Abstract
Otoconia-related vertigo and balance deficits, particularly benign paroxysmal positional vertigo (BPPV), are common. Our recent studies in humans show that, while BPPV prevalence greatly increases with age in both genders, peri-menopausal women are especially susceptible. In the present study, we show that bilateral ovariectomized (OVX) mice have significant balance behavioral deficits, and that estrogen deficiency compromises otoconia maintenance and anchoring by reducing the expression of otoconial component and anchoring proteins. There is ectopic debris formation in the ampulla under estrogen deficiency due to aberrant matrix protein expression. Furthermore, phytoestrogen is effective in rescuing the otoconia abnormalities. By comparing the expression levels of known estrogen receptor (Esr) subtypes, and by examining the otoconia phenotypes of null mice for selected receptors, we postulate that Esr2 may be critical in mediating the effects of estrogen in otoconia maintenance.
Keywords: otoconia, estrogen deficiency, phytoestrogen, menopause, BPPV, estrogen receptor
Introduction
The vestibule of the inner ear senses head motion, which is critical for spatial orientation and bodily balance. Such sense arises from stimulation of the sensory epithelia by the displacement of otoconia in the utricle and saccule, and stimulation of crista by the cupula in the ampulla under motion. Otoconia, composed of proteins and CaCO3 crystals, are much denser than the gel-like cupula. Therefore, dislocation of otoconia from the utricle into the ampulla, or ectopic formation of debris there, can cause abnormal responses of the crista and lead to vertigo and imbalance. In fact, otoconia-related balance disorders and vertigo are a significant health issue that affects over 10 million adults in the USA based on the international consensus of 10 % lifetime prevalence for benign paroxysmal positional vertigo (BPPV) (the Barany Society meeting (von Brevern et al. 2015)). BPPV is believed to be caused by otoconia dislocation, and is the most common cause of vertigo in humans.
Given that BPPV prevalence greatly increases with age (Gananca et al. 2010, Jonsson et al. 2004, Ogun et al. 2014, Ojala and Palo 1991, von Brevern et al. 2007), it is possible that otoconia degeneration can facilitate dislocation. Our recent study has shown that women at peri-menopausal ages are especially susceptible, with a female to male ratio of 3.2:1 in the age group of 40–49 (Ogun et al. 2014). Extensive research has demonstrated the association between estrogen level and bone density in postmenopausal women. Since both bone and otoconia are biomineralized structures, it would not be surprising if the hormonal decline in aging women affect the latter as well. Indeed, Vibert et al. (Vibert et al. 2008) has shown that, in addition to osteopenia/osteoporosis, OVX rats also have otoconia with decreased density and increased particle size compared to those of sham-operated rats. The present study will define the effects of estrogen deficiency on otoconia maintenance and anchoring and provide some mechanistic insight. Due to the intricate anatomy and inaccessibility of the human inner ear, current medical imaging technology does not allow clear visualization of the effects of menopause or estrogen deficiency on otoconia dislocation and degeneration in patients. This study will use a mouse model to investigate the aspects upon which estrogen deficiency affects otoconia, thus address a prevalent clinical problem.
Estrogen receptors (Esr) and the structurally related proteins (Esrr) are present in the inner ear of many species including mice and humans, and some are known to be up- or downregulated depending on the stage of development, maturation, and pregnancy, suggesting that estrogen may have an effect on hearing and balance during various stages of life (Charitidi et al. 2009; Hawkins et al. 2007; Motohashi et al. 2010; Sajan et al. 2007; Simonoska et al. 2009a). Lack of estrogen in humans is the main characteristic of Turner syndrome, and the patients commonly develop an early presbycusis, balance problems, and osteoporosis (El-Mansoury et al. 2009; Stenberg et al. 2002). Mutations in the Esrrb gene cause autosomal recessive nonsyndromic hearing impairment DFNB35 in humans (Collin et al. 2008). Estrogen replacement therapy in postmenopausal women slows hearing loss (Kilicdag et al. 2004).
To date, five nuclear Esrs/Esrrs have been reported, Esr1 (ERa), Esr2 (ERb), Esrra (ERRa), Esrrb (ERRb), and Esrrg (ERRg). The latter three Esrr are orphan receptors and do not bind estrogen and have no known endogenous ligands. They bind the same DNA elements as Esr, but also bind DNA unrelated to Esr. Esrr appears to be constitutively active and may modulate the activities of Esr, but also have other activities not related to Esr or estrogen (Stein and McDonnell 2006; Vanacker et al. 1999). Additionally, the cytoplasmic membrane-bound, G-protein coupled estrogen receptor 1 (GPER1, aka GPR30) also binds to estradiol with high affinity and leads to activation of numerous intracellular signal pathways (Alexander et al. 2017; Ford et al. 2011; Kim et al. 2015; Prossnitz and Maggiolini 2009). However, controversy exists regarding whether GPR30 function as an estrogen receptor (Otto et al. 2008; Otto et al. 2009; Pedram et al. 2006). Esr1 null mice have normal hearing, and Esrra and Esrrg null mice do not have obvious neurological abnormalities either (Heine et al. 2000; Hess et al. 2000). Although Esr2 null mice initially have normal hearing, they show early presbycusis (Simonoska et al. 2009b) and are more sensitive to acoustic trauma (Meltser et al. 2008). Esrrb conditional null mice are nearly deaf and show circling behavior indicative of balance defects (Chen and Nathans 2007). The present study will also examine which estrogen receptor subtype may underlie the observed effects of estrogen on otoconia.
Materials and Methods
Mice
Female mice were used to obtain the reported results. C57Bl/6 J (C57) mice (Stock No: 000664), heterozygous and homozygous Esr1 (Stock No. 004744), and Esr2 (Stock No. 004745) knockout mice were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA) and maintained in the vivarium at Boys Town National Research Hospital (BTNRH). Because homozygous Esr1 females were infertile and Esr2 females were subfertile, heterozygous females were bred to heterozygous or homozygous males to maintain live colonies. Genotyping primers were 5′-GCTACTTCCATTTGTCACGTCC-3′, 5′-TACGGCCAGTCGGGCATC-3′, and 5′-GTAGAAGGCGGGAGGGCCGGTGTC-3′ for Esr1; 5′-GTTGTGCCAGCCCTGTTACT-3′, 5′-TCACAGGACCAGACACCGTA-3′, and 5′-GCAGCCTCTGTTCCACATACAC-3′ for Esr2.
