Abstract
Spinal muscle cross‐sectional area has been highly associated with spinal pathology. Despite the medium‐high prevalence of spinal pathology in children, there is very limited knowledge regarding muscle size and growth pattern in individuals younger than 20 years of age. The aim of this study is to analyze the change in size and symmetry of spinal muscles (erector spinae, multifidus, psoas and quadratus lumborum) in children 2–20 years of age. We studied reformatted images from 91 abdominal computed tomographic scans of children aged 2–20 years, from an existing imaging dataset. The cross‐sectional area of the muscles was bilaterally measured parallel to the upper endplate of the lumbar vertebrae L3–L5 and at true horizontal for S1. The cross‐sectional area of the upper vertebral endplate was measured at spinal levels L3–L5. Results were analyzed according to six groups based on children's age: 2–4 years (group 1), 5–7 years (group 2), 8–10 years (group 3), 11–13 years (group 4), 14–16 years (group 5) and 17–20 years (group 6). Vertebral endplate and spinal muscles cross‐sectional area increased with age. Two patterns were observed: Endplate, psoas and quadratus lumborum increased up to our 6th oldest age group (17–20), and multifidus and erector spinae reached their largest size in the 5th age group (14–16). The epaxial muscles (erector spinae and multifidus) reached their maximal cross‐sectional area before skeletal maturity (18–21 years of age). The hypaxial muscles (psoas and quadratus lumborum) continued to increase in size at least until spinal maturity. Contributing factors for the differences in developmental pattern between the epaxial and hypaxial muscles might include functional, embryological and innervation factors. In conclusion, this research is the first to describe the cross‐sectional area of spinal muscles in children. Future longitudinal studies are needed for further understanding of muscle development during childhood and adolescence. Level of evidence: level 2b, Retrospective cohort study.
Keywords: endplate, erector spinae, multifidus, paraspinal muscles, psoas major, quadratus lumborum
Introduction
Low back pain (LBP) is a common condition in children and adolescents, with prevalence reported to be as high as 70–80% (Jones & Macfarlane, 2005; Calvo‐Munoz et al. 2013). During the last decade, studies exploring the association between paraspinal muscles, LBP and spinal pathology (Kim et al. 2011; Kalichman et al. 2017) have consistently shown a smaller paraspinal muscle cross‐sectional area (CSA) in chronic LBP subjects (Danneels et al. 2000; Kader et al. 2000; Kim et al. 2011) especially in the multifidus (Hodges et al. 2006; Hides et al. 2010). Vives (2016) suggested that the dynamic soft tissue spinal stabilizers (i.e. the muscles) play a critical role in the maintenance of spinal alignment and that spinal muscle imbalance is associated with spinal asymmetry or scoliosis (Chan et al. 1999; Hyun et al. 2007; Yagi et al. 2011).
It is important to know the main aspects of the normal skeletal muscle structure including development, plasticity and regeneration in order to make a proper clinical, histopathological and genetic diagnosis and to provide new therapeutic approaches (de Rezende Pinto et al. 2015).
Skeletal muscle mass increases during postnatal development through a process of hypertrophy of individual muscle fibers. This process is similar to the adult skeletal muscle response to contractile activity, such as strength exercise, and specific hormones, such as androgens and β‐adrenergic agonists. Muscle hypertrophy occurs when the overall rates of protein synthesis exceed the rates of protein degradation (Schiaffino et al. 2013).
Despite its possible involvement in children's spinal pathology and its critical importance for the development of new methods of evaluation and treatment, knowledge regarding the size and development of spinal muscles in children is limited.
Therefore, the aim of this study is to analyze the pattern of increase in CSA of spinal muscles [erector spinae (ES), multifidus, psoas and quadratus lumborum (QL)] in children 2–20 years of age. This includes: comparing size differences between spinal segments within a particular muscle at a given age group; exploring the pattern of increase in CSA between six age groups, within a specific muscle; defining at what age the muscle reaches its greatest CSA; comparing symmetry/asymmetry of the right and left sides of each muscle; and exploring the relative size of each muscle compared with the size of the vertebral endplate.
Materials and methods
Sample
Using an electronic search tool, 779 abdominal computed tomographic scans (ABCT) scans of children aged 2–20 years at the time of the examination, were retrieved from the hospital Picture Archive Communication System (PACS) (Vue Pacs, Carestream Health, Inc., Rochester, NY, USA). The ABCT scans were performed at the emergency ward for trauma or abdominal pain between 2006 and 2011, at the Edmond and Lily Safra Children's Hospital, Chaim Sheba Medical Center. Scans with positive findings such as hematoma, traumatic organ damage, inflammation, abscess, free fluid or fractures (≈ 35%) that could affect the position of subjects, were excluded from the study. Subjects with evidence of congenital spinal anomalies, spinal alignment disorders, such as scoliosis, spondylolysis or spondylolisthesis, and subjects with known metabolic or chronic illness (≈ 15%) were also excluded. Other exclusion criteria included CT examinations with inadequate sagittal reformations (≈ 20%) and inadequate visualization of the spinal muscles (≈ 30%). A total of 91 scans matched research criteria. All CT scans were performed in the supine position with knees extended. The hospital review board approved the retrospective review of the images.
Examples of CT images are presented in Fig. 1.
Figure 1.
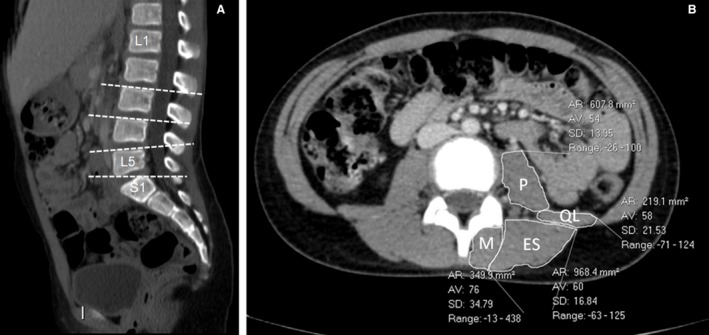
(A) Sagittal reconstructed CT image of a 9‐year‐old girl showing the levels of paraspinal CSA measurements (L3, L4, L5, S1). (B) Cross‐section image at the level of L4 endplate of a 12‐year‐old boy showing the measurements of paraspinal muscles.
CSA measurements
Measurements were performed electronically on a Carestream PACS workstation (Carestream Health), using a freehand region of interest (ROI) drawn around the margins of the muscles and vertebral endplates, following Sions et al. (2017).
The CSA of the muscles was bilaterally measured parallel to the upper endplate of the lumbar vertebrae 3 (L3), 4 (L4) and 5 (L5) similar to the method described by Sions et al. (2016, 2017) and at true horizontal for the first sacral vertebra (S1). This was achieved by employing a sagittal reconstruction of the lumbar–sacral area. (Fig. 1A). Our preliminary exploratory study showed that due to the normal sacral endplate orientation, measuring the spinal muscles in a plane parallel to the endplate would actually provide the oblique CSA of spinal muscles at a higher segment (anywhere between L3 and L5 vertebrae). Therefore at S1, we measured true horizontal/axial images at the level of the posterior superior corner of the sacral endplate. Non‐contractile tissues that could be distinguished from muscle tissue were excluded from the calculation of CSA (Kader et al. 2000; Gildea et al. 2013). The CSA of the upper vertebral endplate was measured at spinal level L3–L5.
Repeatability and reliability of CSA measurements of trunk muscles from CT scans have been reported previously (Keller et al. 2003). One author (S.S.) experienced in CT evaluation took all measurements. To assess the intra‐observer reliability of the measurements, 20 cross‐sections were measured twice, 4 weeks apart. The intraclass correlation coefficients (ICC) were ICC = 0.95 (P < 0.001) for the CSA of vertebral endplate, and 0.79 < ICC < 0.98 (P < 0.001) for the CSA of the measured muscles.
