Abstract
Anthropogenic influences on the environment have been become a focal point for many social and political endeavors. With an ever-increasing rate of new contaminants being introduced into the environment every year, regulatory policies have begun to shift to prevention rather than mitigation. However, current in vivo testing strategies, in addition to ethical considerations, are too expensive and time consuming to adequately screen potential contaminants within a realistic timeframe. As a result, in vitro testing on cell cultures has been identified as an ideal alternative testing strategy for emerging contaminants. In the context of ecotoxicology, in vitro testing has had limited use particularly with marine invertebrates like the marine mussel Mytilus edulis mainly due to difficulties in establishing longer term cell cultures and cell lines. The aim of this study was to define an optimal technique (extraction and maintenance) for establishing a primary cell culture on M. edulis hemocytes that could be used for screening contaminants.
Keywords: Primary cell culture, Mytilus edulis, Contaminants, In vitro screening
Introduction
Anthropogenic influences are an ever-increasing source of pollution in the environment with many ecosystems suffering negative influences from a wide range of contaminants from multiple sources. Ecotoxicology and environmental regulation is focused on addressing this issue by quantifying the ecological risk associated with the discharge of contaminants into aquatic systems (Domart-Coulon et al. 2000). The mixtures of contaminants already present in ecosystems however make it difficult to determine what mitigation efforts are appropriate leading to environmental policy efforts, like the precautionary principle, to shift focus to developing strategies for prevention and requiring chemicals to be screened prior to use. In addition to this, it is difficult to measure the toxicity of anthropogenic pollutants discharged into the marine environment, leading to an emphasis on laboratory testing to characterize the environmental risk of contaminants (Le Pennec and Le Pennec 2001). In vivo testing on marine organisms have been historically used to establish strong evidence of toxicological impact and characterize the mechanisms of toxicity of chemicals in order to predict adverse effects on the ecosystem (Canesi et al. 2007; Domart-Coulon et al. 2000; Jimeno-Romero et al. 2017). With the rapid rate that advances are being made in materials science and drug development it is unfeasible to test all the new products being created using conventional ecotoxicological testing strategies (Domart-Coulon et al. 2000; Judson et al. 2013). As a result, there is a growing need to quickly prescreen products for regulatory compliance, to quickly identify products that pose the greatest environmental risk and to focus in vivo testing on products that require comprehensive testing. In addition to this, there is also growing public concern over the use of organisms for the screening of chemicals which is applying pressure to implement the 3R’s (Replace, Reduce and Refine) to ecotoxicology and develop in vitro techniques (Fenwick et al. 2009).
In vitro testing strategies can provide a means in which contaminants can be quickly screened, allowing for the identification of chemicals that require more in-depth analysis through in vivo testing. It has also been previously proposed that results from in vitro testing can be useful in predicting acute in vivo toxicity (National Institute of Environmental and Health Sciences 2001). This testing strategy is also in line with the development of a high throughput screening (HTS) test strategy, which takes advantage of advances in technology to improve the rate in which in vitro testing can be conducted (Barrick et al. 2017). HTS techniques are currently being considered by regulatory organizations like Organization for Cooperation and Economic Co-operation and Development (OECD) for use in regulatory policies (OECD Environment Directorate 2017), with the aim of establishing an intelligent testing strategy (ITS) to identify high risk products and prioritizing research on how to manage these risks (Stone et al. 2014). By developing a means to quickly screen a large number of chemicals using high throughput in vitro testing it becomes possible to link physical and chemical properties to ecological effects (Barrick et al. 2017). To investigate the application of this testing strategy for the marine environment, a suitable test organism needs to be selected and there needs to be clear understanding of technique used to establish in vitro testing strategy.
