Abstract
Nanoparticles (NPs) are widely used in various domestic products and their usage is constantly increasing which in turn can raise several environmental health issues. Like other abiotic stresses, nanomaterials also affect the growth of crop plants. Solanum melongena is a common vegetable crop grown in the tropics and subtropics regions with medicinal properties. In this study, S. melongena was analyzed for its response to three commercially important metallic nanoparticles, namely NiO, CuO, and ZnO, at four different concentrations (100, 250, 500 and 1000 mg/L). The growth of the eggplant seedlings was suppressed by all the NPs in a concentration-dependent manner and among them, NiO was shown to be more toxic as it suppressed the root and shoot growth effectively. Total chlorophyll contents were decreased in the NP-treated plants compared to control plants. Significant changes were found in the secondary metabolites such as anthocyanins, total phenolic and total flavonoid contents in the NP-treated plants. A dose-dependent increase in the reactive oxygen species (ROS) generation was noticed in the NP-treated plants which are evidenced by the 4-nitro blue tetrazolium chloride (NBT) and 3,3′-diamiobenzidine (DAB) histochemical staining. The DNA damage imposed by the NP in the seedlings of eggplants may be due to the elevated ROS and MDA (malondialdehyde) production. NiO NP was found to be more toxic comparable to CuO and ZnO NPs in the present study. Apart from the toxic effects, nanoparticles also showed profound effects on the production of important secondary metabolites such as phenolics and flavonoid compounds.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1386-9) contains supplementary material, which is available to authorized users.
Keywords: Nanoparticles, Eggplant, Reactive oxygen species, Malondialdehyde, Anthocyanin, Phenolics, Flavonoids
Introduction
Nanometer-sized metal particles have attracted intensive attention due to their wide application in biomedical, agriculture, electronics, telecommunications, and renewable energy (Ma et al. 2015). Their unique features and properties allow them to be used in various fields such as reproductive science, disease prevention, conversion of agricultural and food wastes to energy through nanobioprocessing, chemical sensors and plant treatment using various nanocides (Nair et al. 2010). On the other hand, this expanding field does raise concerns about the toxicity and environmental impact of nanomaterials. Nanofertilizers can be used effectively on crops for controlled release of chemicals, thereby enhancing target activity and plant growth. Atmospheric deposition, agricultural application, rain erosion, and surface runoff are the main pathways through which nanoparticles (NPs) enter the soil. Their weak migration ability gradually will result in the accumulation of NPs in soil with the advance in time. This could result in changes in the soil-based food crop quality and yield (Priester et al. 2012).
The effect of NPs on plants depends on the plant species, seed size, growth stage, growth medium and the nanoparticle-coating material (Yang et al. 2017). Nanoparticles have been found to exert profound changes on the physiological indices in plants, namely the germination percentage, root elongation, biomass and leaf number (Lee et al. 2010). Nanoparticles are found to change the content of amino acids, fatty acids, non reducing sugars and phenolics in plants (Rico et al. 2014). Production of plant hormones can be influenced by NPs (Yang et al. 2017). Rice seedlings, when exposed to carbon nanotubes, demonstrated decreased phytohormone concentrations (Hao et al. 2016). Studies on TiO2 NPs in cucumber leaves showed the increased content of total chlorophyll and catalase (CAT) and decreased ascorbate peroxidase (APX) content in leaves (Servin et al. 2013). Rare earth oxide NPs (Gd2O3, La2O3, CeO2 and Yb2O3) when added to roots at high concentrations was found to exert detrimental effects on plant growth in radish, tomato, cabbage, cucumber, lettuce, rape, wheat, and corn (Ma et al. 2010; López-Moreno et al. 2010). Nickel oxide (NiO) NP has been reported to induce cellular oxidative stress by a strong increase in reactive oxygen species (ROS) formation (Oukarroum et al. 2009). NiO NP treatment enhanced the activities of antioxidant enzymes, namely CAT, superoxide dismutase (SOD), and glutathione (GSH) in tomato (Faisal et al. 2013). Magaye et al. (2012) reported that NiO NPs can easily be transported into biological systems and hence can induce both cytotoxic and genotoxic effects. Zinc (Zn) is an essential micronutrient crucial for plant development. It is an essential component of a majority of plant enzymes and proteins and forms a part of structural ions in transcription factors (Broadley et al. 2007). Zinc in higher doses is toxic to plants (Broadley et al. 2007). Zinc nanoparticles exhibit biological activities such as antibacterial, antifungal, antioxidant (Santhoshkumar et al. 2017), anti-diabetic, anti-inflammatory, wound healing (Agarwal et al. 2017), anti-corrosive and UV filtering properties. They have potential application in drug delivery and diagnosis of diseases (Santhoshkumar et al. 2017). The photocatalytic activity of zinc oxide (ZnO) NPs promotes the level of ROS production under irradiation with energy at/or above its band gap energy and induces phytotoxicity (Ma et al. 2013). Oryza sativa when exposed to ZnO NPs showed a reduction in the leaf surface area (Salah et al. 2015). Phytotoxicity studies with ZnO NPs on Lolium perenne (rye-grass) show that concentrated ZnO NPs absorbed on the root surface and in the rhizosphere solution caused potential effects on the plant’s growth (Lin and Xing 2008). The potent antibiotic activity of nano-sized copper oxides (CuO) has found wide application in nanobiocide products available in the markets (De Rosa et al. 2010; Nair et al. 2010). CuO NP can cross plant cell walls and cell membranes, thereby distributing themselves around nucleus and organelles, forming agglomerates within the root cells (Yuan et al. 2016). Detrimental effects of nano-sized CuO on plants include symptoms such as growth inhibition (Perreault et al. 2014a), modified antioxidant enzyme activity (Kim et al. 2012; Dimkpa et al. 2012), metabolic disturbances (Hong et al. 2015), macromolecular damages (Shaw and Hossain 2013), photosynthesis interferences (Perreault et al. 2014b), decreased chlorophyll content (Shi et al. 2011, 2014) and induced DNA lesions (Atha et al. 2012). The toxicogenomic effects of CuO have been attributed to the Cu2+ ions released from CuO NPs (Tang et al. 2016). Studies in zucchini and maize showed that CuO NPs did not affect the germination but suppressed root elongation (Stampoulis et al. 2009; Wang et al. 2012). Yasmeen et al. (2015) have reported that wheat (Triticum aestivum) seeds when soaked in copper NPs and inoculated in distilled water resulted in increased shoot growth but with severe reduction in seedling vigor. CuO NPs can induce DNA damage by the accumulation of oxidatively modified, mutagenic DNA lesions (7,8-dihydro-8-oxoguanine; 2,6-diamino-4-hydroxy-5-formamidopyrimidine; 4,6-diamino-5-formamidopyrimidine) (Areeba et al. 2016). CuO NPs, when exposed to wheat plants, inhibited their growth and changed the structure of the roots (Dimkpa et al. 2012; Tang et al. 2016). CuO NPs were found to significantly reduce the germination rate and biomass of rice seeds, fresh weights and root length of Arabidopsis seedlings (Shaw and Hossain 2013).
In this study, the most economically viable vegetable crop Eggplant (Solanum melongena) was exposed to different concentrations of various metallic nanoparticles such as NiO, CuO and ZnO NP to investigate their growth stimulative and the toxic effects in eggplant. Growth parameters, chlorophyll and anthocyanin levels, were determined in response to NP treatments. Furthermore, antioxidant potential, ROS generation, and genotoxicity were investigated in eggplants with response to nanoparticle treatment. The influence of NPs on the contents of the total phenolics and flavonoids was also examined.
Materials and methods
Seed treatment and data observation
Surface sterilization of S. melongena seeds was done using 70% ethanol and 0.1% mercuric chloride as described previously by Ray et al. (2011). The seeds were rinsed well with distilled water after each treatment to remove traces of ethanol and mercuric chloride. Metal oxide nanoparticles such as ZnO (18 nm, 99.95%), CuO (25–55 nm, 99%) and NiO NPs (10–20 nm, 99%) were obtained from US research Nanomaterials. Four different concentrations (100, 250, 500 and 1000 mg/L) of nanoparticles were added aseptically to culture vessels lined with sterile filter paper. The seedling germination was inhibited by all the respective bulk materials such as CuCl2, ZnSO4, NiSO4 at > 150 mg/L concentration. However, except NiO NP, no significant growth suppression was found in the CuO and ZnO NP treatment up to 250 mg/L. Therefore, we proceeded further to study the impact of metal oxide nanoparticles at various higher concentrations. Around 20–30 seeds were inoculated in each concentration of nanoparticles and kept for 15 days, at 25 °C under a 16/8-h photoperiod. Similar experiments with distilled water were conducted as a control. After 15 days, seedlings were harvested. Seedling growth in terms of shoots and root length was recorded using a ruler.
Total chlorophyll estimation
The total chlorophyll content of NP (NiO, CuO, and ZnO)-treated and control seedlings of eggplants was estimated as described previously by Ma et al. (2013). Using a scalpel, the harvested fresh seedlings (50 mg) were cut into small bits into eppendorfs. To each Eppendorf, 95% (v/v) ethanol was added until the samples were soaked completely. This was left undisturbed for 3 days under dark conditions at 4 °C. The resulting supernatant was recorded at 664.2 and 648.6 nm. The following formula was used for total chlorophyll estimation, total chlorophyll = Chl a + Chl b, Chl a = 13.36 A664.2 − 5.19 A648.6, Chl b = 27.43 A648.6 − 8.12 A664.2. Total chlorophyll content was expressed as milligram per gram per fresh weight (FW).
