Abstract
The human hand is well known for its unique dexterity which is largely facilitated by a highly mobile, long and powerful thumb that enables both tool manufacturing and use, a key component of human evolution. The bonobo (Pan paniscus), the closest extant relative to modern humans together with the chimpanzee (Pan troglodytes), also possesses good manipulative capabilities but with a lower level of dexterity compared with modern humans. Despite the close phylogenetic relationship between bonobos and humans, detailed quantitative data of the bonobo forelimb musculature remains largely lacking. To understand how morphology may influence dexterity, we investigated the functional anatomy of the bonobo hand using a unique sample of eight bonobo cadavers, along with one chimpanzee and one human (Homo sapiens) cadaver. We performed detailed dissections of unembalmed specimens to collect quantitative datasets of the extrinsic and intrinsic hand musculature, in addition to qualitative descriptions of the forelimb muscle configurations, allowing estimation of force‐generating capacities for each functional group. Furthermore, we used medical imaging to quantify the articular surface of the trapeziometacarpal joint to estimate the intra‐articular pressure. Our results show that the force‐generating capacity for most functional groups of the extrinsic and intrinsic hand muscles in bonobos is largely similar to that of humans, with differences in relative importance of the extensors and rotators. The bonobo thumb musculature has a lower force‐generating capacity than observed in the human specimen, but the estimated maximal intra‐articular pressure is higher in bonobos. Most importantly, bonobos show a higher degree of functional coupling between the muscles of the thumb, index and lateral fingers than observed in humans. It is conceivable that differentiation and individualization of the hand muscles rather than relative muscle development explain the higher level of dexterity of humans compared with that of bonobos.
Keywords: muscles, Pan paniscus, primate anatomy, thumb
Introduction
The thumb plays a key role in the functioning of the primate hand, known for its unprecedented dexterity. The modern human (Homo sapiens) hand is a prime example of such dexterity, with a thumb fully devoted to manipulation. Despite the fact that non‐human primates use their hands in locomotion and manipulation, many also show advanced manipulative abilities, used in grooming and for processing food [e.g. capuchins (Spinozzi et al. 2004), orangutans, chimpanzees, and bonobos (Colell et al. 1995; Furuichi & Thompson, 2008)] or for making and using tools [e.g. capuchins (Fragaszy et al. 2004; Visalberghi et al. 2009), gorillas (Breuer et al. 2005), macaques (Gumert et al. 2011), chimpanzees, and bonobos (Jordan, 1982; Toth et al. 1993; Takeshita & Walraven, 1996; Boesch et al. 2009)]. As the primate hand displays many varieties of phenotypes where form and function of the thumb are closely correlated, understanding these phenotypes in closely related primate species may facilitate the interpretation of function in an evolutionary context. Despite the great importance of the thumb in human evolution, key to the unique human dexterity, a complete understanding of the adaptive signals and form–function relationship in the primate thumb is lacking.
Within the extant primates, the bonobo (Pan paniscus) is one of the modern human's closest relatives, sharing approximately 98.7% of their genetic blueprint with modern humans (Prüfer et al. 2012). The common Homo‐Pan ancestor lived 7–13 million years ago (Young et al. 2015), while the split in the genus Pan appears to have happened between 2 and 1 million years ago (Prado‐Martinez, 2013; Kuhlwilm et al. 2016). Both bonobos and chimpanzees possess a very diverse locomotor repertoire, but the thumb is predominantly used during arboreal locomotion (e.g. vertical climbing and quadrumanous scrambling), as their thumb is relatively short compared with the other fingers so that it is not involved during knuckle‐walking. During manipulation and locomotion, they are capable of using fine precision grips, in‐hand manipulation and forceful power grips, similar to humans (Christel et al. 1998; Crast et al. 2009; Feix et al. 2015; Bardo et al. 2016). Additionally, they are capable of thumb opposition, which seems to be facilitated by the saddle‐shaped surfaces of the trapeziometacarpal (TMC) joint, as seen in modern humans (Marzke, 1997). Bonobos and chimpanzees favour precision grips in picking up small objects in which the tip of the thumb makes contact with the radial aspect of the index finger, from the distal to the proximal phalanx (Butterworth & Itakura, 1998; Christel et al. 1998). However, they do not always use their thumb for grasping small objects, whereas modern humans always use their thumb for precision gripping (Pouydebat et al. 2009). When grasping large objects, bonobos and chimpanzees mostly use a power grip (Pouydebat et al. 2009), but this does not provide the same accurate control as the power grip in modern humans (Marzke et al. 1992). In addition, both bonobos and chimpanzees are capable of using tools, a feature that has been observed both in captivity (Jordan, 1982; Toth et al. 1993; Takeshita & Walraven, 1996) and in their natural environment (Ingmanson, 1998; Neufuss et al. 2017).
Whereas the anatomy of the chimpanzee hand has been studied in detail, based on dissections of approximately 50 arm specimens (e.g. Tuttle, 1969; Marzke, 1997; Thorpe et al. 1999; Carlson & Lowe, 2006; Oishi et al. 2009; Myatt et al. 2012; Almécija et al. 2015; Lesnik et al. 2015), information about the bonobo hand musculature is limited (Miller, 1952; Diogo et al. 2017a,b). Most previous studies have focused only on hand proportions, and external morphology of the hand and finger bones (Inouye, 1992; Alba et al. 2003; Tocheri et al. 2008; Almécija et al. 2015), whereas detailed quantitative data on the surrounding soft‐tissue are largely missing in the literature.
In this study, we describe and quantify the extrinsic and intrinsic hand musculature of bonobos. We hypothesize that the bonobo hand, and specifically the thumb, musculature is relatively well developed in terms of volume and force‐generating capacity, possibly comparable to humans. Furthermore, we expect morphological deviations from the human configuration that account for differences in the manipulative capabilities of the hand. Here we investigate whether such deviations are present, and whether they concern (i) quantitative differences in muscle volume and force‐generating capacity and/or (ii) qualitative differences, such as muscle paths and other changes in muscle configuration, that have functional implications.
Materials and methods
Specimen selection
The hand and/or forearm of nine (sub)adult bonobos were obtained from different European zoos. All animals died of natural causes and were sampled opportunistically. The sample details are provided in Table 1. All specimens were stored at −18 °C shortly after death and kept frozen until they were CT‐scanned and dissected. Medical imaging (CT scanning) was obtained for all nine specimens. Muscle data were obtained for eight of the nine animals [specimen Pp4 (Lomela; MIG12‐29745517) was fixed in formaldehyde prior to freezing and it was impossible to dissect this specimen due to tissue dryness in total]. Ten samples were dissected [forearm and hand (8/10) or only hand (2/10)], of which two samples of two specimens were dissected as part of the Bonobo Morphology Initiative which took place at the University of Antwerp in January 2016. Specimen Pp2 had been disarticulated at the elbow joint such that not all of the extrinsic muscles could be quantified. The specimen has therefore been included in the qualitative study of the muscles present, but it has been excluded from the quantitative analysis, as total muscle mass could not be determined.
Table 1.