Marine bivalves have a long history of being used in biomonitoring studies due their high filtration rate, ability to bioconcentrate contaminants, widespread distribution and abundance (Canesi et al. 2012; Hanana et al. 2011). This makes them ideal targets for the development and integration of in vitro testing strategies in ecotoxicological risk assessment (Domart-Coulon et al. 2000). As suspension feeders, Mytilus species posses highly developed endocytic and phagocytic mechanisms, which can lead to the uptake of particles that may otherwise not interact with the biology in an ecosystem (Canesi et al. 2012; Katsumiti et al. 2014a, b). In addition to this, Mytilus species may accumulate contaminants into concentrations that can adversely impact the ecosystem, making Mytilus species ideal candidates for the development of in vitro testing strategy. Historically a lot of work has been conducted on developing cell culture testing strategies for mussel cells (Table 1). Many of these cell cultures are focused on maintaining primary cell cultures as the establishment of cell lines has not been successful, which can be attributed to the low speed of cell proliferation in mussel hemocyte cells and the lack of knowledge regarding specific growth factors (Cao et al. 2003; Daugavet and Blinova 2015; Mothersill and Austin 2000).
Table 1.
List of publications from independent laboratories that have developed primary cell cultures on Mytilus species
| Publication | Gómez-Mendikute and Cajaraville (2003) | Cao et al. (2003) | Katsumiti et al. (2014a, b) | Katsumiti et al. (2014a, b) | Tanguy et al. (2013) | Daugavet and Blinova (2015) | Bouki et al. (2013) | Faucet et al. (2004) |
|---|---|---|---|---|---|---|---|---|
| Species | Mytilus galloprovincialis | Mytilus galloprovincialis | Mytilus galloprovincialis | Mytilus galloprovincialis | Mytilus edulis | Mytilus edulis | Mytilus galloprovincialis | Mytilus galloprovincialis |
| Culture tissue | Hemocytes | Hemocytes | Hemocytes | Gills | Hemocytes | Mantle cells | Hemocytes | Digestive gland |
| Microplate (wells) | 96 | – | 96 | 96 | 24 | 4; 25; 96 | – | – |
| Concentration seeded (cells/mL) | 2 × 105 | – | 2 × 105 | 5 × 105 | 1 × 106 | – | 1 × 106 | – |
| Cleaning solution | – | – | – | Saline solution: 10 units/mL bacitracin; 400 unit/mL polyxin B; 300 units/mL penicilling G; 300 units/mL streptomycin; 50 µg/mL amphotericin B; 50 units/mL nystatin | – | – | – | Physiological saline solution: 20 mM HEPES, 436 mM NaCl, 10 mM KCl, 10 mM CaCl2, 8 mM MgSO4, 40 MgCl2 |
| Anti aggregation solution | Alseve buffer: 20.8 g/L glucose; 8 g/L sodium citrate 3.36 g/L EDTA; 22.5 g/L NaCL | 10 g/L NaCl; 24 g/L Tris; 0.15% v/v HCl 1 N; 7 g/L EDTA | 10 g/L NaCl; 24 g/L tris; 0.15% v/v HCl 1 N; 7 g/L EDTA | – | – | Alseve buffer: 20.8 g/L glucose; 8 g/L sodium citrate 3.36 g/L EDTA; 22.5 g/L NaCL | – | |
| Culture media | Eagle’s Basal Medium | Leibovitz L-15 | Eagle’s basal medium | Leibovitz L-15 | Leibovitz L-15 | Leibovitz L-15 | Leibovitz L-15 | Leibovitz L-15 |
| pH | 7 | 7.4 | 7.4 | – | 7.4–7.5 | 7 | 7.3 | |
| Temperature (°C) | 15 | 18 | 18 | 16 | – | 15 | 18 | |
| Osmolarity (mOSM/kg) | 1050 | 1000 | 1040 | 1040 | 990 | – | 1000 | – |
| Salt supplements | 20.2 g/L NaCl; 0.54 g/L KCl; 0.6 g/L CaCl2; 1 g/L MgSO4; 1 g/L MgCl2 | – | – | 20.2 g/L NaCl; 0.54 g/L KCl; 0.6 g/L CaCl2; 1 g/L MgSO4; 83 g/L MgCl2–6H2O | 18.05 g/L NaCl; 0.29 g/L KCl; 1.2 g/L CaCl2–2H2O; 4.28 g/L MgSO4–7H2O; 5.48 g/L MgCl2–6H2O; 2.86 g/L HEPES | 20.2 g/L NaCl; 0.54 g/L KCl; 0.6 g/L CaCl2; 1 g/L MgSO4; 1 g/L MgCl2–6H2O | 20.45 g/L NaCl; 0.52 g/L mM KCl; 0.44 g/L CaCl2; 0.97 g/L MgSO4; 3.8 MgCl2 | |