Statistical analysis
The study sample was divided into six groups based on children's age: 2–4 years (group 1), 5–7 years (group 2), 8–10 years (group 3), 11–13 years (group 4), 14–16 years (group 5) and 17–20 years (group 6), similar to Shefi et al. (2013). Group 1 included children from 2 years of age to 4 years and 364 days, and so forth.
Descriptive statistics were used to characterize the vertebral body superior endplate and spinal muscles CSA in each age group. We used one‐way anova to compare the CSA of spinal muscles of the different age groups and a paired t‐test to compare the CSA of spinal muscles of right and left side at each spinal level. Bonferroni correction for multiple comparisons was applied. To compare the muscle CSA between the different spinal levels we used general linear model analysis, with and without subdivisions within age groups. Plots were created to represent the results graphically.
Results
Each age group consisted of 9–21 individuals (Table 1).
Table 1.
Demographic data of the research subjects
| Age group (years) | Subjects n | Boys n | Girls n | Average age ± SD |
|---|---|---|---|---|
| 2–4 | 9 | 6 | 3 | 3.3 ± 0.8 |
| 5–7 | 21 | 16 | 5 | 5.9 ± 0.8 |
| 8–10 | 21 | 17 | 4 | 8.9 ± 0.8 |
| 11–13 | 13 | 11 | 2 | 12.2 ± 0.8 |
| 14–16 | 12 | 10 | 2 | 15.2 ± 0.7 |
| 17–20 | 15 | 12 | 3 | 18.5 ± 1.1 |
Vertebral endplate
The CSA of the vertebral endplates of the lumbar vertebrae L3–L5 significantly increased with age. The overall increase in area was 252% (L5), 245% (L4) and 254% (L3) (Table 2, Fig. 2).
Table 2.
CSA (cm2) of vertebral endplate of L3–L5 from 2 to 20 years of age
| Age group (years) | Measurement | n | Mean | SD | SE | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| 2–4 | L5 endplate | 9 | 5.66 | 1.17 | 0.44 | 3.78 | 7.32 |
| L4 endplate | 9 | 5.63 | 0.95 | 0.35 | 3.87 | 6.81 | |
| L3 endplate | 9 | 5.64 | 1.06 | 0.40 | 3.93 | 6.97 | |
| 5–7 | L5 endplate | 21 | 7.79 | 1.06 | 0.23 | 5.81 | 10.30 |
| L4 endplate | 21 | 7.35 | 1.04 | 0.23 | 5.50 | 9.89 | |
| L3 endplate | 21 | 7.05 | 0.97 | 0.21 | 5.08 | 9.45 | |
| 8–10 | L5 endplate | 21 | 9.83 | 1.16 | 0.25 | 8.06 | 12.20 |
| L4 endplate | 21 | 9.31 | 0.98 | 0.21 | 7.08 | 11.80 | |
| L3 endplate | 21 | 8.90 | 1.31 | 0.29 | 6.46 | 11.50 | |
| 11–13 | L5 endplate | 13 | 11.53 | 1.90 | 0.53 | 9.10 | 14.60 |
| L4 endplate | 13 | 11.35 | 1.98 | 0.55 | 8.26 | 15.20 | |
| L3 endplate | 13 | 10.88 | 2.06 | 0.57 | 6.98 | 13.80 | |
| 14–16 | L5 endplate | 12 | 13.56 | 2.68 | 0.77 | 7.83 | 17.30 |
| L4 endplate | 12 | 13.15 | 2.47 | 0.72 | 7.22 | 16.30 | |
| L3 endplate | 12 | 13.19 | 2.28 | 0.66 | 8.25 | 15.90 | |
| 17–20 | L5 endplate | 15 | 14.24 | 2.02 | 0.52 | 12.10 | 18.10 |
| L4 endplate | 15 | 14.33 | 1.83 | 0.47 | 11.50 | 17.42 | |
| L3 endplate | 14 | 13.84 | 1.55 | 0.42 | 11.40 | 16.32 |
SD, standard deviation; SE, standard error.
Figure 2.
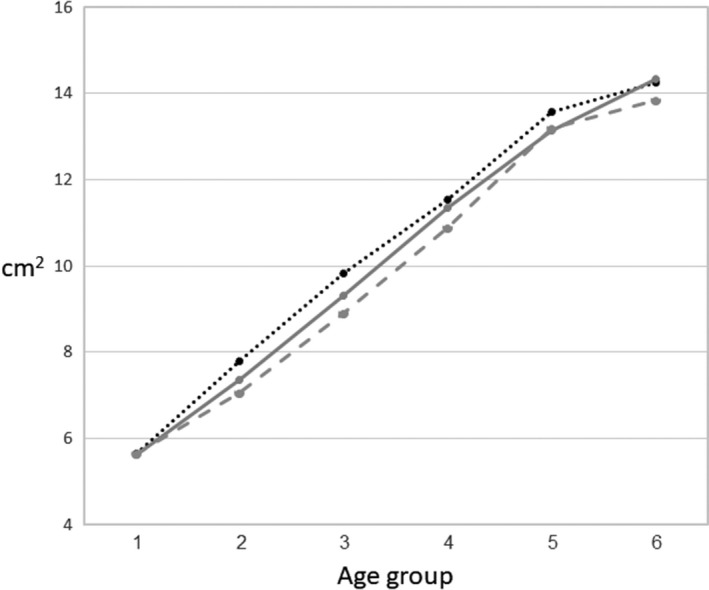
Changes in mean vertebral endplate CSA from the younger to the older age group.
Erector spinae
The CSA of ES significantly increased with age at all spinal levels. The overall increase between the younger (1) and the older (6) age groups was 300% (S1), 352% (L5), 415% (L4) and 445% (L3).
The general linear model indicated significant differences between spinal levels of ES CSA (P < 0.001). In all age groups, the largest CSA was at L3 and the lowest at S1. Post hoc analysis showed that there were significant differences between all spinal levels at all age groups.
The CSA of S1 showed a gradual increase between the young and the old age groups. The CSA of L3–L5 increased until the 5th age group, but there was very little or no increase in CSA between the 5th and the 6th age groups (Table 3, Fig. 3A).
Table 3.