Determination of malondialdehyde (MDA)
The procedure of Heath and Packer (1968) was followed for the measurement of malondialdehyde (MDA) content in seedlings using the thiobarbituric acid (TBA) test. Approximately 100 mg of NP-treated and control seedling tissues was ground to a fine powder using liquid nitrogen. The samples were homogenized and extracted with 1 mL of 1% (w/v) trichloroacetic acid (TCA). Centrifugation was done at 13,000×g for 15 min and 0.5 mL of the resultant extract was added to 1 mL of 20% (w/v) TCA and 1 mL of 0.5% (w/v) TBA solution. The extract was heated at 95 °C for 30 min followed by centrifugation at 10,000×g for 10 min. The resultant supernatant absorbance was recorded at 532 and 600 nm. The amount of MDA was calculated using the formula C¼ = D/EL. The content of MDA was expressed as micromoles units per g/FW.
Determination of anthocyanin content
Anthocyanin concentration was determined as described previously by Baskar et al. (2015). Fifty micrograms of NP-treated and untreated S. melongena seedlings was powdered with liquid nitrogen. The powdered samples were mixed with 1 mL of 1% (v/v) HCl in methanol and incubated in the dark at 4 °C overnight. In this extract, 1 ml of 500 µL chloroform and 500 µL distilled H2O were added and centrifuged at 13,000×g for 2 min at 4 °C, and the absorbances of the supernatant were recorded at 530 and 657 nm by UV–Vis spectrophotometer. Anthocyanin quantity was determined using A530 − 1/4 A657 formula and the results were presented as milligram per grams FW.
Antioxidant capacity
The antioxidant capacity of nanoparticle-treated and control samples was determined by diphenyl-2-picriylhydrazil (DPPH) radical scavenging assay using the method described by Brand-Williams et al. (1995). Total phenolic contents (TPC) were determined by Folin–Ciocalteu spectroscopic method using gallic acid as standard and the results were represented as mg gallic acid equivalent (GAE) per gram of sample (mg GAE/g). Total flavonoid content (TFC) estimation was done following the calorimetric method of Bao et al. (2005) with catechin as standard. The results of TFC were expressed as mg catechin equivalent (CE) per gram (mg CE/g).
In vivo ROS detection
The roots of eggplant seedlings from NP-treated (NiO, CuO and ZnO) and control samples were cut with a scalpel followed by nitro blue tetrazolium chloride (NBT) and 3,3′-diaminobenzidine (DAB) histochemical staining (Kumar et al. 2014) for the qualitative detection of ROS generation. Staining was performed for 2 h duration with 0.5 mg/mL NBT solution and 8 h duration with 2 mg/mL DAB solution. Decolourisation was performed using boiling ethanol for 2 h after DAB staining. The stained root samples of NP treated and control were placed on a white sheet and the images were taken using a digital camera (Canon, Japan).
DNA extraction and electrophoresis
DNA fragmentation assay was performed to detect the apoptotic DNA cleavage in the eggplant seedlings exposed to different concentrations of NiO, CuO and ZnO nanoparticles. Approximately 100 mg of NP-treated and control seedling tissues was ground to a fine powder with a mortar and pestle. Cetyl trimethyl ammonium bromide (CTAB) method was employed to isolate DNA from the NP-treated and control samples (Rogers and Bendich 1989). The ground samples were treated with CTAB buffer and incubated at 65 °C for 20 min. This was followed by centrifugation and the addition of phenol:chloroform:isoamyl alcohol (25:24:1). After centrifugation, ice-cold isopropanol was added to precipitate the DNA. Finally, DNA was dissolved in TE buffer after ethanol wash. The extracted genomic DNA (5 µg) was run on an agarose gel stained with ethidium bromide and visualized under UV transilluminator.
Statistical analysis
All samples were analyzed in triplicates. Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (P < 0.05) with the help of SPSS ver. 17 statistical package program. The values are expressed as means of triplicate analysis of the samples (n = 3) ± SD.
Results and discussion
Nanoparticles play a profound role in influencing plant growth and development. They have been found to impact several factors such as seed germination, biomass production, shoot growth, root growth, physiological and biochemical activities (Siddiqi and Husen 2017). Hence the present work focused on the phytotoxicity of three important metal oxide nanoparticles, such as NiO, CuO and ZnO NPs, recording their enhancive and inhibitive effects on S. melongena seedlings. This edible fruit is a good source of dietary fiber, vitamin B1, and copper and is widely used in the cuisine of many countries.
Effects of metal oxide NP on the growth characteristics of eggplant seedlings
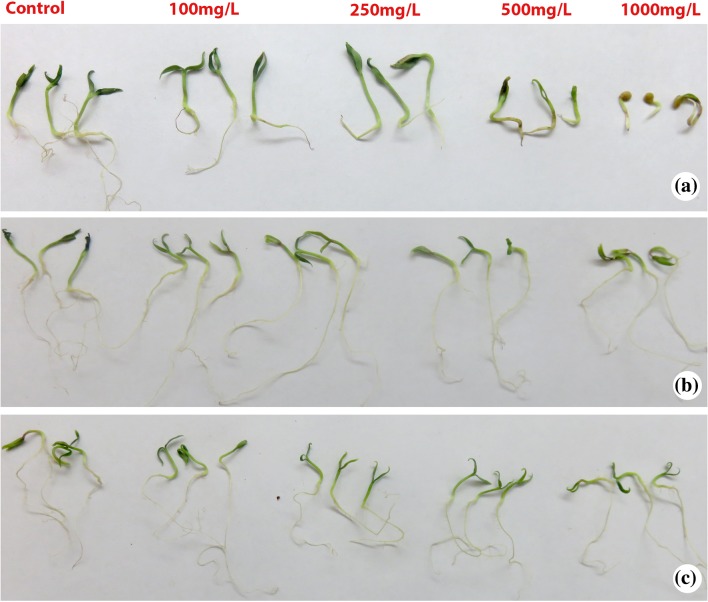
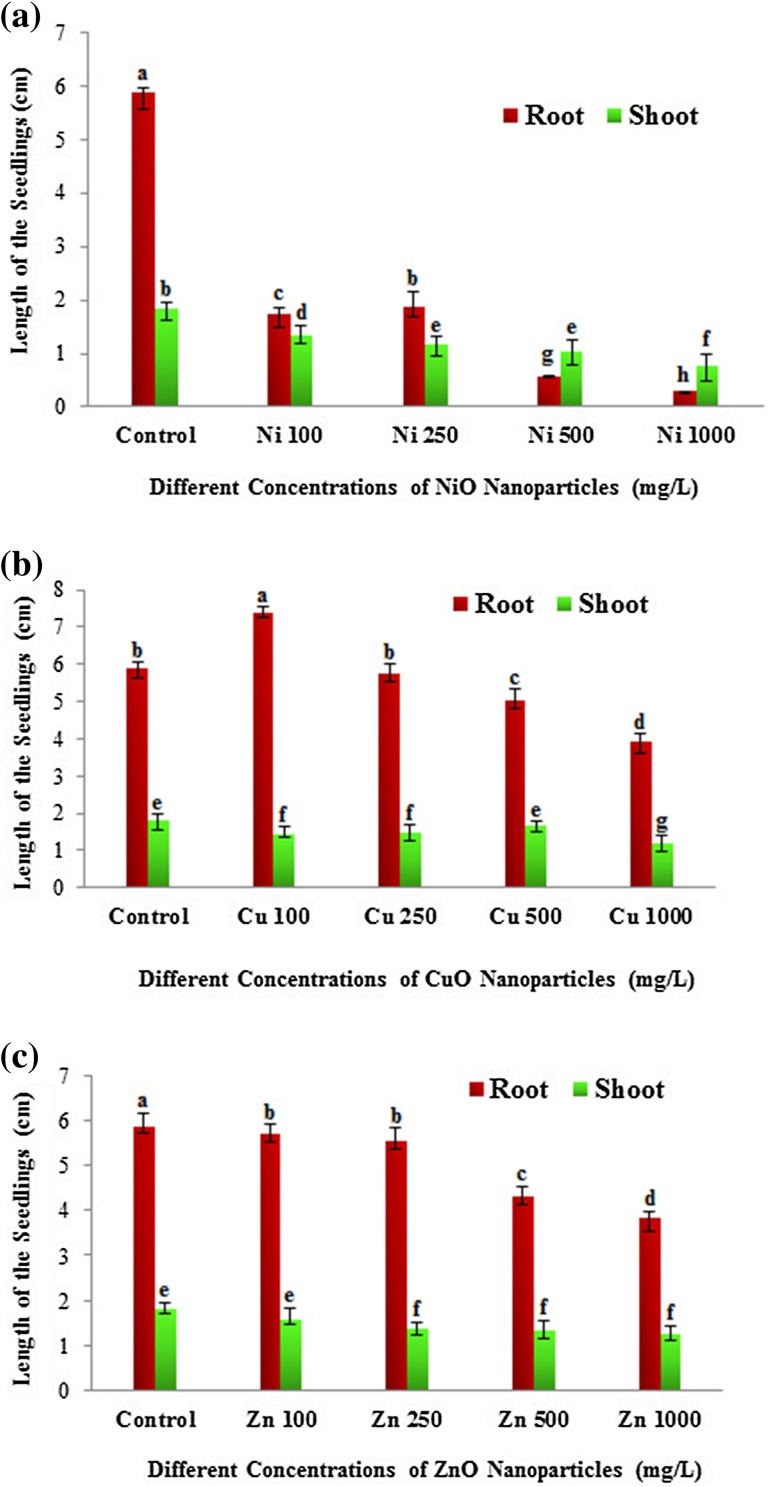
The eggplant seedlings were treated with NiO, CuO and ZnO NPs at different sub-lethal concentrations (100, 250, 500 and 1000 mg/L) to study various physiological and biochemical parameters. The plant root and shoot growth of treated and control eggplant seedlings were evaluated after 2 weeks to investigate the possible growth retardation effects of nanoparticles. There was a significant decline in seed germination and plant growth with elevated concentrations of nanoparticles. Interestingly, at 100 mg/L lower concentration of CuO NP root and shoot length of the eggplant seedlings was higher compared to control plants (Figs. 1, 2). The increased length of the seedlings at this concentration indicates the growth promoting ability of CuO NP at lower concentrations (Fig. 2b). However, at higher concentrations (500 and 1000 mg/L) CuO NP exhibited the repression of shoot and root length (Figs. 1, 2). In accordance with our results, a significant reduction in shoot growth and biomass was observed at higher concentrations (200 and 500 mg/L) of CuO NP in Vigna radiata (Nair et al. 2014). In Brassica juncea, ZnO NP showed dose-dependent growth inhibition and a significant inhibition of root and shoot length was found in higher concentration (1000 and 1500 mg/L) (Rao and Shekhawat 2014). In our study, ZnO NP revealed dose-dependent growth retardation. The root and shoot length of eggplant seedlings were sequentially decreased from lower to higher concentrations of ZnO NP (Fig. 2c). In the case of NiO NP, all the four concentrations showed suppression of root and shoot length with maximum inhibition of seedlings in terms of root and shoot growth at the highest concentration (1000 mg/L) compared to control (Fig. 2a). In agreement with our results, Faisal et al. (2013) found that the root growth profile of tomato was inhibited in a dose-dependent manner. Although all the NP showed growth inhibition, NiO NP displayed significant toxicity to the eggplant seedlings.
