Abstract
Accumulating evidence suggests a relationship between the gut microbiota and the development of obesity, indicating the potential of probiotics as a therapeutic approach. Bifidobacterium breve B-3 has been shown to exert anti-obesity effects in high-fat diet-induced obese mice. In the present study, the anti-obesity effects of the consumption of B. breve B-3 by healthy pre-obese (25 ≤ BMI < 30) adults were investigated in a randomized, double-blind, placebo-controlled trial (trial registration: UMIN-CTR No. 000023919; preregistered on September 2, 2016). Eighty participants were randomized to receive placebo or B. breve B-3 capsules (2 × 1010 CFU/day) daily for 12 weeks. The visceral fat area significantly increased at weeks 4 and 8 in the placebo group only; no significant change was observed in the B-3 group. Body fat mass and percent body fat were significantly lower in the B-3 group than in the placebo group at weeks 8 and 12 (p<0.05, ANCOVA adjusted with baseline values). Although no significant differences were observed in blood parameters between the groups, the intake of B. breve B-3 slightly decreased triglyceride levels and improved HDL cholesterol from the baseline. No serious adverse effects were noted in either group. These results suggest that the probiotic strain B. breve B-3 has potential as a functional food ingredient to reduce body fat in healthy pre-obese individuals.
Keywords: Bifidobacterium breve, pre-obese, body fat reduction, randomized controlled trial
INTRODUCTION
Obesity is defined as abnormal or excessive fat accumulation and causes lifestyle-related metabolic disorders such as hypertension, dyslipidemia, and diabetes [1]. Even though the consumption of energy-dense foods and a low energy requirement for physical activity are the main factors leading to obesity, recent evidence indicates that the gut microbiota affects energy homeostasis and fat storage in adipocytes and is closely associated with obesity [2,3,4].
The relationship between the gut microbiota and obesity may be explained by the metabolic endotoxemia hypothesis, which links the gut microbiota to low-grade inflammation and further metabolic disorders [5]. In experimental animals and humans, obesity and metabolic disorders have been associated with an impaired gut barrier, which may lead to the increased translocation of endotoxins, particularly in connection with a Western diet [6,7,8]. Previous studies reported that the Gram-negative bacterial outer membrane component, lipopolysaccharide (LPS), was responsible for the early onset of inflammation, insulin resistance, obesity, and diabetes [9]. Sun et al. showed that the levels of the LPS-binding protein (LBP), a marker of subclinical endotoxemia, were significantly higher in overweight/obese individuals than in normal weight individuals (geometric mean 27.6 vs. 10.0 µg/ml; p<0.001) in addition to high-sensitivity C-reactive protein (hCRP), IL-6, adiponectin, and leptin levels [10]. The feeding of bifidobacteria reversed metabolic endotoxemia and improved gut integrity and associated metabolic changes in mice [5, 11]. Therefore, the administration of bifidobacteria may be a promising strategy for the prevention of metabolic endotoxemia and treatment of metabolic diseases.
The relationship between the composition of the microbiota and obesity has been reported as a higher ratio of Firmicutes to Bacteroidetes in obese subjects than in lean subjects [12]. A recent study on obese children obtained findings that were consistent with a similar gut microbiota composition [13]; however, not all human trials showed similar findings [14]. In addition, the fecal abundance of the genus Bifidobacterium was found to be significantly lower in obese subjects than in lean subjects [15,16,17]. A meta-analysis also indicated that the gut microbiota of an obese group was consistently and significantly depleted of bifidobacteria [18]. These lines of evidence imply that this probiotic bifidobacterial strain is the best candidate to exert anti-obesity effects [18]. However, the anti-obesogenic effects of Bifidobacterium spp. are known to be species- or even strain-specific [14], and the magnitude of the effects depend on human conditions. Thus, the anti-obesity effects of probiotics need to be clarified in clinical trials using a specific strain in well-defined subjects.
Bifidobacterium breve B-3 is one of the promising anti-obesogenic strains. We previously reported that the administration of this strain suppressed body weight gain and visceral fat deposition in a dose-dependent manner and improved serum levels of total cholesterol, glucose, and insulin in diet-induced obese mice [19]. In humans, the daily intake of capsules containing a lyophilized powder of B. breve B-3 at a dose of 50 billion colony-forming units (CFU)/day reduced body fat mass [20]; however, some subjects were receiving medication for diabetes, hypertension, or hyperlipidemia.
In the present study, a randomized, double-blinded, placebo-controlled trial was conducted to investigate whether the consumption of capsules containing lyophilized live B. breve B-3 powder exerts reducing effects on abdominal adiposity and affects the body composition in healthy pre-obese subjects without diabetes, hypertension, hyperlipidemia, or any other disorders. We also evaluated blood parameters associated with obesity, such as blood lipids, blood sugar, liver function, and inflammation markers (hCRP and LBP), in order to assess the effects of B. breve B-3 on metabolic function and evaluate its safety.