CSA (cm2) of right and left erector spinae from 2 to 20 years of age
| Age group (years) | n | Spinal level | Right | Left | Significancea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | SE | Min. | Max. | Mean | SD | SE | Min. | Max. | ||||
| 2–4 | 9 | S1 | 1.03 | 0.27 | 0.10 | 0.49 | 1.48 | 1.12 | 0.29 | 0.10 | 0.73 | 1.76 | ns |
| 9 | L5 | 3.40 | 0.86 | 0.33 | 2.17 | 5.01 | 3.54 | 0.93 | 0.35 | 2.21 | 5.23 | ns | |
| 9 | L4 | 3.63 | 0.81 | 0.31 | 2.13 | 4.58 | 3.65 | 0.83 | 0.31 | 2.41 | 4.66 | ns | |
| 9 | L3 | 3.49 | 0.82 | 0.30 | 2.00 | 4.65 | 3.86 | 0.83 | 0.31 | 2.44 | 4.93 | P = 0.01 | |
| 5–7 | 17 | S1 | 1.49 | 0.43 | 0.10 | 0.71 | 2.41 | 1.64 | 0.62 | 0.15 | 0.41 | 2.78 | ns |
| 21 | L5 | 4.63 | 1.16 | 0.25 | 2.75 | 6.68 | 4.87 | 1.14 | 0.24 | 3.21 | 7.13 | ns | |
| 21 | L4 | 5.22 | 1.18 | 0.25 | 2.35 | 7.32 | 5.52 | 1.17 | 0.26 | 3.13 | 7.61 | ns | |
| 21 | L3 | 5.32 | 1.13 | 0.25 | 3.03 | 6.86 | 5.70 | 1.14 | 0.25 | 3.12 | 7.85 | P = 0.01 | |
| 8–10 | 21 | S1 | 1.95 | 0.62 | 0.15 | 1.11 | 3.35 | 2.13 | 0.84 | 0.20 | 0.97 | 3.91 | ns |
| 21 | L5 | 5.59 | 0.94 | 0.21 | 3.76 | 7.14 | 5.92 | 1.26 | 0.28 | 4.14 | 8.15 | P = 0.05 | |
| 21 | L4 | 6.36 | 0.89 | 0.20 | 4.68 | 7.91 | 6.86 | 1.34 | 0.29 | 5.14 | 9.27 | P = 0.02 | |
| 21 | L3 | 7.05 | 1.29 | 0.28 | 5.25 | 9.54 | 7.14 | 1.19 | 0.26 | 5.08 | 9.18 | ns | |
| 11–13 | 13 | S1 | 2.43 | 0.84 | 0.30 | 1.20 | 4.05 | 2.38 | 0.84 | 0.30 | 1.25 | 3.78 | ns |
| 13 | L5 | 8.48 | 2.53 | 0.70 | 3.92 | 13.70 | 8.65 | 1.71 | 0.47 | 6.59 | 12.90 | ns | |
| 13 | L4 | 9.31 | 2.33 | 0.65 | 5.55 | 14.00 | 10.25 | 2.68 | 0.74 | 7.06 | 16.20 | P = 0.04 | |
| 13 | L3 | 10.82 | 3.73 | 1.04 | 5.68 | 19.00 | 10.67 | 3.49 | 0.97 | 5.87 | 18.40 | ns | |
| 14–16 | 12 | S1 | 3.21 | 1.41 | 0.50 | 1.91 | 6.21 | 2.76 | 0.93 | 0.33 | 1.18 | 3.99 | ns |
| 12 | L5 | 12.70 | 4.59 | 1.32 | 5.78 | 20.10 | 12.60 | 4.89 | 1.41 | 4.44 | 22.90 | ns | |
| 12 | L4 | 13.95 | 4.00 | 1.15 | 5.69 | 21.80 | 14.42 | 3.79 | 1.09 | 5.65 | 21.10 | ns | |
| 12 | L3 | 14.70 | 4.05 | 1.17 | 4.63 | 20.50 | 16.08 | 4.48 | 1.29 | 4.90 | 22.70 | ns | |
| 17–20 | 15 | S1 | 3.48 | 1.42 | 0.54 | 2.03 | 6.20 | 2.91 | 0.67 | 0.25 | 2.18 | 4.07 | ns |
| 15 | L5 | 11.81 | 2.49 | 0.64 | 7.57 | 16.09 | 12.65 | 3.80 | 0.98 | 6.51 | 20.80 | ns | |
| 14 | L4 | 14.94 | 3.04 | 0.79 | 10.20 | 21.10 | 15.32 | 3.88 | 1.00 | 9.67 | 23.90 | ns | |
| 14 | L3 | 16.25 | 4.27 | 1.14 | 9.86 | 22.93 | 16.41 | 4.56 | 1.21 | 8.76 | 23.6 | ns | |
SD, standard deviation; SE, standard error.
Significance of difference between right and left CSA.
Figure 3.
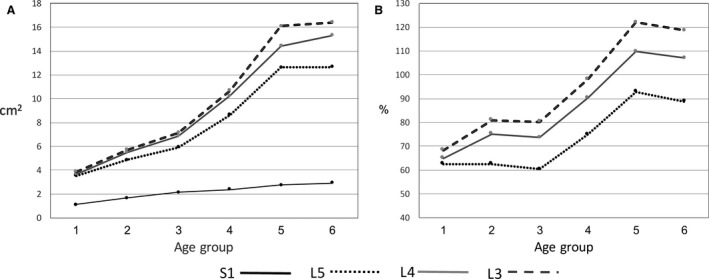
Changes in mean erector spine CSA (A) and mean erector spine relative size (B) from the younger to the older age groups.
There was no difference between the CSA of the left and right erector spinae when applying the Bonferroni correction for multiple comparisons (P ≤ 0.002).
The increase in the erector spinae CSA relative to vertebral endplate CSA (ES‐CSA/endplate CSA*100) is shown in Fig. 3B. At the L3–L5 levels the increase of CSA of erector spinae exceeded that of the vertebral body endplates, with most of the change occurring between the 3rd and 4th groups. There was no change in relative muscle CSA between the 5th and the 6th age groups.
Multifidus
The CSA of multifidus significantly increased with age at all spinal levels. The overall increase between the younger (1) and older (6) age groups was 419% (S1), 391% (L5), 360% (L4) and 362% (L3). The general linear model indicated significant differences between spinal levels of multifidus CSA (P < 0.001). In all age groups, the largest CSA of multifidus was at spinal level L5 or S1 and the lowest in L3. Post hoc analysis showed that there were no significant differences between L5 and S1, but all other comparisons showed significant differences between spinal levels.
At S1, the CSA continuously increased from the young (1) and the old (6) age groups. At L3–L5, multifidus CSA increased until the 5th age group, but there was very little or no increase in CSA between the 5th and the 6th age groups (Table 4, Fig. 4A).
Table 4.
CSA (cm2) of the right and left multifidus, from 2 to 20 years of age
| Age group (years) | n | Level | Right | Left | Significancea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | SE | Min. | Max. | Mean | SD | SE | Min. | Max. | ||||
| 2–4 | 9 | S1 | 2.14 | 0.53 | 0.19 | 1.39 | 2.89 | 2.27 | 0.59 | 0.22 | 1.37 | 3.11 | ns |
| 9 | L5 | 2.24 | 0.59 | 0.22 | 1.17 | 3.13 | 2.21 | 0.47 | 0.18 | 1.52 | 3.00 | ns | |
| 9 | L4 | 1.80 | 0.40 | 0.15 | 1.43 | 2.51 | 1.84 | 0.55 | 0.20 | 1.33 | 2.87 | ns | |
| 9 | L3 | 1.53 | 0.29 | 0.11 | 1.21 | 2.14 | 1.49 | 0.38 | 0.15 | 1.05 | 2.42 | ns | |
| 5–7 | 17 | S1 | 3.31 | 0.61 | 0.15 | 2.39 | 4.78 | 3.40 | 0.64 | 0.13 | 2.52 | 4.92 | ns |
| 21 | L5 | 3.47 | 0.67 | 0.15 | 2.42 | 4.66 | 3.50 | 0.68 | 0.15 | 2.24 | 5.20 | ns | |
| 21 | L4 | 2.84 | 0.64 | 0.14 | 1.35 | 4.01 | 2.71 | 0.48 | 0.11 | 1.64 | 3.58 | ns | |
| 21 | L3 | 2.38 | 0.59 | 0.13 | 1.30 | 3.62 | 2.36 | 0.53 | 0.12 | 1.36 | 3.21 | ns | |
| 8–10 | 21 | S1 | 4.53 | 0.59 | 0.13 | 3.22 | 5.70 | 4.50 | 0.64 | 0.14 | 3.21 | 5.56 | ns |
| 21 | L5 | 4.57 | 0.76 | 0.17 | 3.36 | 6.66 | 4.31 | 0.73 | 0.16 | 3.12 | 6.78 | P = 0.005 | |
| 21 | L4 | 3.53 | 0.65 | 0.14 | 2.36 | 4.66 | 3.53 | 0.63 | 0.14 | 2.60 | 5.05 | ns | |
| 21 | L3 | 2.89 | 0.60 | 0.13 | 1.89 | 3.92 | 2.83 | 0.52 | 0.12 | 1.94 | 4.07 | ns | |
| 11–13 | 13 | S1 | 6.77 | 1.84 | 0.51 | 4.48 | 10.40 | 6.46 | 1.89 | 0.52 | 3.96 | 9.92 | ns |
| 13 | L5 | 6.10 | 1.79 | 0.50 | 3.91 | 10.20 | 6.01 | 2.16 | 0.60 | 3.50 | 11.00 | ns | |
| 13 | L4 | 4.83 | 1.82 | 0.51 | 3.31 | 9.56 | 4.41 | 1.86 | 0.52 | 2.43 | 9.42 | P = 0.002 | |
| 13 | L3 | 3.42 | 1.02 | 0.28 | 2.17 | 5.53 | 3.49 | 0.95 | 0.26 | 2.20 | 5.54 | ns | |
| 14–16 | 12 | S1 | 7.90 | 1.75 | 0.51 | 2.99 | 9.60 | 8.11 | 2.03 | 0.59 | 2.63 | 11.00 | ns |
| 12 | L5 | 8.13 | 2.16 | 0.62 | 2.94 | 12.10 | 8.31 | 2.44 | 0.71 | 2.60 | 12.80 | ns | |
| 12 | L4 | 6.66 | 2.05 | 0.59 | 2.17 | 9.72 | 6.51 | 2.15 | 0.62 | 1.75 | 10.60 | ns | |
| 12 | L3 | 5.57 | 2.15 | 0.62 | 2.66 | 9.90 | 5.51 | 1.94 | 0.56 | 2.75 | 9.30 | ns | |
| 17–20 | 15 | S1 | 9.10 | 1.86 | 0.50 | 5.75 | 11.70 | 9.41 | 2.36 | 0.63 | 4.82 | 12.39 | ns |
| 15 | L5 | 8.75 | 1.72 | 0.44 | 4.86 | 11.80 | 8.67 | 1.59 | 0.41 | 5.16 | 11.20 | ns | |
| 14 | L4 | 6.59 | 1.94 | 0.50 | 3.08 | 9.65 | 6.53 | 2.10 | 0.54 | 3.08 | 10.9 | ns | |
| 14 | L3 | 5.50 | 1.59 | 0.43 | 2.04 | 8.56 | 5.46 | 1.36 | 0.36 | 2.24 | 8.31 | ns | |
SD, standard deviation; SE, standard error.