Fig. 1.
The figure showing the morphological features of Solanum melongena seedlings treated with different concentrations (100, 250, 500 and 1000 mg/L) of a nickel oxide (NiO) nanoparticles, b copper oxide (CuO) nanoparticles, c zinc oxide (ZnO) nanoparticles
Fig. 2.
Effects of metal oxide nanoparticles (NiO, CuO and ZnO) on the root and shoot growth of the Solanum melongena seedlings. a Total length of root and shoot (cm) of S. melongena at different concentrations (100, 250, 500 and 1000 mg/L) of nickel oxide (NiO), b copper oxide (CuO), c zinc oxide (ZnO) nanoparticles. Each value in the graph is represented as mean ± SD (n = 3). Significantly different values (P < 0.05) showed in different alphabet letters
Effects of metal oxide NP on total chlorophyll content
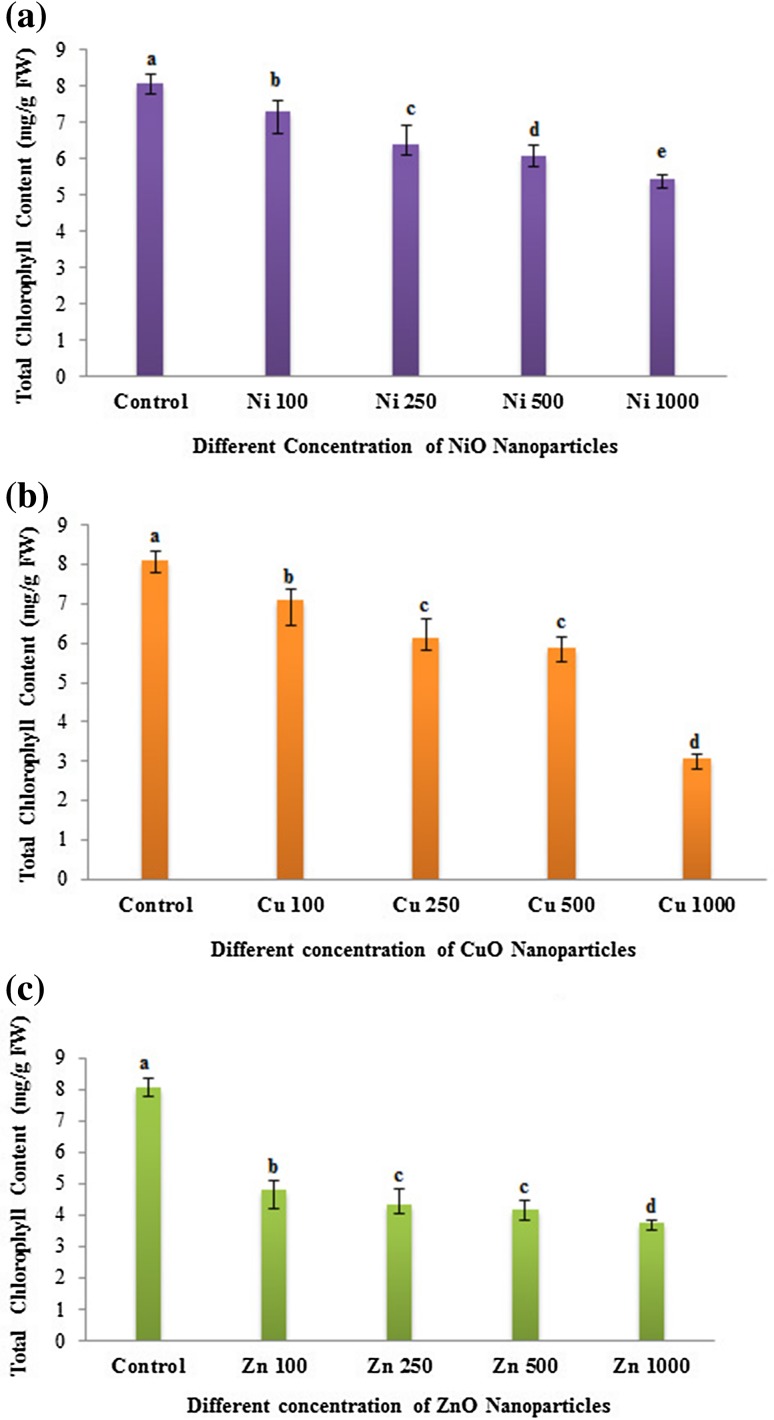
Several previous studies reported that nanoparticle exposure significantly altered the total chlorophyll content and photosynthetic performance in various plants. In our study, the exposure of metal oxide NP (NiO, CuO and ZnO) to eggplant seedlings showed a decline in the total chlorophyll content. The reduction in chlorophyll content varied between the NP treatment and their concentrations (Fig. 3). In the case of NiO and CuO NP treatment, chlorophyll contents exhibited a gradual decrease in a dose-dependent manner compared to control plants (Fig. 3a, b). A strong decline in chlorophyll content was found at the highest concentration (1000 mg/L) in CuO NP treatment. Compared to NiO and CuO NPs, ZnO NP treatment recorded reduction in total chlorophyll contents for all the tested concentrations with a significant decline in the chlorophyll reduction at even the lowest concentration of 100 mg/L (Fig. 3c). In agreement with our results, NiO, CuO and ZnO NPs were shown to decrease the chlorophyll contents and photosynthetic performance in various crops, including aquatic plants in a concentration-dependent manner (Gong et al. 2011; Nair and Chung 2015; Rao and Shekhawat 2014). It has been reported that in ZnO NP-treated Arabidopsis plants, the expression of chlorophyll biosynthesis genes and photosystem-related genes were reduced, exhibiting reduced chlorophyll a and b in treated plants (Wang et al. 2016). Loss of chlorophyll is associated with irreversible damage of the chloroplast structure due to ROS formation impacted by combining of electrons with molecular oxygen (Foyer et al. 1994). Thus, nanoparticle could affect the crop yield by influencing the net rate of photosynthesis caused by reduced chlorophyll contents.
Fig. 3.