MATERIALS AND METHODS
Participants
This randomized, double-blind, placebo-controlled study was performed between September 2016 and December 2016 at the Shinagawa Season Terrace Health Care Clinic in Tokyo, Japan. Prior to the study, participants were recruited by the contract research organization KSO Co., Ltd. (Tokyo, Japan) via the internet and were subjected to body composition measurements, blood tests, urinary tests, and background questionnaires for screening. Eligible participants were healthy pre-obese adults. The inclusion criteria were between 20 and 64 years and a body mass index (BMI) of 25 or more but less than 30 kg/m2 (25 ≤ BMI < 30 kg/m2), which was considered to be pre-obese according to the criteria of WHO [21]. The exclusion criteria were as follows: taking medication for gastrointestinal diseases, diabetes, hypertension, or dyslipidemia that may affect outcome measures; a history of receiving treatment for serious disorders (i.e., cardiovascular diseases, liver diseases, digestive disorders, endocrine metabolic diseases, and sleep apnea syndrome); serious allergies to medicine and food; pregnancy or lactation; heavy smoking; heavy drinking; irregular lifestyles; and judgment as inappropriate for the trial, such as due to disease, by the physician in charge. Participants provided written informed consent before study initiation.
Procedure
After the assessment for eligibility, participants were randomized to receive B-3 or placebo capsules. A computer-generated randomization scheme was used that utilized random permuted blocks of 4 participants stratified by gender, age, BMI, and the abdominal fat area to ensure the balanced assignment of participants to each group. Randomization was performed by a concealed study coordinator. Participants and observers were both blinded to group allocation. Double-blinding was achieved by labeling the test foods with an identification number only. The blinding code was retained by the concealed study coordinator and opened after completion of the study. The study period consisted of a 2-week run-in period followed by a 12-week ingestion period. Participants were subjected to physical measurements and a medical interview 0, 4, 8, and 12 weeks after the initiation of the intervention. At 0 and 12 weeks, blood and urine tests were conducted. Participants recorded the intake of the test foods, dietary constituents, medication, drinking, lifestyle changes, and exercise in a diary. The diary was assessed by a dietitian at every 4-week visit. Participants were instructed not to change their regular lifestyles, diet, or exercise and were prohibited from consuming foods, beverages, and supplements that lower body fat, blood glucose, or cholesterol as well as fermented food and supplements with probiotic bacteria because these foods may affect the body composition.
The primary outcome measure was the change in the abdominal visceral fat area at week 12. Secondary outcome measures included changes in the body composition (body weight, fat mass, fat percentage, BMI, and subcutaneous fat area), waist circumference, blood parameters (blood lipids, blood glucose, liver function, and inflammatory parameters), and the composition of the gut microbiota. The safety of test food ingestion was evaluated by blood tests, urine tests, and a diagnostic interview by a physician.
A minimum sample size of 80 participants was calculated as necessary to detect a 10 ± 15 cm2 (mean ± SD) change in the visceral fat area between the groups at week 12 at a 5% significance level with 80% power. A dropout rate of 10% was expected.
The study protocol was reviewed and approved by the Review Board of the Ethical Committee of Nihonbashi Cardiology Clinic (Tokyo, Japan). The protocol was in accordance with the Declaration of Helsinki. This clinical trial was preregistered in the UMIN Clinical Trials Registry on September 2, 2016 (UMIN 000023919).
Probiotic capsules
The test food was capsules containing the lyophilized powder of live B. breve B-3 (10 billion CFU per capsule). The placebo capsule mainly contained corn starch and did not have any bifidobacteria. Participants were instructed to ingest 2 capsules (20 billion CFU/day for the B-3 group) within 30 minutes of a meal daily for 12 weeks. The B-3 capsule was confirmed to contain the required CFU during the intervention period and to be identical in terms of appearance, smell, and weight to the placebo capsule. Sixty B-3 or placebo capsules were distributed at each visit at 0, 4, 8, and 12 weeks, and the capsules remaining at each visit were recovered from the participants.
Measurements and body composition
The abdominal visceral fat area and subcutaneous fat area were measured with a DUALSCAN HDS-2000 dual bioelectrical impedance analysis (BIA) instrument (Omron Healthcare, Kyoto, Japan). The principles of intra-abdominal fat area measurements obtained with the dual BIA instrument were described in detail previously [22, 23]. In order to avoid interference caused by hydration status in results, fasting morning measurements were performed with minimal consumption of water. Measurements were performed in triplicate, and the mean values were utilized for analyses. Body weight, fat mass, and fat percentage were measured by the bioelectrical impedance method using an InBody 770 medical-grade body composition analyzer (InBody Japan, Tokyo, Japan). BMI was also automatically calculated by the InBody 770 using a manually measured value for height. Waist circumference was measured at the midpoint between the lower margin of the rib and the top of the iliac crest.
Blood and urine tests
Blood and urine tests were performed at 0 and 12 weeks. The following blood parameters were analyzed by LSI Medience Corporation (Tokyo, Japan): total cholesterol, low-density lipoprotein cholesterol (LDL-cholesterol), high-density lipoprotein cholesterol (HDL-cholesterol), triglycerides, glycoalbumin, fasting blood glucose, hemoglobin A1c (HbA1c), insulin, aspartate transaminase (AST), alanine aminotransaminase (ALT), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GTP), hCRP, LBP, white blood cells (WBCs), red blood cells (RBCs), hemoglobin, hematocrit, platelets, total protein, albumin, lactate dehydrogenase (LDH), bilirubin, creatine kinase (CK), urea nitrogen, creatinine, uric acid, sodium, chloride, potassium, calcium, inorganic phosphorus, magnesium, and serum iron. HOMA-IR was calculated as insulin (μU/ml) × fasting blood glucose (mg/dl) / 405. The following urine parameters were also analyzed by LSI Medience Corporation: urine protein, sugar, urobilinogen, bilirubin, ketone bodies, and occult blood.