| FBS/FCS (%) | 5 | – | – | 10 | 2 | 10 | – | |
| Glucose (%) | – | 10 | – | – | 10 | – | – | – |
| Gentamicin (%) | 1 | 4 | 1 | – | – | 4 | 4 | – |
| Penicillin (units/mL) | 100 | – | 100 | – | 100 | 100 | 1% | |
| Streptomycin (µg/mL) | 100 | – | 100 | – | 100 | 100 | 1% | |
| Neomycin (µg/mL) | – | – | 100 | – | – | – | – | |
| Kanamycin (µg/mL) | – | – | 100 | – | – | – | – | |
| Amphotericin B (µg/mL) | 0.1 | – | 0.1 | – | 2.5 | 0.1 | – |
–: denotes information not provided or not applicable
Hemocytes from Mytilus species are commonly used in in vitro studies due to: the role in the innate immunity of the organisms, the relative ease in which they can be extracted and the comparative sterility of the extraction (Canesi et al. 2007; Gomez-Mendikute et al. 2002; Katsumiti et al. 2014a, b). In addition to this, Mytilus hemocytes display similar structure and function to mammalian immune cells, which can provide a useful basis for comparison in regulatory studies (Canesi et al. 2012). There are however key differences that make it difficult to establish long term cell cultures on hemocytes, which has led to many ecotoxicology studies focus on short term exposure to chemicals when using hemocytes (Canesi et al. 2012; Gómez-Mendikute and Cajaraville 2003; Katsumiti et al. 2014a, b). For example, when hemocytes form aggregations for clotting they do not form extracellular fibers, are reversible and can reenter the circulatory system as a result (Chen 1992). In cell culture this can also lead to the spontaneous formation of aggregations which can make it difficult to establish a uniform cell plating for ecotoxicology studies (Chen 1992). Hemocytes also begin to lose functionality after 2–4 days in culture, limiting their application for chronic toxicity studies (Cao et al. 2003; Rioult et al. 2013; Yoshino et al. 2013). In addition to this, hemolymph contains multiple cell types which are often classified according to morphology or histochemical differences. However, some discrepancies of the number of each cell types present have been reported. The cell types also vary in their attachment efficiencies, which can make difficult to identify what cell types are actually being tested when using a primary cell culture for ecotoxicological testing (Chen 1992). Despite these challenges, primary cell cultures on hemocytes can provide useful insights as a technique to rapidly prescreen industrial products in order identify which products pose the most environmental concern and require in vivo testing strategies.
One of the challenges in the establishing a primary culture for Mytilus species however is the ability to maintain a sterile cell culture. As filter feeders, mussels can retain water and host a wide array of microparasites that can limit the ability to establish a clean culture (Mothersill and Austin 2000). As a result, a mixture of antibiotics are often implemented when establishing primary cell cultures for Mytilus hemocytes. Different antibiotic supplements are usually proposed to determine the minimum number of antibiotics required to maintain a sterile cell culture. Gentamycin (1%), is often used for gram-negative bacteria (Gómez-Mendikute and Cajaraville 2003; Katsumiti et al. 2014a, b) and Penicillin (100 units/mL) and Streptomycin (100 µg/mL) are often used together for gram positive and gram-negative bacteria (Bouki et al. 2013; Buffet et al. 2014; Cao et al. 2003; Daugavet and Blinova 2015; Faucet et al. 2004; Alberto Katsumiti et al. 2014a, b). Amphotericin B, also used to account for contamination by fungi and yeast is used in varying concentrations (Cao et al. 2003; Daugavet and Blinova 2015).