Dose-dependent effects of three different nanoparticles (NiO, CuO and ZnO) on total chlorophyll content (mg/g FM) in Solanum melongena. a Total chlorophyll content of S. melongena at different concentrations of nickel oxide (NiO) nanoparticles (100, 250, 500 and 1000 mg/L). b Copper oxide (CuO) nanoparticles (100, 250, 500 and 1000 mg/L). c Zinc oxide (ZnO) nanoparticles (100, 250, 500 and 1000 mg/L). Each value in the graph is represented as mean ± SD (n = 3). Significantly different values (P < 0.05) showed in different alphabet letters
Determination of metal oxide NP-induced malondialdehyde (MDA) production
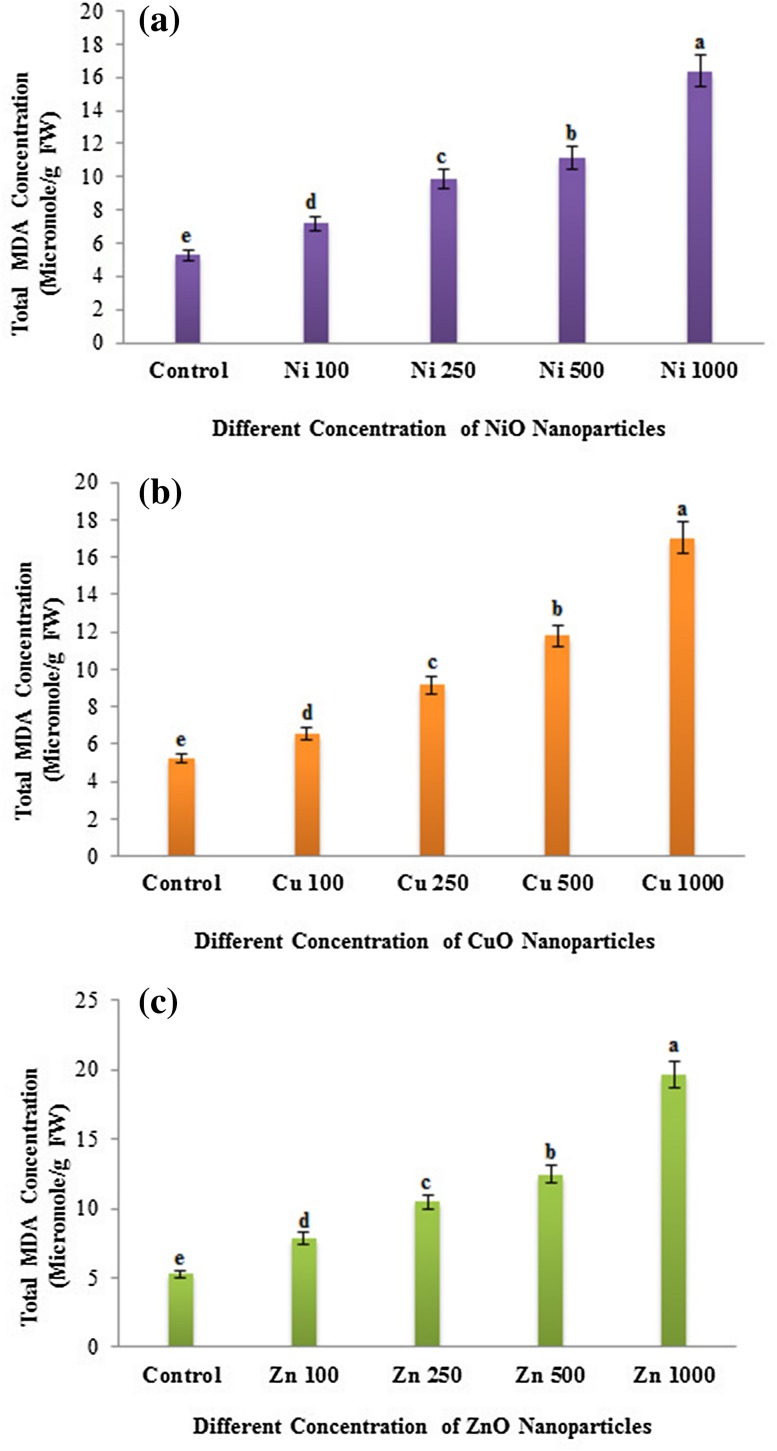
In the present study, we observed the formation of MDA in eggplant seedlings with response to NP treatments. The increased accumulation of lipid peroxide in treated samples compared to control is an indication of enhanced ROS production. A concentration-dependent enhancement in MDA formation was found in all the NP (NiO, CuO and ZnO)-treated eggplant seedlings (Fig. 4). Significant MDA level was found in the ZnO NP-treated seedlings, followed by CuO and NiO NP treatment (Fig. 4). Rao and Shekhawat (2014) reported a dose-dependent enhancement of MDA formation in the root, shoot and leaf tissues of B. juncea plants upon exposure to ZnO NP. Nair and Chung (2015) also reported an increased lipid peroxidation at high concentrations of CuO NP-treated B. juncea seedlings. In our study, a sequential increase in MDA generation was found in the CuO NP treatment from lower to higher concentrations. Thus, all the three metal oxide nanoparticles could induce dose-dependent MDA accumulation in the eggplant seedlings.
Fig. 4.
Dose-dependent effects of three different nanoparticles (NiO, CuO and ZnO) on concentration of MDA (Micromole/g FM) in Solanum melongena. a Concentration of MDA in S. melongena at different concentrations of nickel oxide (NiO) nanoparticles (100, 250, 500 and 1000 mg/L), b copper oxide (CuO) nanoparticles (100, 250, 500 and 1000 mg/L), c zinc oxide (ZnO) nanoparticles (100, 250, 500 and 1000 mg/L). Each value in the graph is represented as mean ± SD (n = 3). Significantly different values (P < 0.05) showed in different alphabet letters
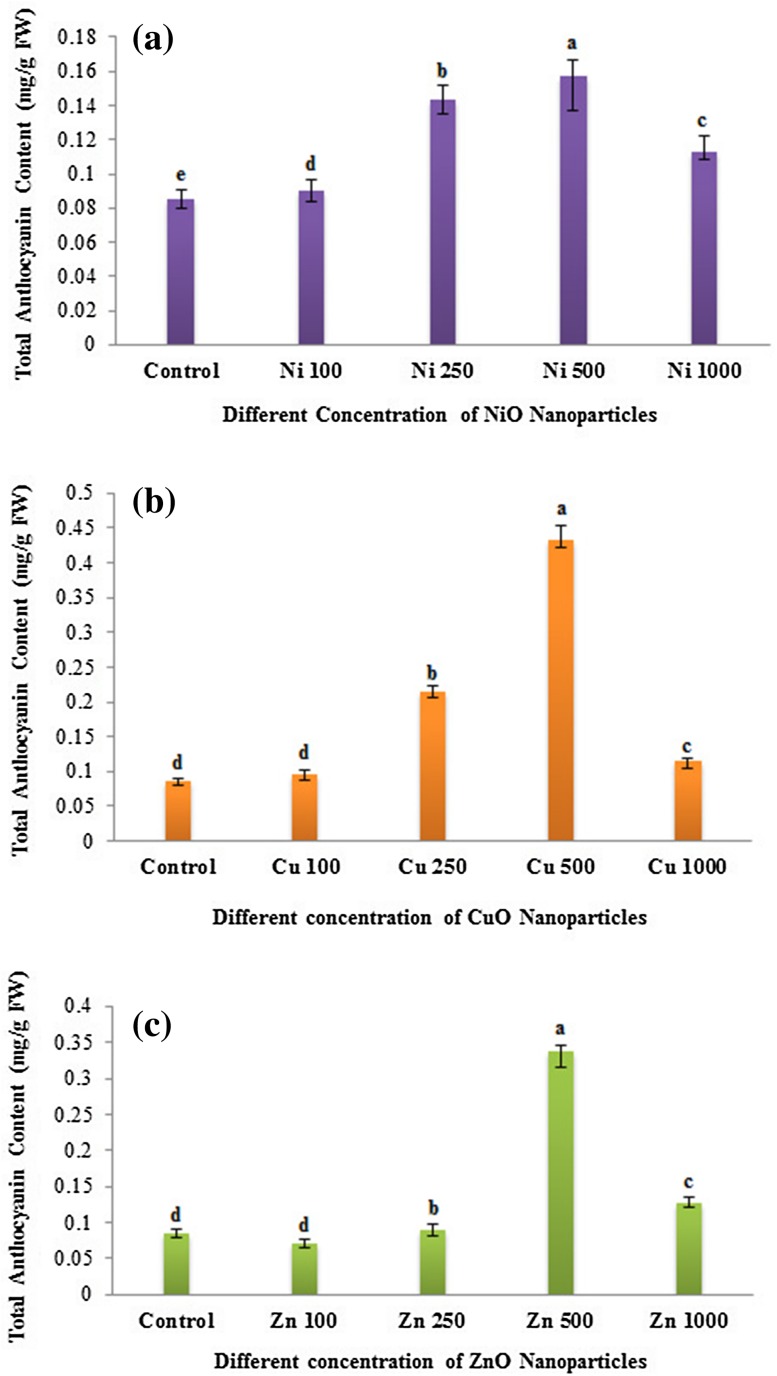
Effects of metal oxide NP on anthocyanin production
Anthocyanins are stress-responsive molecules, protecting plants from damage induced by ROS. Anthocyanin production was measured in both the NP-treated (NiO, CuO and ZnO) and untreated seedlings of eggplants. In all the three NPs used, changes in anthocyanin content were directly proportional to the concentration of NPs used up to 500 mg/L (Fig. 5). However, anthocyanin contents showed a decreased in all the NP-treated eggplant seedlings at 1000 mg/L concentration. Interestingly, CuO NP treatment revealed the accumulation of significant levels of total anthocyanin followed by ZnO and NiO NP treatment (Fig. 5). Similarly, an enhanced anthocyanin production was noticed in the Arabidopsis plants treated with CuO NP (Nair and Chung 2014). However, compared to control, a slightly decreased anthocyanin content was found at lower concentration (100 mg/L) followed by an increase at higher concentration (250 and 500 mg/L) of ZnO NP-treated eggplants. In accordance with our results, the exposure of ZnO NP resulted in a slight decrease of anthocyanin production at 100 ppm compared to control and then increased at 300 and 500 ppm in the potato plants (Raigond et al. 2017). Therefore, the enhanced anthocyanin production on NP exposure (up to 500 mg/L) might serve for the protection against the oxidative stress induced by the nanoparticles (Thiruvengadam et al. 2015).
Fig. 5.