Specimen details
| Code | Subject identifier | Sex | Age | Injury | Sample | Origin |
|---|---|---|---|---|---|---|
| Pp1 |
Dzeetaa
11957872 |
F |
Adult 31 years |
TMC | R hand | Royal Zoological Society of Antwerp, Belgium |
| Pp2 |
Zorbaa
8365526 |
M |
Adult 35 years |
MCP1 | L forearm and hand | Wilhelma Zoo, Stuttgart, Germany |
| Pp3 | X | ? | Adult | – | L hand | Royal Zoological Society of Antwerp, Belgium |
| Pp4 |
Lomela MIG12‐29745517 |
F |
Adult 17 years |
– | No dissection, only CT scanning | Royal Zoological Society of Antwerp, Belgium |
| Pp5 |
Jasiri 15295295 |
F |
Sub‐adult 8 years |
– | L+R forearm and hand | Royal Zoological Society of Antwerp, Belgium |
| Pp6 |
Kidogo MIG12‐27564614 |
M |
Adult 25 years |
– | L+R forearm and hand | Royal Zoological Society of Antwerp, Belgium |
| Pp7 |
Ludwig MIG12‐29882197 |
M |
Adult 32 years |
DP2‐3‐4 | R forearm and hand | Frankfurt Zoo, Germany |
| Pp8 |
Kirembo SB:177 |
M | Adult 24 years | DP2 and 4 | R forearm and hand |
La Vallée des Singes, Le Gureau, France |
| Pp9 |
Hermiena
27641621 |
F |
Adult 39 years |
– | L forearm and hand | Wilhelma Zoo, Stuttgart, Germany |
| Pt1 |
Marlene 208210000495828 |
F |
Adult 42 years |
– | R forearm and hand | Burger's Zoo, Arnhem, The Netherlands |
| Hs1 | 692 | M |
Adult 60 years |
– | L forearm and hand | University of Leuven, Kortrijk, Belgium |
DP, distal phalanx; F, female; L, left; M, male; MCP, metacarpophalangeal joint; R, right; TMC, trapeziometacarpal joint.
Wild born.
Some cadaver hands showed musculoskeletal injuries (4/10) (Table 1. In two specimens, several distal phalanges were missing, either entirely (Pp7R: DP3) or partially (Pp7R: DP2 and 4; Pp8R: DP2 and 4). Soft tissue at the level of the fingers had already healed pre‐mortem, but the extent of the scar tissue indicates repeated damage to the digits. In two other specimens, there was evidence of a dislocation at the metacarpophalangeal joint (Pp2L: MCP1) or TMC (Pp1R).
For comparison, anatomical data from a fresh‐frozen chimpanzee (Pan troglodytes; Pt1) and a human cadaver (Homo sapiens, Hs1) are included. The chimpanzee specimen was obtained from Burger's Zoo, Arnhem (The Netherlands) and the human specimen was obtained via the Human Body Donation Programme of the university. Both specimens were also dissected and CT‐scanned.
CT scanning and image segmentation
Prior to dissection, the entire hand of each specimen (either the left or the right hand) was CT‐scanned at the local hospital (AZ Groeninge, Kortrijk, Belgium) using a 64‐slice Discovery HD 750 CT scanner [GE Healthcare, Little Chalfont, UK; display field of view (DFOV): 250 mm, slice thickness: 0.625 mm, voxel size: 0.150 mm3, 100 kV, 180 mA, 512 × 512].
The CT images were segmented manually using mimics software (Mimics for Research 18.0, Materialise, Leuven, Belgium) and 3D surface models of the trapezium and first metacarpal (MC1) were reconstructed to be able to measure the articular area of the TMC joint. The articular area of the trapezium and MC1 was determined by manually delineating the border of the articular facet on each 3D bone model using 3‐matic software (Materialise). The articular area of the trapezium and MC1 were obtained from five bonobo specimens (Pp5–9), from the chimpanzee (Pt1) and human (Hs1), and were used to estimate TMC joint pressure (see below).
Dissection procedure
The specimens were stored in freezers (−18 °C) and were thawed at room temperature 24–48 h prior to the dissections. All muscles were isolated one by one and their origin and insertion were determined, using the same protocol as in previous anatomical studies (Vereecke et al. 2005; Channon et al. 2009). A complete dissection of the left and/or right forearm and hand was performed for five bonobo specimens (Pp5–9), but only one side per animal was included in the quantitative analysis. In addition, the left or right hands of three bonobo specimens (Pp1–3) were also dissected. As such, the extrinsic hand musculature in five specimens and the intrinsic hand musculature in eight specimens could be quantified and incidences for presence/absence of muscles could be obtained for all dissected arms (10 arms/hands from 8 bonobos).
For each muscle, the following parameters were measured: (i) muscle volume (V); (ii) fascicle length (FL), which is the approximate length of the muscle fibres; and (iii) pennation angle (PA), the average angle of the muscle fibres relative to the force‐generating axis. Length measurements were taken to the nearest 0.1 mm with digital callipers (Mitutoyo, UK, accurate to 0.01 mm) and muscle volume was determined to the nearest 0.1 mL by submersion in physiological saline solution (0.9% NaCl). Muscles were cut lengthwise along the tendon to determine muscle fascicle length and pennation angle. Digital photographs of the muscles were taken, and pennation angle and fascicle length were measured using Fiji software (Schindelin et al. 2012). The data provided for fascicle length and pennation angle are average values of at least three independent measurements taken on different places along the muscle belly.
Data analysis
Muscles were categorized into functional groups to facilitate comparison (Table 2). Physiological cross‐sectional area (PCSA) of a muscle was calculated using Eq. (1).
| (1) |
Table 2.
Functional muscle groups
| Muscle group | Muscle | Abbreviation | Crossing TMC jointa |
|---|---|---|---|
| Extrinsic hand muscles | |||
| Wrist flexors |
m. flexor digitorum superficialis m. flexor digitorum profundus m. flexor carpi radialis m. flexor carpi ulnaris m. palmaris longus m. brachioradialis |
FDS FDP FCR FCU PL BR |
Only FDP1, variable |
| Wrist extensors |
m. extensor digitorum m. extensor carpi radialis longus m. extensor carpi radialis brevis m. extensor digiti minimi m. extensor carpi ulnaris m. extensor indicis |
ED ECRL ECRB EDM ECU EI |
Variable |
| Arm rotators |
m. pronator teres m. pronator quadratus m. supinator |
PT PQ SUP |
|
| Thumb |
m. abductor pollicis longus m. extensor pollicis longus |
APL EPL |
Only APL I X |
| Intrinsic hand muscles | |||
| Thenar |
m. flexor pollicis brevis m. abductor pollicis brevis m. adductor pollicis m. opponens pollicis |
FPB APB ADP OPP |
X X X X |
| Intermediate |
m. intermetacarpalis I, II, III, IV m. flexor brevis profundi III, IV, V, VI, VII, IIX, IX m. interosseous dorsalis I, II, III m. lumbricalis II, III, IV, V |
IM FBP IOD LUMB |
|
| Hypothenar |
m. palmaris brevis m. abductor digiti minimi m. flexor digiti minimi m. opponens digiti minimi |
PB ADM FDM ODM |
|
The PCSA of the muscles that are consistently crossing the TMC joint were included in the estimation of TMC joint pressure.
However, we chose to omit pennation angle from the PCSA equation because: (i) pennation angle is difficult to measure accurately during dissections, (ii) the in vitro measurements are not fully representative of the pennation angles in vivo given that pennation angles change during muscle contraction and (iii) the pennation angle of most muscles ranges between 0° and 30°, the cosine of which ranges between 1 and 0.87, having only a minor influence on PCSA calculation. Therefore, Eq. (2) was used in our final analysis.
| (2) |
To obtain an estimate of the force‐generating capacity of a muscle (Fmax), PCSA was multiplied by 0.3 MPa, i.e. the maximal isometric stress of vertebrate muscle (Wells, 1965; Medler, 2002). The force‐generating capacity was calculated for the extrinsic thumb muscles and thenar muscles (for a definition see Table 2). To obtain an estimate of the maximal compressive force occurring in the bonobo TMC joint compared with the chimpanzee and human TMC joint, we calculated the total force‐generating capacity of the muscles that cross the TMC joint (sum of PCSA values multiplied by 30 Nm−2). By dividing the total force‐generating capacity by articular area of the trapezium (i.e. surface of the distal facet), we estimated the pressure occurring at the joint. These values were acquired for each specimen individually (n = 5; only for the specimens for which both extrinsic and intrinsic hand muscles could be quantified). Average and standard deviations were calculated for the bonobo (based on the five pressure estimates) to allow comparison with the chimpanzee and human data.