Dietary constituents
Participants maintained a detailed record of their diet for 3 consecutive days before each visit: at weeks 0, 4, 8, and 12. A dietitian analyzed dietary records to assess the intake of total energy, protein, fat, carbohydrate, and dietary fiber using Excel Eiyokun ver. 8.0 (Kenpakusha Co., Ltd., Tokyo, Japan).
Statistical analysis
SAS software version 9.3 (SAS Institute, Cary, NC, USA) was used for statistical analyses on body composition and blood parameters. Variables that did not follow a normal distribution were analyzed after a natural log transformation. For clarity, the values in the tables are presented according to the original scale. Baseline characteristics were compared with Fisher’s exact test for categorical variables and a two-sample T-test for continuous variables. Regarding primary and secondary outcomes, a comparison was performed between experimental groups using an analysis of covariance (ANCOVA) adjusted for the baseline (week 0). Within-group changes at each time point (weeks 4, 8, and 12) from the baseline (week 0) were analyzed using a one-sample t-test. A p-value <0.05 was considered to be significant. The magnitude of effects were calculated and expressed as η2=SS effect / SS total, and the effect size was considered to be small (η2=0.01), medium (η2=0.06), or large (η2=0.14) [24].
RESULTS
Participant baseline characteristics and dietary constituents
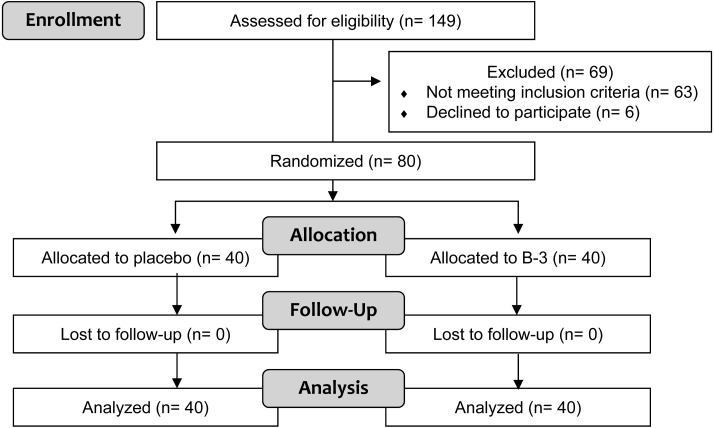
Following assessment for eligibility of 149 volunteers who expressed interest in participating in the study for eligibility, 80 healthy pre-obese participants were allocated to be 2 groups, the placebo and B-3 groups, as shown in the participant flow diagram (Fig. 1). No participants dropped out after the initiation of the study, and all participants completed the protocol. Participant backgrounds, including body weight, BMI, the abdominal fat area, body fat, and muscle mass, were not significantly different between the placebo and B-3 groups at baseline (Table 1). No significant differences were observed in dietary constituents between the groups, and no significant changes in total energy, protein intake, fat intake, carbohydrate intake, or dietary fiber intake were observed at weeks 0, 4, 8, or 12 from the baseline (Table 2). Intake rates were 99.9% (the placebo group) and 99.8% (the B-3 group). There were no contraventions with respect to compliance.
Fig. 1.
Participant flow diagram.
Table 1. Baseline information of participants.
| Placebo (N=40) | B-3 (N=40) | ||
|---|---|---|---|
| Sex (male, female) | 37, 3 | 37, 3 | |
| Age | 45.6 ± 8.5 | 45.4 ± 9.8 | |
| Anthropometric measures | |||
| Height (cm) | 170.4 ± 7.0 | 170.5 ± 5.9 | |
| Body weight (kg) | 80.3 ± 7.1 | 80.6 ± 6.8 | |
| BMI (kg/m2) | 27.6 ± 1.2 | 27.7 ± 1.2 | |
| Waist circumference (cm) | 96.2 ± 5.6 | 96.6 ± 4.9 | |
| Abdominal fat areas | |||
| Visceral fat area (cm2) | 106.1 ± 20.7 | 106.3 ± 20.3 | |
| Subcutaneous fat area (cm2) | 233.4 ± 40.9 | 233.2 ± 36.2 | |
| Whole body fat area (cm2) | 339.6 ± 54.2 | 339.6 ± 47.1 | |
| Body composition | |||
| Body fat mass (kg) | 23.6 ± 4.2 | 22.9 ± 3.1 | |
| Body fat (%) | 29.5 ± 5.4 | 28.5 ± 3.9 | |
| Muscle mass (kg) | 53.6 ± 6.8 | 54.5 ± 6.2 | |
Continuous variables are shown as means ± SD.