Under anesthesia using ketamine (200 mg/kg b. wt.) and xylazine (5 mg/kg b. wt.), bilateral ovariectomy (OVX) was performed on 2-month-old naïve female C57 mice, and age-matched sham-operated mice (in which surgery was performed but ovaries were left intact) were used as controls. Morphological and molecular analyses were done at various time points after surgery as indicated in the reported data. All animal procedures were approved by the Institutional Animal Care and Use Committee at BTNRH and/or the University of Nebraska at Lincoln in accordance with federal and international guidelines.
Rotarod Test
A four-lane rotarod (Rotamex-5, Columbus Instruments, Columbus, OH, USA) was used in our experiments. OVX and sham mice were tested for five consecutive days with three trials a day. All tests were carried out within the same time window of the day (between 1 and 4 pm) to control for possible variations introduced by circadian rhythms. Prior to the test each day, a single adaptive session was done during which each mouse was placed on the rotarod at a constant speed (5 rpm) for 60 s. Then the mice were tested on the accelerating rod (5–20 rpm over 3 min) for three consecutive sessions, separated by 10-min resting periods. A laser sensor detected if the animal fell from the rod and the time and speed of rotation at falling were recorded. The average latency of fall (time the mice remained on the rotarod) was calculated from 9 to 10 animals and plotted for each trial and each group.
Measurement of Vestibular Evoked Potentials
Vestibular evoked potentials (VsEPs) recording methods were as published previously (Jones et al. 1999; Jones et al. 2004; Vijayakumar et al. 2017) and are briefly described here. Animals were anesthetized by intraperitoneal injection with a ketamine (180 mg/kg b. wt.)/xylazine (20 mg/kg b. wt.) mixture. Depth of anesthesia was monitored and maintenance doses of anesthetic were administered as needed during physiological recording. Body core temperature was maintained at 37.0 ± 0.2 °C using a homeothermic heating blanket and rectal thermocouple (FHC, Inc., Bowdoin, ME, USA). Vestibular stimuli were delivered by securing the head using non-invasive spring clip to a voltage-controlled mechanical shaker. Linear acceleration pulses (17 pulses/s, 2 ms duration) ranged from +6 dB to −18 dB re = 1.0 g/ms (where 1 g = 9.8 m/s2) adjusted in 3 dB steps were presented along the naso-occipital cranial axis. Needle electrodes were placed posterior to the left pinna and at the ventral neck or hip (inverting and ground electrodes, respectively). Stainless-steel wire was placed subcutaneously at the nuchal crest served as noninverting electrode. Ongoing electroencephalographic activity was amplified (200,000×), filtered (300–3000 Hz, −6 dB amplitude points), and digitized (1024 points, 10 μs/pt). Two hundred fifty-six primary responses were averaged and replicated for each VsEP waveform. VsEP intensity series were collected beginning at the maximum stimulus level (i.e., +6 dB re = 1.0 g/ms) with and without acoustic masking, then descending in 3 dB steps to − 18 dB re = 1.0 g/ms. A broad-band forward masker (50–50,000 Hz, 97 dB SPL) was presented during VsEP measurements to verify absence of cochlear responses (Jones and Jones 1999). Peak latencies (measured in milliseconds), peak-to-peak amplitudes (measured in microvolts), and thresholds (measured in dB re = 1.0 g/ms) were obtained from the VsEP waveforms and quantified.
Microdissection
Animals were anesthetized with ketamine (200 mg/kg) and xylazine (5 mg/kg) and then decapitated. Inner ears were dissected in phosphate-buffered saline (PBS) for the reported experiments.
For fluorescent immunostaining, the inner ears were fixed in 4 % paraformaldehyde, decalcified in 0.25 M EDTA (pH7.4) overnight, dehydrated in 30 % sucrose prepared in PBS, embedded in O.C.T. compound at below − 20 °C, and sectioned at 9 μm.
For real-time quantitative RT-PCR (qRT-PCR), fresh epithelial tissues from the utricle, saccule, ampulla, semi-circular canal, and cochlea were dissected in PBS containing RNasin (Promega, Madison, WI, USA), and were collected in Trizol Reagent (Invitrogen, Carlsbad, CA, USA) for RNA preparation.
Scanning Electron Microscopy
Animals were anesthetized using ketamine (200 mg/kg) and xylazine (5 mg/kg), perfused through the left ventricle with artificial endolymph (125 mM KCl, 25 mM KHCO3, 5 mM HEPES, 2 mM CaCl2, 1 mM NaCl), followed by 20–30 ml fixative (2.5 % glutaraldehyde +3 mM CaCl2 in 0.1 M sodium cacodylate, pH 7.2). Utricles and saccules were dissected out of the inner ear in artificial endolymph and the roof of the utricle and saccule was removed using fine scissors. Tissues were immersion-fixed for 24 h in the same fixative, washed three times with artificial endolymph, and post-fixed in 1 % osmium tetroxide in 0.1 M sodium cacodylate (pH 7.2) for 1 h. Samples were then dehydrated, critical point dried in CO2, mounted, sputter coated with gold palladium and viewed on a Jeol (JSM-848) scanning electron microscope at 15 kV.
Sizes of 9–12 otoconia crystals in the central and peripheral regions of the utricles of C57 and Esr2 null mice were measured using open-source software ImageJ (NIH). Statistical analysis was done using unpaired, two-sample Student’s t test assuming equal variances (Microsoft Office Excel 2010).