There are however limitations associated with the use of antibiotics, primarily the fact that they can impact the viability of cells and reduce attachment efficiency. In particular the use of antifungal products are known to have toxic properties for marine invertebrate cells with a previous study able to clearly show an effect of amphotericin B on cell viability, an antifungal, on M. galloprovincialis hemocytes (0.1, 0.5 and 1 µg/mL) (Cao et al. 2003; Mothersill and Austin 2000). Despite this, various laboratories have developed different cocktails of antibiotics, with amphotericin B commonly used to supplement culture media. Previous ecotoxicology studies on M. edulis hemocytes in ecotoxicology however do not report the technique used when accounting for potential contamination and selecting antibiotic supplements to be used.
Previously published studies using Mytilus hemocytes vary in the technique used to establish the primary cell culture and rarely go into detail. This includes extraction method for collecting hemocytes, type of plastic for the plating, the optimization of antibiotics for the study and the characterization of what types of cells are present in the cell culture. As a result, this study had two main aims: (1) to clearly investigate and define the best method to establish a stable primary cell culture on M. edulis hemocytes and (2) to characterize the cell types that are retained in cell culture.
Materials and methods
Specimens
Mussels were collected from a relatively clean site Saint-Cast-le-Guildo (48°37′48″N 2°15′24″W), previously identified as suitable for experimental research (Chevé et al. 2014). Mussels were cleaned of epibionts and acclimated in 30 PSU artificial seawater (ASW (Tropic Marin, Orgeval, France), 0.5 mussels/L) to lab conditions for 2 days, as recommended by the international council for exploration of the sea (ICES), at 15 °C prior to experimentation (Leverett and Thain 2013).
Extraction of hemolymph, plating and cell culture medium
Extraction of hemolymph was performed using a 23-gauge (30 mm), 2 mL syringe (St-Jean et al. 2003) filled with 0.1 mL of artificial seawater (30 PSU) and three methods were tested: (1) cutting open the mussel and directly extracting from the posterior adductor muscle, (2) gently prying open the shell using a scalpel, inserting the syringe in the dorsal-anterior of the animal and extracting the hemolymph at 45° angle oriented towards posterior adductor muscle and (3) inserting a scalpel in ventral-anterior side of the animal, slowly moving the scalpel towards the posterior and inserting the syringe at a 90° angle into the posterior adductor muscle. Cell concentration was measured through trypan blue exclusion using a hemocytometer and the total amount of hemocytes collected from each extraction technique was determined using a hemocytometer and the following formula:
The cleanliness was analyzed by qualitatively noting contaminants and presence of debris under a confocal microscope.
To determine what plate type the cells was most effective for retaining the cells, four plate types were selected and seeded with equivalent cell concentrations to: a 35 mm petri dish (VWR (Fontenay-sous-Bois, France) 734–2137), a 35 mm petri dish with a cover glass slip, a tissue culture (TC) treated 12 multi-well plate, (VWR 734–2354), and a TC treated 96 multi-well plate, (Falcon (Boulogne-Billancourt, France) 353916). Hemolymph (1 × 106 cells/mL) was added to each plate and cells were allowed to adhere for 30 min. After 30 min, cell attachment was tested by aspirating the hemolymph and refreshing the media with PBS adjusted for use with marine organisms (0.5 M NaCl, 4 mM KH2PO4, 10 mM Na2HPO4, 1100 mOSM) and qualitatively observing cell densities and visual extension of pseudopods, indicating attachment (Le Marrec-Croq et al. 1999; Quinn et al. 2009).
