Abstract
Permanent loss of auditory nerve (AN) fibers occurs with increasing age and sound overexposure, sometimes without hair cell damage or associated audiometric threshold elevation. Rodent studies suggest effects of AN damage on central processing and behavior, but these species have limited capacity to discriminate low-frequency speech-like sounds. Here, we introduce a new animal model of AN damage in an avian communication specialist, the budgerigar (Melopsittacus undulatus). The budgerigar is a vocal learner and speech mimic with sensitive low-frequency hearing and human-like behavioral sensitivity to many complex signals including speech components. Excitotoxic AN damage was induced through bilateral cochlear infusions of kainic acid (KA). Acute KA effects on cochlear function were assessed using AN compound action potentials (CAPs) and hair cell cochlear microphonics (CMs). Long-term KA effects were assessed using auditory brainstem response (ABR) measurements for up to 31 weeks post-KA exposure. KA infusion immediately abolished AN CAPs while having mild impact on the CM. ABR wave I, the far-field AN response, showed a pronounced 40–75 % amplitude reduction at moderate-to-high sound levels that persisted for the duration of the study. In contrast, wave I latency and the amplitude of wave V were nearly unaffected by KA, and waves II–IV were less reduced than wave I. ABR thresholds, calculated based on complete response waveforms, showed no impairment following KA. These results demonstrate that KA exposure in the budgerigar causes irreversible AN damage, most likely through excitotoxic injury to afferent fibers or synapses as in other species, while sparing ABR thresholds. Normal wave V amplitude, assumed to originate centrally, may persist through compensatory mechanisms that restore central response amplitude by downregulating inhibition. Future studies in this new animal model of AN damage can explore effects of this neural lesion, in isolation from hair cell trauma and threshold elevation, on central processing and perception of complex sounds.
Keywords: auditory brainstem response, central gain, cochlear microphonic, compound action potential
INTRODUCTION
Each human cochlea contains ~ 30,000 auditory nerve (AN) fibers at birth that innervate the inner hair cells of the organ of Corti (Spoendlin and Schrott 1989). These fibers, which provide the only path for auditory information to the CNS, are lost with age and acoustic overexposure. Age-related AN degeneration progresses steadily at a rate of 1000–2000 fibers per decade of life (Otte et al. 1978; Makary et al. 2011). Noise-induced AN loss occurs following hair cell death (Spoendlin 1984) and also as a potential consequence of glutamate excitotoxicity at hair cell afferent synapses (Young 2013). Both age- and noise-related AN degeneration can occur alongside hair cell damage, in which case audiometric threshold shifts occur, or in the absence of hair cell damage (Schuknecht and Gacek 1993).
AN loss without hair cell damage is intriguing, because lesions of this type might impair complex sound perception yet be undetectable with a clinical audiogram (Schuknecht and Woellner 1953; Schuknecht 1994; Kujawa and Liberman 2009; Lin et al. 2011; Makary et al. 2011; Bharadwaj et al. 2014; Liberman and Kujawa 2017). AN degeneration could potentially cause central processing deficits that impair complex-sound perception in noise, but few studies have tested this hypothesis. Profound ouabain-induced AN damage (> 95 % fiber loss) in mice leads to compensatory increases in central neural excitability at the midbrain level and especially in the auditory cortex (Chambers et al. 2016). Behavioral consequences of AN damage are less explored but include impairment of acoustic startle reflexes in ouabain-treated mice (Chambers et al. 2016) and in rats with modest noise-induced neuropathy (Lobarinas et al. 2017). In contrast, pure-tone detection thresholds measured using operant conditioning are unaffected by ouabain damage in mice (Chambers et al. 2016).
Existing behavioral studies of AN degeneration have mostly been conducted in rodents with insensitive low-frequency hearing (Heffner 1980; Koay et al. 2002) and limited capacity to discriminate complex speech-like sounds. New animal models with more human-like behavioral sensitivity to speech components can provide additional insight, by identifying specific aspects of complex sound perception impacted by AN damage and changes in central coding responsible for perceptual deficits. The budgerigar (Melopsittacus undulatus) is a small parrot (psittacine) with sensitive low-frequency hearing (Dooling and Saunders 1975) and the capacity to regenerate hair cells following cochlear injury as in other bird species (Corwin and Cotanche 1988; Ryals and Rubel 1988; Hashino et al. 1992). Budgerigars are lifelong vocal learners with the ability to imitate human speech. Behavioral thresholds in budgerigars are as sensitive as human thresholds for amplitude-modulation detection (Dooling and Searcy 1981; Carney et al. 2013; Henry et al. 2016) and discrimination of many simple and complex sounds including synthesized vowels (Dooling et al. 1989, 1995; Dooling and Brown 1990; Henry et al. 2017; reviewed in Dooling et al. 2000). Furthermore, neurophysiological studies show that the budgerigar auditory pathway encodes complex sounds based on principles shared between birds and mammals (Henry et al. 2016, 2017), at least up to the midbrain level. Midbrain neurons in both taxa exhibit band-pass modulation tuning of average discharge rate in response to envelope fluctuation frequency (Woolley and Portfors 2013). A budgerigar model of AN degeneration can provide new insight into effects on perception and central processing.
Kainic acid (KA) is a glutamate analog that damages AN afferent fibers through excitotoxicity as in cases of noise overexposure, but without damage to hair cells (Pujol et al. 1985; Juiz et al. 1989; Puel et al. 1998). Previous studies in chicken show that KA infusion into the perilymph causes permanent AN damage while leaving hair cells functionally intact (Sun et al. 2000, 2001). Our goal here was to determine whether the same KA effects occur in the budgerigar, as part of a larger effort to develop a new animal model of AN damage/synaptopathy with human-like auditory perceptual capabilities. We recorded AN compound action potentials (CAPs) and hair cell cochlear microphonics (CMs) in budgerigars before and immediately following KA infusion to characterize acute KA effects on cochlear function. Auditory brainstem responses (ABRs) were recorded prior to infusions and at multiple time points thereafter, for up to 31 weeks, to assess longitudinal changes in wave I amplitude and ABR thresholds. ABR wave I provides a measure of summed AN activity (Brittan-Powell et al. 2002). ABR thresholds were used as an indirect measure of hair cell status based on previous studies (Woolley et al. 2001; Harding et al. 2002; Harding and Bohne 2004; Saunders 2010). The results show that KA exposure in budgerigars causes pronounced and irreversible AN damage without threshold impairment.
MATERIALS AND METHODS
Procedures were performed in young (4–12 months old) budgerigars (M. undulatus) of both sexes (four females; four males) and approved by the University of Rochester Committee on Animal Resources. All procedures, which included head-post surgeries, ABR recordings, and cochlear infusions, were conducted in anesthetized animals. Anesthesia was induced with ketamine (3–5 mg/kg) and dexmedetomidine (0.08–0.1 mg/kg, subcutaneous) and maintained during longer procedures through slow infusion of ketamine (6–10 mg/kg/h), dexmedetomidine (0.16/0.2 mg/kg/h), and lactated Ringer’s solution (30–50 ml/kg/h). Animals were positioned on a warming pad (Adroit HTP-1500, Loudon, TN, USA) with their body covered by a towel. Body temperature was monitored with a thermocouple placed against the breast muscle and remained at 38–40 °C. Breathing rate was monitored using a thermistor-based sensor positioned near the nares. Birds were given atipamezole (0.5 mg/kg subcutaneous) following all procedures, to reverse effects of dexmedetomidine, and allowed to recover in a heated chamber until fully alert. The analgesic carprofen (1 mg/kg, subcutaneous) was given prior to surgeries and once daily for 2 to 3 days thereafter.
In the first surgical procedure, birds were implanted with a head post that facilitated positioning of the animal during subsequent ABR recordings and cochlear infusions. Head-post surgeries were conducted under anesthesia as described previously. An area of the skull ~ 10 mm in diameter was exposed with an incision, cleared of tissue, and etched with phosphoric acid gel. The head post was anchored to the skull using M0.6 stainless steel machine screws and light-curing dental composite material (Kerr Vertise Flow; Orange, CA, USA). Anchor screws were advanced through pre-drilled pilot holes to the level of the dura. The screw nearest the vertex had a gold pin attached, for use in electrophysiological recordings. Cochlear infusions were performed bilaterally in each animal, with left and right ears treated during different procedures 4 weeks apart to avoid extended periods of anesthesia. ABR recordings were conducted prior to infusions (three–four sessions) and at multiple time points following the second infusion, for up to 31 weeks post-exposure (seven–nine sessions).