Immunohistochemistry
Frozen tissue sections were blocked in PBS containing 5 % BSA + 0.25 % Triton-X-100 at room temperature for 30 min, and incubated in 5 % BSA + 0.25 % Triton-X-100 at 4 °C overnight with appropriate dilutions of one of the antibodies for otoconial proteins: rabbit-derived polyclonal anti-mouse Oc90 (1:500) (Zhao et al. 2007), rabbit-derived polyclonal anti-mouse Otolin (1:100) (Zhao et al. 2007), mouse-derived monoclonal anti-KSPG (1:200) (Chemicon international Inc., Temecula, CA, USA; Cat# MAB2022, Lot# 0607035591), rabbit-derived polyclonal anti-mouse α-tectorin (gift from Dr. Richardson (Legan et al. 2000)). Specificity of these antibodies was confirmed by Western blotting as described in the quoted publication or on the stated supplier’s websites. Pre-immune (Oc90, Otolin) or non-immune (the remaining ones) sera instead of primary antibodies were used in some sections as negative controls.
After 3 washes in PBS, Alexa-488 or 568 (Molecular Probes, Carlsbad, CA) conjugated secondary antibodies were added at a dilution of 1:600, together with DAPI (Sigma-Aldrich, St. Louis, MO, USA) at a dilution of 1:10,000, and incubated at room temperature for 1 h in the dark. Slides were mounted in Fluoromount-G and pictures were taken using a Zeiss Axio Observer Z1 inverted microscope equipped with an AxioCam MRm camera and with GFP, DsRed, and DAPI filter sets. To further match the background signals between two sections under comparison, the brightness and contrast of some images were slightly adjusted (< 15 %) using Zeiss AxioVision SE64 Rel. 4.9.1 Software.
To minimize measurement variation, all tissue sections under comparison were processed strictly under the same conditions (e.g., identical immunostaining procedures, identical microscope scanning parameters, the same number of fluorescent exposures and same degree of contrast enhancement). Cross sections that covered both the striola (central) and peri-macular regions were analyzed to take into consideration possible intensity differences caused by the position and type of cells and sections. Three or more animals were examined for each age and tissue type.
Real-Time Quantitative RT-PCR
Total RNA was prepared using the Trizol Reagent (Invitrogen, Carlsbad, CA, USA), and reverse transcription (RT) was carried out with a 1:1 mixture of oligo (dT) and random hexamer primers using the SuperScript III first-strand synthesis system (Invitrogen). Probe and primer sets for qPCR were purchased from Applied Biosystems as TaqMan Gene Expression assays (Applied Biosystems, Foster City, CA, USA), and probes were designed to minimize detection of genomic DNA (e.g., probes spanned 2 or more exons). Also, reactions without reverse-transcriptase were included. Amplification was done according to the manufacturer’s protocol with minor modifications based on cDNA samples and primer/probe conditions. Forty cycles of amplification was achieved in a 96-well plate consisting of TaqMan probe and primer set, TaqMan Universal PCR Master Mix and cDNA (~ 50 ng). The standard mode of an ABI Prism 7900HT Sequence Detection System (Applied Biosystems) was used, and β-actin (Actb) was amplified in parallel as an endogenous control for the quantification of the relative gene expression between samples. The cycle number at which the reaction crosses a predetermined cycle threshold (CT) was identified for each gene, and the expression value of each target gene relative to Actb was determined using the Eq. 2-ΔΔCT.
The probe sequences were:
Esr1—GCATGATGAAAGGCGGCATACGGAA (ABI i.d. Mm00433149_m1)
Esr2—ACCTTGCCTGTAAACAGAGAGACCC (ABI i.d. Mm01281854_m1)
Gpr30—AACGCCACGGCACAGATCAGGACAC (ABI i.d. Mm01194814_g1)
Actb—TTACTGAGCTGCGTTTTACACCCTT (ABI i.d. Mm00607939_s1).
Data Analysis
Reported data represent the mean values ± standard deviation. Microsoft Office Excel 2010 (Microsoft Corp., USA) was used to perform the statistical analyses. Statistical significance was determined by unpaired, two-sample Student’s t test assuming equal variances for rotarod tests and otoconia size comparison, one way ANOVA with Bonferroni correction for qRT-PCR, and multivariate analysis of variance (MANOVA) with Bonferroni correction for latencies, amplitudes and thresholds of VsEPs. Significance was set at the level of p < 0.05.
Results
OVX Mice Had Significant Balance Deficits
In order to examine the effects of estrogen deficiency on the balance function of mice, we performed rotarod tests of OVX and age-matched sham-operated mice. At 10 months (10 M) of age (8 M post-surgery), OVX mice (n = 10) stayed on the accelerating rotarod for a significantly shorter time as compared with sham mice (n = 9) [Fig. 1, Day 1: t(19) = 2.13, P = 0.047; Day 2: t(19) = 2.46, P = 0.023; Day 3: t(18) = 1.91, P = 0.072; Day 4: t(17) = 1.90, P = 0.074; Day 5: t(17) = 1.62, P = 0.124].
Fig. 1.
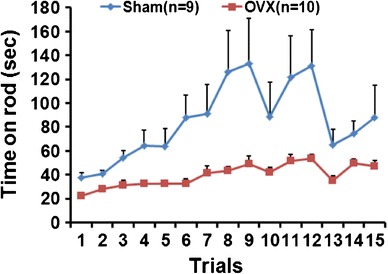
Rotarod performance of OVX and sham mice. OVX mice (n = 10) stayed on the rotarod for a significantly shorter time when compared with age-matched sham controls (n = 9). Mice were tested on the rotarod at 5–20 rpm over 3 min for three trials per day for five consecutive days. The time on the rod of three trials was averaged for each day in each group, and each day’s average was compared independently between the two groups
The vestibular function of OVX and age-matched sham-operated mice were further evaluated by measuring VsEPs. However, there were no significant differences in latencies, amplitudes, or thresholds between OVX and sham groups (data not shown).