Leibovitz (L-15) culture medium (Sigma, Lyon, France) was selected as the base medium for the study as recommended by (Cao et al. 2003). L-15 medium was adjusted using the following salt supplements: 20 g/L sodium chloride (NaCl), 0.54 g/L potassium chloride (KCl), 0.3 g/L calcium chloride (CaCl2), 0.5 g/L magnesium sulphate (MgSO4) and 1.95 g/L magnesium chloride (MgCl2) as specified in the literature (Cao et al. 2003; Chatziargyriou and Dailianis 2010). The adjusted L-15 medium was then filtered through 0.22 µm under a PSM class II flowhood to sterilize the solution. Culture medium was then prepared by adding fetal bovine serum (10%) (Sigma) and glucose (10%) (Sigma).
Primary cell culture
All cell culture procedures were carried out under a PSM class II flowhood (MSC-Advantage). All glassware and equipments were autoclaved and wiped with 70% ethanol before use. All solutions were adjusted to a pH of 7 before use and were sterilized by autoclaving and filtering the solutions through a 0.22 µm filter. On the day of the extraction, mussels were sterilized in 1L of bleach solution (0.026% v/v) prepared in autoclaved ultra-pure water for 10 min as recommended in Droguet (2006). This step was done in order to sterilize the mussels and reduce the risk of contamination due to contaminants residing on the shell. After 10 min, mussels were then placed in autoclaved ASW for 10 min prior to hemocyte extraction.
Mussels were then removed from ASW and the shells were sterilized with 70% ethanol and allowed to dry (Droguet et al. 2012). A scalpel was then gently inserted into the ventral-anterior side of the animal to drain retained water that could potentially contaminate the cell culture. The scalpel was then slowly moved towards the center-posterior of the animal to create an opening to insert a needle into the posterior adductor muscle. Hemolymph was then aspirated by a 2 mL syringe containing 0.1 mL of Alseve (ALS) buffer (20.8 g/L glucose, 8 g/L sodium citrate, 3.36 g/L EDTA, 22.5 g/L NaCl, pH 7.0). ALS buffer was used to reduce cell aggregations and promote a more uniform cell suspension. After aspirating the hemolymph from 5 mussels the needle was removed and the hemolymph was filtered through a 70 µm filter into a 50 mL falcon tube and maintained at 4 °C. The filter was used to remove debris and cell aggregations, allowing for a more homogenous suspension. This operation was repeated until hemolymph was collected from 40 mussels.
After hemolymph was collected, the total volume was recorded. Cell concentration and cell viability in the hemolymph was determined using trypan blue exclusion. Hemolymph was then diluted to 1 × 106 cells/mL using ALS solution. 200 µL of hemolymph was then seeded into a 96-well microplate (2 × 105 cells/well) which was placed into an incubator at 18 °C to allow cells to adhere for 30 min.
While the cell attachment was occurring, cell culture medium was prepared using the 7 following conditions: (1) culture medium with no antibiotic supplement; (2) culture medium with gentamycin (1%); (3) culture medium with gentamycin (1%), penicillin (100 units/mL) and streptomycin (100 µg/mL); (4) culture medium with gentamycin (1%), penicillin (100 units/mL), streptomycin (100 µg/mL) and amphotericin B (0.1 µg/mL); (5) culture medium with gentamycin (1%), penicillin (100 units/mL) streptomycin (100 µg/mL) and amphotericin B (0.2 µg/mL); (6) culture medium with gentamycin (1%) penicillin (100 units/mL), streptomycin (100 µg/mL) and amphotericin B (0.3 µg/mL); (7) culture medium with gentamycin (1%) penicillin (100 units/mL), streptomycin (100 µg/mL) and amphotericin B (0.3 µg/mL) and tylosin (1 µL/mL) (all antibiotics were purchased from Sigma). After the 30-min attachment period, hemolymph was slowly aspirated and replaced with 200 µL of cell culture medium supplemented with the different antibiotics. Each test condition was tested in triplicate with blank wells containing only medium to verify that no environmental contamination was occurring.