Dose-dependent effects of three different nanoparticles (NiO, CuO and ZnO) on total anthocyanin content (mg/g FM) in Solanum melongena. a Total Anthocyanin content of S. melongena at different concentrations (100, 250, 500 and 1000 mg/L). a Nickel oxide (NiO) nanoparticles, b copper oxide (CuO) nanoparticles, c zinc oxide (ZnO) nanoparticles. Superscript letters with same alphabets are non-significant and those with different alphabets indicates that they are significantly different (P < 0.05) analyzed by Duncan’s multiple range test
Effects of metal oxide NP on antioxidant capacity in eggplants
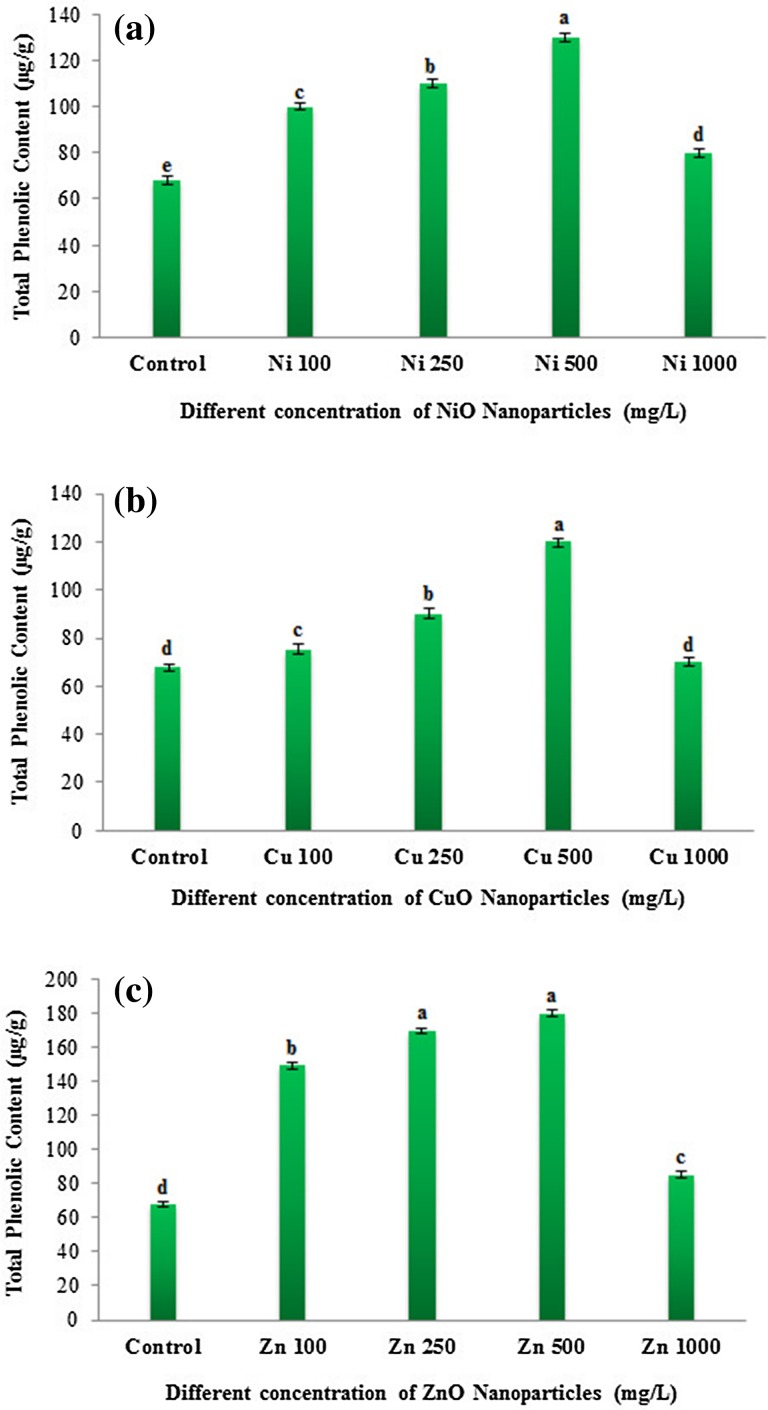
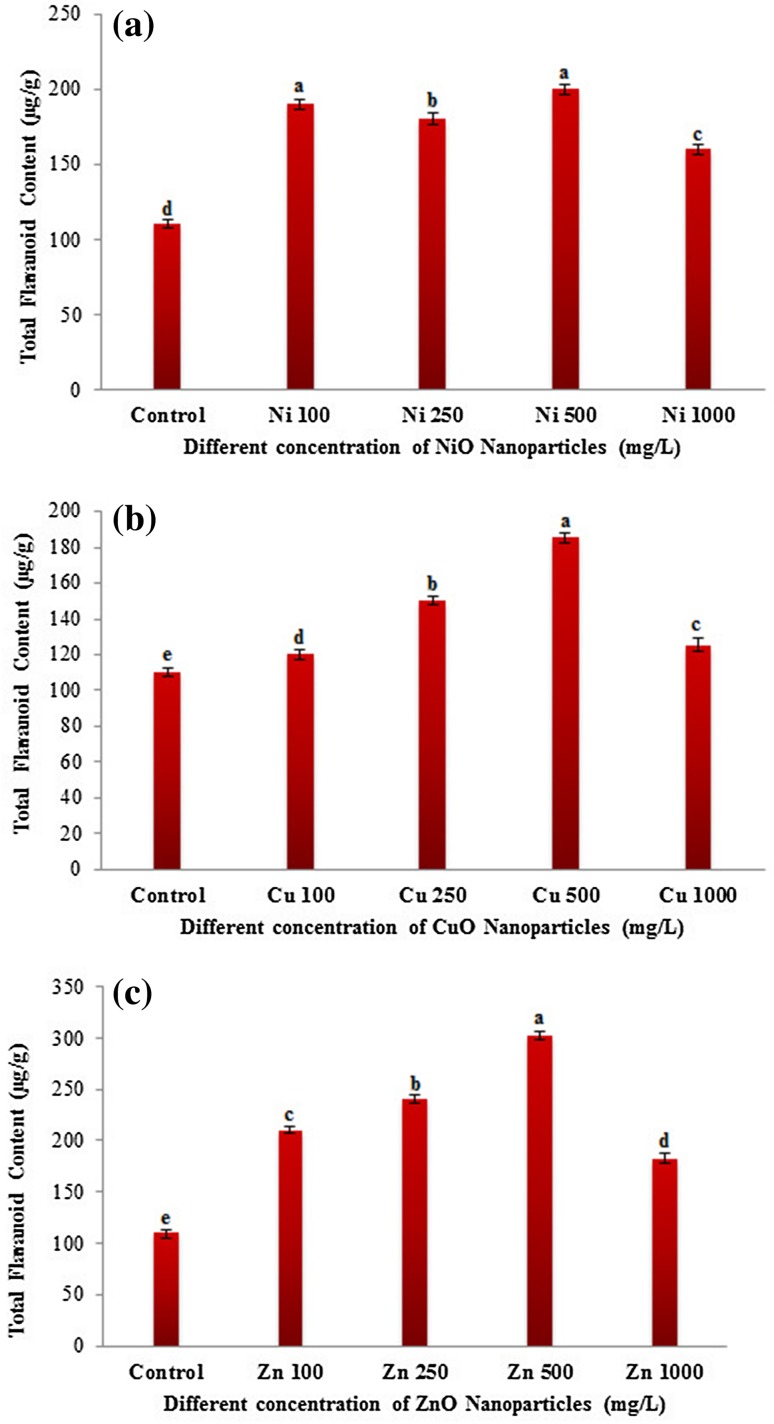
Nanoparticles have been reported to cause oxidative stress in plants (Rico et al. 2013) owing to their interaction with the cellular components resulting in the formation of ROS (Krishnaraj et al. 2012). Phenolic compounds, flavonoids, and antioxidant enzymes act as defense molecules in mitigating oxidative stress caused by excess ROS (Corral-Diaz et al. 2014). The widely used radical scavenging assay 2,2-diphenyl-1-picryl hydrazyl (DPPH) was carried out in NP-treated (NiO, CuO and ZnO) and non-treated eggplant seedlings to determine the antioxidant potential. The observed radical scavenging values (%) were lesser in NP-treated plants than that of control plants (Fig. S1). Antioxidant capacity followed a decreasing order with the increasing concentration of NP treatment. Among the different NP treatment, ZnO NP showed higher DPPH activity compared to NiO and CuO NPs (Fig. S1). DPPH activity was more pronounced at lower concentrations (100, 250 mg/L) of ZnO NP-treated eggplant seedlings (Fig. S1c). However, at higher (1000 mg/L) concentrations all NPs reported lower DPPH activity. NiO at 1000 mg/L showed the least antioxidant capacity compared to CuO and ZnO NP treatment (Fig. S1). These results suggest that lower radical scavenging activity observed in NiO and CuO NPs indicate that they are more toxic compared to ZnO NP. The previous study by Zafar et al. (2016) in Brassica nigra also reported that at lower concentrations ZnO NP showed an increased DPPH activity followed by a decrease at higher concentrations. Flavonoids and phenolic acids are highly correlated with the total antioxidant activity as they are potent antioxidants (Zhao et al. 2014). Total phenolic contents (TPC) at all the four concentrations of NP treatment were found to be higher than the control eggplants. A gradually increasing order of TPC values with increasing NP concentration was observed in NiO, CuO and ZnO NPs till 500 mg/L and then declined at 1000 mg/L (Fig. 6). A significant reduction in the TPC contents was observed at 1000 mg/L of all the NP-treated eggplant seedlings. TPC contents were higher in ZnO (180 µg/g) treatment followed by NiO (130 µg/g) and CuO NP (120 µg/g) (Fig. 6). Similar to TPC, total flavonoid content (TFC) was also higher in all NP-treated samples compared to control (Fig. 7). TFC level followed a concentration-dependent increase up to 500 mg/L in CuO and ZnO NPs (Fig. 7). A drastic reduction in the TFC was found at 1000 mg/L concentration of all the NP investigated in this study. TFC contents were higher in ZnO (302 µg/g) followed by NiO (200 µg/g) and CuO NP (185 µg/g)-treated eggplant seedlings at 500 mg/L. Previous studies have also shown that TPC and TFC contents were significantly altered in several plants treated with the nanoparticles in a dose-dependent manner. In line with our results, Zafar et al. (2016) also reported the TPC and TFC contents were enhanced in the ZnO NP-treated B. nigra plants in a concentration-dependent manner. Similarly, the bulk and nano-ZnO, and CuO treatment was shown to increase the contents of TPC and TFC in Glycyrrhiza glabra seedlings (Oloumi et al. 2015). They concluded that nano- and bulk-sized ZnO and CuO could affect the secondary metabolite production. Furthermore, the contents of flavonoids and phenolics were increased in Amaranthus caudatus L. when treated with the biologically synthesized silver nanoparticles and they suggested that antioxidant activity and phytochemical contents can be enhanced by nanoparticles in a dose-dependent manner (Azeez et al. 2017). The above results clearly indicate that NPs could have an impact on the secondary metabolite production.