Estrogen Deficiency in Adult Female Mice Led to Abnormal Otoconia, and Phytoestrogen Could Largely Rescue the Defects
Scanning electron microscopy (SEM) revealed abnormalities in both otoconia morphology and anchoring that indicated possible degeneration or insufficient maintenance of OVX otoconia. As shown in Fig. 2b, OVX otoconia had deep wrinkles and peanut-like shapes (indicated by arrows), whereas the sham-operated controls were normal with smooth surfaces and barrel shapes with pointed, 3-facaded ends. There were fused and giant OVX otoconia (indicated by asterisks and hollow arrow, respectively, in Fig. 2e), a sign of degeneration, as well as unusually tiny crystals, especially in the peripheral utricular region adjacent to the ampulla (denoted by arrowheads in Fig. 2e, h), further suggesting degeneration. OVX otoconia appeared less tightly packed together, especially the tiny crystals, which appeared loose and were easy to fall into the ampulla region (arrowhead in Fig. 2h). We have previously shown that most of the outer surface of normal WT otoconia is organic matrix (Zhao et al. 2007), and others have shown that the surface has filaments (Hallworth et al. 1995) that are likely anchoring proteins. Hence, the irregular surface structures of OVX otoconia may be an indication of altered protein expression, and may lead to less secure crystal attachment and increased propensity for dislocation, a hallmark feature causing BPPV. Dietary supplementation with genistein and daidzein (300 ppm each for 4 months) largely rescued the otoconia abnormalities caused by OVX (Fig. 2c, f). The phytoestrogen rescue appeared to be more effective on otoconia morphology than fallen debris in the ampulla (Fig. 2c vs. 2i).
Fig. 2.
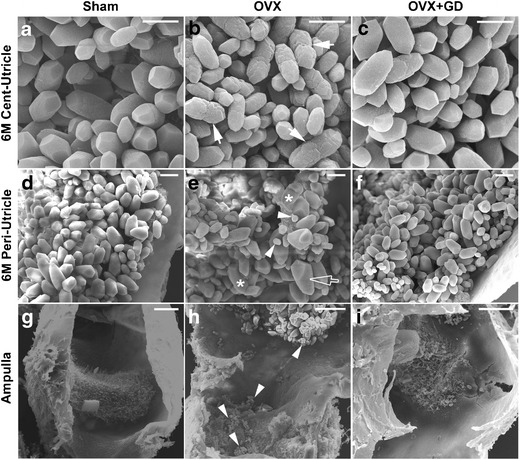
Estrogen deficiency compromises otoconia maintenance and anchoring, and phytoestrogen is largely effective in rescuing such abnormalities. Arrows in (b) denote deep wrinkles, asterisks in (e) denote fused otoconia, hollow arrow points to giant otoconia, and arrowheads in (e, h) indicate unusually small crystals that are loose and easy to fall into the ampulla of the OVX mice. Surgery was always done at 2-months (2 M) of age. Peri- and Cent- indicate peripheral and central regions of the utricle. GD Genistein and Daidzein (herein as phytoestrogen). Mice in a–f and i were 6 months old, and g, h 15 months old. Scale bars: a–c, 5 μm; d–f, 10 μm; g–i, 50 μm
Estrogen Deficiency in Adult Females Led to Insufficient Expression or Incorporation of Otoconial Proteins
We next examined whether estrogen deficiency compromises otoconia maintenance and anchoring by reducing protein expression. Among the nine known matrix proteins and five anchoring proteins, we examined the important ones (Oc90, Otolin, Sc1, α-tectorin, KSPG, Otogelin). Most of these genes were no longer expressed or only had extremely low mRNA levels in adults despite the presence of the proteins; therefore, we focused on examining changes in the proteins levels using fluorescent immunostaining. There were reduced matrix proteins in the OVX otoconia, as represented by reduced signals of Otolin and Oc90 (Fig. 3). Since Oc90 and Otolin interact with each other (Yang et al. 2011) to form the otoconia matrix, and absence of Oc90 in the null mice leads to undetectable Otolin and largely inorganic CaCO3 crystals with surface folds (Zhao et al. 2007), reduced Oc90 and Otolin could have caused the wrinkles in the OVX otoconia in Fig. 2b. Besides being an otoconia component, Otolin is also a component of the anchoring filament (Murayama et al. 2005), so its reduction likely caused the loose otoconia observed in Fig. 2h. There were no apparent changes between the OVX and sham otoconia in the signals of other proteins examined.
Fig. 3.
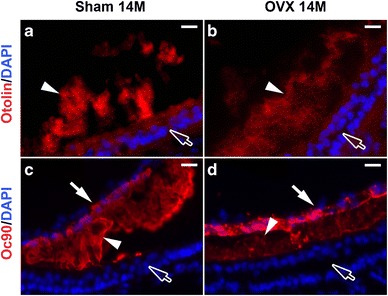
Estrogen deficiency leads to reduced Otolin (a, b) and Oc90 (c, d) in OVX otoconia. Arrow heads indicate otoconia; arrows in c and d point to the roof (partially collapsed due to sectioning); hollow arrows point to the sensory epithelium. Scale bars 20 μm
Estrogen Deficiency in Adult Females Led to Increased Debris near the Crista
Immunohistochemical analysis also revealed numerous ectopic particles near the OVX crista, which was absent or minimal in age-matched sham or un-operated control mice. The debris stained positive for otoconia proteins Oc90, KSPG, Otolin, and unexpectedly, α-tectorin (Fig. 4b, d, f and h). There was upregulation of the cellular signals of these proteins in the ampullar epithelial cells, which were either absent or much weaker in age-matched sham mice. Increased expression of these proteins in the OVX ampulla can potentially cause ectopic calcification, as these proteins have been shown to cause matrix calcification in cell culture (Xu et al. 2010; Yang et al. 2011). While some of the ectopic debris near the crista is likely otoconia dislocated from the utricle, some of it may be formed in situ given the aberrant expression of otoconial proteins there.
Fig. 4.