The cell cultures were inspected daily to verify the cell quality through cell shape via pseudopod extension and formation of aggregations in culture using a confocal microscope (Olympus CK 2). Visual presence of contamination and pH change in the media using phenol red (2.7 × 10−5 M) where a decrease in pH, indicating bacterial contamination, changes in the coloration of the media to yellow.
Cell viability
A calibration curve was established using a serial dilution of hemocytes (0.25 × 104–4 × 105 cells/well). Medium was aspirated from hemocytes and wells were washed twice with 100 µL of PBS (1100 mOSM). 100 µL of Alamar Blue (Invitrogen, Paris, France), prepared following the producer’s instructions (10% v/v) in PBS (1100 mOSM), was added to each well. Absorbance values were immediately measured at 570 and 600 nm using a Tecan sunrise spectrometer. The microplate was then covered with aluminum foil and placed in the incubator at 18 °C. Absorbance values were then measured after 30 min. Dye reduction was then calculated using the following formula:
where λ1 = 570 nm, λ2 = 600 nm, A = absorbance of wells with hemocytes, A′ = absorbance of blanks, ξoxλ2 = molar extinction coefficient of oxidized alamar at 600 nm, ξoxλ1 = molar extinction coefficient of oxidized alamar at 570 nm, ξredλ1 = molar extinction coefficient of reduced alamar at 570 nm, ξredλ2 = molar extinction coefficient of reduced alamar at 600 nm.
The stability of the cell culture was then analyzed by measuring cellular metabolism of cells retained within the wells of the microplate through Alamar Blue dye reduction. Cellular metabolism was measured every day, up to 3 days in culture with 6 wells seeded for each day. This duration was selected as previous literature has identified that hemocytes begin to lose functional activity after 3 days in culture (Cao et al. 2003). The duration of the test was also chosen to be representative of a 24-h toxicity assay (Katsumiti et al. 2016). For the whole duration of the experiment, media were not changed in order to reduce the risk of external contamination and to maintain the microenvironment established by the cells (Mothersill and Austin 2000). Results were analyzed using the previously described formula for Alamar Blue and expressed relative to the start of the experiment.
Flow cytometry analysis
Crude hemolymph was extracted from 15 mussels using the previously described method that inserted the scalpel into the ventral-anterior side of the organism. To clean the hemolymph, half of the hemolymph was centrifuged at 500 g for 5 min. The hemolymph was extracted and the cells were suspended in an equal volume of PBS-(1100 mOSM). To assess the evolution of cell types retained in cell culture, hemocytes were placed in cell culture overnight using the cell culture medium supplemented with the following antibiotics: gentamycin (1%), penicillin (100 units/mL), streptomycin (100 µg/mL) and amphotericin B (0.1 µg/mL). After 24 h in culture, cells were detached using trypsin. The cells were then pelleted by centrifugation at 500 g for 5 min, washed twice with PBS (1100 mOSM) and suspended in PBS (1100 mOSM).
All three hemocyte samples were analyzed through flow cytometry using a FACSCanto cytometer (BD Biosciences, Rungis, France) and were measured with a 488 nm laser. Cytometry settings were optimized using a practice sample to determine which settings were most appropriate for analysis. 2 different sizes of fluorescent beads, 2 and 3 µm, were added to determine the size of cells present. Each analysis consisted of 100,000 events. Characterization of cell populations present in the suspension was performed through the Forward light scatter (FSC), to determine size, and side light scatter (SSC), to determine granulometry, using the settings defined in García-García et al. (2008).
Data analysis
All data were expressed as mean ± standard deviation. For statistical analysis, experimental results compared with corresponding control values using Kruskal–Wallis nonparametric analysis. All statistical analysis was conducted using the R Studio. A p value of < 0.05 was considered to be statistically significant.