Values in bold are significant after adjusting for Bonferroni (P ≤ 0.002).
Significance of difference between right and left CSA.
Figure 4.
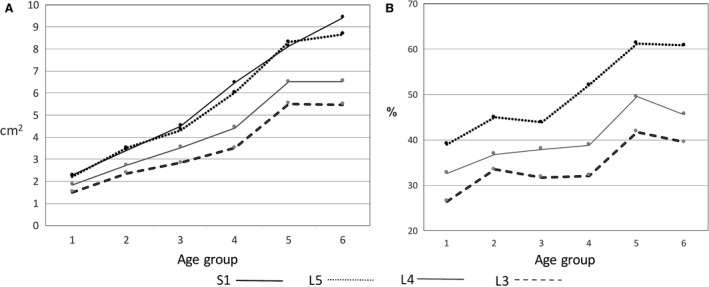
Changes in mean multifidus CSA (A) and mean multifidus relative size (B) from the younger to the older age groups.
When applying the Bonferroni correction for multiple comparisons (P ≤ 0.002), there was no difference in multifidus CSA between the left and right side at any spinal level at all age groups, with one exception. The CSA of the right multifidus is larger than the left one in L4 of the 4th group (11–13 years) (Table 4).
The relative increase in multifidus CSA in relation to vertebral endplate CSA (multifidus‐CSA/endplate CSA*100) is shown in Fig. 4B. At all spinal levels (L3, L4 and L5) the relative increase of multifidus exceeded that of the vertebral body endplates, with most of the change occurring between the 1st and 2nd age groups and between the 4th and 5th groups. It is worth noting that the relative muscle CSA of the 6th group at L3–L4 is slightly lower than that of the 5th group.
Psoas major
The CSA of psoas significantly increased with age group at all spinal levels. The overall increase between the younger (1) and the older (6) age groups was 422% (S1), 480% (L5), 476% (L4) and 477% (L3).
The general linear model indicated significant differences between spinal levels of CSA (P < 0.001). In all age groups, the largest CSA was at spinal level L5 and the lowest at L3. Post hoc analysis showed that there were no significant differences between L4 and S1, but all other comparisons showed significant differences between spinal levels.
At all spinal levels, psoas CSA increased until the oldest (6) age group (Table 5, Fig. 5A). When applying the Bonferroni correction for multiple comparisons (P ≤ 0.002), there was no difference in psoas CSA between the left and right side at any spinal level in any age group, with two exceptions. The CSA of the right psoas is larger than the left at L4 in the 2nd age group (5–7 years) and at L3 in the 3rd age group (8–10 years) (Table 5). The relative increase in psoas CSA in relation to vertebral endplate CSA (psoas‐CSA/endplate CSA*100) is shown in Fig. 5B. The psoas CSA nearly doubled its size relative to vertebral endplate CSA at spinal levels L3–L5.
Table 5.
CSA (cm2) of the right and left psoas, from 2 to 20 years of age
| Age group (years) | n | Spinal level | Right | Left | Significancea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | SE | Min. | Max. | Mean | SD | SE | Min. | Max. | ||||
| 2–4 | 9 | S1 | 3.23 | 0.45 | 0.17 | 2.50 | 3.99 | 3.21 | 0.54 | 0.19 | 2.57 | 4.04 | ns |
| 9 | L5 | 3.51 | 0.63 | 0.23 | 2.87 | 4.54 | 3.34 | 0.52 | 0.18 | 2.70 | 4.31 | ns | |
| 9 | L4 | 2.99 | 0.70 | 0.25 | 2.28 | 3.91 | 2.92 | 0.60 | 0.23 | 2.32 | 3.91 | ns | |
| 9 | L3 | 2.26 | 0.49 | 0.17 | 1.47 | 3.03 | 2.06 | 0.44 | 0.16 | 2.0 | 3.01 | ns | |
| 5–7 | 17 | S1 | 4.71 | 0.91 | 0.20 | 2.63 | 6.31 | 4.57 | 0.89 | 0.19 | 2.59 | 6.18 | ns |
| 21 | L5 | 5.19 | 1.07 | 0.24 | 2.95 | 6.89 | 4.96 | 0.911 | 0.24 | 2.96 | 6.75 | P = 0.04 | |
| 21 | L4 | 4.35 | 0.76 | 0.17 | 2.54 | 5.70 | 4.14 | 0.78 | 0.17 | 2.08 | 5.33 | P = 0.002 | |
| 21 | L3 | 3.53 | 0.76 | 0.17 | 2.05 | 4.47 | 3.19 | 0.60 | 0.13 | 1.78 | 4.06 | ns | |
| 8–10 | 21 | S1 | 6.04 | 1.18 | 0.26 | 3.99 | 9.11 | 5.91 | 1.31 | 0.29 | 3.94 | 8.46 | ns |
| 21 | L5 | 7.02 | 1.51 | 0.33 | 4.20 | 11.10 | 6.53 | 1.41 | 0.30 | 3.92 | 9.05 | P = 0.01 | |
| 21 | L4 | 5.77 | 1.28 | 0.28 | 3.48 | 8.84 | 5.59 | 1.30 | 0.28 | 3.56 | 8.26 | ns | |
| 21 | L3 | 4.39 | 0.96 | 0.21 | 3.22 | 6.23 | 3.97 | 0.95 | 0.20 | 2.29 | 5.59 | P = 0.001 | |
| 11–13 | 13 | S1 | 8.05 | 2.55 | 0.71 | 4.90 | 14.90 | 7.66 | 2.14 | 0.59 | 4.57 | 13.00 | ns |
| 13 | L5 | 10.01 | 3.91 | 1.09 | 5.32 | 20.70 | 9.28 | 3.09 | 0.86 | 5.12 | 16.60 | ns | |
| 13 | L4 | 8.48 | 2.89 | 0.80 | 5.23 | 14.60 | 7.81 | 2.27 | 0.63 | 4.64 | 12.50 | P = 0.009 | |
| 13 | L3 | 6.29 | 2.12 | 0.59 | 4.23 | 12.00 | 5.95 | 1.68 | 0.47 | 3.97 | 10.2 | ns | |
| 14–16 | 12 | S1 | 10.62 | 4.11 | 1.19 | 3.10 | 15.40 | 10.42 | 3.41 | 0.99 | 3.80 | 14.70 | ns |
| 12 | L5 | 13.60 | 4.24 | 1.22 | 5.21 | 19.00 | 13.33 | 3.81 | 1.11 | 4.81 | 18.30 | ns | |
| 12 | L4 | 12.34 | 3.42 | 0.99 | 4.10 | 17.10 | 12.45 | 3.48 | 1.01 | 4.33 | 17.20 | ns | |
| 12 | L3 | 9.10 | 2.65 | 0.77 | 3.47 | 13.00 | 8.98 | 2.68 | 0.78 | 2.74 | 13.20 | ns | |
| 17–20 | 15 | S1 | 13.63 | 2.33 | 0.60 | 10.72 | 18.20 | 13.60 | 2.51 | 0.67 | 9.71 | 20.2 | ns |
| 15 | L5 | 16.46 | 3.38 | 0.87 | 11.63 | 23.40 | 16.42 | 3.84 | 0.94 | 10.71 | 24.20 | ns | |
| 14 | L4 | 14.07 | 3.11 | 0.80 | 8.77 | 19.50 | 14.14 | 3.22 | 0.83 | 9.22 | 19.40 | ns | |
| 14 | L3 | 10.27 | 2.53 | 0.67 | 6.03 | 16.00 | 10.36 | 3.53 | 0.94 | 4.67 | 17.70 | ns | |
SD, standard deviation; SE, standard error.