Auditory Brainstem Response Recording Procedures
ABR recordings were conducted in a double-walled acoustic isolation booth (Industrial Acoustics; 2 × 2.1 × 2.2 m) lined with 5-cm thick sound-absorbing foam (Pinta Acoustic, Minneapolis, MN, USA). Birds were anesthetized as described previously and placed in a stereotaxic apparatus located on a foam-covered table. The apparatus held the bird’s head 9 cm above the table with the rostral surface of the skull facing downward. A free-field loudspeaker (Polk Audio MC60, Baltimore, MD, USA) was located 45 cm from the bird and 25 cm above the table surface, directed toward the stereotaxic apparatus. Acoustic stimuli were generated in MATLAB (The MathWorks, Natick, MA, USA; 50 kHz sampling frequency) and converted to analog (National Instruments PCIe-6251, Austin, TX, USA) prior to power amplification (Crown D-75A, Elkhart, IN, USA) and presentation by the loudspeaker. Sound levels were calibrated based on the output of a 1/4-inch precision microphone (Brüel and Kjær type 4938, Marlborough, MA, USA) in response to steady-state tones. Electrophysiological activity was recorded differentially using the vertex screw as the noninverting electrode. Subdermal platinum needle electrodes (Grass F-E2; Natus Manufacturing, Gort, Co. Galway, Ireland) positioned behind the left and right ears served as inverting (reference) and ground electrodes, respectively. Electrophysiological activity was amplified (50 k) and bandpass filtered (30–10,000 Hz; Grass IP511, West Warwick, RI, USA) prior to digital sampling (31.25 kHz; National Instruments PCIe-6251) and storage on the computer hard drive.
ABRs were recorded in response to clicks and tones with frequencies of 1, 2, and 3 kHz. Clicks were square waves with duration of 0.1 ms. Tones had 1-ms, cosine-squared onset and offset ramps and were 15 ms in duration. ABR recording sessions were ~ 30 min long and started with a block of click stimuli of varying levels. Three blocks of tones were then presented in random sequence, followed by a second block of clicks. Within each block, clicks or tones were presented 19.2/s in repeating sequences of alternating polarity. Sequences consisted of six stimuli of increasing level, from 30 to 80 dB SPL, in 10-dB steps. Click sound levels were defined based on the peak-equivalent method, i.e., the sound pressure level of a steady-state 1000-Hz tone with the same peak-to-baseline amplitude as the transient. A total of 300 stimuli were presented for each level–polarity combination. ABR waveforms were calculated as the average response to stimuli of both polarities (600 presentations).
ABR wave I amplitude was measured as the absolute voltage difference between the first major negative deflection of the response and the preceding baseline (Brittan-Powell et al. 2002) (Fig. 1, red). The preceding baseline (averaged from 1 ms before stimulus onset to 0.5 ms after) was used as the reference voltage rather than the subsequent peak to minimize possible contribution of early brainstem activity to the measurement. Wave I latency was measured relative to stimulus onset at the ear canal. Wave V was identified as the slower, negative deflection occurring ~ 10 ms after wave I (Fig. 1, blue). Wave V amplitude was measured as the mean voltage of the preceding and subsequent positive peaks minus the voltage of this negative deflection. Additionally, ABR amplitude was quantified as the root-mean-square (RMS) energy of the response over analysis time windows encompassing wave I (1 ms before to 1 ms after), waves II–IV (1–6 ms after wave I), and wave V (6–16 ms after wave I). RMS energy was calculated after first subtracting the mean voltage in the analysis window.
Fig. 1.
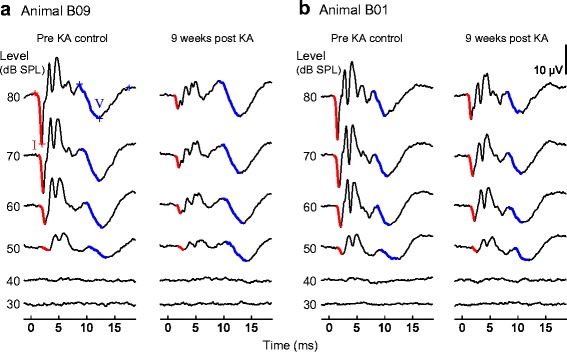
Representative auditory brainstem responses (ABRs) recorded before and after kainic acid (KA) infusion. Waveforms are presented for animals B09 (a) and B01 (b). Each ABR is the average response to 600 click stimuli of alternating polarity. Time is expressed relative to stimulus arrival at the ear canal. ABRs are arranged from top to bottom in order of decreasing stimulus level, with waves I and V outlined in red and blue, respectively. ABRs recorded before KA infusion (“pre-KA control”) and 8–9 weeks thereafter (“post-KA”) show that KA reduces wave I amplitude at moderate-to-high sound levels, has minimal impact on wave V amplitude, and does not impair the ABR threshold
ABR thresholds were estimated based on an analysis of complete response waveforms (0–20 ms following stimulus onset). Each ABR was initially scored as visible (at least one ABR wave present) or not visible by an experienced observer, and its RMS energy computed over the first 20 ms of the response. The ABR threshold was determined from an analysis of visible ABRs as the sound level at which the response energy exceeded 0.75 μV, i.e., approximately 2.5–3 times the typical noise level in the averaged physiological recordings (median 0.273 μV; interquartile range 0.251–0.313 μV). Noise level was calculated as the average RMS energy of 1000 null responses, where each null response was the mean of 600, 20-ms waveform segments drawn randomly from the raw physiological recording. ABR thresholds were calculated by either linear interpolation or extrapolation, by up to 10 dB, of the unsmoothed ABR energy by sound level function. Extrapolation was required for 23.3 % of thresholds and was typically performed over less than a few decibels. In practice, this procedure yielded thresholds that were consistent with visual inspection of the waveforms. Threshold resolution could be increased with a smaller step size (e.g., 5 dB) but appeared acceptable based on our observation that click thresholds repeated 15–20 min apart within the same session were generally within 2 dB of each other. Across 20 sessions for which repeated click-ABR thresholds were available, the absolute difference in the threshold had a median value of 0.98 dB and interquartile range from 0.24 to 1.99 dB.
Cochlear Infusions
Bilateral cochlear infusions were conducted under anesthesia in the same experimental apparatus used for ABR recordings. Left and right ears were infused separately, 4 weeks apart during different procedures. The basal, high-frequency end of the cochlea was exposed near its input from the middle ear system based on surgical procedures described by Konishi (1964). Briefly, the skin overlying the crossing of the horizontal and posterior semicircular canals was incised, and the neck muscle bluntly separated at this location and spread apart with fine-wire hooks to reveal the canals through the semi-translucent skull. A flap of bone was created in the area ventral to the horizontal canal and rostral to the posterior canal using fine forceps and folded up to expose the middle ear space and dome-shaped bony prominence of the cochlear base. Note that the ampullae of the horizontal and posterior canals are visible with this approach, but not the columella or round window due to obstruction by the external auditory meatus. A 150-μm opening was made in the cochlear prominence using gentle pressure on a small hand drill, thus allowing access to the underlying perilymphatic space.
Infusions were made through a 35-gauge needle with a blunted tip, advanced ~ 0.2 mm into the cochleostomy under stereotaxic control, and coupled to a 10-μL syringe through flexible tubing. Infusions were conducted over 90 s of either (1) 2.5 μL of 1-mM KA in Hanks’ balanced salt solution (HBSS, Sigma-Aldrich H8264; Saint Louis, MO, USA) or (2) the same volume of HBSS without KA. This formulation of HBSS contains 138 mM NaCl, 5.33 mM KCl, 1.66 mM CaCl2, 0.41 mM MgCl2, and 5.6 mM glucose, similar to avian and mammalian perilymph (Sauer et al. 1999). The 1-mM KA dosage was selected in an attempt to produce moderate-to-severe AN damage, based on prior findings in chicken that 0.5 mM KA causes mild-to-moderate AN loss whereas 5 mM KA produces severe or profound damage (Sun et al. 2000, 2001). Excess solution was absorbed with paper points, and the bony flap was then closed over the skull opening. Neck muscles were moistened with saline solution and returned to their original position, and the overlying skin closed with tissue adhesive (Vetbond, 3M; Saint Paul, MN, USA). Infusion procedures were typically completed within 1–1.5 h. Birds were able to stand and eat within 1–2 h of the procedure and showed a head tilt toward the infused ear that recovered over several days.