Table 2. Daily nutrition intake in the B-3-supplemented and placebo groups during the intervention.
| 0 week |
4 weeks |
8 weeks |
12 weeks |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Energy (kcal/day) | Placebo | 2,028 | 450 | 2,009 | 564 | 1,959 | 580 | 1,922 | 518 |
| B-3 | 1,940 | 404 | 1,959 | 427 | 1,966 | 466 | 1,885 | 492 | |
| Protein (g/day) | Placebo | 76.3 | 20.6 | 74.3 | 23.2 | 72.8 | 22.4 | 73.7 | 22.0 |
| B-3 | 70.0 | 15.4 | 70.8 | 16.2 | 73.1 | 15.4 | 68.3 | 14.8 | |
| Fat (g/day) | Placebo | 68.5 | 18.7 | 68.1 | 23.7 | 66.0 | 24.3 | 65.2 | 21.9 |
| B-3 | 65.0 | 19.7 | 69.8 | 21.9 | 68.0 | 26.1 | 62.1 | 21.2 | |
| Carbohydrates (g/day) | Placebo | 260.7 | 68.6 | 258.7 | 70.9 | 253.2 | 76.6 | 243.7 | 69.2 |
| B-3 | 255.4 | 54.5 | 248.0 | 52.2 | 251.0 | 55.5 | 250.8 | 66.0 | |
| Fiber (g/day) | Placebo | 10.6 | 3.6 | 10.5 | 3.7 | 10.7 | 3.5 | 10.5 | 3.7 |
| B-3 | 10.5 | 2.6 | 10.7 | 3.3 | 10.4 | 3.4 | 11.1 | 4.4 | |
Body composition
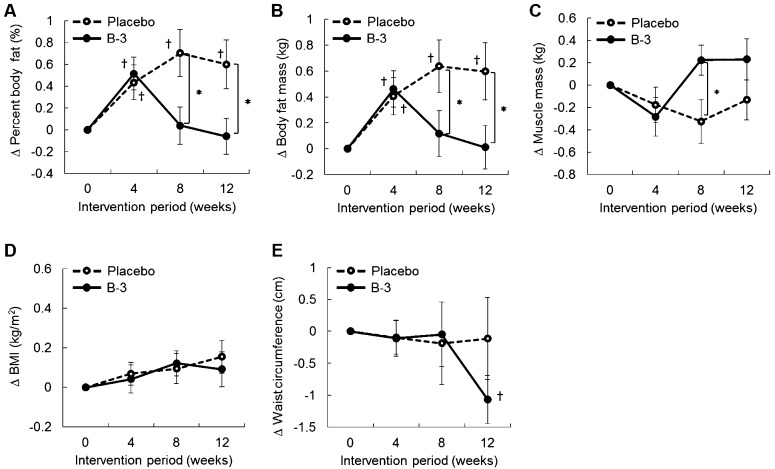
Percent body fat significantly increased from the baseline in the placebo group from weeks 4 to 12 (Fig. 2A). Percent body fat in the B-3 group was significantly lower at weeks 8 and 12 than in the placebo group, and the effect size was medium (p=0.02, η2=0.074, for week 8; p=0.02, η2=0.068, for week 12) (Fig. 2A). Body fat mass in the B-3 group was significantly lower at weeks 8 and 12 than in the placebo group, and the effect size was small to medium (p=0.04, η2=0.053, for week 8; p=0.03, η2=0.060, for week 12) (Fig. 2B). Muscle mass in the placebo group slightly decreased during the intervention period, while that in the B-3 group started to increase after week 8. Muscle mass showed a significant between-group difference at week 8 with a medium effect size (p=0.03, η2=0.062) (Fig. 2C). Waist circumference in the B-3 group was significantly lower at week 12 than at the baseline (p<0.01) (Fig. 2E). No significant change was observed in BMI (Fig. 2D).
Fig. 2.
Transition of changes in (A) percent body fat, (B) body fat mass, (C) muscle mass, (D) BMI, and (E) waist circumference during the intervention period.
The data are presented as means ± SE.
*Significant difference between the placebo and B-3 groups analyzed by ANCOVA adjusted for the baseline (p<0.05).
†Significant within-group difference from the baseline analyzed by a one-sample t-test (p<0.05).
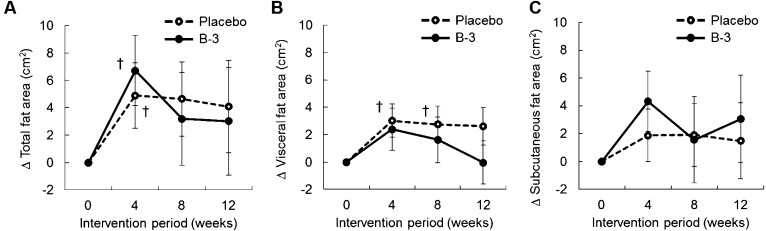
Changes in the abdominal fat area are shown in Fig. 3. The total fat area was significantly increased from the baseline in both groups at week 4 (Fig. 3A). The visceral fat area in the placebo group was significantly increased from the baseline at weeks 4 and 8 (p=0.02 and p=0.045, respectively) (Fig. 3B). No significant change was observed for visceral fat mass in the B-3 group or for the subcutaneous fat area in both groups (Fig. 3B, 3C).
Fig. 3.
Transition of changes in (A) total fat area, (B) visceral fat area, and (C) subcutaneous fat area during the intervention period.
The data are presented as means ± SE.
†Significant within-group difference from the baseline analyzed by a one-sample t-test (p<0.05).
In a stratified analysis with mildly obese participants with a visceral fat area that was less than 100 cm2 (placebo, n=15; B-3, n=19) at week 12, percent body fat was slightly lower in the B-3 group (−0.65%, p=0.08) than in the placebo group, whereas waist circumference was significantly lower in the B-3 group (−3.3 cm, p=0.01) than in the placebo group.