Results
Extraction and plating
Cutting the mussel open was able to achieve a relatively clean extraction of hemocytes but yielded a much lower cell concentration when compared to the other two testing strategies (Table 2). Inserting the syringe at a 45° made it difficult to accurately target the posterior adductor muscle and as a result, seawater retained by the organism was collected during extraction. Under the microscope, mobile parasites were visibly and resulted in a contaminated sample. By inserting the scalpel in the anterior-ventral side of the organism it allowed for the draining of the water retained by the mussel prior to extracting the hemolymph, resulting in a much cleaner extraction of the hemolymph. The insertion of the syringe at a 90° angle also improved the reliability of targeting the posterior adductor muscle.
Table 2.
Different extraction techniques tested for the acquisition of hemocytes
| Extraction technique | ||||
|---|---|---|---|---|
| Technique for opening shell | Orientation of syringe | Solution in syringe | Total cells extracted (Cells/mL) | Additional comments |
| Completely cut open mussel | Directly into posterior adductor mussel | 0.1 mL of artificial sea water | 1.22 × 105 | Process yields reliable but lower cell extraction. Technique is slower than keeping mussel alive |
| Insert scalpel dorsal-anterior and slowly move towards posterior | Insert syringe at a 45° angle oriented toward the posterior-dorsal side of the organism | 0.1 mL of artificial sea water | 4 × 105 | Orientation results in extraction of sea water, major source of contamination. Difficult to reliably target posterior adductor mussel |
| Insert scalpel anterior-ventral and slowly move towards posterior | Insert syringe at a 90° angle at the posterior-dorsal side of the organism | 0.1 ml of artificial sea water | 4.5 × 105 | Extraction technique reliably targets posterior adductor mussel. Solution is cleaner than the 45° angle technique |
Each condition was measured for total cell extracted as well as cleanliness of the sample
Of the four plate types tested attachment and pseudopod extension only occurred for the 35 mm plate with the glass slip and in the 96-well plate (Table 3). Minimal cell retention was observed in both the 35 mm plate without the glass slip as well as the 24-well plate.
Table 3.
Different culture vessels used to test hemocyte attachment
| Plating technique | |||
|---|---|---|---|
| Culture vessel | Surface area (cm2) | Optimal medium volume (mL) | Cell attachment |
| 35 mm petri dish (Terumo (Guyancourt, France) 601–5412) | 8 | 2 | – |
| 35 mm petri dish (Terumo 601–5412) + glass slip | 8 | 2 | + |
| Corning 12-well plate (734–2334) | 1.9 | 0.5 | – |
| Corning 96-well plate (734–1376) | 0.32 | 0.2 | + |
Cells were visually observed to identify if attachment occurred (+: attachment occurred; −: cells did not attach)
Primary cell culture
Despite the caution used in sterilizing the shells of the mussels and draining seawater retained by the organism when extracting hemocytes, contamination continued to be a prevalent issue in many of the conditions tested (Table 4). Contamination was not observed in blank plates containing only culture medium, suggesting the contamination is inherently present in the mussel rather than due to external contamination. The most prevalent contamination observed was fungal contamination, which was consistently observed after 3 days in culture. To address this, amphotericin B was used to maintain the sterility of the culture. Higher concentrations of amphotericin B however was found to reduce cell attachment and resulted in rounded and poorly attached cells, indicating poor health. Conditions with amphotericin B could be maintained up to 7 days until contamination by yeast and mycoplasma was observed. Mycoplasma were able to be accounted for with the addition of 1 µL/mL of tyolisn but resulted in poorly attached cells and the presence of lots of debris, suggesting apoptosis.
Table 4.