Compound Action Potential and Cochlear Microphonic Recording Procedures
CAP and CM measurements were made immediately before and after KA infusion of each ear using an 80-μm tungsten wire advanced into the cochleostomy until in contact with the perilymph. A needle electrode in the neck muscle was used as a grounded reference for these recordings. The CAP (Fig. 2a; thick lines) was evoked primarily with click stimuli. The CM (Fig. 2a; thin lines, right) was evoked using 4-kHz tones because this frequency minimizes the contribution of AN phase locking to the CM and thus provides a fairly selective measure of hair cell function. While the impact of KA on AN phase locking was also of interest, these effects were considered better explored in a future study of AN single-fiber response properties. Four kilohertz was also chosen because this stimulus frequency was found to elicit large CM responses. Stimuli were presented over the same level range used in ABR experiments, but with 100 presentations for each level–polarity combination and with lower amplification (10 k). Tone duration was 5 or 15 ms. The CAP was calculated as the average response to stimuli of both polarities. The CM was calculated as the average of the response to the positive-polarity stimulus and the inverted response to the negative-polarity stimulus. CAP amplitude was measured as the voltage difference between the N1 and P1 components of the response waveform (Fig. 2a, left). CAP thresholds were estimated with the same method described for ABRs, as the sound level predicted to evoke a 4-μV response. The peak-to-peak amplitude of the CM was measured from the Fourier transform of 4 ms of the steady-state tone response. In general, the scope of CAP/CM recordings was limited to help minimize the overall duration of infusion procedures.
Fig. 2.
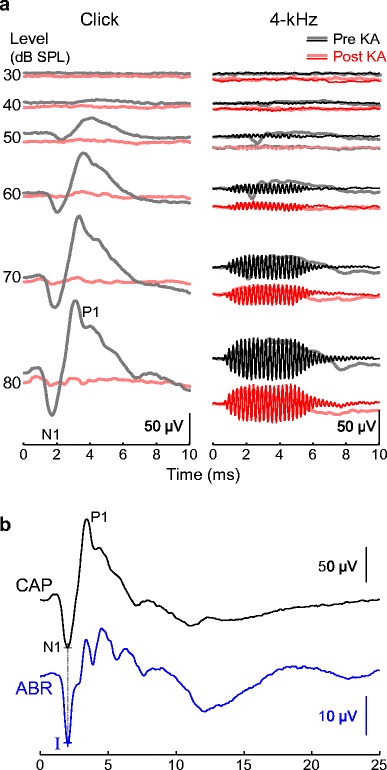
Representative cochlear potentials measured before and immediately following KA infusion. a Cochlear potentials evoked by clicks (left) and 4-kHz tones (right), recorded from the perilymph. Click responses show the compound action potential (CAP) of the auditory nerve. Tone responses show either the CAP and summating potential (SP; thick lines) or the cochlear microphonic (CM; thin lines) depending on whether mean responses to both stimulus polarities are added or subtracted, respectively, prior to division by two. KA selectively abolishes the CAP whereas the CM is reduced by 14.1 % in this example. Recording parameters were not optimal for the SP, but reduction appears intermediate. b Comparison of click-evoked CAP and ABR waveforms. Wave I of the ABR coincides with CAP N1. Time is expressed relative to stimulus onset at the ear canal
Statistical Analyses
Statistical analyses were conducted in R (version 3.4.1) using linear mixed-effects models (Bates et al. 2015). Dependent variables included cochlear response amplitude (CAP or CM), ABR wave I amplitude, ABR wave V amplitude, ARB wave I latency, and ABR threshold. Subject intercepts were modeled as a random effect. Within-subject fixed effects included KA exposure status (pre- vs. post-infusion), stimulus type (click, 1-, 2-, or 3-kHz tone), and sound level (50, 60, 70, or 80 dB SPL). Interactions were included between fixed effects and dropped when not significant (p < 0.05) in order of decreasing p value. Degrees of freedom for F tests were calculated based on the Satterthwaite approximation. Visual inspection of model results showed that residuals were normally distributed after log transformation of wave I and wave V amplitudes.
RESULTS
Kainic Acid Immediately Abolishes Auditory Nerve CAPs
Cochlear recordings were made both before and immediately following KA infusion in nine ears of six budgerigars. CAPs evoked by clicks and 4-kHz tones prior to infusion showed a prominent N1-P1 deflection from 2 to 4 ms following stimulus onset (Fig. 2a; thick gray lines). CM responses exhibited sustained oscillations at the 4-kHz stimulus frequency (Fig. 2a, right). CAP and CM amplitude increased with increasing sound level. The CAP threshold for click stimuli prior to KA was 49.22 ± 4.59 dB SPL (mean ± SD). CAPs were essentially abolished following KA infusion in eight of nine ears, even at 80 dB SPL precluding estimating of a threshold shift, and reduced by 87 % in the remaining ear (Fig. 2a, left; Fig. 3a, left). In contrast, CMs were either unaffected by KA or partly reduced in amplitude (Fig. 2a, right; Fig. 3a, right). The mean amplitude change across sound levels from 60 to 80 dB SPL was − 22.60 ± 4.01 dB for the click-evoked CAP (mean ± SD; 92.6 % reduction) and − 2.90 ± 3.26 dB for the CM (28.4 % reduction; Fig. 3b). These results show that immediately following infusion, KA selectively impairs neural responses over potentials associated with hair cell activity. A sustained summating potential (SP) was also noted in response to alternating polarity, 4-kHz tones that persisted for the duration of the stimulus (Fig. 2a, right; thick lines). Recording parameters were not optimized for the SP, but slight reduction of this response following KA rather than elimination suggests that the SP was perhaps primarily generated by hair cells and partly neural in origin.
Fig. 3.
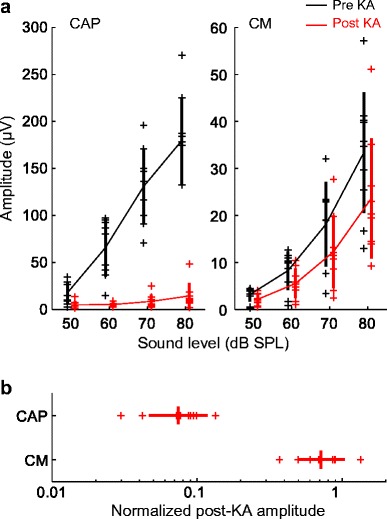
Immediate KA effects on cochlear potentials. a CAP and CM amplitude are shown for nine ears (six animals) as a function of stimulus level, before and immediately following KA infusion. Thick vertical lines indicate means ± SD. b Post-KA amplitude of CAPs and CMs in nine ears, expressed as a proportion of the pre-exposure baseline amplitude in the same animal and averaged across sound levels from 60 to 80 dB SPL. KA nearly abolishes the CAP and mildly reduces CM amplitude
A mixed-model analysis of cochlear response amplitude before and after KA infusion showed a significant effect of response type (CM vs. click-evoked CAP; F1,25.7 = 30.35, p < 0.0001) due to lower amplitude of the CM compared to CAPs. The dependent variable was the mean log-transformed response amplitude for each ear, across stimulus levels from 60 to 80 dB SPL. Effects of KA exposure (F1,24.7 = 101.78, p < 0.0001) and the interaction between KA exposure and response type (F1,24.7 = 57.53, p < 0.0001) were also significant model factors. The interaction was driven by a stronger negative effect of KA on CAPs than on the CM. Pairwise comparisons of least-squares means from the full model showed that KA caused a significant reduction for CAP amplitude (− 23.6 ± 1.91 dB; mean difference ± SE; t24.5 = − 12.37, p < 0.0001) but not CM amplitude (− 3.30 ± 1.87 dB; t24.8 = − 1.79, p = 0.086). In contrast, a reduced model including only CM data showed that the amplitude reduction following KA was statistically significant (− 3.05 ± 1.07 dB [mean difference ± SE]; t8.2 = − 2.85, p = 0.021). Thus, in the minutes immediately following infusion, KA dramatically reduced AN activity while also causing a minor decrease in hair cell responses. The reason for mild CM reduction was not explored, but possible explanations include cooling of hair cells during KA infusions (Schermuly and Klinke 1985) or perhaps contraction of the columellar (middle ear) muscle.
KA Causes Persistent Reduction of ABR Wave I Amplitude
ABRs were recorded longitudinally for 12–31 weeks in five animals to track auditory recovery from KA excitotoxicity over time. ABR wave I is the far-field representation of the AN CAP (Fig. 2b) (Brittan-Powell et al. 2002), whereas ABR threshold shifts generally indicate hair cell injury (Woolley et al. 2001; Harding et al. 2002; Harding and Bohne 2004; Saunders 2010). ABR waveforms evoked by clicks contained four to five prominent waves within 15 ms of stimulus onset (Fig. 1). Wave I was clearly identifiable in most recordings, both before and after bilateral infusions of KA, as the first prominent negative deflection of the response. Wave V was also typically visible as a negative deflection occurring ~ 10 ms after wave I. The neural origin of wave V was not explored, but its longer latency in comparison to midbrain neural responses in this species (typically 4–5 ms; unpublished observations) suggests possible generation at the forebrain level (Hall 1992). Representative click ABR waveforms recorded before and 9 weeks following bilateral KA infusion illustrate the main findings of the study (Fig. 1). KA causes marked, persistent reduction of wave I amplitude at moderate-to-high sound levels (≥ 60 dB SPL), has minimal effect on ABR wave V, and does not impair ABR thresholds.