Blood parameters for efficacy
The transitions of blood parameters from weeks 0 to 12 are summarized in Table 3. No significant differences were observed in blood parameters between the groups. Significant differences from the baseline were observed for the values of glycoalbumin (12.7 to 12.9%, p<0.01) in the placebo group and AST (24.8 to 25.5 U/l, p=0.049) and ALT (34.3 to 36.4 U/l, p=0.03) in the B-3 group. HDL-cholesterol slightly increased (46.4 to 48.2 mg/dl, p=0.058) from the baseline in the B-3 group, and triglycerides slightly decreased (176.8 to 150.4 mg/dl, p=0.053) from the baseline in the B-3 group. We did not observe significant changes in the inflammation markers, hCRP and LBP.
Table 3. Changed values of blood parameter from the baseline.
| Placebo | B-3 | ||
|---|---|---|---|
| Blood lipids | |||
| Δ | Total cholesterol (mg/dl) | −2.1 ± 18.7 | 3.0 ± 15.4 |
| Δ | LDL-cholesterol (mg/dl) | −2.1 ± 16.1 | 3.5 ± 16.6 |
| Δ | HDL-cholesterol (mg/dl) | 1.8 ± 6.7 | 1.8 ± 5.7† |
| Δ | Triglyceride (mg/dl)‡ | −0.8 ± 63.4 | −26.3 ± 83.5† |
| Blood sugar | |||
| Δ | Glycoalbumin (%) | 0.2 ± 0.3* | 0.1 ± 0.4 |
| Δ | Fasting blood glucose (mg/dl) | 0.9 ± 5.9 | 0.5 ± 7.8 |
| Δ | HbA1c (%) | −0.02 ± 0.15 | −0.03 ± 0.16 |
| Δ | HOMA-IR | −0.22 ± 2.34 | −0.18 ± 1.58 |
| Liver function | |||
| Δ | AST (U/l)‡ | −1.0 ± 11.7 | 0.6 ± 7.8* |
| Δ | ALT (U/l)‡ | 0.0 ± 14.2 | 2.0 ± 15.3* |
| Δ | ALP (U/l) | 5.0 ± 20.2 | −2.2 ± 16.7 |
| Δ | γ-GTP (U/l)‡ | −0.9 ± 19.2 | −1.2 ± 11.5 |
| Inflammation markers | |||
| Δ | hCRP (mg/dl)‡ | 0.076 ± 0.361 | −0.012 ± 0.186 |
| Δ | LBP (μg/ml)‡ | 0.21 ± 3.79 | −0.33 ± 1.67 |
Values are means ± SD.
Within-group comparisons: *p<0.05; †p<0.1.
‡Analysis was performed after logarithmic transformation of the values.
Blood and urine tests for the safety evaluation
During the trial period of this study, no serious adverse events were observed in participants. Among 8 mild adverse events, such as the common cold, headache, or muscular pain, 6 were observed in the placebo group, and 2 common colds were noted in the B-3 group. The symptoms observed in the B-3 group were considered by the physician to be unrelated to test food ingestion.
The results of the blood, biochemical, and urine tests are shown in Table 4. No significant changes were observed in blood parameters including WBCs, RBCs, hemoglobin, and platelets. In the B-3 group, albumin, uric acid, and calcium significantly decreased from the baseline, while creatinine and chloride significantly increased from the baseline. In contrast, in the placebo group, creatinine and uric acid significantly decreased, while chloride significantly increased. However, these changes in blood parameters were within normal ranges.
Table 4. Changed values of hematology, biochemical, and urine parameters from the baseline.
| Placebo | B-3 | |
|---|---|---|
| Δ WBC (/ml) | –128 ± 694 | –5 ± 1158 |
| Δ RBC (× 104/ml) | 2.9 ± 15.8 | 4.0 ± 15.4 |
| Δ Hemoglobin (g/dl) | 0.0 ± 0.6 | 0.0 ± 0.4 |
| Δ Hematocrit (%) | –0.4 ± 2.4 | –0.5 ± 2.3 |
| Δ Platelets (× 104/ml) | 0.3 ± 2.0 | 0.4 ± 1.6 |
| Δ LDH (U/l) | –2.4 ± 17.8 | –0.6 ± 13.2 |
| Δ Bilirubin (mg/dl)‡ | 0.0 ± 0.24 | 0.1 ± 0.30 |
| Δ CK (U/l)‡ | –4.3 ± 53.6 | –56.0 ± 325.6 |
| Δ Total protein (g/dl) | 0.0 ± 0.23 | 0.0 ± 0.24 |
| Δ Albumin (g/dl) | –0.1 ± 0.18 | –0.1 ± 0.16* |
| Δ Urea nitrogen (mg/dl) | 0.2 ± 2.19 | 0.1 ± 2.79 |
| Δ Creatinine (mg/dl) | –0.03 ± 0.07* | –0.02 ± 0.07 |
| Δ Uric acid (mg/dl) | –0.4 ± 0.72* | –0.3 ± 0.55* |
| Δ Sodium (eEq/l) | 0.2 ± 1.6 | 0.4 ± 1.5 |
| Δ Chloride (mEq/l) | –0.2 ± 2.2 | 0.8 ± 1.8* |
| Δ Potassium (mEq/l) | 0.1 ± 0.32* | 0.0 ± 0.29 |
| Δ Calcium (mg/dl) | 0.1 ± 0.29 | –0.1 ± 0.25* |
| Δ Phosphorus (mg/dl) | 0.0 ± 0.39 | –0.1 ± 0.35 |
| Δ Magnesium (mg/dl) | 0.1 ± 0.12* | 0.0 ± 0.12 |
| Δ Serum Iron (mg/dl) | –5.8 ± 35.0 | 9.3 ± 35.9 |
| Δ Urine pH‡ | –0.1 ± 0.61 | –0.1 ± 0.68 |
| Δ Urine specific gravity | –0.001 ± 0.008 | 0.000 ± 0.009 |
Values are means ± SD.
