]
- Howard V, Reed MG (2005) Unbiased stereology: Three‐dimensional measurement in microscopy, pp. 277 2nd edn Milton Park: Garland Science/BIOS Scientific Publishers. [Google Scholar]
- Hughes RL, Hall LS (1988) Structural adaptations of the newborn marsupial In: The Developing Marsupial. Models for Biomedical Research. (eds Tyndale‐Biscoe CH, Janssens PA.), pp. 8–27. Berlin: Springer. [Google Scholar]
- Krause WJ, Leeson CR (1975) Postnatal development of the respiratory system of the opossum. II. Electron microscopy of the epithelium and pleura. Acta Anat 92, 28–44. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Frappell PB (2001) Convection requirement is established by total metabolic rate in the newborn tammar wallaby. Resp Physiol 126, 221–231. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Frappell PB, Mortola JP (2002) Mechanics of the respiratory system in the newborn tammar wallaby. J Exp Biol 205, 533–538. [DOI] [PubMed] [Google Scholar]
- Makanya AN, Mortola JP (2007) The structural design of the bat wing web and its possible role in gas exchange. J Anat 211, 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makanya AN, Tschanz SA, Haenni B, et al. (2007) Functional respiratory morphology in the newborn quokka wallaby (Setonix brachyurus). J Anat 211, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mess AM, Ferner KJ (2010) Evolution and development of gas exchange structures in Mammalia: the placenta and the lung. Respir Physiol Neurobiol 173, 74–82. [DOI] [PubMed] [Google Scholar]
- Miller NJ, Orgeig S, Daniels CB, et al. (2001) Postnatal development and control of the pulmonary surfactant system in the tammar wallaby Macropus eugenii . J Exp Biol 204, 4031–4042. [DOI] [PubMed] [Google Scholar]
- Mortola JP (2015) Generalities of gas diffusion applied to the vertebrate blood–gas‐barrier In: The vertebrate blood–gas barrier in health and disease (ed. Makanya AN.), pp. 1–14. Cham: Springer. [Google Scholar]
- Mortola JP, Frappell PB, Woolley PA (1999) Breathing through the skin in a newborn marsupial. Nature 397, 660. [DOI] [PubMed] [Google Scholar]
- Mover‐Lev H, Minzberg H, Ar A (1998) Is there a significant gas exchange through the skin of the shrew, Crocidura russula monacha? Physiol Zool 71, 407–413. [DOI] [PubMed] [Google Scholar]
- Randall D, Gannon B, Runciman S, et al. (1984) Gas transfer by the neonate in the pouch of the tammar wallaby, Macropus eugenii In: Respiration and Metabolism of Embryonic Vertebrates. Perspectives in Vertebrate Science, vol 3. (ed.Seymour RS.), pp 423–436. Dordrecht: Dr Junk Publ. [Google Scholar]
- Renfree MB (2006) Society for Reproductive Biology Founders’ Lecture 2006 Life in the pouch: womb with a view. Reprod Fertil Dev 18, 721–734. [DOI] [PubMed] [Google Scholar]
- Ribbons KA, Baudinette RV, McMurchie EJ (1989) The development of pulmonary surfactant lipids in a neonatal marsupial and the rat. Respir Physiol 75, 1–10. [DOI] [PubMed] [Google Scholar]
- Ross KFA (1953) Cell shrinkage caused by fixatives and paraffin‐wax embedding in ordinary cytological preparations. Q J Microsc Sci 94, 125–139. [Google Scholar]
- Runciman SIC (1994) Cardiovascular and respiratory development in the tammar wallaby, Macropus eugenii. Diss. Med.: Flinders University of South Australia. [Google Scholar]
- Runciman SIC, Gannon BJ, Baudinette RV (1995) Central cardiovascular shunts in the perinatal marsupial. Anat Rec 243, 71–83. [DOI] [PubMed] [Google Scholar]
- Runciman SIC, Baudinette RV, Gannon BJ (1996) Postnatal development of the lung parenchyma in a marsupial: the tammar wallaby. Anat Rec 244, 193–206. [DOI] [PubMed] [Google Scholar]
- de Saint‐Aubain ML (1982) The morphology of amphibian skin vascularization before and after metamorphosis. Zoomorphology 100, 55–63. [Google Scholar]
- Simpson SJ, Flecknoe SJ, Clugston RD, et al. (2011) Structural and functional development of the respiratory system in a newborn marsupial with cutaneous gas exchange. Physiol Biochem Zool 84, 634–649. [DOI] [PubMed] [Google Scholar]
- Spearman RIC (1968) Epidermal keratinization in the salamander and a comparison with other amphibia. J Morphol 125, 129–143. [DOI] [PubMed] [Google Scholar]
- Stephenson A, Adams JW, Vaccarezza M (2017) The vertebrate heart: an evolutionary perspective. J Anat 231, 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stücker M, Struk A, Altmeyer P, et al. (2002) The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. J Physiol 538, 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szdzuy K, Zeller U (2009) Lung and metabolic development in mammals: contribution to the reconstruction of the marsupial and eutherian morphotype. J Exp Zool 312B, 555–578. [DOI] [PubMed] [Google Scholar]
- Szdzuy K, Zeller U, Renfree MB, et al. (2008) Postnatal lung and metabolic development in two marsupial and four eutherian species. J Anat 212, 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndale‐Biscoe CH, Renfree MB (1987) Reproductive Physiology of Marsupials. Cambridge: Cambridge Press. [Google Scholar]
- Whitford WG, Hutchinson VH (1965) Gas exchange in salamanders. Physiol. Zool 38, 228–242. [Google Scholar]