Results
Observations on bonobo hand musculature
Extrinsic hand musculature
The origin, insertion, and function of all extrinsic hand muscles are listed in Supporting Information Table S1. Differences regarding the origin and insertion between the specimens (n = 8) are indicated in the Table, but the most conspicuous differences are discussed below.
The m. flexor carpi radialis (FCR) originates from the medial epicondyle of the humerus (8/8). The FCR inserts either on the base of MC1 (3/8) or MC2 (5/8), and in the case of the latter it may also extend towards MC3 with an additional tendon (1/8) or tendon slip (1/8) from MC2.
The m. palmaris longus (PL) originates from the medial epicondyle of the humerus. It inserts radially on the radial palmar aponeurosis and connects to the fascia of the m. abductor pollicis brevis (APB) (8/8). Occasional fusion with the FCR (3/8) is observed.
The m. flexor digitorum superficialis (FDS) in bonobos usually consists of three muscle bellies, one for digit 2 (FDS II: 7/8), one for digits 3 and 4 (FDS III–IV: 8/8) and one for digit 5 (FDS V: 6/8). However, occasionally FDS II (1/8) and FDS V (2/8) may also be fused with the FDS III–IV belly. In most specimens, the FDS II shows a distinctive double muscle‐tendon unit (MTU) configuration (5/8) (Fig. 1). FDS II originates from the medial epicondyle of the humerus (8/8) and from the proximal ulna (1/8) and inserts on the intermediate phalanx of digit 2 (8/8) with an occasional cross‐over tendon to the FDS III tendon (3/8). FDS III–IV originates from both the medial epicondyle (8/8) and proximal radius (7/8). In one specimen, FDS III–IV originates from the ulna instead of the radius. Its individual tendons insert on the intermediate phalanges of the digits 3 and 4 (8/8). FDS V originates from the medial epicondyle of the humerus (8/8) or from the radius (1/8) and inserts on the intermediate phalanx of digit 5 (7/8). In one specimen, FDS V inserts on the distal phalanx.
Figure 1.

Photo of the m. flexor digitorum superficialis to digit 2 (FDS II), showing serial MTU organization.
The m. flexor digitorum profundus (FDP) is separated into two muscle bellies. One head (FDP I–II) originates from the shaft of the radius – between the m. supinator (SUP) and m. pronator quadratus (PQ) – and inserts onto the distal phalanx of digits 1 and 2 (Fig. 2). The other head (FDP III–IV–V) originates from the interosseous membrane and the shaft of the ulna and inserts on the distal phalanx of digits 3, 4 and 5. In one specimen, FDP I and V are absent, consequently the m. lumbricalis to the fifth digit (LUMB IV) is absent as well. Additionally, there is an extra tendon from FDP III–IV to the base of the lumbrical inserting on digit 2 (LUMB I).
Figure 2.
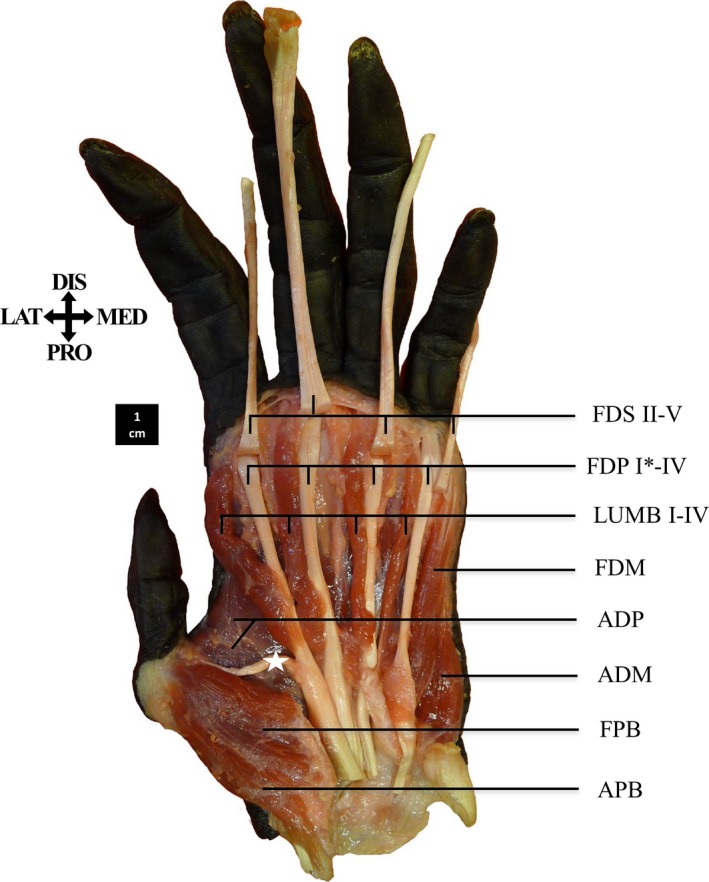
Palmar view of the superficial flexor muscles of the fingers. The m. opponens pollicis (OPP) and m. opponens digiti minimi (ODM) are not visible here. The m. flexor digitorum profundus has a mutual tendon going to the distal phalanx of the thumb (*; FDP I) and a tendon to digit 2 (FDP II).
The m. flexor pollicis longus (FPL) is not present as a separate muscle in bonobos. Instead, a tendon, here described as the FDP I tendon, splits from the FDP II tendon and inserts onto the distal phalanx of the thumb (7/8) (Fig. 2).
The m. abductor pollicis longus (APL) consists of two proximally fused muscle bellies, each with its own insertion (8/8). Both originate from the interosseous membrane and the posterior side of the shaft of the radius and ulna, and are sometimes proximally fused with the m. supinator (3/8). One tendon (APL I) always inserts on the base of the MC1 (8/8), but the second insertion (APL II) is variable. Most frequently it inserts on the trapezium (7/8), occasionally with an additional insertion on the pre‐pollex (1/8), the dorsal ligament of the thumb (1/8) or the MC1 base (1/8) (Fig. 3). The APL II tendon can also insert solely on the pre‐pollex (1/8), a sesamoid bone present in 7/8 of the specimens, located at the base of the thumb, generally articulating with the scaphoid and trapezium.
Figure 3.
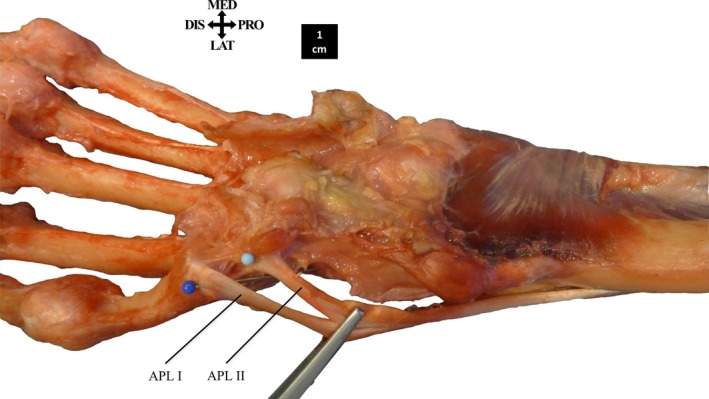
The insertion of m. abductor pollicis longus (APL). The tendon of APL I always inserts on the base of the MC1. The tendon of APL II inserts on the trapezium.
The m. brachioradialis (BR) invariably originates from the supracondylar ridge of the humerus and inserts onto the styloid process of the radius. The tendon either inserts directly onto the styloid process (5/8) or onto the shaft of the radius proximal to the styloid, continuing to the styloid process (2/8). In one specimen, the tendon is split in two distally, with one slip inserting on the styloid process and the other slip inserting adjacent to the groove of the APL.