Within-group comparisons: *p<0.05.
‡Analysis was performed after logarithmic transformation of the values.
DISCUSSION
The composition of the gut microbiota differs between lean and obese subjects and is recognized as a therapeutic target of obesity. In a meta-analysis of randomized controlled trials to examine the effects of probiotic supplementation on the body composition in overweight (BMI 25–29.9 kg/m2) and obese (BMI ≥30 kg/m2) subjects, five studies reported changes in percent body fat, and the pooled estimate showed that percent body fat was significantly lower in the intervention groups (−0.60%) than in the control groups, with low heterogeneity between the studies [25]. These five studies, including our previous study, showed a 0.7-kg reduction in fat mass with the intake of B. breve B-3 at a dose of 50 billion CFU/day for 12 weeks [20]. In the present study, the intake of B. breve B-3 at a dose of 20 billion CFU/day for 12 weeks significantly reduced percent body fat and body fat mass in pre-obese participants. The differences in percent body fat and body fat mass between the groups were 0.7% and 0.6 kg, respectively (Fig. 2A, 2B), which are consistent with the findings of the abovementioned meta-analysis [25] and our previous study. We confirmed that nutrient intake did not significantly differ between the groups during the study period because an excessive food intake is a major factor leading to obesity. These results suggest that B. breve B-3 is a promising probiotic strain for body fat reductions in healthy pre-obese individuals. To the best of our knowledge, B. breve B-3 is the only bifidobacterial strain to have induced significant reductions in body fat in a randomized controlled trial without lifestyle changes, such as calorie restrictions, or any combination with other pre/probiotics.
Regarding body fat in the abdominal cavity, visceral adipose tissue is anatomically, physiologically, and prognostically different from subcutaneous adipose tissue [26]. Since excess visceral fat shows a stronger correlation with metabolic disorders than excess subcutaneous fat, we investigated changes in visceral fat area as a primary outcome. However, significant differences in the visceral fat area were not observed between the groups in the present study despite significant body fat reductions. Several clinical trials have reported the positive effects of probiotic strains on visceral fat reductions. The consumption of fermented milk containing Lactobacillus gasseri SBT2055 for 12 weeks significantly reduced the visceral fat area in adults with visceral fat accumulation (81.2–178.5 cm2) [27]. Furthermore, the consumption of fermented milk containing B. animalis ssp. lactis GCL2505 for 12 weeks was also recently reported to significantly reduce the visceral fat area in healthy subjects with BMIs ranging between 23 and 30 [28]. Visceral fat deposition in the participants of the present study (Table 1) was less than that in previous studies (127.3 ± 24.6 cm2 in the SBT2055 group in the study of Kadooka et al., 133.4 ± 29.6 cm2 in the GCL2505 group in the study of Takahashi et al.) [27, 28]. Smaller visceral fat deposits may be one reason for the undetectable change in the visceral fat area in the present study.
In the present study, triglyceride levels slightly decreased, while HDL-cholesterol slightly improved from the baseline in the B-3 group (Table 3). Some probiotics have been reported to affect serum lipid levels in humans [29]. In diet-induced obese mice, B. breve B-3 supplementation improved the serum levels of total cholesterol [19]. A DNA microarray analysis also revealed that the administration of B. breve B-3 upregulated the gene expression of pathways involved in lipid metabolism including the Cyp4a family and Cyp7A1a in the liver of a mouse model of diet-induced obesity [30]. Cyp4a family members play roles in the induction of fatty acid oxidation or energy expenditure in the liver [31], and Cyp7A1a is the rate limiting enzyme in the synthesis of bile salt from cholesterol [32]. Transgenic mice overexpressing Cyp7a1 in the liver were found to be resistant to high-fat diet-induced obesity, fatty liver, and insulin resistance [33]. Hepatic Cyp7A1 was shown to be regulated by the intestinal farnesoid X receptor (FXR) in combination with fibroblast growth factor 15 (FGF15) [34, 35]. Sayin et al. demonstrated that the gut microbiota regulated the expression of FGF15 in the ileum and Cyp7A1 in the liver via FXR-dependent mechanisms and altered the size and composition of the total bile acid pool in their rederivation study on germ-free FXR-deficient mice [36]. The effects of probiotic administration were investigated and found to induce hepatic bile acid synthesis via downregulation of the FXR-FGF15 axis in mice [37]. Based on these findings, the induction of fatty acid oxidation, energy expenditure, and bile acid synthesis via upregulation of Cyp7A1 expression in the liver appear to explain improved lipid metabolism and body fat reductions caused by the consumption of B. breve B-3.