Values in bold are significant after adjusting for Bonferroni (P ≤ 0.002).
Significance of difference between right and left CSA.
Figure 5.
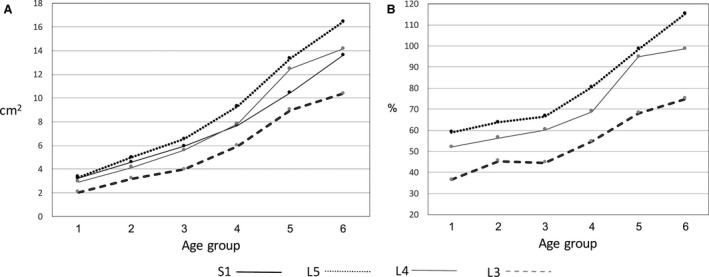
Changes in mean psoas CSA (A) and mean psoas relative size (B) from the younger to the older age groups.
Quadratus lumborum
The muscle mass of QL was not found at spinal level S1 and occasionally found at spinal level L5. Therefore, we only calculated the CSA of QL for spinal levels L3 and L4. The CSA of QL increased significantly with age. The overall increase in size between the younger (1) and the older (6) age groups was 443% (L4) and 412% (L3).
The general linear model indicated significant differences of QL CSA between spinal levels (P < 0.001). In all age groups, the largest CSA was at the spinal level at L4 and the lowest at L3 (Fig. 6A).
Figure 6.
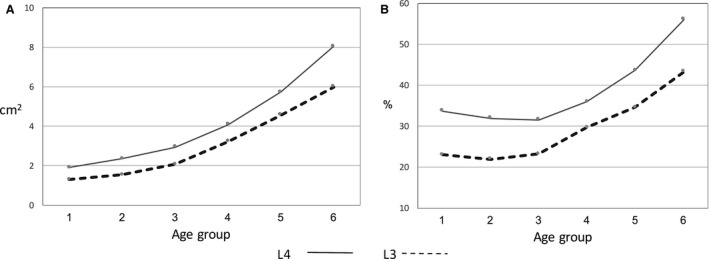
Changes in mean quadratus lumborum CSA (A) and mean quadratus lumborum relative size (B) from the younger to the older age groups.
The pattern of change in CSA at L3 and L4 was similar: muscle CSA increased until the older age group (6). There was no difference between the right and the left sides in CSA at L3–L4 (Table 6).
Table 6.
CSA (cm2) of the right and left quadratus lumborum, from 2 to 20 years of age
| Age group (years) | n | Spinal level | Right | Left | Significancea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | SE | Min. | Max. | Mean | SD | SE | Min. | Max. | ||||
| 2–4 | 9 | L4 | 1.94 | 0.63 | 0.24 | 1.15 | 3.01 | 1.89 | 0.60 | 0.22 | 1.35 | 3.09 | ns |
| 9 | L3 | 1.39 | 0.41 | 0.16 | 0.74 | 1.97 | 1.30 | 0.48 | 0.18 | 0.70 | 2.14 | ns | |
| 5–7 | 21 | L4 | 2.38 | 0.46 | 0.10 | 1.60 | 3.50 | 2.34 | 0.46 | 0.10 | 1.42 | 3.36 | ns |
| 21 | L3 | 1.63 | 0.48 | 0.10 | 1.00 | 3.13 | 1.54 | 0.45 | 0.10 | 1.03 | 2.69 | ns | |
| 8–10 | 21 | L4 | 3.36 | 0.81 | 0.33 | 1.79 | 5.04 | 2.93 | 0.86 | 0.39 | 1.77 | 5.26 | P = 0.01 |
| 21 | L3 | 2.17 | 0.58 | 0.13 | 1.47 | 3.65 | 2.06 | 0.75 | 0.16 | 1.14 | 3.81 | ns | |
| 11–13 | 13 | L4 | 4.34 | 1.53 | 0.42 | 2.28 | 7.35 | 4.07 | 1.31 | 0.36 | 2.26 | 5.99 | ns |
| 13 | L3 | 3.54 | 1.51 | 0.45 | 2.02 | 7.02 | 3.22 | 1.41 | 0.43 | 1.64 | 6.10 | ns | |
| 14–16 | 12 | L4 | 5.64 | 1.97 | 0.57 | 2.12 | 8.92 | 5.72 | 1.50 | 0.43 | 2.50 | 7.79 | ns |
| 12 | L3 | 4.55 | 1.63 | 0.47 | 1.36 | 7.54 | 4.56 | 1.49 | 0.43 | 1.38 | 7.31 | ns | |
| 17–20 | 14 | L4 | 7.86 | 2.59 | 0.69 | 3.16 | 12.60 | 8.03 | 2.37 | 0.63 | 3.83 | 11.26 | ns |
| 14 | L3 | 5.94 | 2.37 | 0.63 | 2.87 | 10.90 | 5.99 | 2.27 | 0.61 | 2.57 | 11.10 | ns | |
SD, standard deviation; SE, standard error.
Significance of difference between right and left CSA.
The increase in QL CSA relative to vertebral endplate CSA is shown in Fig. 6B. At all spinal levels (L3 and L4) the size increase of QL exceeded that of the vertebral body endplates. Most of the change was between the 3rd and the 6th age groups.
Discussion
In this secondary analysis study of an existing ABCT imaging series, we describe the change in CSA of the lumbar spinal muscles in children aged 2–20 years. To the best of our knowledge, this is the first article to publish the CSA of spinal muscles of younger age groups. We found that vertebral endplate and spinal muscle CSA increased with age. Two patterns were observed: Endplate, psoas and QL increased up to our oldest age group (17–20), and multifidus and ES reached their largest size in the 5th age group (14–16). We have also found that the increase in CSA of spinal muscles is greater than the increase of vertebral endplate. We found symmetry between the right and the left CSA of spinal muscles at all age groups.
Change in muscle CSA
The increase in CSA of spinal muscles showed two different patterns:
Epaxial muscles – multifidus and ES, which act primarily on the axial skeleton, increased in size from the 1st to the 5th age group, where they reached their maximal or near maximal size (age 14–16 years), before skeletal maturity (18–21 years, as defined by Sanders et al. 2017 and Uraoka et al. 2018). It should be noted, however, that other definitions, based on datasets of different ethnicities, exist.
Hypaxial muscles – psoas and QL, which act primarily on the pelvis and lower limb, increased in size until the last age group (17–20 years). We also found that vertebral endplates CSA increased in size until the last age group as previously shown (Stokes & Windisch, 2006).