The m. extensor carpi radialis longus (ECRL) and m. extensor carpi radialis brevis (ECRB) are clearly separated in the bonobo specimens. The ECRL usually inserts onto the base of MC2 (5/8) but can insert onto MC1 as well (3/8). The ECRB inserts onto the dorsal side of the MC3 base (8/8) and can also be connected to the mm. intermetacarpales (IM) I and II (3/8).
The m. extensor digitorum (ED) originates from the lateral epicondyle of the humerus and is fused proximally with the m. extensor carpi ulnaris (ECU) (8/8). Its four differentiated muscle bellies are fused proximally to a varying degree. In most cases, each individual tendon inserts on its respective distal phalanx, after forming the extensor mechanism with the m. lumbricalis and mm. interossei (see intrinsic hand musculature) (7/8). Additionally, some tendons may interconnect between the digits (5/8). The ED IV and ED V muscle bellies may be completely fused; here, a single tendon splits into two distally to insert onto digits 4 and 5 (1/8). On occasion, the ED V and EDM tendons also are fused, together inserting on the extensor mechanism of digit 5 (1/8) (Fig. 4).
Figure 4.
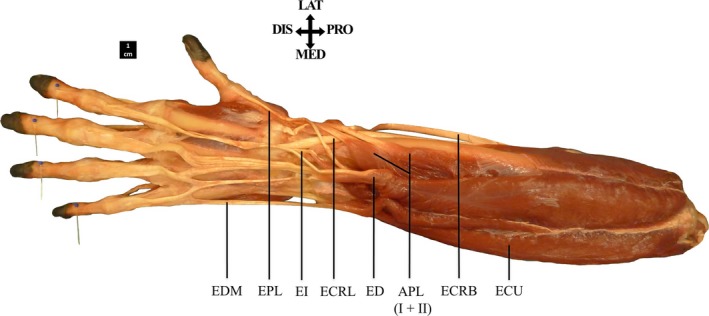
Dorsal view of the extrinsic extensor muscles. The muscle bellies of the m. extensor digitorum (ED) and m. extensor digiti minimi (EDM) are fused proximally. The tendon of ED V is fused with the EDM tendon, together inserting on the extensor mechanism of digit 5.
The m. extensor indicis (EI) inserts distally to the m. extensor pollicis longus (EPL) on the ulnar shaft; both muscles may be fused proximally (2/8). The EI has an underdeveloped tendon relative to other forearm muscles and its insertion is variable. It may insert dorsally on the proximal phalanx of the index finger (5/8) or dorsally on the MC2 base (1/8). On occasion, the EI tendon splits in two distally, with one slip inserting on the MCP2 and the other on the MCP3 joint (1/8). Furthermore, the EI may have two distinct tendons, one inserting on the proximal phalanx of digit 2, the other on that of digit 4 (1/8). The m. extensor pollicis brevis (EPB) as found in humans is not present in bonobos (8/8).
The m. extensor digiti minimi (EDM) originates from the lateral epicondyle of the humerus accompanied by the ECU (7/8) and is sometimes fused with the m. extensor digitorum (ED) proximally (2/8). The EDM tendon inserts either on the extensor mechanism of digit 5 together with the tendon of ED V (3/8) or on the proximal phalanx of digit 5 (4/8). In one specimen, in addition to its insertion on digit 5, two short tendons inserting on the extensor retinaculum were observed. The EDM may also be absent in its entirety (1/8).
Intrinsic hand musculature
The origin, insertion, and function of all intrinsic hand muscles are listed in Supporting Information Table S2. Differences regarding the origin and insertion between the specimens (n = 10) are indicated in the Table, but the most important differences are discussed below (see also Fig. 2).
In the majority of our specimens, each m. intermetacarpalis (IM I–IV) is fused with the m. flexor brevis profundi (FBP) of the respective digit (FBP III, V, VI and VIII) to form the mm. interossei dorsales (IOD I–IV), common with the human configuration (7/10) (Fig. 5). A minority displayed the ancestral non‐human primate configuration of separated IM and FBP muscles (1/10) or an intermediate configuration where only one or two IOD are present, and the other muscles are separated (2/10). These bonobo specimens thus show a continuum between the non‐human primate configuration and the configuration associated with modern humans. A detailed visualization of individual specimen's hand muscle configurations (i.e. IM and FPB, or IOD) is reported in the Supporting Information Fig. S1.
Figure 5.
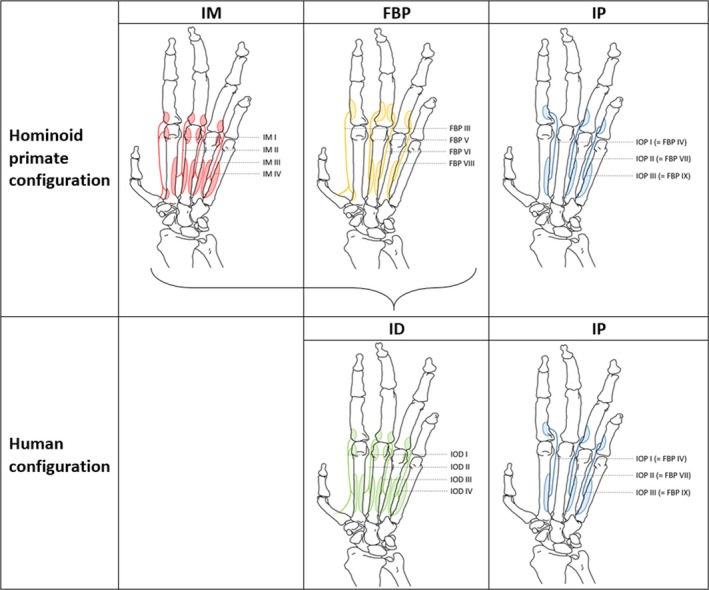
Human vs. hominoid primate configuration of the mm. intermetacarpales (IM), mm. flexores breves profundi (FBP), mm. interossei dorsales (IOD) and mm. interossei palmares (IOP). In modern humans, the IM and FBP are fused to form the IOD.
The m. abductor pollicis brevis (APB) originates from the flexor retinaculum in all specimens. However, additional origins from the shaft of MC3 (5/10) or pre‐pollex (2/10) as well as fusions with the m. opponens pollicis (OPP) (3/10) and/or m. flexor pollicis brevis (FPB) (2/10) are present among the specimens. The APB inserts onto the radial sesamoid bone of the MCP1 joint but variations such as insertion on MC1 base (1/10) may occur.
The m. flexor pollicis brevis (FPB) consists of a single MTU (10/10), in contrast to humans, where a deep (FPB profundum) and a superficial (FPB superficiale) head can be distinguished. Additionally, the FPB may be fused proximally with the APB and OPP (2/10). The FPB originates from the flexor retinaculum and inserts onto the APB tendon, which in turn inserts onto the radial sesamoid bone of the MCP1 joint (10/10).
The m. opponens pollicis (OPP) originates from the flexor retinaculum similar to the origin of the APB and FPB and inserts onto the radial side of the MC1 shaft (10/10), occasionally continuing onto the APB tendon (2/10) or onto the radial sesamoid of MC1 directly (1/10). Sometimes, it also can be fused with either APB (1/10) or both APB and FPB (2/10).
The m. adductor pollicis (ADP) consists of an oblique and transverse head, both of which insert onto the ulnar sesamoid bone of the MCP1 joint. The oblique head most commonly originates from the palmar base of MC3 (7/10) or from the base of MC2 and MC3 (3/10). The transverse head often originates from the palmar side of the entire MC3 (6/10), with additional attachments on the head of MC2 (1/10) or MC4 (1/10). However, several variations on the site of origin of the transverse head were observed, originating from the contrahens raphe of MC3 and MC4 (2/10) or with an origin from the entire MC4 (1/10). In two specimens, a m. adductor pollicis accessorius (APA) was observed, consisting of a small bundle of muscle fibres originating distally from the contrahens raphe near the head of MC2 and inserting on the ulnar side of the MC1.