For click stimuli presented at moderate-to-high sound levels (60–80 dB SPL), post-infusion ABRs showed a pronounced reduction in wave I amplitude followed by minimal recovery over the first 3 to 4 weeks post-infusion (Fig. 4). No further recovery was noted from 4 to 31 weeks post-infusion in any animal, consistent with a permanent KA lesion of the AN. The long-term change in wave I amplitude was calculated based on recordings made 6 or more weeks following infusion. For click stimuli presented at 80 dB SPL, the reduction in wave I amplitude ranged from 40 to 75 % (Fig. 5). Note that wave I amplitude was not reduced following two sham infusions of HBSS in one animal (Fig. 4; × symbols showing data from B07).
Fig. 4.
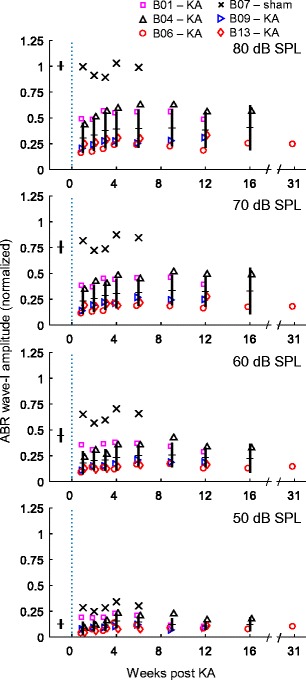
Variation over time in click-evoked wave I amplitude following KA infusion. Measurements were normalized in each animal by dividing by the pre-exposure, 80 dB SPL control value. Different symbols indicate responses of five KA-treated animals and one sham-control animal (see legend, top). Horizontal ticks intersected by a vertical line indicate across-subject means (± 1 SD) of the KA group. KA causes a pronounced decrease in ABR wave I amplitude at moderate-to-high sound levels followed by limited recovery over the first 3–4 weeks post-infusion. No change in wave I amplitude occurs beyond 4 weeks post-infusion, consistent with permanent and stable AN damage in these animals. The long-term average reduction of wave I ranged from 40 to 75 % across animals. No effect of bilateral sham infusions was noted in the control animal
Fig. 5.
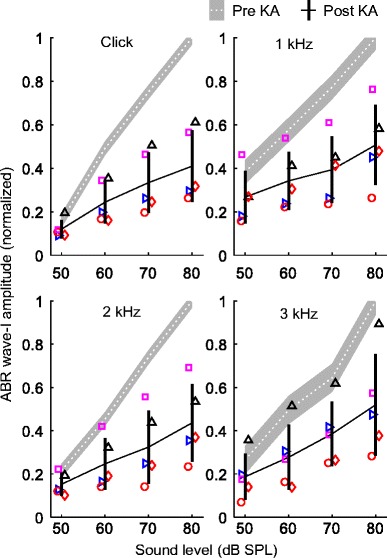
Long-term change in click- and tone-evoked wave I amplitude following KA infusion. Each panel compares wave I amplitude before and ≥ 6 weeks following KA infusion (means ± 1 SD), for a different stimulus type. Stimulus type (click or tone frequency) is given at the top of the panel. The gray-shaded region indicates mean wave I amplitude (± 1 SD) prior to KA. Measurements were normalized in each animal by dividing by the pre-exposure, 80 dB SPL control value. Different symbols indicate observations from different experimental animals as in Fig. 4. Changes in wave I amplitude following KA infusion were similar between clicks and tones
In addition to clicks, ABRs were also evoked using tone stimuli with frequencies of 1–3 kHz. Wave I amplitude prior to KA infusion was greater in response to clicks (16.11 ± 2.43 μV) and 2-kHz tones (12.23 ± 1.27 μV) than for 1-kHz (6.03 ± 0.89 μV) and 3-kHz tones (8.59 ± 3.05 μV; mean ± SD at 80 dB SPL; Fig. 5). A mixed-model analysis of log-transformed wave I amplitude showed a significant negative effect of KA exposure (F1,120.8 = 196.15, p < 0.0001) that increased in severity with increasing sound level (exposure × sound level; F3,119.9 = 6.39, p = 0.0005; i.e., percent reduction of wave I increased with increasing sound level). The interaction between exposure status and stimulus type was not significant (F3,116.9 = 0.82, p = 0.49), suggesting similar KA effects on clicks and tones in this frequency range. Effects of stimulus type (click/tone frequency; F3,119.9 = 61.12, p < 0.0001), sound level (F3,119.9 = 185.16, p < 0.0001), and the stimulus type by sound level interaction (F9,119.9 = 3.45, p = 0.0008) were also significant factors in the model.
ABR wave I latency decreased with increasing sound level and was shortest for click stimuli, intermediate for 2- and 3-kHz tones, and longest for 1-kHz tones (Fig. 6). This pattern likely reflects combined influences of stimulus level and spectral content on AN first-spike latency (Heil and Irvine 1997). Measurements of wave I latency made 6 or more weeks following bilateral KA infusion were generally similar to pre-exposure control values, suggesting minimal effect of the exposure. A mixed-model analysis of mean wave I latency across recordings before or after KA infusion showed a small negative effect of KA exposure (i.e., latency decreased by 0.037 ± 0.017 ms (mean ± SE); F1,133.7 = 4.55, p = 0.035) that did not vary with stimulus type (exposure × stimulus type; F3,117.1 = 2.25, p = 0.086) or with sound level (exposure × sound level; F3,117.1 = 0.47, p = 0.70). Main effects of stimulus type (F3,132.1 = 461.41, p < 0.0001) and sound level (F3,132.1 = 518.37, p < 0.0001) were significant factors in the model. The interaction between stimulus type and sound level was not significant (F9,117.1 = 1.56, p = 0.14).
Fig. 6.
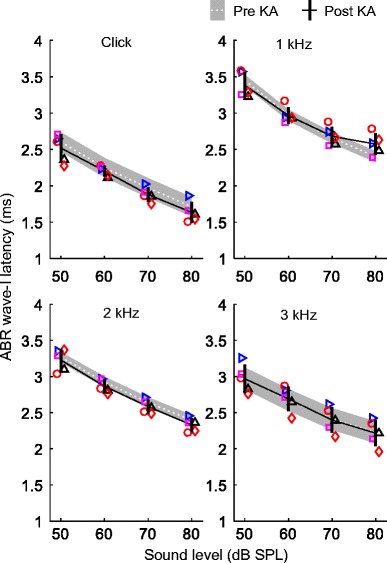
Long-term change in click- and tone-evoked wave I latency following KA infusion. Latency is expressed relative to stimulus arrival at the ear canal. Each panel compares wave I latency before and ≥ 6 weeks following KA infusion (means ± 1 SD), for a different stimulus type. Stimulus type is given at the top of the panel. Different symbols indicate observations from different experimental animals as in previous figures. KA had minimal impact on wave I latency
KA Does Not Impair ABR Thresholds
ABR thresholds were estimated based on an analysis of complete response waveforms from 0 to 20 ms post-stimulus onset. Thresholds were determined before and after KA infusion as the sound level at which the RMS energy of the ABR exceeded a criterion level of 0.75 μV. ABR energy by sound level functions was consistent across different recording sessions in the same animal, both before and after KA exposure (Fig. 7a, black and red, respectively). ABR thresholds measured prior to KA exposure ranged from 35 to 45 dB SPL and were slightly lower for 1-kHz tones (35.70 ± 1.89 dB SPL) than for 2-kHz tones (41.16 ± 1.53 dB SPL), 3-kHz tones (40.20 ± 4.86 dB SPL), and click stimuli (41.76 ± 1.10 dB SPL; means ± SD). Post-exposure ABR thresholds were generally within ± 5 dB of pre-exposure control values (Fig. 7b), consistent with minimal effect of KA exposure on audiometric sensitivity. Click thresholds based on wave I alone showed a similar pattern (pre-KA, 45.82 ± 2.85 dB SPL; post-KA, 49.33 ± 4.01 dB SPL; means ± SD across animals). A mixed-model analysis of whole-waveform ABR thresholds showed a significant effect of stimulus type (F3,24.2 = 6.78, p = 0.0018) due to lower threshold for 1-kHz tones than other stimulus types. The dependent variable was the mean ABR threshold of each bird before or six or more weeks following KA exposure. The main effect of exposure status (pre- vs. post-KA infusion; F1,26.1 = 3.06, p = 0.092) and interaction between exposure status and stimulus type (F3,24.2 = 0.31, p = 0.82) were not significant. The finding of no substantial change in ABR thresholds following KA infusion suggests that hair cells and the structures of the sensory epithelium recovered following KA infusion.
Fig. 7.