Blood inflammation markers were not affected by the consumption of B. breve B-3 in the present study. The levels of hCRP and LBP were lower in the B-3 group (mean values of 0.13 mg/l for hCRP and 7.5 µg/ml for LBP) than in previous studies. Sun et al. demonstrated that hCRP differed with the obese status and that the blood level of LBP correlated with the odds ratio for metabolic syndrome, type 2 diabetes, and insulin resistance (mean values of 1.38 mg/l for hCRP and 27.6 µg/ml for LBP) [10]. The mean value of hCRP was 7-fold higher (0.98 µg/ml) in our previous study in which we observed a significant reduction in hCRP with the consumption of B. breve B-3 at a dose of 50 billion CFU/day for 12 weeks [20]. These findings suggest that the inflammatory condition related to metabolic endotoxemia was mild in the healthy population. Although BMI is an indicator of the amount of body fat, it does not differentiate adiposity types, function, or metabolic implications [38]. Circulating endotoxin levels need to be added to inclusion criteria for assessing the effects of probiotics on endotoxemia and chronic inflammation accompanied by obesity.
We observed significant between-group difference in muscle mass at week 8 (Fig. 2C). Although recent studies have suggested the presence of a “gut-muscle axis,” whereby gut microbiota may act as the mediator of the effects of nutrition on muscle cells [39, 40], little is known about the clinical evidence [39, 41]. In the present study, we instructed participants not to change their regular lifestyle or exercise. However, we did not assess measurable parameters for the physical activity of participants. Since physical activity and exercise are critical factors for increases and decreases of muscle mass, further study is needed to explore the effect of B. breve B-3 on muscle mass through stringent evaluation of physical activity.
We investigated the safety of B. breve B-3 consumption as well as its efficacy on the body composition in the present study. Probiotic microorganisms are commonly from the genera Lactobacillus and Bifidobacterium and are generally considered to be safe [42]. In the present study, no adverse effects attributed to the administration of B. breve B-3 were observed, and the changes noted (cf. blood and urinary parameters) were within normal ranges. Thus, the present study suggests that there were no safety concerns with respect to intake of B. breve B-3.
There were some limitations in the present study. Variations in each participant’s physical activity may have influenced energy expenditure and body composition. We instructed participants to not change their regular lifestyle or exercise and asked them to record lifestyle changes and exercise in a diary. However, we did not investigate changes in physical activity using measurable parameters such as steps as measured with a pedometer. Thus, variations in each participant’s physical activity may have affected the results of the present study. Furthermore, the results of the present study cannot be extrapolated to patients, such as those with severe adiposity (BMI >30), diabetes, hyperlipidemia, and childhood obesity, because the participants were healthy pre-obese adults. Although the anti-obesity effects of probiotics in fermented milk were close to baseline at the 4-week follow-up visit in a previous study [43], we did not conduct a follow-up analysis in the present study. Therefore, a follow-up analysis is needed in order to evaluate the persistence of effectiveness.
In conclusion, the present study demonstrated that the daily consumption of B. breve B-3 at a dose of 20 billion CFU/day for 12 weeks exerted significant reducing effects on body fat in healthy pre-obese adults without any adverse effects. Therefore, a strain of B. breve B-3 is safe for a healthy population and may be useful for preventing body fat accumulation and related metabolic disorders in pre-obese individuals.
Acknowledgments
KSO Co., Ltd. supported the work of this clinical trial as a contract research organization.
REFERENCES
- 1.Guilherme A, Virbasius JV, Puri V, Czech MP. 2008. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbaum M, Knight R, Leibel RL. 2015. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab 26: 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101: 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tremaroli V, Bäckhed F. 2012. Functional interactions between the gut microbiota and host metabolism. Nature 489: 242–249. [DOI] [PubMed] [Google Scholar]
- 5.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. 2007. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50: 2374–2383. [DOI] [PubMed] [Google Scholar]
- 6.Stenman LK, Lehtinen MJ, Meland N, Christensen JE, Yeung N, Saarinen MT, Courtney M, Burcelin R, Lähdeaho ML, Linros J, Apter D, Scheinin M, Kloster Smerud H, Rissanen A, Lahtinen S. 2016. Probiotic with or without fiber controls body fat mass, associated with serum zonulin, in overweight and obese adults-randomized controlled trial. EBioMedicine 13: 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kallio KA, Hätönen KA, Lehto M, Salomaa V, Männistö S, Pussinen PJ. 2015. Endotoxemia, nutrition, and cardiometabolic disorders. Acta Diabetol 52: 395–404. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. 2014. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol 14: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. 2007. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772. [DOI] [PubMed] [Google Scholar]
- 10.Sun L, Yu Z, Ye X, Zou S, Li H, Yu D, Wu H, Chen Y, Dore J, Clément K, Hu FB, Lin X. 2010. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care 33: 1925–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Xiao G, Yao Y, Guo S, Lu K, Sheng Z. 2006. The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma 61: 650–657. [DOI] [PubMed] [Google Scholar]