The hypothenar muscles [m. palmaris brevis (PB), m. abductor digiti minimi (ADM), m. flexor digiti minimi (FDM), m. opponens digiti minimi (ODM)] have a constant configuration similar to that seen in humans. However, a strong fusion of ADM, FDM and ODM was observed in one specimen.
Quantification of bonobo hand muscles
A detailed documentation of the quantitative muscle parameters discussed below is provided in Supporting Information Table S3. An overview of both muscle fascicle length and pennation angle of the bonobo hand muscles can be found in Supporting Information Figs S2 and S3).
Functional muscle groups
The PCSA of the functional muscle groups as a percentage of total forelimb muscle PCSA is depicted in Fig. 6 for each of the dissected specimens (5 bonobos, 1 chimpanzee and 1 human). Bonobos have an average flexor/extensor ratio of 3 : 1 with the PCSA of the forearm flexors making up on average 39.3% (SD: 2.6%) of the total forearm muscle PCSA, whereas the extensors on average only make up 13.2% (SD: 2.3%). The chimpanzee has a flexor/extensor ratio of about 2.1 : 1 with the flexors PCSA amounting to 35.2% and the extensors PCSA to 16.7% of the total forearm muscle PCSA. The human specimen has a flexor/extensor ratio of 1.3 : 1, 35.4% of the forearm muscle PCSA comprising flexors and 27.0% extensors. The rotators take up a greater proportion of the total forearm muscle PCSA in bonobos and chimpanzee (20.6 and 25.6%, respectively) compared with the human specimen (14.0%).
Figure 6.
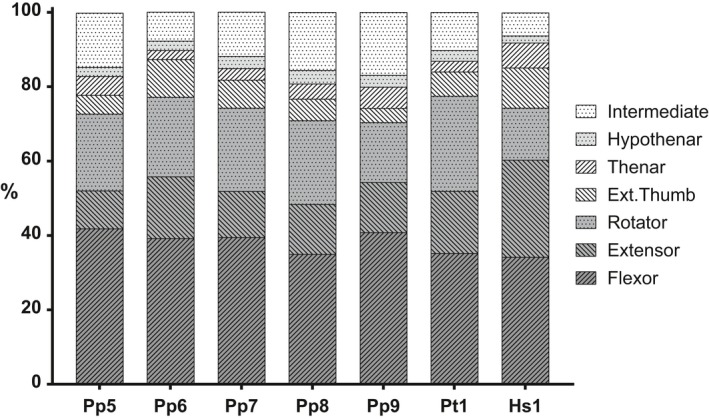
Functional muscle group PCSA as a percentage of total forelimb PCSA in bonobos (Pp), chimpanzee (Pt) and human (Hs).
If we look at the intrinsic hand muscles as a percentage of total forearm muscle PCSA, we observe that these, on average, make up 20.5% in bonobos, 16.1% in the chimpanzee and 14.8% in the human specimen (Fig. 7C). The configuration of the intrinsic hand muscles differs markedly between Pan and Homo, with a dominant development (% PCSA) of the intermediate hand muscles in Pan (~ 60%) and a similar development of the thenar and intermediate hand muscles in Homo (~ 42%).
Figure 7.
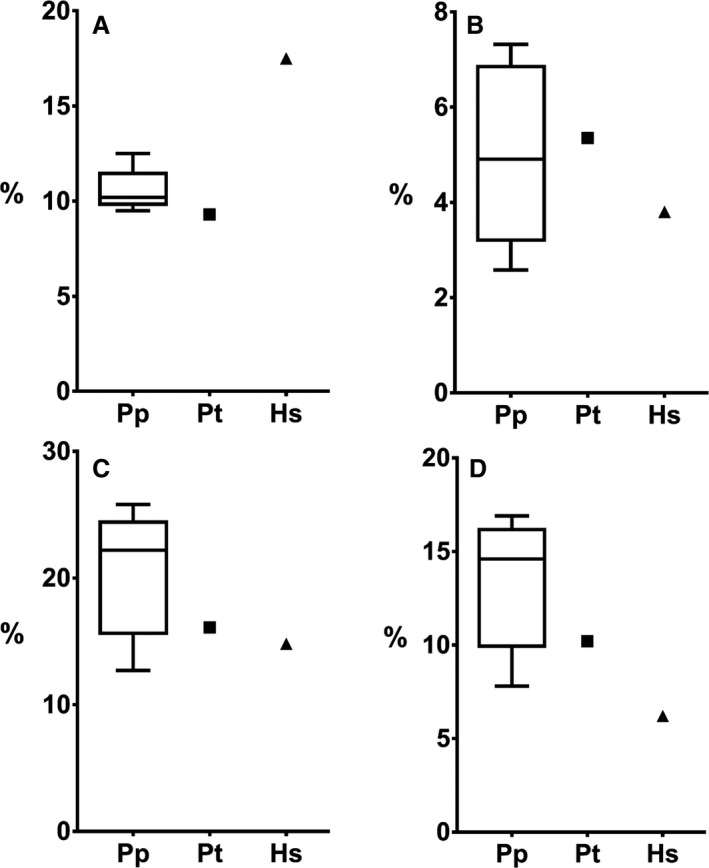
A comparison of the PCSA of (A) thumb muscles, (B) APL, (C) intrinsic hand muscles and (D) intermediate hand muscles, as a percentage of total PCSA in bonobos (Pp), chimpanzee (Pt) and human (Hs).
Thumb muscles
The muscles that move and stabilize the thumb are composed of the extrinsic thumb muscles (APL, EPB, EPL, FPL), the thenar muscles (OPP, APB, FPB, ADP), and the first dorsal interosseous muscle (IOD I). The proportion of the thumb muscle PCSA as a percentage of total forearm muscle PCSA is, on average, 10.6% in bonobos, and 9.3% and 17.5% in, respectively, the chimpanzee and human specimen (Fig. 7A). Furthermore, the percentage of thenar musculature in proportion to the intrinsic hand muscles is on average 25.1% in bonobos, 19.3% in the chimpanzee, and 45.8% in the human. The APL, in bonobos, takes up 5% of the total forearm muscle PCSA, similar to that found in the chimpanzee (5.3%). The relative contribution of the APL is lower in the human specimen (3.8%), even when including the EPB (4%), but falls within the large range observed for the bonobo specimens. In Pan, however, the APL appears to be the most important muscle within the thumb musculature, accounting for on average 47% of thumb muscle PCSA in bonobos and 58% in the chimpanzee; in contrast, the contribution in Homo is much lower (21%) (Fig. 7B).
Finally, by dividing the maximal force‐generating capacity of the muscles crossing the TMC joint by the trapezial articular area, an estimate of the maximal pressure at the TMC joint was obtained. The pressure estimate is on average to 3.0 MPa for the bonobos, 3.2 MPa for the chimpanzee and 2.6 MPa in the human (Table 3).
Table 3.
Estimated trapeziometacarpal joint pressure
| TMC pressure | Pp5 | Pp6 | Pp7 | Pp8 | Pp9 | Bonobo average | Chimp Pt1 | Human Hs1 |
|---|---|---|---|---|---|---|---|---|
| Surface area (mm2) | 100.7 | 106.4 | 145.0 | 130.0 | 138.1 | 124.0 | 132.7 | 178.9 |
| PCSA (mm2) | 899.1 | 1827.2 | 1635.1 | 930.8 | 750.7 | 1208.6 | 1401.8 | 1563.5 |
| Force (N) | 269.7 | 548.2 | 490.5 | 279.3 | 225.2 | 362.6 | 420.5 | 469.1 |
| Pressure (MPa) | 2.7 | 5.2 | 3.4 | 2.2 | 1.6 | 3.0 | 3.2 | 2.6 |
Discussion
This study identifies important features of the hand musculature in bonobos in comparison with the chimpanzee and human configuration, based on the detailed dissection of a unique sample of bonobo specimens. Three major findings are: (i) the high variability in bonobo hand musculature, (ii) the well‐developed thumb musculature and (iii) the presence of functional coupling between muscles.