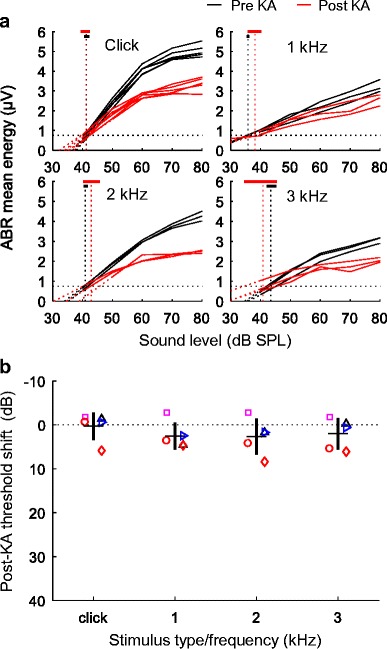
Shifts in ABR threshold following KA infusion. a ABR energy as a function of stimulus sound level, before and after KA exposure. Representative functions are shown for each stimulus type in one animal (B09; see Fig. 1 (left) for waveforms), before (three sessions) and six or more weeks following KA exposure (three sessions). Thresholds were calculated as the sound level at which ABR energy exceeded 0.75 μV. Vertical dashed lines indicate mean thresholds before and after KA infusion. Thick horizontal lines show the range of thresholds observed across sessions. b Mean threshold shifts (± 1 SD) are plotted as a function of stimulus type (click, or tone frequency). Different symbols indicate observations from different experimental animals as in previous figures. KA infusion did not impair thresholds of click- or tone-evoked ABRs
KA Has Minimal Impact on ABR Wave V
ABR wave V was monitored in response to click stimuli for 12–16 weeks following bilateral KA exposure in four animals (Fig. 8). Wave V measurements were not possible in the fifth animal prior to KA infusion and during the early recovery period due to high-pass filtering of these recordings at 300 Hz (vs. 30 Hz in other recordings), which greatly attenuated wave V. Wave V was also noted in response to tones, but not analyzed further due to substantial overlap with other shorter latency waves associated with stimulus offset. For click stimuli presented at moderate-to-high sound level (≥ 60 dB SPL), wave V amplitude showed a slight reduction after infusion followed by complete or partial recovery in some animals. Wave V was not reduced for lower level clicks, but showed a slight increase following exposure in two animals. Thus, despite substantial reduction of wave I amplitude, KA infusion had minimal impact on ABR wave V. A mixed-model analysis of log-transformed wave V amplitude showed no effect of KA exposure (F1,21 = 3.22, p = 0.087) or interaction between exposure status and sound level (F3,21 = 0.74, p = 0.54). The dependent variable was the mean log-transformed response amplitude of each animal across sessions before or 6 or more weeks following KA exposure. The main effect of sound level was the only significant factor in the model (F3,21 = 29.57, p < 0.0001).
Fig. 8.
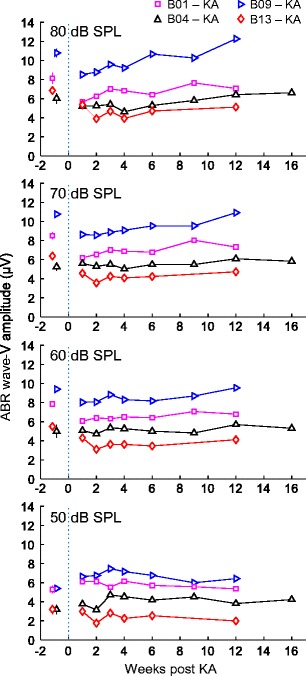
Variation over time in click-evoked wave V amplitude following KA infusion. Different symbols indicate observations from different experimental animals as in previous figures. Wave V amplitude decreases slightly following KA infusion and recovers partially or completely by 4–6 weeks post-infusion
Effects of KA on ABR-wave amplitudes were also assessed using an RMS-based approach that did not require identifying specific peaks. In addition to waves I and V, this method allowed us to analyze KA effects on mid-latency response components thought to reflect brainstem/midbrain activity (i.e., waves II–IV). Analyses were limited to click ABRs. In contrast to wave V, mid-latency waves were reduced following KA but to a lesser extent than wave I (Fig. 9). Thus, the negative impact of KA on neural response amplitude appeared greatest for wave I, intermediate for mid-latency waves, and minimal for wave V. A mixed model analysis of log-transformed response amplitude showed a significant negative effect of KA exposure (F1,18.2 = 26.96, p < 0.0001) that varied across ABR waves (F2,17.0 = 5.93, p = 0.011). The dependent variable in this analysis was mean log-transformed response RMS amplitude across levels from 60 to 80 dB SPL. The effect of KA was strongest for wave I (− 8.1 ± 1.83 dB; mean difference ± SE; t17.4 = − 5.32, p = 0.0001), intermediate for mid-latency waves (− 5.1 ± 1.53 dB; t17.4 = − 3.33, p = 0.004), and not significant for wave V (− 0.8 ± 1.53 dB; t17.4 = − 0.53, p = 0.60). The main effect of ABR wave was not significant (F2,17.0 = 1.59, p = 0.23).
Fig. 9.
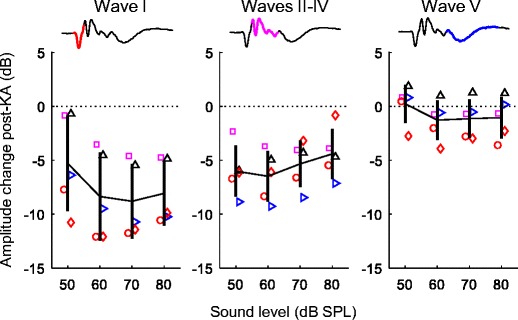
Long-term change following KA in the root-mean-square (RMS) amplitude of wave I, waves II–IV, and wave V. Waveforms (top) show the region of the response used for amplitude calculation. Mean amplitude change was quantified based on click ABRs recorded six or more weeks following KA infusion and is plotted as a function of sound level. Different symbols indicate observations from different experimental animals as in previous figures. Error bars indicate ± 1 SD. Amplitude reduction following KA is greatest for wave I, intermediate for waves II–IV, and insignificant for wave V
DISCUSSION
The present study assessed the effects of KA excitotoxicity on cochlear function in the budgerigar using CAPs, CMs, and ABRs. KA exposure immediately abolished the AN CAP while having mild impact on CM amplitude. Longitudinal ABR measurements showed a pronounced 40–75 % reduction in ABR wave I amplitude at moderate-to-high sound levels that persisted for the duration of the study (i.e., up to 31 weeks). In contrast, KA had minimal impact on wave I latency or the amplitude of wave V and did not impair ABR thresholds. Waves II–IV were moderately reduced in amplitude by KA. This pattern of results is consistent with selective damage to AN afferents (e.g., synaptopathy) followed by increased central gain (Hickox and Liberman 2014; Chambers et al. 2016; Hickox et al. 2017).
CAP abolishment in the minutes following KA infusion indicates a dramatic reduction of synchronous AN onset activity. Persistent reduction of ABR wave I for more than 6 months thereafter is most likely caused by permanent damage to AN afferents innervating tall hair cells of the avian cochlear epithelium. KA is a potent neuroexcitatory agent that, at sufficient concentration, induces neural death through overstimulation of glutamate receptors. In chicken, infusion of 5-mM KA leads to immediate swelling and breakage of afferent hair cell synapses followed by irreversible loss of nearly all AN cell bodies in the cochlear ganglion (Sun et al. 2001). Many remaining neurons have abnormal morphology, with vacuoles between the soma and distal neurite process or apparent disintegration of the myelin sheath. These neuroanatomical changes are accompanied by profound reduction of AN CAPs, without recovery, for the duration of measurements (i.e., up to 20 weeks) (Sun et al. 2000). Less concentrated, 0.3-mM KA also reduces CAP amplitude, but responses partly recover over several weeks before stabilizing at ~ 50 % of the pre-exposure control value (Sun et al. 2000). Chickens exposed to this lower KA dose show no clear loss of AN cell bodies, but an increase in the proportion of abnormal neurons which could be functionally disconnected from hair cells as in mammals following moderate noise overexposure (Kujawa and Liberman 2009; Furman et al. 2013).
ABR wave I reduction was probably not associated with hair cell damage considering that CM amplitude showed only mild reduction immediately following KA exposure. In previous studies, KA-treated chickens showed no impairment of CM amplitude or distortion product otoacoustic emissions (DPOAEs), and no change in hair cell morphology beyond damage to afferent synapses (Sun et al. 2000, 2001). These results suggest that wave I impairment in budgerigars reflects a permanent AN lesion rather than a secondary consequence of hair cell dysfunction. The extent to which wave I reduction reflects loss of AN fibers versus diminished AN onset-response synchrony is less clear. However, note that our wave I latency analysis suggested minimal change in average AN onset-response timing.