Fig. 6.
Dose-dependent effects of three different nanoparticles (NiO, CuO and ZnO) on total phenolic content (µg/g) in Solanum melongena. Total phenolic content in S. melongena at different concentrations (100, 250, 500 and 1000 mg/L) of a nickel oxide (NiO) nanoparticles, b copper oxide (CuO) nanoparticles, c zinc oxide (ZnO) nanoparticles. Each value in the graph is represented as mean ± SD (n = 3). Significantly different values (P < 0.05) showed in different alphabet letters
Fig. 7.
Dose-dependent changes in the total flavonoid content (µg/g) in Solanum melongena treated with the three different metal oxide nanoparticles (NiO, CuO and ZnO). a Total flavonoid content in S. melongena at different concentrations of nickel oxide (NiO) nanoparticles (100, 250, 500 and 1000 mg/L), b copper oxide (CuO) nanoparticles (100, 250, 500 and 1000 mg/L), c zinc oxide (ZnO) nanoparticles (100, 250, 500 and 1000 mg/L). Each value in the graph is represented as mean ± SD (n = 3). Significantly different values (P < 0.05) showed in different alphabet letters
Detection of metal oxide NP-induced ROS generation
Plants respond to various stresses, including abiotic and biotic stresses through the production of reactive oxygen species (H2O2, OH−, and O2− molecules). The ROS will be effectively scavenged by the innate-evolved plants’ enzymatic and nonenzymatic antioxidant mechanisms. Several previous studies reported that diverse factors, namely size and shape, solubility and particle dissolution, metal ions released from metal and metal oxide NPs, biotransformation of NPs, light, etc., may cause the ROS production and phytotoxicity (Yang et al. 2017). In this study, NBT and DAB histochemical stainings were performed in the roots of eggplant seedlings exposed to the metal oxide NP (NiO, CuO, and ZnO NP) for visual detection of the presence of O2− and H2O2 radicals. ROS generation was increasing with increasing concentrations of nanoparticles. A concentration-dependent increased dark blue coloration was noticed in the roots of NP-treated plants after staining with NBT (Fig. S2). Similarly, NP-treated and control plant roots stained with the DAB solution resulted in a gradual increase in the dark brown color formation in NP-treated roots exhibiting a dose-dependent phenomenon (Fig. S3). Among the different NP used, NiO NP treatment enhanced the ROS production even at low concentrations (100 mg/L) compared to CuO and ZnO NP which is evident from the NBT and DAB staining. Followed by the NiO, CuO recorded higher ROS production compared to ZnO NP-treated plant roots. In accordance with our results, NiO was shown to induce ROS production in tomato in a concentration-dependent manner, which was detected through 2′,7′-dichlorofluorescein diacetate (DCFH-DA) staining. Similarly, ZnO NP-treated Allium cepa roots exhibited a concentration-dependent increase in ROS generation which was detected in vivo by staining the roots with DCFH-DA (Ahmed et al. 2017). Nair et al. (2014) also reported a concentration-dependent ROS generation in the Vigna radiata L. seedlings treated with the CuO NP.
Determination of metal oxide NP-induced genotoxicity
Nanoparticles have been reported to cause DNA strand break (Rim et al. 2013) and chromosomal aberrations (Ghosh et al. 2012). Nanoparticle-mediated genotoxicity is primarily triggered by ROS-mediated oxidative stress. Mitochondrial depolarization, down-regulation of DNA repair genes, chromosomal instability, damage of cellular components, oxidative stress-induced apoptosis, and mitosis inhibition are some of the factors involved in NP-induced toxicity (Rim et al. 2013). It has been reported that abiotic stresses induce DNA damage in various plants (Kumari et al. 2009). DNA laddering assay was performed for the qualitative determination of the extent of DNA damage in the seedlings of eggplants treated with different metal oxide (NiO, CuO and ZnO) nanoparticles. Compared to untreated plants, NP-treated eggplants revealed the presence of DNA damage (Fig. S4). The genomic DNA of control plant samples was undamaged as evidenced by a thick band on the agarose gel while DNA damage was found in all the NP-treated samples at all the concentrations used which indicated these engineered nanoparticles could induce DNA damage. In agreement with our results, DNA damage imposed by the various nanoparticles in several plants has been detected through several techniques which include DNA fragmentation assay, tunnel assays and RAPD (Baskar et al. 2015; Thiruvengadam et al. 2015; Lee et al. 2013).
Conclusion
In the present investigation, we examined the eggplants (15-day-old seedlings) responses to different metal oxide NPs such as CuO, ZnO, and NiO at various concentrations. A strong suppression of plant root and shoot growth was observed in all the tested NP in a dose-dependent manner. NiO NP was found to repress the shoot and root growth even at the lowest concentration (100 mg/L). A similar concentration-dependent decrease in the total chlorophyll content was observed in the NP-treated seedlings with ZnO showing the significant reduction in chlorophyll contents. The decreased anthocyanin accumulation was found at highest concentrations (1000 mg/L) of NP-treated samples. The increase in anthocyanin levels at lower concentrations of NP exhibits significant tolerance against oxidative stress. Furthermore, the nonenzymatic antioxidants such as flavonoids and phenolics were altered in NP-treated plants and these changes occurred depending on the concentrations of NP used. The increased intracellular ROS formation and MDA production were prominent at higher concentrations (500 and 1000 mg/L) of metal oxide NP treatments. ROS generation in the roots of NP-treated S. melongena seedlings was evident with DAB and NBT histochemical staining. Altogether, our results suggest that the higher concentration (500 and 1000 mg/L) of all the subjected metal oxide nanoparticles induces phytotoxicity via the inhibition of growth features in eggplants which is greatly correlated with the detected different physiological and biochemical changes. It is also suggested that further studies would be necessary to study the effects of NP-induced phytotoxicity at different growth stages of plants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 Figure S1: Concentration-dependent changes in the total radical scavenging activity (%) of the seedlings of Solanum melongena treated with three different nanoparticles (NiO, CuO and ZnO). a) Total radical scavenging activity (%) of S.melongena at different concentrations (100 mg/L, 250 mg/L, 500 mg/L and 1000 mg/L) of (a) Nickel oxide (NiO) nanoparticles, (b) Copper Oxide (CuO) nanoparticles, (c) Zinc oxide (ZnO) nanoparticles. Each value in the graph is represented as mean ± SD (n = 3). Significantly different values (P < 0.05) showed in different alphabet letters (JPG 118 kb)
Supplementary material 2 Figure S2: Figure showing the in vivo generation of gradual reactive oxygen species (ROS) formation in the roots of Solanum melongena seedlings treated with different concentrations (100 mg/L, 250 mg/L, 500 mg/L and 1000 mg/L) of (a) Nickel oxide (NiO) (b) Copper oxide (CuO) (c) Zinc oxide (ZnO) nanoparticles were examined using Nitroblueterazolium (NBT) staining (JPG 2476 kb)
Supplementary material 3 Figure S3: Dose-dependent effects of metal oxide nanoparticales (NiO, CuO and ZnO) on reactive oxygen species (ROS) generation in the two week old Solanum melongena roots at different concentrations (100 mg/L, 250 mg/L, 500 mg/L and 1000 mg/L) (a) Nickel oxide (NiO) nanoparticles (b) Copper oxide (CuO) nanoparticles (c) Zinc oxide (ZnO) nanoparticles was determined using 3,3’-diaminobenzidine (DAB) staining (JPG 3274 kb)
Supplementary material 4 Figure S4: Dose dependant effects of metal oxide nanoparticles (NiO, CuO and ZnO) on DNA fragmentation analysis of Solanum melongena treated with the three different concentrations such as 100 mg/L, 250 mg/L, 500 mg/L and 1000 mg/L. DNA fragmentation analysis of (a) Nickle oxide (NiO) nanoparticles, Lane 1- control, Lane 2- NiO NP (100mg/L), Lane 3- NiO NP (250 mg/L), Lane 4- NiO NP (500 mg/L), Lane 5 – NiO NP (1000 mg/L), (b) Copper oxide (CuO) nanoparticles, Lane 1- CuO NP (100mg/L), Lane 2- CuO NP (250 mg/L), Lane 3- CuO NP (500 mg/L), Lane 4- CuO NP (1000 mg/L), Lane 5 – control, (c) Zinc oxide (ZnO) nanoparticles, Lane 1- ZnO NP (100mg/L), Lane 2- ZnO NP (250 mg/L), Lane 3- ZnO NP (500 mg/L), Lane 4- ZnO NP (1000 mg/L), Lane 5 – control (JPG 907 kb)