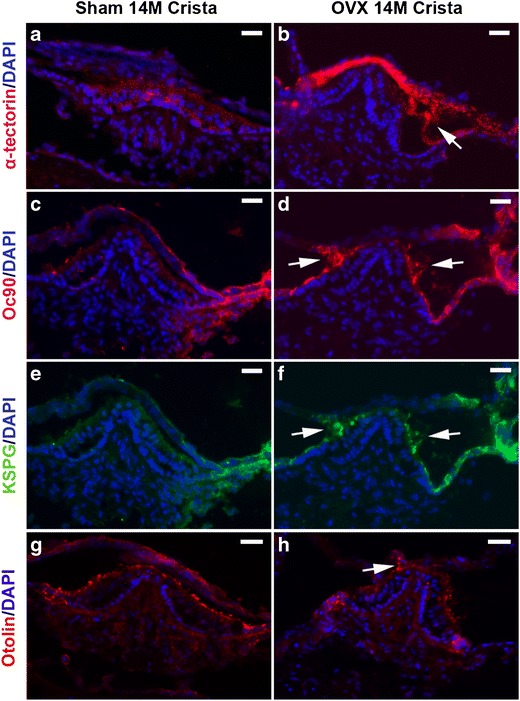
The OVX ampulla has ectopic debris near the crista that stained positive for α-tectorin, Oc90, KSPG, and Otolin (indicated by arrows in b, d, f and h). There is upregulation of the cellular signals of these proteins in the ampulla. Scale bars 25 μm
Identification of Estrogen Receptors Mediating the Effects of Estrogen on Otoconia
In order to identify which estrogen receptor(s) mediates estrogen signaling in otoconia maintenance, we examined the expression of the known receptor subtypes in different inner ear epithelial tissues in OVX and sham mice. Since Esrra, Esrrb, and Esrrg are orphan receptors that do not bind estrogen, we focused on the main receptors Esr1, Esr2, and GPR30. In both OVX and sham mice, Esr2 was the main receptor expressed in the utricle and saccule (ANOVA F(3,8) = 191.95, P = 8.6 × 10−8, Bonferroni correction P = 0.00027 vs. the ampulla, P = 9.9 × 10−5 vs. the canals, and P = 0.00046 vs. the cochlea in sham mice; F(3,8) = 406.65, P = 4.4 × 10−9, Bonferroni correction P = 0.00032 vs. the ampulla, P = 2.4 × 10−5 vs. the canals, and P = 7.8 × 10−5 vs. the cochlea in OVX mice), whereas GPR30 was the predominant receptor expressed in the cochlea (ANOVA F(3,8) = 176.74, P = 1.2 × 10−7, Bonferroni correction P = 0.0001 vs. the utricle + saccule, P = 0.00012 vs. the ampulla, and P = 0.019 vs. the canals in sham mice; ANOVA F(3,8) = 1018.06, P = 1.3 × 10−9, Bonferroni correction P = 1.5 × 10−5 vs. the utricle + saccule, P = 0.00037 vs. the ampulla, and P = 1.7 × 10−5 vs. the canals in OVX mice) (Fig. 5). At embryonic stages, GPR30 was the predominant receptor in all inner ear epithelial tissues (data not shown). The expression of Esr1 was either extremely low or negligible in all inner ear epithelia at embryonic, postnatal, or adult stages. Based on these data, we postulate that Esr2 may be critical in mediating the effects of estrogen in otoconia maintenance. This is further supported by our observation that Esr2 null mice have similar otoconia abnormalities compared to those of OVX mice, but Esr1 null mice have less severe otoconia defects (Fig. 6). In addition, the sizes of otoconia crystals in Esr2 null mice are larger than those in C57 mice in both the central (Cent-U) and peripheral (Peri-U) regions of the utricle (Fig. 6c vs. 6a; 6f vs. 6d, Fig. 6g, Cent-U, t(22) = −5.19, P = 3.3 × 10−5 when compared with C57; Peri-U, t(18) = −3.07, P = 0.0065 when compared with C57).
Fig. 5.
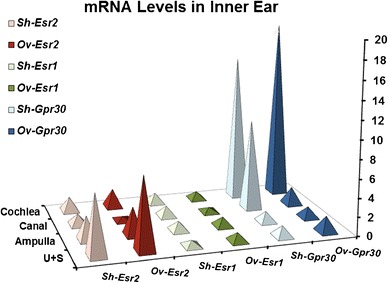
Expression of estrogen receptors in adult inner ear tissues and the effects of OVX on the expression by qRT-PCR. Ov, OVX mice; Sh, sham-operated mice; U + S, utricle + saccule. The utricle/saccule expression level of sham Esr1 relative to Actb was set to 1 (n = 3 each)
Fig. 6.
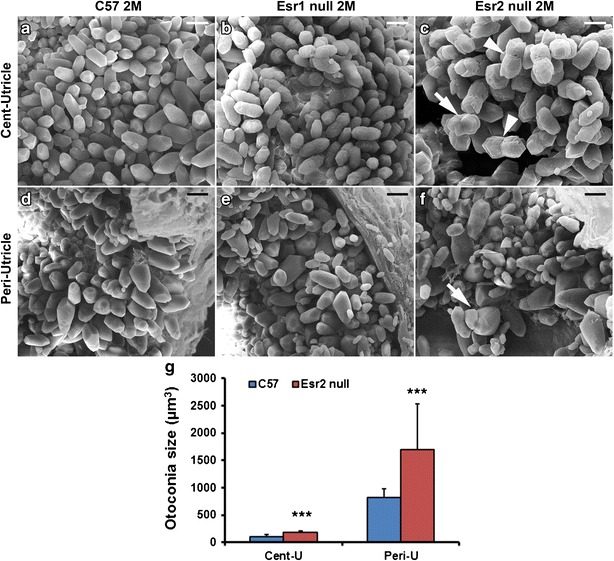
Otoconia abnormalities are more severe in the utricle of Esr2 null mice (c, f) than that of Esr1 null mice in both the center (b vs. c) and periphery of the tissue (e vs. f). Arrowhead in c denotes the wrinkled, peanut-shaped otoconial crystal and arrows in c and f denote fused otoconia. Sizes of otoconial crystals in the central and peripheral regions of the utricle (denoted as Cent-U and Peri-U, respectively) in Esr2 null and C57 mice were measured and compared in (g). Cent-U, t(22) = −5.19, P = 3.3 × 10−5 when compared with C57; Peri-U, t(18) = −3.07, P = 0.0065 when compared with C57. Scale bars: 5 μm in a–c; 10 μm in d–f
Discussion
Otoconia-related balance deficits and vertigo/dizziness are common, yet the molecular etiology of these deficits/diseases is completely unknown in humans. In fact, most genes and proteins responsible for otoconia formation, maintenance, and anchoring have been discovered only in the most recent decades. Our recent findings suggest a strong effect of estrogen changes in causing BPPV in humans (Ogun et al. 2014), and the present study has identified the aspects upon which estrogen deficiency affects otoconia. Furthermore, the present study used phytoestrogen to rescue such vestibular abnormalities for the first time, thus potentially solving a profound clinical problem.