Our finding that ABR thresholds were not impaired following KA exposure also suggests normal hair cell function. Prior studies show that audiometric thresholds are nearly always elevated following hair cell damage (Henderson et al. 1983; Schuknecht 1994; Harding et al. 2002; Harding and Bohne 2004; Saunders 2010; Salvi et al. 2017). In birds, extensive hair cell loss followed by regeneration causes permanent threshold shifts of 20 dB or more, putatively due to residual abnormality in the orientation of stereocilia bundles (Marean et al. 1998; Woolley et al. 2001; Woolley and Rubel 2002; Dooling et al. 2006; Ryals et al. 2013). Note that ABR threshold shifts provide an indirect measure of hair cell status due to minimal contribution of hair cell-generated activity (e.g., CM or SP) to response waveforms. Otoacoustic emission or chronic CM recordings could be used to more directly assess hair cell status (Sun et al. 2000).
KA effects observed here and in chicken are similar to those reported in mammals. In guinea pigs, KA infusion immediately abolishes AN CAPs but has no impact on putative hair cell responses including the CM and SP (Bledsoe et al. 1981). Impairment of the CAP is associated with severe swelling of afferent hair cell synapses (Pujol et al. 1985), and in rats, with permanent loss of 34 % of spiral ganglion neurons within 10 days post-infusion (Juiz et al. 1989). KA application to the round window in chinchillas also decreases CAP amplitude, with no impact on hair cell morphology or on DPOAE amplitude (Zheng et al. 1996, 1997). CAP amplitude in chinchillas recovers to within 20 % of normal at 30 days post-KA infusion, possibly to a greater extent than in other species following excitotoxic injury (Zheng et al. 1999).
The avian cochlea is tonotopic but anatomically somewhat different than in mammals, raising the question of how loss of AN afferent synapses might be distributed across different regions of the hair cell epithelium. The cochlear duct is uncoiled in birds and relatively short, with a length of 2.5 mm in the budgerigar (Manley et al. 1993). As many as 30 hair cells span the width of the epithelium at apical locations, with no clear delineation between distinct subtypes (Takasaka and Smith 1971). Instead, avian hair cells show a graded transition between “tall” and “short” morphology (defined based on height to width ratio) across the width of the sensory epithelium. Short hair cells are located above the free basilar membrane and, like outer hair cells, amplify sound-induced vibrations through active force generation (Beurg et al. 2013). Tall hair cells are located on the “neural” half of the epithelium, closer to the AN ganglion across its width, and serve as the primary target for AN afferents (Takasaka and Smith 1971; Gleich 1989; Smolders et al. 1995; Köppl et al. 2000). KA is hypothesized to damage afferent synapses in this tall hair cell region, along the full basal-to-apical extent of cochlea based on similar percent wave I reduction across stimulus frequencies.
Percent reduction of ABR wave I in KA-exposed budgerigars was greatest at high sound levels. The same pattern in mammals has been linked to disproportionate loss of high-threshold AN fibers with low spontaneous rate (SR) (Furman et al. 2013; Liberman and Liberman 2015). While the reason for this pattern in budgerigars is unknown, the same mechanism could apply considering that AN thresholds in birds vary by 50 dB or more at the same frequency and are inversely correlated with SR (Sachs et al. 1974; Manley et al. 1985; Salvi et al. 1992; Smolders et al. 1995). Further single-unit studies are needed to test this hypothesis.
AN degeneration has been reported previously in birds with increasing age (Ryals and Westbrook 1988) and following hair cell regeneration. Quail overexposed to noise show acute hair cell loss followed by near-complete regeneration of the sensory epithelium over several weeks (Ryals and Rubel 1988). In contrast to the recovery observed for hair cells, partial loss of AN ganglion cells is first evident after 30 days and increases to 25–30 % loss at 90 days post-overexposure (Ryals et al. 1989). Thus, degeneration of AN cell bodies can lag behind hair cell/synaptic injury by weeks or months, as in mammals (Kujawa and Liberman 2009; Viana et al. 2015). Behavioral changes following hair cell regeneration in birds are complex and sometimes transient (Dooling et al. 1997, 2006; Marean et al. 1998; Ryals et al. 2013) and may reflect contributions from both residual hair cell dysfunction and partial AN loss.
CAP measurements made immediately following KA exposure suggested similar damage to both ears in most animals. Possible asymmetry of KA-induced damage was not investigated at later time points and could be difficult to quantify considering that monaurally presented sounds effectively stimulate both cochleae in birds due to the interaural sound pathway (Calford 1988; Larsen et al. 2006). One possible solution would be to implant chronic CAP electrodes for recording electrophysiological activity from each ear independently. Alternatively, one cochlea could be surgically ablated to eliminate uncertainty over the origin of remaining ABR activity. Ultimately, asymmetry will be quantified in these animals through histological studies at the conclusion of an ongoing behavioral study.
Our finding that two sham infusions in one animal did not reduce ABR wave I amplitude suggests that the impairment observed in KA-treated budgerigars was not due to infusion trauma. While some uncertainty remains due to the low number of sham surgeries performed, previous studies have found that infusion of perilymph-like solution has little or no lasting impact on cochlear thresholds and input–output functions (Bledsoe et al. 1981; Sun et al. 2000; Wang and Olson 2016). Furthermore, note that none of the KA-treated animals showed any evidence of ABR threshold shifts, as would be expected following infusion-related trauma to the middle or inner ear.
ABR wave V was unaffected by KA, and waves II–IV were less reduced than wave I. These results suggest the possibility of compensatory mechanisms that restore central response amplitude despite diminished peripheral input to the CNS (increased “central gain”). Compensation of this kind has not been shown previously in the avian auditory system to our knowledge and should be confirmed with more direct measures of central response amplitude, but occurs in mammals. Mice with profound AN degeneration due to ouabain show extensive recovery of multi-unit responses in primary auditory cortex and, to a lesser extent, at the midbrain processing level within 30 days of injury (Chambers et al. 2016). Similarly, in chinchillas, local field potentials in the auditory cortex recover completely following ototoxic injury to inner hair cells, while midbrain responses recover partially (Salvi et al. 2017). In both cases, recovery of cortical response amplitude is thought to reflect downregulation of inhibitory signaling pathways that unmask the weakened excitatory drive to auditory cortex (Salvi et al. 2017; Resnik and Polley 2017). Downregulation of inhibitory signaling could arise through reduced GABAA release or changes in receptor binding/subunit composition (Caspary et al. 2008; Wang et al. 2011).
The perceptual consequences of AN damage is a topic of active debate and investigation (Mehraei et al. 2016; Plack et al. 2016; Prendergast et al. 2017). ABRs are routinely used to detect audiometric threshold shifts, and hence, our finding of no ABR threshold impairment following KA infusion suggests that the behavioral audiogram may be preserved as well (Henderson et al. 1983). While mice with ouabain-induced AN degeneration show some impairment of acoustic-startle reflexes, audiometric thresholds based on operant conditioning are normal (Chambers et al. 2016). Similarly, in cats, partial sectioning of the AN produces no behavioral threshold elevation unless neural survival falls below 20 % (Schuknecht and Woellner 1953). Effects of AN damage on perception of complex signals are less clear. Diminished behavioral sensitivity to envelope fluctuations seems possible following AN damage (Bharadwaj et al. 2014, 2015), due to downregulation of inhibitory pathways that shape neural responses to envelope structure (Caspary et al. 2002; Nelson and Carney 2004, 2007). In contrast, a recent model based on signal detection theory predicts nearly undetectable changes in complex sound discrimination, even in cases of substantial neural loss (Oxenham 2016). Finally, increased central gain has been implicated as a potential contributor to tinnitus in human subjects with a normal audiogram (Schaette and McAlpine 2011; Gu et al. 2012).
In conclusion, KA exposure in budgerigars resulted in pronounced reduction of ABR wave I amplitude, consistent with permanent damage to the AN. KA had little impact on wave V, suggesting partial recovery of central processing, and did not elevate ABR thresholds. This pathology could potentially cause supra-threshold processing deficits, particularly in noise, without elevation of audiometric thresholds, i.e., “hidden hearing loss” (Schaette and McAlpine 2011; Bharadwaj et al. 2014, 2015). Given the widely studied psychoacoustic abilities of this traditional behavioral model species, further studies in trained budgerigars might provide new insight into AN-degeneration effects on perception and neural encoding of complex sounds.
Acknowledgements
This research was supported by the National Institutes of Health grant R00 DC013792.