High variability of bonobo hand musculature
Both the qualitative and quantitative analysis of the bonobo hand musculature indicate a high variation among individuals. From the qualitative analysis we report incidences of all observed configurations for all dissected specimens, also including left and right hands from the same animal. We found a particularly high degree of variability in the configuration of the intermediate hand muscles as well as many, often small, variations in the site of insertion of the extrinsic hand muscles, most notable the long flexors (FDS, FDP) and EI muscle. Similarly, the quantitative analysis, which only includes unilateral sampling and a smaller sample size (n = 5), yields marked intraspecific variations in muscle volume and PCSA. We focus on muscle PCSA as this is the most functionally relevant parameter, being strongly correlated with force‐generating capacity of a muscle (Marzke & Marzke, 2000; Vereecke et al. 2005). However, analyses on muscle mass show similar results. We speculate that this high interindividual variation in the soft‐tissue configuration of the bonobo hand might be an indication that this region is under only mild selective pressure, and/or that the functional implications of these variations are limited. In contrast, variability in the bonobo thenar and extrinsic thumb muscles is relatively small. Such consistency may suggest that these muscles are more strictly regulated by selective pressure.
Despite the variability observed in the bonobo hand muscles, we were able to identify some diverging general trends for the muscle configuration between Pan and Homo. For example, when we look at the relative proportion of the different functional muscle groups, we observe a similar organization in the bonobo and chimpanzee specimens which deviates from the human configuration. Most importantly, the proportion of wrist extensors is increased in Homo relative to Pan, a trait potentially linked to tool use due to the importance of wrist extension during tool‐making (Williams et al. 2010); in turn, Pan has a stronger development of the forearm rotators. The large amount of rotators has also been shown in previous anatomical studies on great apes (e.g. Thorpe et al. 1999; Myatt et al. 2012) and can be explained by the importance of pro‐supination movements during arboreal locomotion of bonobos and chimpanzees. Wrist flexors show a similar relative development in Pan and Homo, in agreement with previous studies (Tuttle, 1969; Thorpe et al. 1999). Other functional group ratios (e.g. wrist flexor to extensor ratio, thenar to intrinsic hand musculature) were found to be in agreement with Tuttle's results as well, despite the dissimilarity in methods used, Gorilla gorilla being included in Pan, and without the inclusion of any bonobos (Tuttle, 1969). The large amount of (wrist and finger) flexors in Pan is most likely due to their involvement in arboreal locomotion. Consequently, the emphasis on flexors may restrict extension of the wrist, favouring knuckle‐walking over palmigrade quadrupedalism during terrestrial locomotion. Additionally, the recruitment of wrist flexors as shock absorbers during knuckle‐walking (Simpson et al. 2018) may further reinforce the prominence of flexor muscles in the forearm of knuckle‐walkers. Another example that may reflect differences in locomotion is found in the proportion of intrinsic hand muscle PCSA. Bonobos, on average, possess a somewhat larger proportion of intrinsic hand muscles compared with humans, although humans fall within the large range of bonobos (Fig. 7C). The difference between bonobos and humans is, however, most pronounced for the relative development of the intermediate hand muscles, which account for on average 13.6% of the total PCSA in bonobos and merely 6.4% in humans (Fig. 7). This, too, may be explained in the context of locomotion, either arboreal in the form of grasping, which is of major importance for vertical climbing and clambering, or terrestrial knuckle walking, where the intermediates might play an important role (Susman & Stern, 1980), although these two are not mutually exclusive.
Differences in forelimb musculature between bonobo and chimpanzee are limited. This has already been indicated in a recent publication by Diogo and colleagues that points to an evolutionary stasis in the Pan clade using soft‐tissue characters to underline the low divergence between chimpanzees and bonobos (Diogo et al. 2017b). One of the three divergent characters in the forelimb musculature described is the different configuration of the intermediate hand muscles in chimpanzees and bonobos. Our dissections indicate that, contrary to the statements of Diogo et al. (2017b), bonobos can have distinct intermetacarpales common to the configuration found in chimpanzees. Rather than invariably presenting the human configuration with four dorsal interossei (fusion of FBP III, V, VI, VIII with the intermetacarpales I–IV) and three palmar interossei (FBP IV, VII, IX), bonobos display all kinds of variations, and these variations can also occur between the left and right hands of one individual. The high variability seen in the organization of the intermediate hand muscles of bonobos suggests that the functional implications are limited and that this trait cannot be used as a divergent character of bonobos.
Bonobo thumb musculature
This study shows that bonobos possess a well‐developed thumb musculature on par with that of humans. While the relative PCSA of the thumb muscles in humans is higher, the estimate of the intra‐articular pressure to which the TMC is subjected is higher in bonobos (and chimpanzees). Moreover, this estimate is likely an underestimation of the actual maximal pressure in the bonobo as (i) the contribution of the FDP I is not accounted for in bonobos, whereas FPL is included in the human pressure estimate, and (ii) in some bonobo specimens, the FCR and/or ECRL also cross the TMC joint and can therefore also generate compressive forces at this joint. Despite the strong thumb musculature of bonobos, they do have a lower level of dexterity compared with modern humans (Kivell, 2015; Bardo et al. 2016; Neufuss et al. 2017). A simple correlation between the force‐generating capacity of the thumb muscles and dexterity does not apply; in addition to size, muscle configuration (and motor control) plays an important role.
One of the more explicit examples of differences in muscle configuration between humans, bonobos and chimpanzees can be found in the diverging morphology of the extrinsic thumb flexor. In chimpanzees, this flexor is present in the form of a vestigial tendon coming from the FDP II tendon that inserts onto the distal phalanx of the thumb, as observed both in our chimpanzee specimen and as reported in the literature (see figure 19 in Tuttle 1969), but it can also be absent (Susman, 1998). In bonobos, however, we see that the tendon of the FDP to digit 1 is well developed, with similar dimensions as the tendons acting on other digits (Fig. 2) and as the human FPL. In humans, the FDP I has differentiated into a separate muscle, the FPL, and its presence has been linked to the unique dexterity of modern humans (Marzke, 1997; Wolfe et al. 2006; Skinner et al. 2015). The muscle is important for precision control and manipulation, and appears to be particularly active during power squeeze grips, rather than during precision grips (Kivell, 2015). Although gibbons also have a distinct FPL (Susman, 1998), it has been posited that the presence of the FPL in modern humans fulfils the specific functional requirements of the thumb to be able to perform a variety of complex functions (Tocheri et al. 2008; Skinner et al. 2015). We believe that the configuration found in bonobos, with a stout tendon to the thumb and a shared muscle belly for FDP I and II, has important functional consequences regarding individual finger control and dexterity.