Extensive studies have shown that skeletal muscle is a highly adaptive tissue that responds to exercise, nutrient supply, innervation and endocrine factors with alterations in fiber composition and size (Bayline et al. 2001; Piccirillo et al. 2014; Kalichman et al. 2016). Therefore, the differences in patterns between the epaxial and hypaxial muscles may be due to functional, embryonic and innervation factors.
Functional differences
Epaxial muscles act mostly on the spine. Both muscles are involved in spinal extension and in keeping the erect posture of the spine during sitting, standing and walking. These muscles provide mechanical stability and play an important role in controlling the movement of the lumbar spine, as well as in stabilizing the spine during arm and leg movements (Macintosh & Bogduk, 1986; Dickstein et al. 2004; Hansen et al. 2006). In healthy individuals, the epaxial muscles contain a high proportion of slow‐twitch fibers (Type I), reflecting their role in maintaining posture (Demoulin et al. 2007; Cornwall et al. 2011). However, in contrast, Hesse et al. (2013) indicate similar proportions of slow and fast twitch fibers, so the relevance of the difference in proportion of fibers is questionable.
Hypaxial muscles are involved not only in spinal stabilization and motion but also in lower limb function. The psoas muscle flexes the hip joint during the swing phase of walking, during stair climbing, running, kicking and other activities (Hesse et al. 2013). The human psoas major muscle has a predominance of type IIA muscle fibers, whereas type I muscle fibers had the largest CSA (Arbanas et al. 2009). The fiber type composition of the psoas major muscle indicates its dynamic and postural functions, which supports the fact that it is the main flexor of the hip joint (dynamic function) and stabilizer of the lumbar spine, sacroiliac and hip joints (postural function) (Arbanas et al. 2009). The QL is involved with lateral bending of the lumbar spine or in lifting the pelvis (hiking) during single limb support (Adams et al. 2006).
Differences in embryonic development
The large majority of skeletal muscles develop from somites, which form in craniocaudal sequence as metameric blocks of paraxial mesoderm on both sides of the neural tube and notochord. The dorsolateral parts of the somites differentiate into dermomyotomes. The epaxial myotome forms the ‘intrinsic’ back muscles dorsal to the transverse processes of the vertebrae (including ES and multifidus, among others), whereas the hypaxial myotome forms the muscles of the body wall (including psoas and QL) and limbs (Mekonen et al. 2016).
Differences in muscular innervation
Branches of the lumbar dorsal rami innervate the epaxial muscles, whereas branches of the lumbar ventral rami innervate the hypaxial muscles (Bogduk, 1983). Interactions between motoneurons and muscles influence many aspects of neuromuscular development in all animals (Bayline et al. 2001) and therefore might contribute to the differences in muscle growth.
Interestingly, Crawford et al. (2016a, 2016b) found that degenerative changes of the psoas, multifidus and ES start as early as 20 years of age. Both Dahlqvist et al. (2017) and Crawford et al. (2016b) reported a different rate of compositional changes between muscles. They found that the paraspinal muscles (ES and multifidus) have a significantly higher age‐related increase in fat infiltration compared with psoas. The higher rate of fat infiltration in the paraspinal muscles may be related to the fact that these muscles reach their maximal CSA at an earlier age (14–16 years) compared with the psoas (17–20 years). Dahlqvist et al. (2017) argued that the age‐related increase in paraspinal fat infiltration did not correlate with body mass index (BMI) or physical activity, suggesting it might be a developmental phenomenon that may be difficult to modify with interventions.
Muscle CSA
The largest muscular CSA is important, as it is related to the maximal force generation of the muscle (Close, 1972; Brand et al. 1986; Narici, 1999). The capacity of a muscle to generate force is directly proportional to the number and size of the muscle fibers within it (Akagi et al. 2009). A larger CSA implies a greater ability to generate force (Close, 1972; Brand et al. 1986; Narici, 1999). It is important to note that muscle composition, i.e. the amount of muscle fibers relative to fat infiltration, is another important component of force generation, but this is not in the scope of the current study. Future study should explore fat infiltration in spinal muscles of young individuals. Our results indicate that the largest CSA of each muscle is at different levels: L3 for ES; L4 for QL; L5 for psoas; and L5/S1 for multifidus. These results are in agreement with published data for an adult population (Table 7). We can conclude that across the developmental process the largest CSA of the spinal muscles stays at the same spinal level. Epaxial muscles are segmental multipennate, with fibers of different lengths (Cornwall et al. 2011). Therefore, it is possible that CSA represents the level where these muscles are most active – multifidus at the lower lumbar spine (L5–S1) and ES at the middle lumbar spine (L3).
Table 7.
Spinal muscle CSA in sub‐adult and adult population
| Research | Modality | Participants | Segments | Level of measurement | Position | Mult. CSA (cm2) (SD) | ES CSA (cm2) (SD) | Psoas CSA (cm2) (SD) | QL CSA (cm2) (SD) |
|---|---|---|---|---|---|---|---|---|---|
| (Chaffin et al. 1990) | CT | 96 women age, 40–63 years | L2 | Intervertebral disc | S, flexed knees | L, 5.9 (1.7) R, 5.8 (1.5) | L, 3.3 (1.6) R, 3.0 (0.7) | ||
| L3 | L, 8.3 (1.9) R, 8.3 (1.9)) | L, 4.5 (1.4) R, 4.1 (1.2) | |||||||
| L4 | L, 9.8 (2.2) R, 9.8 (2.0) | L, 4.5 (1.3) R, 4.6 (1.0) | |||||||
| (Danneels et al. 2000) | CT | 23 healthy volunteers | L3 | Superior endplate | S | 4.7 (1.4) | |||
| L4 | Inferior end plate | 6.3 (1.4) | |||||||
| L4 | 9.0 (1.5) | ||||||||
| (McGill et al. 1993) | MRI | 15 young men, age 25 (3.6) years | L3 | Intervertebral disc | S | L, 4.7 (2.7) R, 4.5 (2.7) | L, 29.3 (3.8) R, 28.3 (4.6) | L, 15.9 (2.9) R, 15.9 (3.7) | L, 7.5 (1.7) R, 7.0 (2.1) |
| L4 | L, 22.3 (4.8) R, 21.5 (5.4) | L, 18.2 (2.7) R, 18.6 (3.5) | L, 6.3 (2.5) R, 7.3 (2.5) | ||||||
| L5 | L, 9.9 (3.3) R, 9.0 (3.4) | L, 15.9 (2.4) R, 16.0 (2.0) | |||||||
| (Niemelainen et al. 2011) | MRI | 126 adult male | L3 | Center of the disc | S | L, 6.9 R, 7.3 | L, 19.7 R, 19.6 | ||
| L4 | L, 9.5 R, 10.1 | L, 15.3 R, 14.3 | |||||||
| L5 | L, 10.8 R, 11.1 | L, 10.4 R, 9.4 | |||||||
| (Hides et al. 2010) | MRI | 54 professional AFL players | L3 | Center of the disc | S, flexed knees | 23–25 | 8.2–9.9 | ||
| L4 | |||||||||
| (Hsu et al. 2015) | MRI | 31 young adults 18–35 years | L4 | S | 7.1 (3.2) | 15.9 (7.3) | 13.7 (5.9) | 6.3 (3.0) | |
| L5 | 6.8 (3.0) | 8.0 (3.7) | 11.3 (4.9) | 3.7 (3.5) | |||||
| (Hides et al. 2008a) | US | 16 young male elite cricketers | L2 | Between lamina and spinous process | P | 2.8 (1.1) | |||
| L3 | 4.3 (1.5) | ||||||||
| L4 | 6.5 (2.2) | ||||||||
| L5 | 8.0 (1.7) | ||||||||
| (Stokes et al. 2005) | US | 68 females | L4 | P | 5.6 (1.3) | ||||
| 52 males | L5 | 6.7 (1.0) | |||||||
| L4 | 7.9 (1.9) | ||||||||
| L5 | 8.9 (1.7) | ||||||||
| Current study | CT | 15 young males and females age 17–20 years | L3 | Superior end plate | S | L, 5.5 (1.4) R, 5.5 (1.6) | L, 16.4 (4.6) R, 16.3 (4.3) | L, 10.4 (3.5) R, 10.3 (2.5) | L, 6.0 (2.3) R, 5.9 (2.4) |
| L4 | L, 6.5 (2.1) R, 6.6 (2.9) | L, 15.3 (3.9) R, 14.9 (3.0) | L, 14.1 (3.8) R, 14.0 (3.0) | L, 8.0 (2.4) R, 7.9 (2.6) | |||||
| L5 | L, 8.7 (1.6) R, 8.8 (1.7) | L, 12.7 (3.8) R, 11.8 (2.5) | L, 16.4 (3.8) R, 16.5 (3.8) | ||||||
| S1 | Horizontal | L, 9.4 (2.4) R, 9.1 (1.4) | L, 2.9 (0.7) R, 3.5 (1.4) | L, 13.6 (2.5) R, 13.6 (2.3) |
AFL, Australian football league; CT, computed tomography; ES, erector spinae; L, left; MRI, magnetic resonance imaging; Mult, multifidus; P, prone; QL, quadratus lumborum; R, right; S, supine; US, ultrasonography.