Our data show that estrogen deficiency leads to insufficient maintenance of utricular and saccular otoconia and aberrant calcification in the ampulla, which can partially be rescued by dietary supplement of phytoestrogen (Fig. 2). Otoconia degeneration can potentially cause imbalance, not only because it mediates insufficient or inappropriate sensory input to the utricle and saccule, but also because degenerating otoconia are loose and prone to dislocation into the ampulla/canals, as shown in Fig. 2. This notion also explains the profound aging effect on BPPV occurrence (Gananca et al. 2010; Jonsson et al. 2004; Ogun et al. 2014; Ojala and Palo 1991; von Brevern et al. 2007). As aged inner ears are known to have otoconia degeneration (Anniko et al. 1984; Igarashi et al. 1993; Jang et al. 2006; Lim 1984; Ross et al. 1976; Takumida and Zhang 1997), it is highly likely that otoconia degeneration accompanies BPPV occurrence.
Further investigation demonstrates that there is insufficient expression of otoconial proteins, which underlies the otoconia degeneration in the OVX utricle and saccule, and concurrent ectopic expression of these proteins at unwanted sites (the ampulla), which likely contributes to ectopic calcification in OVX mice (Figs. 2, 3, and 4). This apparent paradox could be explained by the differential expression of estrogen receptor subtypes in these tissues (Fig. 5), which are coupled to different downstream signaling pathways, resulting in opposite effects of the hormone. Opposite effects of estrogen receptors in other tissues have been reviewed by Charitidi K et al. (Charitidi et al. 2009). Studies in other types of soft tissues, mostly cardiovascular tissues, show that ectopic calcification is an active, complex, and regulated process that exhibits intriguing similarities to normal calcification (Fitzpatrick et al. 2003; Rajamannan et al. 2003).
Our data suggest that Esr2 may play a major role in mediating the effects of estrogen in the vestibule (Fig. 6). Esr2 null mice are constitutive knockout (conditional mutants in all epithelial cell types are not available), so at present we cannot differentiate whether the observed otoconia defects in the null mice are due to abnormal development or maintenance. Given the expression (albeit at a low level) of Esr2 in both embryonic and adult stages, we postulate that both stages are affected. Esr2 null mice are prone to age-related hearing loss (tested at 12 months old) despite the initial normal hearing at a young age (3 months old), and the mice have severe loss of hair cells and ganglia in the cochlea at older ages (Simonoska et al. 2009b), therefore, we predict that the mice will have age-related otoconia and neurosensory (hair cells and ganglia) degeneration in the vestibule as well. Previous reports show that estrogen and receptors affect endolymph ion homeostasis and inner ear function through regulation of gene expression. For example, estrogen can directly modulate the activities of ion channels/pumps (Lee and Marcus 2001). The hearing and balance problems in Esrrb conditional null mice likely arise from abnormal endolymph production as a result of partial to nearly complete loss of transcripts for the following genes: Pendrin (Slc26a6) (a Cl−/I−/HCO3− transporter), K+ channels (Kcnq1, Kcne1), Na+/K+ transporters (Atp1b2, Atp1a1), a Na+/K+/Cl− transporter Slc12a2, and a regulator of Na+/Cl− symporter Wnk4 (lysine-deficient protein kinase 4) (Chen and Nathans 2007). As ion channels/pumps play important roles in otoconia formation and maintenance (for review see (Lundberg et al. 2015)), the levels and functional state of estrogen and receptors may affect otoconia homeostasis from this aspect as well, in addition to their direct effects on otoconia protein expression.
Intriguingly, the histological and molecular changes in the OVX inner ear did not result in significant changes in VsEPs. While linear VsEP is a good method to directly measure the functional state of the utricle and saccule, it does not provide information on that of the ampulla, nor can it detect subtle deficits in the former organs (Jones et al. 2004). Rotarod tests did show that OVX mice stayed on the accelerating rotarod for a significantly shorter time as compared with age-matched sham-operated mice. It is possible that additional sources (e.g., a weakened neuromuscular system under estrogen deficiency) could have contributed to the observed poorer performance on the rotarod. However, the rotarod test only requires minimal muscle strength, because the mice are standing on the rod, so it is more of a test for balance and coordination. We believe that otoconial degeneration affects the canal (ampulla) function via a BPPV-type effect, with which the symptoms are not constant but are triggered by certain head movements. Regardless, the current findings can be useful information for further research and for clinical remediation.
Conclusion
Our data show that estrogen deficiency leads to deficits in balance behaviors and otoconia maintenance and anchoring. Esr2 is likely an important contributor in mediating the effects of estrogen in the adult vestibule. We also show that phytoestrogen can potentially rescue such abnormalities caused by estrogen deficiency, although future studies are needed to determine the optimal dose. Since phytoestrogen appears to be somewhat protective against cancer, or at least not carcinogenic like estrogen (for review see (Pelekanou and Leclercq 2011)), our finding can be served as a starting point for future studies to provide a novel and safer strategy for treating menopause-triggered BPPV.