Functional coupling
Functional coupling between muscles results in a concerted action. The association between thumb and index finger flexion enables bonobos to move digits 1 and 2 independently from digits 3, 4 and 5, which might contribute to differences in grasping capability, particularly in precision gripping, in which the thumb and index finger play a crucial role (Christel et al. 1998). A similar fusion was also observed between EPL and EI in two bonobo specimens, which points to a developmental relation between these neighbouring muscles. A fusion between EPL and EI is also found in some New World monkeys, such as Alouatta fusca and Saguinus geoffroyi, forming a structure referred to as ‘extensor pollicis et indicis longus’ (Aversi‐Ferreira et al. 2010). Such a configuration might lead to joined extension of thumb and index finger. Functional coupling like this may be crucial to executing certain coordinated hand movements, but it also complicates individual digit mobility and thus dexterity. In modern humans, common chimpanzees, and our other bonobo specimens, the EPL inserts exclusively on digit I and is not fused to the EI, resulting in a functional dissociation between the extension of the thumb and the index finger. This individualization is found more often in the more dextrous primates, with humans as a prime example. With the distinct FPL, EPL and EI configurations, we see this in the human thumb especially. It is therefore very likely that the increased amount of functional coupling found in bonobos compared with humans rather than representing a difference in muscle development, plays a major role in the difference in dexterity.
Critical considerations
Our findings are based on detailed dissections of eight bonobo specimens obtained from different European zoos. Although this is the largest series of bonobos that has been dissected so far, it remains a relatively small and heterogeneous sample (age, sex, body size). It is not possible to evaluate the effect of age and sex in the current dataset, but no apparent differences were observed for the subadult specimen (female of 8 years, unfused growth plates) or between the male and female specimens. This is to be expected for a species with a low sexual dimorphism (Coolidge & Shea, 1982; Zihlman & Bolter, 2015). To allow for comparison between specimens of different sizes (total forelimb muscle mass ranges from 500 to 1100 g), we used total forelimb PCSA as a normalizing factor. Additionally, as our specimens originate from various zoos, the effect of captivity on muscle development should not go unremarked. Furthermore, for interspecific comparison we have made use of only one chimpanzee, and one human specimen. These were included as representatives of their species, an indication of how the bonobo relates to its close relatives. Therefore, no significant conclusions on interspecific variations between these species can be made. This has to be kept in mind while interpreting these data. Despite these limitations, we were able to fully document the bonobo forelimb musculature, both qualitatively and quantitatively, using a consistent protocol on a unique series of unembalmed bonobo cadavers. Not only is this research important to generate a general view of the bonobo anatomy, but in combination with in vivo research and behavioural studies, it can be translated to complete form–function relations of the thumb. This will provide important insights into the form–function relation of the thumb in modern humans and aid accurate interpretation of hominin fossil remains.
Conclusions
This study shows that the bonobo forelimb musculature displays a relatively high variability and although the muscles of the hand and thumb are well developed, they show an increased amount of functional coupling compared with humans. It is likely that the strong differentiation and individualization of the hand muscles in humans, rather than relative muscle development, explains the higher dexterity compared with bonobos.
Author contributions
E.E.V. conceived the study; E.E.V., T.vL. and M.J.M.V. further designed the study; J.S. provided the specimens and assisted in data collection; T.vL., M.J.M.V., F.D.K. and E.E.V. performed the dissections; T.vL., M.J.M.V. and E.E.V. analysed the data and wrote the manuscript; all authors reviewed and approved the article. T.vL. and M.J.M.V. are joint first authors on this publication.
Supporting information
Fig. S1. Configuration of intermediate hand muscles of each bonobo specimen.
Fig. S2. Muscle fascicle lengths of the bonobo specimens (n = 5).
Fig. S3. Muscle pennation angles of the bonobo specimens (n = 5).
Table S1. Extrinsic hand muscles: origin, insertion and function.
Table S2. Intrinsic hand muscles: origin, insertion and function.
Table S3. Anatomical dataset of all dissected specimens.
Acknowledgements
The authors thank the Bonobo Morphology Initiative organized by Dr Nauwelaerts and Dr Pereboom from the Centre for Research and Conservation (Royal Zoological Society Antwerp, Belgium) in January 2016 that enabled access to a large part of the bonobo specimens and allowed dissection of these specimens by an international team of anatomists. We are also grateful to the different zoos which provided additional primate specimens: Jean‐Pascal Guéry (La Vallée des Singes, Romagne, France), Christina Geiger (Zoo Frankfurt, Germany), Martina Balz (Wilhelma Zoo, Stuttgart, Germany) and Constanze Mager (Burgers’ Zoo, Arnhem, The Netherlands). Furthermore, we thank Dr Olivier Vanovermeire and Henk Lacaeyse from the Medical Imaging Department, AZ Groeninge (Kortrijk, Belgium) for the CT‐scanning of the specimens. Funding for this project was obtained from KU Leuven (project number: C14/16/082).
References
- Alba DM, Moya‐Sola S, Kohler M (2003) Morphological affinities of the Australopithecus afarensis hand on the basis of manual proportions and relative thumb length. J Hum Evol 44, 225–254. [DOI] [PubMed] [Google Scholar]
- Almécija S, Smaers JB, Jungers WL (2015) The evolution of human and ape hand proportions. Nat Commun 6, 7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aversi‐Ferreira TA, Diogo R, Potau JM, et al. (2010) Comparative anatomical study of the forearm extensor muscles of Cebus libidinosus (Rylands et al., 2000; primates, cebidae), modern humans, and other primates, with comments on primate evolution, phylogeny, and manipulatory behavior. Anat Rec 293, 2056–2070. [DOI] [PubMed] [Google Scholar]
- Bardo A, Borel A, Meunier H, et al. (2016) Behavioral and functional strategies during tool use tasks in bonobos. Am J Phys Anthropol 161, 125–140. [DOI] [PubMed] [Google Scholar]
- Boesch C, Head J, Robbins MM (2009) Complex tool sets for honey extraction among chimpanzees in Loango National Park, Gabon. J Hum Evol 56, 560–569. [DOI] [PubMed] [Google Scholar]
- Breuer T, Ndoundou‐Hockemba M, Fishlock V (2005) First observation of tool use in wild gorillas. PLoS Biol 3, 2041–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth G, Itakura S (1998) Development of precision grips in chimpanzees. Dev Sci 1, 39–43. [Google Scholar]
- Carlson NS, Lowe NK (2006) CenteringPregnancy: a new approach in prenatal care. MCN Am J Matern Child Nurs 31, 218–223. [DOI] [PubMed] [Google Scholar]
- Channon AJ, Günther MM, Crompton RH, et al. (2009) Mechanical constraints on the functional morphology of the gibbon hind limb. J Anat 215, 383–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christel MI, Kitzel S, Niemitz C (1998) How precisely do bonobos (Pan paniscus) grasp small objects? Int J Primatol 19, 165–194. [Google Scholar]
- Colell M, Segarra MD, Pi JS (1995) Hand preferences in chimpanzees (Pan troglodytes), bonobos (Pan paniscus), and orangutans (Pongo pygmaeus) in food‐reaching and other daily activities. Int J Primatol 16, 413–434. [Google Scholar]
- Coolidge HJ, Shea BT (1982) External body dimensions of Pan paniscus and Pan troglodytes chimpanzees. Primates 23, 245–251. [Google Scholar]
- Crast J, Fragaszy D, Hayashi M, et al. (2009) Dynamic in‐hand movements in adult and young juvenile chimpanzees (Pan troglodytes). Am J Phys Anthropol 138, 274–285. [DOI] [PubMed] [Google Scholar]