Author Contributions
KSH designed the research, performed the experiments, analyzed the data, and wrote the manuscript. KSA performed the experiments.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Kenneth S. Henry, Phone: (585) 275-4851, Email: kenneth_henry@urmc.rochester.edu
Kristina S. Abrams, Email: kristina_abrams@urmc.rochester.edu
References
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- Beurg M, Tan X, Fettiplace R. A prestin motor in chicken auditory hair cells: active force generation in a nonmammalian species. Neuron. 2013;79:69–81. doi: 10.1016/j.neuron.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj HM, Masud S, Mehraei G, Verhulst S, Shinn-Cunningham BG. Individual differences reveal correlates of hidden hearing deficits. J Neurosci. 2015;35:2161–2172. doi: 10.1523/JNEUROSCI.3915-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG. Cochlear neuropathy and the coding of supra-threshold sound. Front Syst Neurosci. 2014;8:26. doi: 10.3389/fnsys.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe SC, Bobbin RP, Chihal DM. Kainic acid: an evaluation of its action on cochlear potentials. Hear Res. 1981;4:109–120. doi: 10.1016/0378-5955(81)90040-x. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EF, Dooling RJ, Gleich O. Auditory brainstem responses in adult budgerigars (Melopsittacus undulatus) J Acoust Soc Am. 2002;112:999–1008. doi: 10.1121/1.1494807. [DOI] [PubMed] [Google Scholar]
- Calford MB. Constraints on the coding of sound frequency imposed by the avian interaural canal. J Comp Physiol A. 1988;162:491–502. [Google Scholar]
- Carney LH, Ketterer AD, Abrams KS, et al. Detection thresholds for amplitude modulations of tones in budgerigar, rabbit, and human. Adv Exp Med Biol. 2013;787:391–398. doi: 10.1007/978-1-4614-1590-9_43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Palombi PS, Hughes LF. GABAergic inputs shape responses to amplitude modulated stimuli in the inferior colliculus. Hear Res. 2002;168:163–173. doi: 10.1016/s0378-5955(02)00363-5. [DOI] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB. Central gain restores auditory processing following near-complete cochlear denervation. Neuron. 2016;89:867–879. doi: 10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Best CT, Brown SD. Discrimination of synthetic full-formant and sinewave/ra-la/continua by budgerigars (Melopsittacus undulatus) and zebra finches (Taeniopygia guttata) J Acoust Soc Am. 1995;97:1839–1846. doi: 10.1121/1.412058. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Brown SD. Speech perception by budgerigars (Melopsittacus undulatus): spoken vowels. Percept Psychophys. 1990;47:568–574. doi: 10.3758/bf03203109. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Lohr B, Dent ML. Hearing in birds and reptiles. In: Dooling RJ, Fay RR, Popper AN, editors. Comparative hearing: birds and reptiles. New York: Springer; 2000. pp. 308–359. [Google Scholar]
- Dooling RJ, Okanoya K, Brown SD. Speech perception by budgerigars (Melopsittacus undulatus): the voiced–voiceless distinction. Percept Psychophys. 1989;46:65–71. doi: 10.3758/bf03208075. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Ryals BM, Dent ML, Reid TL. Perception of complex sounds in budgerigars (Melopsittacus undulatus) with temporary hearing loss. J Acoust Soc Am. 2006;119:2524–2532. doi: 10.1121/1.2171839. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Ryals BM, Manabe K. Recovery of hearing and vocal behavior after hair-cell regeneration. Proc Natl Acad Sci U S A. 1997;94:14206–14210. doi: 10.1073/pnas.94.25.14206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling RJ, Saunders JC. Hearing in the parakeet (Melopsittacus undulatus): absolute thresholds, critical ratios, frequency difference limens, and vocalizations. J Comp Physiol Psychol. 1975;88:1–20. doi: 10.1037/h0076226. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Searcy MH. Amplitude modulation thresholds for the parakeet (Melopsittacus undulatus) J Comp Physiol A. 1981;143:383–388. [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110:577–586. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich O. Auditory primary afferents in the starling: correlation of function and morphology. Hear Res. 1989;37:255–267. doi: 10.1016/0378-5955(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Gu JW, Herrmann BS, Levine RA, Melcher JR. Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. JARO—J Assoc Res Otolaryngol. 2012;13:819–833. doi: 10.1007/s10162-012-0344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JW., III . Handbook of auditory evoked responses. 1992. Auditory evoked response measurement practices; p. 267. [Google Scholar]
- Harding GW, Bohne BA. Temporary DPOAE level shifts, ABR threshold shifts and histopathological damage following below-critical-level noise exposures. Hear Res. 2004;196:94–108. doi: 10.1016/j.heares.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA, Ahmad M. DPOAE level shifts and ABR threshold shifts compared to detailed analysis of histopathological damage from noise. Hear Res. 2002;174:158–171. doi: 10.1016/s0378-5955(02)00653-6. [DOI] [PubMed] [Google Scholar]
- Hashino E, Tanaka Y, Salvi RJ, Sokabe M. Hair cell regeneration in the adult budgerigar after kanamycin ototoxicity. Hear Res. 1992;59:46–58. doi: 10.1016/0378-5955(92)90101-r. [DOI] [PubMed] [Google Scholar]
- Heffner HE. Hearing in glires: domestic rabbit, cotton rat, feral house mouse, and kangaroo rat. J Acoust Soc Am. 1980;68:1584–1599. [Google Scholar]
- Heil P, Irvine DR. First-spike timing of auditory-nerve fibers and comparison with auditory cortex. J Neurophysiol. 1997;78:2438–2454. doi: 10.1152/jn.1997.78.5.2438. [DOI] [PubMed] [Google Scholar]
- Henderson D, Hamernik RP, Salvi RJ, Ahroon WA. Comparison of auditory-evoked potentials and behavioral thresholds in the normal and noise-exposed chinchilla. Audiology. 1983;22:172–180. doi: 10.3109/00206098309072780. [DOI] [PubMed] [Google Scholar]
- Henry KS, Abrams KS, Forst J, Mender MJ, Neilans EG, Idrobo F, Carney LH. Midbrain synchrony to envelope structure supports behavioral sensitivity to single-formant vowel-like sounds in noise. JARO—J Assoc Res Otolaryngol. 2017;18:165–181. doi: 10.1007/s10162-016-0594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Neilans EG, Abrams KS, Idrobo F, Carney LH. Neural correlates of behavioral amplitude modulation sensitivity in the budgerigar midbrain. J Neurophysiol. 2016;115:1905–1916. doi: 10.1152/jn.01003.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox AE, Larsen E, Heinz MG, Shinobu L, Whitton JP. Translational issues in cochlear synaptopathy. Hear Res. 2017;349:164–171. doi: 10.1016/j.heares.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol. 2014;111:552–564. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juiz JM, Rueda J, Merchán JA, Sala ML. The effects of kainic acid on the cochlear ganglion of the rat. Hear Res. 1989;40:65–74. doi: 10.1016/0378-5955(89)90100-7. [DOI] [PubMed] [Google Scholar]
- Koay G, Heffner RS, Heffner HE. Behavioral audiograms of homozygous medJ mutant mice with sodium channel deficiency and unaffected controls. Hear Res. 2002;171:111–118. doi: 10.1016/s0378-5955(02)00492-6. [DOI] [PubMed] [Google Scholar]
- Konishi M. Effects of deafening on song development in two species of juncos. Condor. 1964;66:85–102. [Google Scholar]
- Köppl C, Wegscheider A, Gleich O, Manley GA. A quantitative study of cochlear afferent axons in birds. Hear Res. 2000;139:123–143. doi: 10.1016/s0378-5955(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen ON, Dooling RJ, Michelsen A. The role of pressure difference reception in the directional hearing of budgerigars (Melopsittacus undulatus) J Comp Physiol A Neuroethol Sensory, Neural, Behav Physiol. 2006;192:1063–1072. doi: 10.1007/s00359-006-0138-1. [DOI] [PubMed] [Google Scholar]
- Liberman LD, Liberman MC. Dynamics of cochlear synaptopathy after acoustic overexposure. J Assoc Res Otolaryngol. 2015;16:205–219. doi: 10.1007/s10162-015-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Kujawa SG. Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res. 2017;349:138–147. doi: 10.1016/j.heares.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. JARO—J Assoc Res Otolaryngol. 2011;12:605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Spankovich C, Le Prell CG. Evidence of “hidden hearing loss” following noise exposures that produce robust TTS and ABR wave-I amplitude reductions. Hear Res. 2017;349:155–163. doi: 10.1016/j.heares.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, et al. Age-related primary cochlear neuronal degeneration in human temporal bones. JARO—J Assoc Res Otolaryngol. 2011;12:711–717. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA, Gleich O, Leppelsack HJ, Oeckinghaus H. Activity patterns of cochlear ganglion neurones in the starling. J Comp Physiol A. 1985;157:161–181. doi: 10.1007/BF01350025. [DOI] [PubMed] [Google Scholar]