Acknowledgements
This study was supported by a Grant (Sanction no. PDF/2016/000750) from the Department of Science and Technology—Science and Engineering Research Board, Government of India.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Agarwal H, Kumar SV, Rajeshkumar S. review on green synthesis of zinc oxide nanoparticles—an eco-friendly approach. Resour Eff Technol. 2017 [Google Scholar]
- Ahmed B, Dwivedi S, Abdin MA, Azam A, Al-Shaeri M, Khan MA, Saquib Q, Al-Khedhairy AA, Musarrat J. Mitochondrial and chromosomal damage induced by oxidative stress in Zn2+ Ions, ZnO-bulk and ZnO-NPs treated Allium cepa roots. Sci Rep. 2017;7:40685. doi: 10.1038/srep40685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Areeba F, Heena T, Asad A, Abdul M, Adnan A, Iffat ZA. Role of nanoparticles in growth and development of plants: a review. Int J Pharm Bio Sci. 2016;7(4):22–37. [Google Scholar]
- Atha DH, Wang H, Petersen EJ, Cleveland D, Holbrook RD, Jaruga P, Dizdaroglu M, Xing B, Nelson BC. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ Sci Technol. 2012;46(3):1819–1827. doi: 10.1021/es202660k. [DOI] [PubMed] [Google Scholar]
- Azeez L, Lateef A, Adebisi SA. Silver nanoparticles (AgNPs) biosynthesized using pod extract of Cola nitida enhances antioxidant activity and phytochemical composition of Amaranthus caudatus Linn. Appl Nanosci. 2017;7:59–66. doi: 10.1007/s13204-017-0546-2. [DOI] [Google Scholar]
- Bao JS, Cai Y, Sun M, Wang G, Cork H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J Agric Food Chem. 2005;53:2327–2332. doi: 10.1021/jf048312z. [DOI] [PubMed] [Google Scholar]
- Baskar V, Venkatesh J, Park SW. Impact of biologically synthesized silver nanoparticles on the growth and physiological responses in Brassica rapa ssp. pekinensis. Environ Sci Pollut Res. 2015;22:17672–17682. doi: 10.1007/s11356-015-4864-1. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytol. 2007;173(4):677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- Corral-Diaz B, Peralta-Videa JR, Alvarez-Parrilla E, Rodrigo-García J, et al. Cerium oxide nanoparticles alter the antioxidant capacity but do not impact tuber ionome in Raphanus sativus (L) Plant Physiol Biochem. 2014;84:277–85. doi: 10.1016/j.plaphy.2014.09.018. [DOI] [PubMed] [Google Scholar]
- De Rosa MC, Monreal C, Schnitzer M, Walsh R, Sultan Y. Nanotechnology in fertilizers. Nat Nanotechnol. 2010;5(2):91. doi: 10.1038/nnano.2010.2. [DOI] [PubMed] [Google Scholar]
- Dimkpa CO, McLean JE, Latta DE, Manangón E, Britt DW, Johnson WP, Boyanov MI, Anderson AJ. CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J Nanopart Res. 2012;14(9):1125. doi: 10.1007/s11051-012-1125-9. [DOI] [Google Scholar]
- Faisal M, Saquib Q, Alatar AA, Al-Khedhairy AA, et al. Phytotoxic hazards of NiO-nanoparticles in tomato: a study on mechanism of cell death. J Hazard Mater. 2013;250–251:318–332. doi: 10.1016/j.jhazmat.2013.01.063. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Descourvières P, Kunert KJ. Protection against oxygen radicals: an important defense mechanism study in transgenic plants. Plant Cell Environ. 1994;17:507–523. doi: 10.1111/j.1365-3040.1994.tb00146.x. [DOI] [Google Scholar]
- Ghosh M, Manivannan J, Sinha S, Chakraborty A, et al. In vitro and in vivo genotoxicity of silver nanoparticles. Mutat Res Genet Toxicol Environ Mutagen. 2012;749(1):60–69. doi: 10.1016/j.mrgentox.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Gong N, Shao K, Feng W, Lin Z, et al. Biotoxicity of nickel oxide nanoparticles and bio-remediation by microalgae Chlorella vulgaris. Chemosphere. 2011;83:510–516. doi: 10.1016/j.chemosphere.2010.12.059. [DOI] [PubMed] [Google Scholar]
- Hao Y, Yu F, Lv R, Ma C, et al. Carbon nanotubes filled with different ferromagnetic alloys affect the growth and development of rice seedlings by changing the C:N ratio and plant hormones concentrations. PLoS One. 2016;11:e0157264. doi: 10.1371/journal.pone.0157264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hong J, Rico CM, Zhao L, Adeleye AS, et al. Toxic effects of copper-based nanoparticles or compounds to lettuce (Lactuca sativa) and alfalfa (Medicago sativa) Environ Sci Proc Impacts. 2015;17(1):177–185. doi: 10.1039/C4EM00551A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee S, Lee I. Alteration of phytotoxicity and oxidant stress potential by metal oxide nanoparticles in Cucumis sativus. Water Air Soil Pollut. 2012;223(5):2799–2806. doi: 10.1007/s11270-011-1067-3. [DOI] [Google Scholar]
- Krishnaraj C, Jagan EG, Ramachandran R, Abirami SM, Mohan N, Kalaichelvan PT. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem. 2012;47(4):651–658. doi: 10.1016/j.procbio.2012.01.006. [DOI] [Google Scholar]
- Kumar D, Yusuf MA, Singh P, Sardar M, Sarin NB, Biosciences JMI. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio Protoc. 2014;4(8):e1108. doi: 10.21769/BioProtoc.1108. [DOI] [Google Scholar]
- Kumari M, Mukherjee A, Chandrasekaran N. Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ. 2009;407(19):5243–5246. doi: 10.1016/j.scitotenv.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Lee CW, Mahendra S, Zodrow K, Li D, et al. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ Toxicol Chem. 2010;29:669–675. doi: 10.1002/etc.58. [DOI] [PubMed] [Google Scholar]
- Lee S, Chung H, Kim S, Lee I. The genotoxic effect of ZnO and CuO nanoparticles on early growth of buckwheat, Fagopyrum esculentum. Water Air Soil Pollut. 2013;224:1668. doi: 10.1007/s11270-013-1668-0. [DOI] [Google Scholar]
- Lin D, Xing B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol. 2008;42(15):5580–5585. doi: 10.1021/es800422x. [DOI] [PubMed] [Google Scholar]
- López-Moreno ML, Rosa GDL, Hernández-Viezcas JA, Castillo-Michel H. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ Sci Technol. 2010;44(19):7315–7320. doi: 10.1021/es903891g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Kuang L, He X, Bai W, et al. Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere. 2010;78:273–279. doi: 10.1016/j.chemosphere.2009.10.050. [DOI] [PubMed] [Google Scholar]
- Ma H, Williams PL, Diamond SA. Ecotoxicity of manufactured ZnO nanoparticles—a review. Environ Pollut. 2013;172:76–85. doi: 10.1016/j.envpol.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Ma C, White JC, Dhankher OP, Xing B. Metal-based nanotoxicity and detoxification pathways in higher plants. Environ Sci Technol. 2015;49:7109–7122. doi: 10.1021/acs.est.5b00685. [DOI] [PubMed] [Google Scholar]
- Magaye R, Zhao J, Bowman L, Ding M. Genotoxicity and carcinogenicity of cobalt nickel- and copper-based nanoparticles. Exp Ther Med. 2012;4(4):551–561. doi: 10.3892/etm.2012.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair PM, Chung IM. Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignification, and molecular level changes. Environ Sci Pollut Res Int. 2014;21(22):12709–12722. doi: 10.1007/s11356-014-3210-3. [DOI] [PubMed] [Google Scholar]
- Nair PMG, Chung IM. Study on the correlation between copper oxide nanoparticles induced growth suppression and enhanced lignifications in Indian mustard (Brassica juncea L. Ecotoxicol Environ Saf. 2015;113:302–313. doi: 10.1016/j.ecoenv.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Nair R, Varghese SH, Nair BG, Maekawa T, et al. Nanoparticulate material delivery to plants. Plant sci. 2010;179(3):154–163. doi: 10.1016/j.plantsci.2010.04.012. [DOI] [Google Scholar]
- Nair PMG, Kim SH, Chung IM. Copper oxide nanoparticle toxicity in mung bean (Vigna radiata L.) seedlings: physiological and molecular level responses of in vitro grown plants. Acta Physiol Plant. 2014;36(11):2947–2958. doi: 10.1007/s11738-014-1667-9. [DOI] [Google Scholar]
- Oloumi H, Soltaninejad R, Baghizadeh A. The comparative effects of nano and bulk size particles of CuO and ZnO on glycyrrhizin and phenolic compounds contents in Glycyrrhiza glabra L. seedlings. Indian J Plant Physiol. 2015;20(2):157–161. doi: 10.1007/s40502-015-0143-x. [DOI] [Google Scholar]