The CSA of all of the muscles in the current study increased more than the CSA of the vertebral endplates. Therefore, the relative CSA of the muscles at the oldest (6) age groups was much larger than that of the younger groups. The relative increase in muscle CSA might indicate that the loads and the stresses they exert on the vertebrae also increase with age.
Muscle CSA asymmetry
There is conflicting evidence regarding asymmetry between the right and left spinal muscles in young healthy adults (McGill et al. 1993; Hides et al. 2010; Niemelainen et al. 2011; Sanchis‐Moysi et al. 2011; Sitilertpisan et al. 2012; Fortin et al. 2013; Gildea et al. 2013). We found very little asymmetry between the left and the right spinal muscles before skeletal maturity. Fortin et al. (2014) report an increase in multifidus asymmetry over a period of 15 years in the adult population, in association with age and body mass index. This may suggest that spinal muscles are symmetric in young populations and become more asymmetric with increased age and weight.
When considering our results, several aspects must be addressed. First, although we excluded abnormal CT scans, we could not accurately define the degree of symptoms in this group of subjects. To ensure that our results were within the normal range we compared them with other publications (see Table 7) and found a close similarity between the oldest group (17–20 years) and previous studies, indicating that our values are similar to those of healthy individuals (Chaffin et al. 1990; McGill et al. 1993; Danneels et al. 2000; Stokes et al. 2005; Stokes & Windisch, 2006; Hides et al. 2008b, 2010; Niemelainen et al. 2011; Hsu et al. 2015). Secondly, the number of children (especially of girls) in our study was limited. Larger‐scale research may provide additional insight into lumbar developmental changes during childhood. Another drawback is our lack of data regarding subject height, weight, BMI and hand preference, all which may affect CSA. Future studies may inspect these aspects as well. Our study cohort represents a local population and may not be representative. Data from other inhabitants around the world are needed to verify whether the results of this study are universal or local. Finally, this is a cross‐sectional study. Longitudinal studies may provide a more precise description of muscle development during childhood and adolescence.
Future research directions include muscle measurements from MRI studies, which are increasingly replacing ABCT studies due to rising concerns overexposure to ionizing radiation and include advanced muscle imaging methods. The research presented herein demonstrates the value inherent in large existing datasets despite the present‐day discouragement of CT examinations in children.
Conclusions
The results of this study indicate that there are two developmental patterns for spinal muscles: the epaxial muscles (ES and multifidus) reach their maximal CSA by the age of 16 years, before skeletal maturity, whereas the CSA of the hypaxial muscles (psoas and QL) continues to increase until at least spinal maturity (18–21 years). Function, embryology and innervation are possible contributing factors in the difference in the developmental patterns. The implications of these differences for LBP, muscle rehabilitation and response to exercise are not known and should be explored.
Another important finding is that spinal muscles CSA are symmetric in a young population and the asymmetry found in older populations is probably associated with increased age and weight.
We found that the increase in CSA of spinal muscles exceeded the increase of the vertebral bodies, which might indicate that the loads and stresses that the muscles exert on the vertebrae increase with age.
This research is the first to describe the CSA of spinal muscles in children. Future studies with larger cohorts can explore gender differences and other factors that might be associated with spinal muscle CSA.
The work was performed at the Department of Diagnostic Imaging, Sheba Medical Center, Tel Hashomer, Israel.
References
- Adams M, Burton K, Bogduk N (2006) The Biomechanics of Back Pain. Vol. 55. Elsevier Health Sciences. [Google Scholar]
- Akagi R, Takai Y, Ohta M, et al. (2009) Muscle volume compared to cross‐sectional area is more appropriate for evaluating muscle strength in young and elderly individuals. Age Ageing 38, 564–569. [DOI] [PubMed] [Google Scholar]
- Arbanas J, Klasan GS, Nikolic M, et al. (2009) Fibre type composition of the human psoas major muscle with regard to the level of its origin. J Anat 215, 636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayline RJ, Duch C, Levine RB (2001) Nerve‐muscle interactions regulate motor terminal growth and myoblast distribution during muscle development. Dev Biol 231, 348–363. [DOI] [PubMed] [Google Scholar]
- Bogduk N (1983) The innervation of the lumbar spine. Spine (Phila Pa 1976), 8, 286–293. [DOI] [PubMed] [Google Scholar]
- Brand RA, Pedersen DR, Friederich JA (1986) The sensitivity of muscle force predictions to changes in physiological cross‐sectional area. J Biomech 19, 589–596. [DOI] [PubMed] [Google Scholar]
- Calvo‐Munoz I, Gomez‐Conesa A, Sanchez‐Meca J (2013) Prevalence of low back pain in children and adolescents: a meta‐analysis. BMC Pediatr 13, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin DB, Redfern MS, Erig M, et al. (1990) Lumbar muscle size and locations from CT scans of 96 women of age 40 to 63 years. Clin Biomech (Bristol, Avon) 5, 9–16. [DOI] [PubMed] [Google Scholar]
- Chan YL, Cheng JCY, Guo X, et al. (1999) MRI evaluation of multifidus muscles in adolescent idiopathic scoliosis. Pediatr Radiol 29, 360–363. [DOI] [PubMed] [Google Scholar]
- Close RI (1972) Dynamic properties of mammalian skeletal muscles. Physiol Rev 52, 129–197. [DOI] [PubMed] [Google Scholar]
- Cornwall J, Stringer MD, Duxson M (2011) Functional morphology of the thoracolumbar transversospinal muscles. Spine (Phila Pa 1976) 36, E1053–E1061. [DOI] [PubMed] [Google Scholar]
- Crawford RJ, Filli L, Elliott JM, et al. (2016a) Age‐ and level‐dependence of fatty infiltration in lumbar paravertebral muscles of healthy volunteers. AJNR Am J Neuroradiol 37, 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford RJ, Volken T, Valentin S, et al. (2016b) Rate of lumbar paravertebral muscle fat infiltration versus spinal degeneration in asymptomatic populations: an age‐aggregated cross‐sectional simulation study. Scoliosis Spinal Disord 11, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist JR, Vissing CR, Hedermann G, et al. (2017) Fat replacement of paraspinal muscles with aging in healthy adults. Med Sci Sports Exerc 49, 595–601. [DOI] [PubMed] [Google Scholar]
- Danneels LA, Vanderstraeten GG, Cambier DC, et al. (2000) CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J 9, 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoulin C, Crielaard JM, Vanderthommen M (2007) Spinal muscle evaluation in healthy individuals and low‐back‐pain patients: a literature review. Joint Bone Spine 74, 9–13. [DOI] [PubMed] [Google Scholar]
- Dickstein R, Shefi S, Marcovitz E, et al. (2004) Anticipatory postural adjustment in selected trunk muscles in poststroke hemiparetic patients. Arch Phys Med Rehabil 85, 261–267. [DOI] [PubMed] [Google Scholar]