- Manley GA, Schwabedissen G, Gleich O. Morphology of the basilar papilla of the budgerigar,Melopsittacus undulatus. J Morphol. 1993;218:153–165. doi: 10.1002/jmor.1052180205. [DOI] [PubMed] [Google Scholar]
- Marean GC, Burt JM, Beecher MD, Rubel EW. Auditory perception following hair cell regeneration in European starling (Sturnus vulgaris): frequency and temporal resolution. J Acoust Soc Am. 1998;103:3567–3580. doi: 10.1121/1.423085. [DOI] [PubMed] [Google Scholar]
- Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, Shinn-Cunningham BG. Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. J Neurosci. 2016;36:3755–3764. doi: 10.1523/JNEUROSCI.4460-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PC, Carney LH. A phenomenological model of peripheral and central neural responses to amplitude-modulated tones. J Acoust Soc Am. 2004;116:2173–2186. doi: 10.1121/1.1784442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PC, Carney LH. Neural rate and timing cues for detection and discrimination of amplitude-modulated tones in the awake rabbit inferior colliculus. J Neurophysiol. 2007;97:522–539. doi: 10.1152/jn.00776.2006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte J, Schuknecht HF, Kerr AG. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. Laryngoscope. 1978;88:1231–1246. doi: 10.1288/00005537-197808000-00004. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ. Predicting the perceptual consequences of hidden hearing loss. Trends Hear. 2016;20:233121651668676. doi: 10.1177/2331216516686768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plack CJ, Léger A, Prendergast G, Kluk K, Guest H, Munro KJ. Toward a diagnostic test for hidden hearing loss. Trends Hear. 2016;20:233121651665746. doi: 10.1177/2331216516657466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G, Guest H, Munro KJ, Kluk K, Léger A, Hall DA, Heinz MG, Plack CJ. Effects of noise exposure on young adults with normal audiograms I: electrophysiology. Hear Res. 2017;344:68–81. doi: 10.1016/j.heares.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel JL, Ruel J, Gervais d’Aldin C, Pujol R. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport. 1998;9:2109–2114. doi: 10.1097/00001756-199806220-00037. [DOI] [PubMed] [Google Scholar]
- Pujol R, Lenoir M, Robertson D, Eybalin M, Johnstone BM. Kainic acid selectively alters auditory dendrites connected with cochlear inner hair cells. Hear Res. 1985;18:145–151. doi: 10.1016/0378-5955(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Resnik J, Polley DB (2017) Fast-spiking GABA circuit dynamics in the auditory cortex predict recovery of sensory processing following peripheral nerve damage. elife 6. 10.7554/eLife.21452 [DOI] [PMC free article] [PubMed]
- Ryals BM, Dent ML, Dooling RJ. Return of function after hair cell regeneration. Hear Res. 2013;297:113–120. doi: 10.1016/j.heares.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Ten Eyck B, Westbrook EW. Ganglion cell loss continues during hair cell regeneration. Hear Res. 1989;43:81–90. doi: 10.1016/0378-5955(89)90061-0. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Westbrook EW. Ganglion cell and hair cell loss in Coturnix quail associated with aging. Hear Res. 1988;36:1–8. doi: 10.1016/0378-5955(88)90133-5. [DOI] [PubMed] [Google Scholar]
- Sachs MB, Young ED, Lewis RH. Discharge patterns of single fibers in the pigeon auditory nerve. Brain Res. 1974;70:431–447. doi: 10.1016/0006-8993(74)90253-4. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Saunders SS, Powers NL, Boettcher FA. Discharge patterns of cochlear ganglion neurons in the chicken. J Comp Physiol A. 1992;170:227–241. doi: 10.1007/BF00196905. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Sun W, Ding DL, Chen GD, Lobarinas E, Wang J, Radziwon K, Auerbach BD. Inner hair cell loss disrupts hearing and cochlear function leading to sensory deprivation and enhanced central auditory gain. Front Neurosci. 2017;10:1–14. doi: 10.3389/fnins.2016.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer G, Richter CP, Klinke R. Sodium, potassium, chloride and calcium concentrations measured in pigeon perilymph and endolymph. Hear Res. 1999;129:1–6. doi: 10.1016/s0378-5955(98)00230-5. [DOI] [PubMed] [Google Scholar]
- Saunders JC. The role of hair cell regeneration in an avian model of inner ear injury and repair from acoustic trauma. ILAR J. 2010;51:326–337. doi: 10.1093/ilar.51.4.326. [DOI] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31:13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermuly L, Klinke R. Change of characteristic frequency of pigeon primary auditory afferents with temperature. J Comp Physiol A. 1985;156:209–211. [Google Scholar]
- Schuknecht HF. Auditory and cytocochlear correlates of inner ear disorders. Otolaryngol Neck Surg. 1994;110:530–538. doi: 10.1177/019459989411000610. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Woellner RC. Hearing losses following partial sectioning of the cochlear nerve. Laryngoscope. 1953;63:441–465. doi: 10.1288/00005537-195306000-00001. [DOI] [PubMed] [Google Scholar]
- Smolders JW, Ding-Pfennigdorff D, Klinke R. A functional map of the pigeon basilar papilla: correlation of the properties of single auditory nerve fibres and their peripheral origin. Hear Res. 1995;92:151–169. doi: 10.1016/0378-5955(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Factors inducing retrograde degeneration of the cochlear nerve. Ann Otol Rhinol Laryngol Suppl. 1984;112:76–82. doi: 10.1177/00034894840930s415. [DOI] [PubMed] [Google Scholar]
- Spoendlin H, Schrott A. Analysis of the human auditory nerve. Hear Res. 1989;43:25–38. doi: 10.1016/0378-5955(89)90056-7. [DOI] [PubMed] [Google Scholar]
- Sun H, Hashino E, Ding DL, Salvi RJ. Reversible and irreversible damage to cochlear afferent neurons by kainic acid excitotoxicity. J Comp Neurol. 2001;430:172–181. doi: 10.1002/1096-9861(20010205)430:2<172::aid-cne1023>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Sun H, Salvi RJ, Ding DL, Hashino E, Shero M, Zheng XY. Excitotoxic effect of kainic acid on chicken otoacoustic emissions and cochlear potentials. J Acoust Soc Am. 2000;107:2136–2142. doi: 10.1121/1.428495. [DOI] [PubMed] [Google Scholar]
- Takasaka T, Smith C. The structure and innervation of the pigeon’s basilar papilla. J Ultrastruct Res. 1971;35:20–65. doi: 10.1016/s0022-5320(71)80141-7. [DOI] [PubMed] [Google Scholar]
- Viana LM, O’Malley JT, Burgess BJ, et al. Cochlear neuropathy in human presbycusis: confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res. 2015;327:78–88. doi: 10.1016/j.heares.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Caspary DM. Inhibitory neurotransmission in animal models of tinnitus: maladaptive plasticity. Hear Res. 2011;279:111–117. doi: 10.1016/j.heares.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Olson ES. Cochlear perfusion with a viscous fluid. Hear Res. 2016;337:1–11. doi: 10.1016/j.heares.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SM, Wissman AM, Rubel EW. Hair cell regeneration and recovery of auditory thresholds following aminoglycoside ototoxicity in Bengalese finches. Hear Res. 2001;153:181–195. doi: 10.1016/s0378-5955(00)00217-3. [DOI] [PubMed] [Google Scholar]
- Woolley SMN, Portfors CV. Conserved mechanisms of vocalization coding in mammalian and songbird auditory midbrain. Hear Res. 2013;305:45–56. doi: 10.1016/j.heares.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SMN, Rubel EW. Vocal memory and learning in adult Bengalese finches with regenerated hair cells. J Neurosci. 2002;22:7774–7787. doi: 10.1523/JNEUROSCI.22-17-07774.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ED. Which neurons survive the glutamate storm? J Neurophysiol. 2013;110:575–576. doi: 10.1152/jn.00292.2013. [DOI] [PubMed] [Google Scholar]
- Zheng XY, Henderson D, Hu BH, McFadden SL. Recovery of structure and function of inner ear afferent synapses following kainic acid excitotoxicity. Hear Res. 1997;105:65–76. doi: 10.1016/s0378-5955(96)00188-8. [DOI] [PubMed] [Google Scholar]
- Zheng XY, Salvi RJ, Fadden SLMC, et al. Recovery of kainic acid excitotoxicity in chinchilla cochlea. Ann N Y Acad Sci. 1999;884:255–269. doi: 10.1111/j.1749-6632.1999.tb08647.x. [DOI] [PubMed] [Google Scholar]
- Zheng XY, Wang J, Salvi RJ, Henderson D. Effects of kainic acid on the cochlear potentials and distortion product otoacoustic emissions in chinchilla. Hear Res. 1996;95:161–167. doi: 10.1016/0378-5955(96)00047-0. [DOI] [PubMed] [Google Scholar]