- Oukarroum A, Schansker G, Strasser RJ. Drought stress effects on photosystem I content and photosystem II thermotolerance analyzed using Chl a fluorescence kinetics in barley varieties differing in their drought tolerance. Physiol Plant. 2009;137(2):188–199. doi: 10.1111/j.1399-3054.2009.01273.x. [DOI] [PubMed] [Google Scholar]
- Perreault F, Popovic R, Dewez D. Different toxicity mechanisms between bare and polymer-coated copper oxide nanoparticles in Lemna gibba. Environ Pollut. 2014;185:219–227. doi: 10.1016/j.envpol.2013.10.027. [DOI] [PubMed] [Google Scholar]
- Perreault F, Samadani M, Dewez D. Effect of soluble copper released from copper oxide nanoparticles solubilisation on growth and photosynthetic processes of Lemna gibba L. Nanotoxicology. 2014;8(4):374–382. doi: 10.3109/17435390.2013.789936. [DOI] [PubMed] [Google Scholar]
- Priester JH, Ge Y, Mielke RE, Horst AM, et al. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. PNAS. 2012;109:2451–2456. doi: 10.1073/pnas.1205431109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raigond P, Raigond B, Kaundal B, Singh B, et al. Effect of zinc nanoparticles on antioxidative system of potato plants. J Environ Biol Lucknow. 2017;38:435–439. doi: 10.22438/jeb/38/3/MS-209. [DOI] [Google Scholar]
- Rao S, Shekhawat GS. Toxicity of ZnO engineered nanoparticles and evaluation of their effect on growth, metabolism and tissue specific accumulation in Brassica juncea. J Environ Chem Eng. 2014;2(1):105–114. doi: 10.1016/j.jece.2013.11.029. [DOI] [Google Scholar]
- Ray BP, Hassan L, Nasiruddin KM. In vitro regeneration of brinjal (Solanum melongena L.) Bangladesh J Agric Res. 2011;36(3):397–406. doi: 10.3329/bjar.v36i3.9268. [DOI] [Google Scholar]
- Rico CM, Hong J, Morales MI, Zhao L, et al. Effect of cerium oxide nanoparticles on rice: a study involving the antioxidant defense system and in vivo fluorescence imaging. Environ Sci Technol. 2013;47(11):5635–5642. doi: 10.1021/es401032m. [DOI] [PubMed] [Google Scholar]
- Rico CM, Lee SC, Rubenecia R, Mukherjee A, et al. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.) J Agric Food Chem. 2014;62(40):9669–9675. doi: 10.1021/jf503526r. [DOI] [PubMed] [Google Scholar]
- Rim KT, Song SW, Kim HY. Oxidative DNA damage from nanoparticle exposure and its application to workers’ health: a literature review. Saf Health Work. 2013;4(4):177–186. doi: 10.1016/j.shaw.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SO, Bendich AJ. Extraction of DNA from plant tissues. In: Gelvin SB, Schilperoort RA, Verma DPS, editors. Plant Molecular Biology Manual. Dordrecht: Springer; 1989. pp. 73–83. [Google Scholar]
- Salah SM, Yajing G, Dongdong C, Jie L, et al. Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci Rep. 2015;5:14278. doi: 10.1038/srep14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhoshkumar J, Kumar SV, Rajeshkumar S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour Eff Technol. 2017 [Google Scholar]
- Servin AD, Morales MI, Castillo-Michel H, Hernandez-Viezcas JA, et al. Synchrotron verification of TiO2 accumulation in cucumber fruit: a possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ Sci Technol. 2013;47:11592–11598. doi: 10.1021/es403368j. [DOI] [PubMed] [Google Scholar]
- Siddiqi KS, Husen A. Plant response to engineered metal oxide nanoparticles. Nanoscale Res Let. 2017;12(1):92. doi: 10.1186/s11671-017-1861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AK, Hossain Z. Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere. 2013;93(6):906–915. doi: 10.1016/j.chemosphere.2013.05.044. [DOI] [PubMed] [Google Scholar]
- Shi J, Abid AD, Kennedy IM, Hristova KR, et al. To duckweeds (Landoltia punctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ Pollut. 2011;59(5):1277–1282. doi: 10.1016/j.envpol.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Peng C, Yang Y, Yang J, et al. Phytotoxicity and accumulation of copper oxide nanoparticles to the Cu-tolerant plant Elsholtzia splendens. Nanotoxicology. 2014;8(2):179–188. doi: 10.3109/17435390.2013.766768. [DOI] [PubMed] [Google Scholar]
- Stampoulis D, Sinha SK, White JC. Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol. 2009;43(24):9473–9479. doi: 10.1021/es901695c. [DOI] [PubMed] [Google Scholar]
- Tang Y, He R, Zhao J, Nie G, et al. Oxidative stress-induced toxicity of CuO nanoparticles and related toxicogenomic responses in Arabidopsis thaliana. Environ Pollut. 2016;212:605–614. doi: 10.1016/j.envpol.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Thiruvengadam M, Gurunathan S, Chung IM. Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.) Protoplasma. 2015;252(4):1031–1046. doi: 10.1007/s00709-014-0738-5. [DOI] [PubMed] [Google Scholar]
- Wang Z, Xie X, Zhao J, Liu X, et al. Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L.) Environ Sci Technol. 2012;46(8):4434–4441. doi: 10.1021/es204212z. [DOI] [PubMed] [Google Scholar]
- Wang X, Yang X, Chen S, Li Q, Wang W, Hou C, Wang S. Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front Plant Sci. 2016;6:1243. doi: 10.3389/fpls.2015.01243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Cao W, Rui Y. Interactions between nanoparticles and plants: phytotoxicity and defense mechanisms. J Plant Int. 2017;12(1):158–169. [Google Scholar]
- Yasmeen F, Razzaq A, Iqbal MN, Jhanzab HM. Effect of silver, copper and iron nanoparticles on wheat germination. Intern J Biosci. 2015;6(4):112–117. doi: 10.12692/ijb/6.4.112-117. [DOI] [Google Scholar]
- Yuan J, He A, Huang S, Hua J, et al. Internalization and phytotoxic effects of CuO nanoparticles in Arabidopsis thaliana as revealed by fatty acid profiles. Environ Sci Technol. 2016;50(19):10437–10447. doi: 10.1021/acs.est.6b02613. [DOI] [PubMed] [Google Scholar]
- Zafar H, Ali A, Ali JS, Haq IU, et al. Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: growth dynamics and antioxidative response. Front Plant Sci. 2016;7:535. doi: 10.3389/fpls.2016.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Peralta-Videa JR, Rico CM, Hernandez-Viezcas JA, Sun Y, Niu G, Gardea-Torresdey JL. CeO2 and ZnO nanoparticles change the nutritional qualities of cucumber (Cucumis sativus) J Agric Food Chem. 2014;62(13):2752–2759. doi: 10.1021/jf405476u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 Figure S1: Concentration-dependent changes in the total radical scavenging activity (%) of the seedlings of Solanum melongena treated with three different nanoparticles (NiO, CuO and ZnO). a) Total radical scavenging activity (%) of S.melongena at different concentrations (100 mg/L, 250 mg/L, 500 mg/L and 1000 mg/L) of (a) Nickel oxide (NiO) nanoparticles, (b) Copper Oxide (CuO) nanoparticles, (c) Zinc oxide (ZnO) nanoparticles. Each value in the graph is represented as mean ± SD (n = 3). Significantly different values (P < 0.05) showed in different alphabet letters (JPG 118 kb)
Supplementary material 2 Figure S2: Figure showing the in vivo generation of gradual reactive oxygen species (ROS) formation in the roots of Solanum melongena seedlings treated with different concentrations (100 mg/L, 250 mg/L, 500 mg/L and 1000 mg/L) of (a) Nickel oxide (NiO) (b) Copper oxide (CuO) (c) Zinc oxide (ZnO) nanoparticles were examined using Nitroblueterazolium (NBT) staining (JPG 2476 kb)
Supplementary material 3 Figure S3: Dose-dependent effects of metal oxide nanoparticales (NiO, CuO and ZnO) on reactive oxygen species (ROS) generation in the two week old Solanum melongena roots at different concentrations (100 mg/L, 250 mg/L, 500 mg/L and 1000 mg/L) (a) Nickel oxide (NiO) nanoparticles (b) Copper oxide (CuO) nanoparticles (c) Zinc oxide (ZnO) nanoparticles was determined using 3,3’-diaminobenzidine (DAB) staining (JPG 3274 kb)
Supplementary material 4 Figure S4: Dose dependant effects of metal oxide nanoparticles (NiO, CuO and ZnO) on DNA fragmentation analysis of Solanum melongena treated with the three different concentrations such as 100 mg/L, 250 mg/L, 500 mg/L and 1000 mg/L. DNA fragmentation analysis of (a) Nickle oxide (NiO) nanoparticles, Lane 1- control, Lane 2- NiO NP (100mg/L), Lane 3- NiO NP (250 mg/L), Lane 4- NiO NP (500 mg/L), Lane 5 – NiO NP (1000 mg/L), (b) Copper oxide (CuO) nanoparticles, Lane 1- CuO NP (100mg/L), Lane 2- CuO NP (250 mg/L), Lane 3- CuO NP (500 mg/L), Lane 4- CuO NP (1000 mg/L), Lane 5 – control, (c) Zinc oxide (ZnO) nanoparticles, Lane 1- ZnO NP (100mg/L), Lane 2- ZnO NP (250 mg/L), Lane 3- ZnO NP (500 mg/L), Lane 4- ZnO NP (1000 mg/L), Lane 5 – control (JPG 907 kb)