- Fortin M, Yuan Y, Battie MC (2013) Factors associated with paraspinal muscle asymmetry in size and composition in a general population sample of men. Phys Ther 93, 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Videman T, Gibbons LE, et al. (2014) Paraspinal muscle morphology and composition: a 15‐yr longitudinal magnetic resonance imaging study. Med Sci Sports Exerc 46, 893–901. [DOI] [PubMed] [Google Scholar]
- Gildea JE, Hides JA, Hodges PW (2013) Size and symmetry of trunk muscles in ballet dancers with and without low back pain. J Orthop Sports Phys Ther 43, 525–533. [DOI] [PubMed] [Google Scholar]
- Hansen L, de Zee M, Rasmussen J, et al. (2006) Anatomy and biomechanics of the back muscles in the lumbar spine with reference to biomechanical modeling. Spine (Phila Pa 1976) 31, 1888–1899. [DOI] [PubMed] [Google Scholar]
- Hesse B, Frober R, Fischer MS, et al. (2013) Functional differentiation of the human lumbar perivertebral musculature revisited by means of muscle fibre type composition. Ann Anat 195, 570–580. [DOI] [PubMed] [Google Scholar]
- Hides J, Stanton W, Freke M, et al. (2008a) MRI study of the size, symmetry and function of the trunk muscles among elite cricketers with and without low back pain. Br J Sports Med 42, 809–813. [DOI] [PubMed] [Google Scholar]
- Hides J, Stanton W, Mcmahon S, et al. (2008b) Effect of stabilization training on multifidus muscle cross‐sectional area among young elite cricketers with low back pain. J Orthop Sports Phys Ther 38, 101–108. [DOI] [PubMed] [Google Scholar]
- Hides J, Fan T, Stanton W, et al. (2010) Psoas and quadratus lumborum muscle asymmetry among elite Australian Football League players. Br J Sports Med 44, 563–567. [DOI] [PubMed] [Google Scholar]
- Hodges P, Holm AK, Hansson T, et al. (2006) Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine (Phila Pa 1976) 31, 2926–2933. [DOI] [PubMed] [Google Scholar]
- Hsu C, Castillo E, Lieberman D (2015) The relationship between trunk muscle strength and flexibility, intervertebral disc wedging, and human lumbar lordosis. Harvard Undergrad Res J 8, 35–41. [Google Scholar]
- Hyun JK, Lee JY, Lee SJ, et al. (2007) Asymmetric atrophy of multifidus muscle in patients with unilateral lumbosacral radiculopathy. Spine (Phila Pa 1976) 32, E598–E602. [DOI] [PubMed] [Google Scholar]
- Jones GT, Macfarlane GJ (2005) Epidemiology of low back pain in children and adolescents. Arch Dis Child 90, 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kader DF, Wardlaw D, Smith FW (2000) Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol 55, 145–149. [DOI] [PubMed] [Google Scholar]
- Kalichman L, Klindukhov A, Li L, et al. (2016) Indices of paraspinal muscles degeneration reliability and association with facet joint osteoarthritis: feasibility study. Clin Spine Surg 29, E465–E470. [DOI] [PubMed] [Google Scholar]
- Kalichman L, Carmeli E, Been E (2017) The association between imaging parameters of the paraspinal muscles, spinal degeneration, and low back pain. Biomed Res Int 2017, 2562957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Gunderson R, Reikeras O, et al. (2003) Reliability of computed tomography measurements of paraspinal muscle cross‐sectional area and density in patients with chronic low back pain. Spine (Phila Pa 1976) 28, 1455–1460. [DOI] [PubMed] [Google Scholar]
- Kim WH, Lee SH, Lee DY (2011) Changes in the cross‐sectional area of multifidus and psoas in unilateral sciatica caused by lumbar disc herniation. J Korean Neurosurg Soc 50, 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintosh JE, Bogduk N (1986) The biomechanics of the lumbar multifidus. Clin Biomech (Bristol, Avon) 1, 205–213. [DOI] [PubMed] [Google Scholar]
- McGill SM, Santaguida L, Stevens J (1993) Measurement of the trunk musculature from T5 to L5 using MRI scans of 15 young males corrected for muscle‐fiber orientation. Clin Biomech (Bristol, Avon) 8, 171–178. [DOI] [PubMed] [Google Scholar]
- Mekonen HK, Hikspoors JP, Mommen G, et al. (2016) Development of the epaxial muscles in the human embryo. Clin Anat 29, 1031–1045. [DOI] [PubMed] [Google Scholar]
- Narici M (1999) Human skeletal muscle architecture studied in vivo by non‐invasive imaging techniques: functional significance and applications. J Electromyogr Kinesiol 9, 97–103. [DOI] [PubMed] [Google Scholar]
- Niemelainen R, Briand MM, Battie MC (2011) Substantial asymmetry in paraspinal muscle cross‐sectional area in healthy adults questions its value as a marker of low back pain and pathology. Spine (Phila Pa 1976) 36, 2152–2157. [DOI] [PubMed] [Google Scholar]
- Piccirillo R, Demontis F, Perrimon N, et al. (2014) Mechanisms of muscle growth and atrophy in mammals and Drosophila. Dev Dyn 243, 201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rezende Pinto WB, de Souza PV, Oliveira AS (2015) Normal muscle structure, growth, development, and regeneration. Curr Rev Musculoskelet Med 8, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis‐Moysi J, Idoate F, Izquierdo M, et al. (2011) Iliopsoas and gluteal muscles are asymmetric in tennis players but not in soccer players. PLoS ONE 6, e22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JO, Qiu X, Lu X, et al. (2017) The uniform pattern of growth and skeletal maturation during the human adolescent growth spurt. Sci Rep 7, 16705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Dyar KA, Ciciliot S, et al. (2013) Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280, 4294–4314. [DOI] [PubMed] [Google Scholar]
- Shefi S, Soudack M, Konen E, et al. (2013) Development of the lumbar lordotic curvature in children from age 2 to 20 years. Spine (Phila Pa 1976) 38, E602–E608. [DOI] [PubMed] [Google Scholar]
- Sions JM, Smith AC, Hicks GE, et al. (2016) Trunk muscle size and composition assessment in older adults with chronic low back pain: an intra‐examiner and inter‐examiner reliability study. Pain Med 17, 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sions JM, Elliott JM, Pohlig RT, et al. (2017) Trunk muscle characteristics of the multifidi, erector spinae, psoas, and quadratus lumborum in older adults with and without chronic low back pain. J Orthop Sports Phys Ther 47, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitilertpisan P, Hides J, Stanton W, et al. (2012) Multifidus muscle size and symmetry among elite weightlifters. Phys Ther Sport 13, 11–15. [DOI] [PubMed] [Google Scholar]
- Stokes IAF, Windisch L (2006) Vertebral height growth predominates over intervertebral disc height growth in adolescents with scoliosis. Spine (Phila Pa 1976) 31, 1600–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes M, Rankin G, Newham DJ (2005) Ultrasound imaging of lumbar multifidus muscle: normal reference ranges for measurements and practical guidance on the technique. Man Ther 10, 116–126. [DOI] [PubMed] [Google Scholar]
- Uraoka H, Higashino K, Morimoto M, et al. (2018) Study of lesions of the lumbar endplate based on the stage of maturation of the lumbar vertebral body: the relationship between skeletal maturity and chronological age. Eur J Orthop Surg Traumatol 28, 183–187. [DOI] [PubMed] [Google Scholar]
- Vives MJ (2016) The paraspinal muscles and their role in the maintenance of global spinal alignment. Another wrinkle in an already complex problem. Spine J 16, 459–461. [DOI] [PubMed] [Google Scholar]
- Yagi M, Akilah KB, Boachie‐Adjei O (2011) Incidence, risk factors and classification of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Spine (Phila Pa 1976) 36, E60–E68. [DOI] [PubMed] [Google Scholar]