Funding Information
This work was supported in part by grants from the National Institutes of Health, DC008603, DC008603-S1 and DC014748 to Y.W.L.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Alexander A, Irving AJ, Harvey J. Emerging roles for the novel estrogen-sensing receptor GPER1 in the CNS. Neuropharmacology. 2017;113:652–660. doi: 10.1016/j.neuropharm.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Anniko M, Ylikoski J, Wroblewski R. Microprobe analysis of human otoconia. Acta Otolaryngol. 1984;97:283–289. doi: 10.3109/00016488409130990. [DOI] [PubMed] [Google Scholar]
- Charitidi K, Meltser I, Tahera Y, Canlon B. Functional responses of estrogen receptors in the male and female auditory system. Hear Res. 2009;252:71–78. doi: 10.1016/j.heares.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Chen J, Nathans J. Estrogen-related receptor beta/NR3B2 controls epithelial cell fate and endolymph production by the stria vascularis. Dev Cell. 2007;13:325–337. doi: 10.1016/j.devcel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Collin RW, Kalay E, Tariq M, Peters T, van der Zwaag B, Venselaar H, Oostrik J, Lee K, Ahmed ZM, Caylan R, Li Y, Spierenburg HA, Eyupoglu E, Heister A, Riazuddin S, Bahat E, Ansar M, Arslan S, Wollnik B, Brunner HG, Cremers CW, Karaguzel A, Ahmad W, Cremers FP, Vriend G, Friedman TB, Riazuddin S, Leal SM, Kremer H. Mutations of ESRRB encoding estrogen-related receptor beta cause autosomal-recessive nonsyndromic hearing impairment DFNB35. Am J Hum Genet. 2008;82:125–138. doi: 10.1016/j.ajhg.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mansoury M, Barrenas ML, Bryman I, Hanson C, Landin-Wilhelmsen K. Impaired body balance, fine motor function and hearing in women with turner syndrome. Clin Endocrinol. 2009;71:273–278. doi: 10.1111/j.1365-2265.2008.03473.x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LA, Turner RT, Ritman ER. Endochondral bone formation in the heart: a possible mechanism of coronary calcification. Endocrinology. 2003;144:2214–2219. doi: 10.1210/en.2002-0170. [DOI] [PubMed] [Google Scholar]
- Ford J, Hajibeigi A, Long M, Hahner L, Gore C, Hsieh JT, Clegg D, Zerwekh J, Oz OK. GPR30 deficiency causes increased bone mass, mineralization, and growth plate proliferative activity in male mice. J Bone Miner Res. 2011;26:298–307. doi: 10.1002/jbmr.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gananca FF, Gazzola JM, Gananca CF, Caovilla HH, Gananca MM, Cruz OL. Elderly falls associated with benign paroxysmal positional vertigo. Braz J Otorhinolaryngol. 2010;76:113–120. doi: 10.1590/S1808-86942010000100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallworth R, Wiederhold ML, Campbell JB, Steyger PS. Atomic force microscope observations of otoconia in the newt. Hear Res. 1995;85:115–121. doi: 10.1016/0378-5955(95)00038-6. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Bashiardes S, Powder KE, Sajan SA, Bhonagiri V, Alvarado DM, Speck J, Warchol ME, Lovett M. Large scale gene expression profiles of regenerating inner ear sensory epithelia. PLoS One. 2007;2:e525. doi: 10.1371/journal.pone.0000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lubahn DB, Zhou Q, Bouma J. Morphologic changes in efferent ductules and epididymis in estrogen receptor-alpha knockout mice. J Androl. 2000;21:107–121. [PubMed] [Google Scholar]
- Igarashi M, Saito R, Mizukoshi K, Alford BR. Otoconia in young and elderly persons: a temporal bone study. Acta Otolaryngol Suppl. 1993;504:26–29. doi: 10.3109/00016489309128117. [DOI] [PubMed] [Google Scholar]
- Jang YS, Hwang CH, Shin JY, Bae WY, Kim LS. Age-related changes on the morphology of the otoconia. Laryngoscope. 2006;116:996–1001. doi: 10.1097/01.mlg.0000217238.84401.03. [DOI] [PubMed] [Google Scholar]
- Jones TA, Jones SM (1999) Short latency compound action potentials from mammalian gravity receptor organs. Hear Res 136:75–85 [DOI] [PubMed]
- Jones SM, Erway LC, Bergstrom RA, Schimenti JC, Jones TA. Vestibular responses to linear acceleration are absent in otoconia-deficient C57BL/6JEi-het mice. Hear Res. 1999;135:56–60. doi: 10.1016/S0378-5955(99)00090-8. [DOI] [PubMed] [Google Scholar]
- Jones SM, Erway LC, Johnson KR, Yu H, Jones TA. Gravity receptor function in mice with graded otoconial deficiencies. Hear Res. 2004;191:34–40. doi: 10.1016/j.heares.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Jonsson R, Sixt E, Landahl S, Rosenhall U. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47–52. [PubMed] [Google Scholar]
- Kilicdag EB, Yavuz H, Bagis T, Tarim E, Erkan AN, Kazanci F. Effects of estrogen therapy on hearing in postmenopausal women. Am J Obstet Gynecol. 2004;190:77–82. doi: 10.1016/j.ajog.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Kim TH, Lee HH. G-protein coupled estrogen receptor (GPER/GPR30) and Women's health. J Menopausal Med. 2015;21:79–81. doi: 10.6118/jmm.2015.21.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Marcus DC. Estrogen acutely inhibits ion transport by isolated stria vascularis. Hear Res. 2001;158:123–130. doi: 10.1016/S0378-5955(01)00316-1. [DOI] [PubMed] [Google Scholar]
- Legan PK, Lukashkina VA, Goodyear RJ, Kossi M, Russell IJ, Richardson GP. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron. 2000;28:273–285. doi: 10.1016/S0896-6273(00)00102-1. [DOI] [PubMed] [Google Scholar]
- Lim DJ. Otoconia in health and disease. A review Ann Otol Rhinol Laryngol Suppl. 1984;112:17–24. doi: 10.1177/00034894840930S404. [DOI] [PubMed] [Google Scholar]