- Diogo R, Shearer B, Potau JM, et al. (2017a) Photographic and Descriptive Musculoskeletal Atlas of Bonobos, 1st edn Cham, Switzerland: Springer International Publishing. [Google Scholar]
- Diogo R, Molnar JL, Wood B (2017b) Bonobo anatomy reveals stasis and mosaicism in chimpanzee evolution, and supports bonobos as the most appropriate extant model for the common ancestor of chimpanzees and humans. Sci Rep 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feix T, Kivell TL, Pouydebat E, et al. (2015) Estimating thumb‐index finger precision grip and manipulation potential in extant and fossil primates. J R Soc Interface 12, 20150176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragaszy D, Izar P, Visalberghi E, et al. (2004) Wild capuchin monkeys (Cebus libidinosus) use anvils and stone pounding tools. Am J Primatol 64, 359–366. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Thompson J (2008) The Bonobos: Behavior, Ecology, and Conservation. New York, USA: Springer. [Google Scholar]
- Gumert MD, Hoong LK, Malaivijitnond S (2011) Sex differences in the stone tool‐use behavior of a wild population of Burmese long‐tailed macaques (Macaca fascicularis aurea). Am J Primatol 73, 1239–1249. [DOI] [PubMed] [Google Scholar]
- Ingmanson EJ (1998) Tool‐using behaviour in wild Pan paniscus: social and ecological considerations In: Reaching Into Thought: The Minds of the Great Apes (eds Russon AE, Bard KA, Parker ST.), p. 464 Cambridge: Cambridge University Press. [Google Scholar]
- Inouye SE (1992) Ontogeny and allometry of African ape manual rays. J Hum Evol 23, 107–138. [Google Scholar]
- Jordan C (1982) Object manipulation and tool‐use in captive pygmy chimpanzees (Pan paniscus). J Hum Evol 11, 35–39. [Google Scholar]
- Kivell TL (2015) Evidence in hand: recent discoveries and the early evolution of human manual manipulation. Philos Trans R Soc B Biol Sci 370, 20150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlwilm M, de Manuel M, Nater A, et al. (2016) Evolution and demography of the great apes. Curr Opin Genet Dev 41, 124–129. [DOI] [PubMed] [Google Scholar]
- Lesnik JJ, Sanz CM, Morgan DB (2015) The interdigital brace and other grips for termite nest perforation by chimpanzees of the Goualougo Triangle, Republic of Congo. Am J Phys Anthropol 157, 252–259. [DOI] [PubMed] [Google Scholar]
- Marzke MW (1997) Precision grips, hand morphology, and tools. Am J Phys Anthropol 102, 91–110. [DOI] [PubMed] [Google Scholar]
- Marzke MW, Marzke RF (2000) Evolution of the human hand: approaches to acquiring, analysing and interpreting the anatomical evidence. J Anat 197, 121–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzke MW, Wullstein KL, Viegas SF (1992) Evolution of the power (‘squeeze’) grip and its morphological correlates in hominids. Am J Phys Anthropol 89, 283–298. [DOI] [PubMed] [Google Scholar]
- Medler S (2002) Comparative trends in shortening velocity and force production in skeletal muscles. Am J Physiol Regul Integr Comp Physiol 283, R368–R378. [DOI] [PubMed] [Google Scholar]
- Miller R (1952) The musculature of Pan paniscus . Am J Anat 91, 183–232. [DOI] [PubMed] [Google Scholar]
- Myatt JP, Crompton RH, Payne‐Davis RC, et al. (2012) Functional adaptations in the forelimb muscles of non‐human great apes. J Anat 220, 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufuss J, Humle T, Cremaschi A, et al. (2017) Nut‐cracking behaviour in wild‐born, rehabilitated bonobos (Pan paniscus): a comprehensive study of hand‐preference, hand grips and efficiency. Am J Primatol 79, 1–16. [DOI] [PubMed] [Google Scholar]
- Oishi M, Ogihara N, Endo H, et al. (2009) Dimensions of forelimb muscles in orangutans and chimpanzees. J Anat 215, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouydebat E, Gorce P, Coppens Y, et al. (2009) Biomechanical study of grasping according to the volume of the object: human versus non‐human primates. J Biomech 42, 266–272. [DOI] [PubMed] [Google Scholar]
- Prado‐Martinez J (2013) Great ape genetic diversity and population history. Nature 499, 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüfer K, Munch K, Hellmann I, et al. (2012) The bonobo genome compared with the chimpanzee and human genomes. Nature 486, 527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda‐Carreras I, Frise E, et al. (2012) Fiji: an open‐source platform for biological‐image analysis. Nat Methods 9, 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SW, Latimer B, Lovejoy CO (2018) Why do knuckle‐walking African apes knuckle‐walk? Anat Rec 301, 496–514. [DOI] [PubMed] [Google Scholar]
- Skinner MM, Stephens NB, Tsegai ZJ, et al. (2015) Human evolution. Human‐like hand use in Australopithecus africanus . Science 347, 395–399. [DOI] [PubMed] [Google Scholar]
- Spinozzi G, Truppa V, Laganà T (2004) Grasping behavior in tufted capuchin monkeys (Cebus apella): grip types and manual laterality for picking up a small food item. Am J Phys Anthropol 125, 30–41. [DOI] [PubMed] [Google Scholar]
- Susman RL (1998) Hand function and tool behavior in early hominids. J Hum Evol 35, 23–46. [DOI] [PubMed] [Google Scholar]
- Susman RL, Stern JT (1980) EMG of the interosseous and lumbrical muscles in the chimpanzee (Pan troglodytes) hand during locomotion. Am J Anat 157, 389–397. [DOI] [PubMed] [Google Scholar]
- Takeshita H, Walraven V (1996) A comparative study of the variety and complexity of object manipulation in captive chimpanzees (Pan troglodytes) and bonobos (Pan paniscus). Primates 37, 423–441. [Google Scholar]
- Thorpe SKS, Crompton RH, Günther MM, et al. (1999) Dimensions and moment arms of the hind‐ and forelimb muscles of common chimpanzees (Pan troglodytes). Am J Phys Anthropol 110, 179–199. [DOI] [PubMed] [Google Scholar]
- Tocheri MW, Orr CM, Jacofsky MC, et al. (2008) The evolutionary history of the hominin hand since the last common ancestor of Pan and Homo. J Anat 212, 544–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth N, Schick KD, Savage‐Rumbaugh ES, et al. (1993) Pan the tool‐maker: investigations into the stone tool‐making and tool‐using capabilities of a bonobo (Pan paniscus). J Archaeol Sci 20, 81–91. [Google Scholar]
- Tuttle RH (1969) Quantitative and functional studies on the hands of the anthropoidea. I. The Hominoidea. J Morphol 128, 309–363. [DOI] [PubMed] [Google Scholar]
- Vereecke EE, D'Aout K, Payne R, et al. (2005) Functional analysis of the foot and ankle myology of gibbons and bonobos. J Anat 206, 453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visalberghi E, Addessi E, Truppa V, et al. (2009) Selection of effective stone tools by wild bearded capuchin monkeys. Curr Biol 19, 213–217. [DOI] [PubMed] [Google Scholar]
- Wells JB (1965) Comparison of mechanical properties between slow and fast mammalian muscles. J Physiol 178, 252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EM, Gordon AD, Richmond BG (2010) Upper limb kinematics and the role of the wrist during stone tool production. Am J Phys Anthropol 143, 134–145. [DOI] [PubMed] [Google Scholar]
- Wolfe SW, Crisco JJ, Orr CM, et al. (2006) The dart‐throwing motion of the wrist. Is it unique to humans? J Hand Surg 32, 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NM, Capellini TD, Roach NT, et al. (2015) Fossil hominin shoulders support an African ape‐like last common ancestor of humans and chimpanzees. Proc Natl Acad Sci 112, 11829–11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihlman AL, Bolter DR (2015) Body composition in Pan paniscus compared with Homo sapiens has implications for changes during human evolution. Proc Natl Acad Sci U S A 112, 7466–7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Configuration of intermediate hand muscles of each bonobo specimen.
Fig. S2. Muscle fascicle lengths of the bonobo specimens (n = 5).
Fig. S3. Muscle pennation angles of the bonobo specimens (n = 5).
Table S1. Extrinsic hand muscles: origin, insertion and function.
Table S2. Intrinsic hand muscles: origin, insertion and function.
Table S3. Anatomical dataset of all dissected specimens.