- 12.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- 13.Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, Berry D. 2017. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ Microbiol 19: 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woting A, Blaut M. 2016. The intestinal microbiota in metabolic disease. Nutrients 8: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. 2010. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 18: 190–195. [DOI] [PubMed] [Google Scholar]
- 16.Kalliomäki M, Collado MC, Salminen S, Isolauri E. 2008. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr 87: 534–538. [DOI] [PubMed] [Google Scholar]
- 17.Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, Martí-Romero M, Lopez RM, Florido J, Campoy C, Sanz Y. 2010. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr 104: 83–92. [DOI] [PubMed] [Google Scholar]
- 18.Angelakis E, Armougom F, Million M, Raoult D. 2012. The relationship between gut microbiota and weight gain in humans. Future Microbiol 7: 91–109. [DOI] [PubMed] [Google Scholar]
- 19.Kondo S, Xiao JZ, Satoh T, Odamaki T, Takahashi S, Sugahara H, Yaeshima T, Iwatsuki K, Kamei A, Abe K. 2010. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci Biotechnol Biochem 74: 1656–1661. [DOI] [PubMed] [Google Scholar]
- 20.Minami J, Kondo S, Yanagisawa N, Odamaki T, Xiao JZ, Abe F, Nakajima S, Hamamoto Y, Saitoh S, Shimoda T. 2015. Oral administration of Bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomised controlled trial. J Nutr Sci 4: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Expert Consultation WHO. WHO Expert Consultation2004. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363: 157–163. [DOI] [PubMed] [Google Scholar]
- 22.Ida M, Hirata M, Odori S, Mori E, Kondo E, Fujikura J, Kusakabe T, Ebihara K, Hosoda K, Nakao K. 2013. Early changes of abdominal adiposity detected with weekly dual bioelectrical impedance analysis during calorie restriction. Obesity (Silver Spring) 21: E350–E353. [DOI] [PubMed] [Google Scholar]
- 23.Takeoka A, Tayama J, Yamasaki H, Kobayashi M, Ogawa S, Saigo T, Kawano H, Abiru N, Hayashida M, Maeda T, Shirabe S. 2016. Intra-abdominal fat accumulation is a hypertension risk factor in young adulthood: a cross-sectional study. Medicine (Baltimore) 95: e5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. 1988. Statistical power analysis for the behavioral sciences, 2nd ed. Lawrence Erlbaum, Hillsdale. [Google Scholar]
- 25.Borgeraas H, Johnson LK, Skattebu J, Hertel JK, Hjelmesaeth J. 2018. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. Obes Rev 19: 219–232. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim MM. 2010. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11: 11–18. [DOI] [PubMed] [Google Scholar]
- 27.Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T. 2010. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 64: 636–643. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi S, Anzawa D, Takami K, Ishizuka A, Mawatari T, Kamikado K, Sugimura H, Nishijima T. 2016. Effect of Bifidobacterium animalis ssp. lactis GCL2505 on visceral fat accumulation in healthy Japanese adults: a randomized controlled trial. Biosci Microbiota Food Health 35: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira DI, Gibson GR. 2002. Effects of consumption of probiotics and prebiotics on serum lipid levels in humans. Crit Rev Biochem Mol Biol 37: 259–281. [DOI] [PubMed] [Google Scholar]
- 30.Kondo S, Kamei A, Xiao JZ, Iwatsuki K, Abe K. 2013. Bifidobacterium breve B-3 exerts metabolic syndrome-suppressing effects in the liver of diet-induced obese mice: a DNA microarray analysis. Benef Microbes 4: 247–251. [DOI] [PubMed] [Google Scholar]
- 31.Hardwick JP. 2008. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol 75: 2263–2275. [DOI] [PubMed] [Google Scholar]
- 32.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. 2000. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6: 517–526. [DOI] [PubMed] [Google Scholar]
- 33.Li T, Owsley E, Matozel M, Hsu P, Novak CM, Chiang JY. 2010. Transgenic expression of cholesterol 7α-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology 52: 678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. 2005. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217–225. [DOI] [PubMed] [Google Scholar]
- 35.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. 2007. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res 48: 2664–2672. [DOI] [PubMed] [Google Scholar]
- 36.Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. 2013. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17: 225–235. [DOI] [PubMed] [Google Scholar]
- 37.Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. 2014. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Reports 7: 12–18. [DOI] [PubMed] [Google Scholar]
- 38.Goyal A, Nimmakayala KR, Zonszein J. 2014. Is there a paradox in obesity? Cardiol Rev 22: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ticinesi A, Lauretani F, Milani C, Nouvenne A, Tana C, Del Rio D, Maggio M, Ventura M, Meschi T. 2017. Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: is there a gut-muscle axis? Nutrients 9: E1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grosicki GJ, Fielding RA, Lustgarten MS. 2018. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: biological basis for a gut-muscle axis. Calcif Tissue Int 102: 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coqueiro AY, de Oliveira Garcia AB, Rogero MM, Tirapegui J. 2017. Probiotic supplementation in sports and physical exercise: Does it present any ergogenic effect? Nutr Health 23: 239–249. [DOI] [PubMed] [Google Scholar]
- 42.Donohue DC. 2006. Safety of probiotics. Asia Pac J Clin Nutr 15: 563–569. [PubMed] [Google Scholar]
- 43.Kadooka Y, Sato M, Ogawa A, Miyoshi M, Uenishi H, Ogawa H, Ikuyama K, Kagoshima M, Tsuchida T. 2013. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br J Nutr 110: 1696–1703. [DOI] [PubMed] [Google Scholar]