- Lundberg YW, Xu Y, Thiessen KD, Kramer KL. Mechanisms of otoconia and otolith development. Dev Dyn. 2015;244:239–253. doi: 10.1002/dvdy.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltser I, Tahera Y, Simpson E, Hultcrantz M, Charitidi K, Gustafsson JA, Canlon B. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest. 2008;118:1563–1570. doi: 10.1172/JCI32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi R, Takumida M, Shimizu A, Konomi U, Fujita K, Hirakawa K, Suzuki M, Anniko M. Effects of age and sex on the expression of estrogen receptor alpha and beta in the mouse inner ear. Acta Otolaryngol. 2010;130:204–214. doi: 10.3109/00016480903016570. [DOI] [PubMed] [Google Scholar]
- Murayama E, Herbomel P, Kawakami A, Takeda H, Nagasawa H. Otolith matrix proteins OMP-1 and Otolin-1 are necessary for normal otolith growth and their correct anchoring onto the sensory maculae. Mech Dev. 2005;122:791–803. doi: 10.1016/j.mod.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Ogun OA, Buki B, Cohn ES, Janky KL, Lundberg YW. Menopause and benign paroxysmal positional vertigo. Menopause. 2014;21:886–889. doi: 10.1097/GME.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala M, Palo J. The aetiology of dizziness and how to examine a dizzy patient. Ann Med. 1991;23:225–230. doi: 10.3109/07853899109148052. [DOI] [PubMed] [Google Scholar]
- Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80:34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Pelekanou V, Leclercq G. Recent insights into the effect of natural and environmental estrogens on mammary development and carcinogenesis. Int J Dev Biol. 2011;55:869–878. doi: 10.1387/ijdb.113369vp. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol Cell Endocrinol. 2009;308:32–38. doi: 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Gersh B, Bonow RO. Calcific aortic stenosis: from bench to the bedside—emerging clinical and cellular concepts. Heart. 2003;89:801–805. doi: 10.1136/heart.89.7.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MD, Peacor D, Johnsson LG, Allard LF. Observations on normal and degenerating human otoconia. Ann Otol Rhinol Laryngol. 1976;85:310–326. doi: 10.1177/000348947608500302. [DOI] [PubMed] [Google Scholar]
- Sajan SA, Warchol ME, Lovett M. Toward a systems biology of mouse inner ear organogenesis: gene expression pathways, patterns and network analysis. Genetics. 2007;177:631–653. doi: 10.1534/genetics.107.078584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoska R, Stenberg A, Masironi B, Sahlin L, Hultcrantz M. Estrogen receptors in the inner ear during different stages of pregnancy and development in the rat. Acta Otolaryngol. 2009;129:1175–1181. doi: 10.3109/00016480802691150. [DOI] [PubMed] [Google Scholar]
- Simonoska R, Stenberg AE, Duan M, Yakimchuk K, Fridberger A, Sahlin L, Gustafsson JA, Hultcrantz M. Inner ear pathology and loss of hearing in estrogen receptor-beta deficient mice. J Endocrinol. 2009;201:397–406. doi: 10.1677/JOE-09-0060. [DOI] [PubMed] [Google Scholar]
- Stein RA, McDonnell DP. Estrogen-related receptor alpha as a therapeutic target in cancer. Endocr Relat Cancer. 2006;13(Suppl 1):S25–S32. doi: 10.1677/erc.1.01292. [DOI] [PubMed] [Google Scholar]
- Stenberg AE, Wang H, Sahlin L, Stierna P, Enmark E, Hultcrantz M. Estrogen receptors alpha and beta in the inner ear of the ‘Turner mouse’ and an estrogen receptor beta knockout mouse. Hear Res. 2002;166:1–8. doi: 10.1016/S0378-5955(02)00310-6. [DOI] [PubMed] [Google Scholar]
- Takumida M, Zhang DM. Electron probe X-ray microanalysis of otoconia in guinea pig inner ear: a comparison between young and old animals. Acta Otolaryngol. 1997;117:529–537. doi: 10.3109/00016489709113433. [DOI] [PubMed] [Google Scholar]
- Vanacker JM, Pettersson K, Gustafsson JA, Laudet V. Transcriptional targets shared by estrogen receptor- related receptors (ERRs) and estrogen receptor (ER) alpha, but not by ERbeta. EMBO J. 1999;18:4270–4279. doi: 10.1093/emboj/18.15.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert D, Sans A, Kompis M, Travo C, Muhlbauer RC, Tschudi I, Boukhaddaoui H, Hausler R. Ultrastructural changes in otoconia of osteoporotic rats. Audiol Neurootol. 2008;13:293–301. doi: 10.1159/000124277. [DOI] [PubMed] [Google Scholar]
- Vijayakumar S, Depreux FF, Jodelka FM, Lentz JJ, Rigo F, Jones TA, Hastings ML. Rescue of peripheral vestibular function in Usher syndrome mice using a splice-switching antisense oligonucleotide. Hum Mol Genet. 2017;26:3482–3494. doi: 10.1093/hmg/ddx234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, Lempert T, Neuhauser H. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. 2007;78:710–715. doi: 10.1136/jnnp.2006.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Brevern M, Bertholon P, Brandt T, Fife T, Imai T, Nuti D, Newman-Toker D. Benign paroxysmal positional vertigo: diagnostic criteria. J Vestib Res. 2015;25:105–117. doi: 10.3233/VES-150553. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhang H, Yang H, Zhao X, Lovas S, Lundberg YW. Expression, functional and structural analysis of proteins critical for otoconia development. Dev Dyn. 2010;239:2659–2673. doi: 10.1002/dvdy.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhao X, Xu Y, Wang L, He Q, Lundberg YW. Matrix recruitment and calcium sequestration for spatial specific otoconia development. PLoS One. 2011;6:e20498. doi: 10.1371/journal.pone.0020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yang H, Yamoah EN, Lundberg YW. Gene targeting reveals the role of Oc90 as the essential organizer of the otoconial organic matrix. Dev Biol. 2007;304:508–524. doi: 10.1016/j.ydbio.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