List of antibiotics tested in media with duration when contamination was observed in the media
| Antibiotics tested | ||||||
|---|---|---|---|---|---|---|
| Gentamycin (%) | Streptomycin (µg/mL) | Penicillin G (µnits/mL) | Amphotericin B | Tylosin (µL/mL) | Time contamination was observed in culture (days) | Additional comments |
| – | – | – | – | – | 1 | Medium color change, significant contamination, medium cloudy and no cells visibly present |
| 1 | – | – | – | – | 1 | Medium color change, cloudy and significant presence of bacterial infection |
| 1 | 100 | 100 | – | – | 3 | Medium, clear but significant fungal contamination and mycoplasma present after 3 days |
| 1 | 100 | 100 | 0.1 | – | 7 | Cells well attached and well dispersed, mycoplasma contamination present |
| 1 | 100 | 100 | 0.2 | – | 7 | Cells well attached, less cells coverage than in 0.1 µg/L amphotericin B, mycoplasma contamination present |
| 1 | 100 | 100 | 0.3 | – | 7 | Cells not well attached, lots of debris present, contamination visible (yeast and mycoplasma) |
| 1 | 100 | 100 | 0.3 | 1 | 7 | Yeast contamination present but minimal, lots of debris present, cells poorly attached |
Cell viability
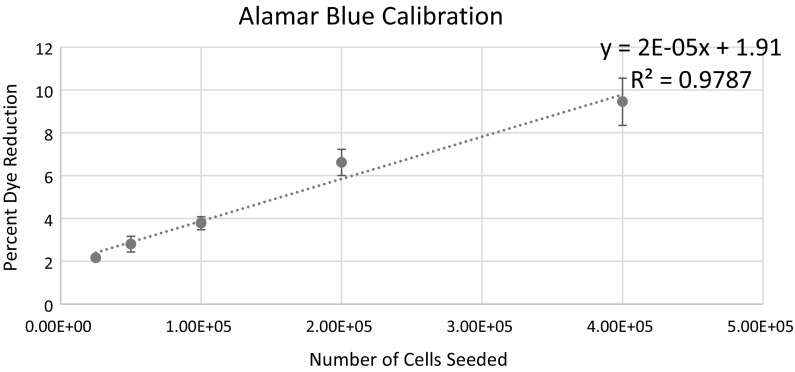
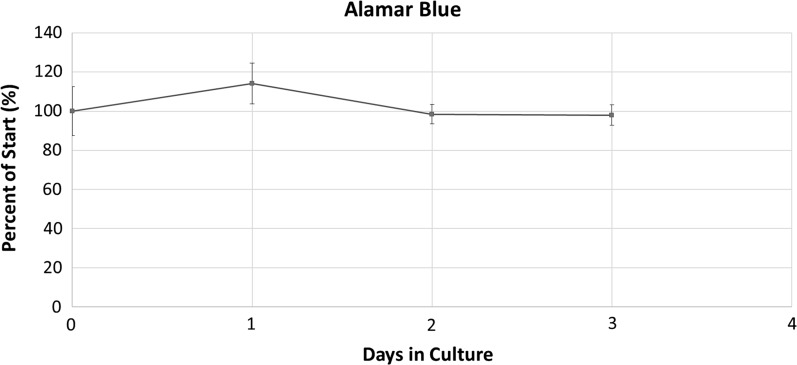
Alamar Blue was able to accurately distinguish between cell concentrations, indicating that is a suitable technique for measuring cell viability (Fig. 1). To analyze the impact of the cocktail of antibiotics on the stability of the cell culture, cellular metabolism was measured via the reduction of Alamar Blue. Cellular metabolism over the duration of the experiment showed no statistically significant declines, suggesting that cellular metabolism was not adversely effected using antibiotics in the cell culture (Fig. 2).
Fig. 1.
Reduction of Alamar Blue dye after incubation with different cell concentrations. Results are expressed as percentage of dye reduced after 30 min of incubation
Fig. 2.
Cell viability measured through Alamar Blue. Results are displayed as percentages relative to the start of the experiment
Flow cytometry analysis
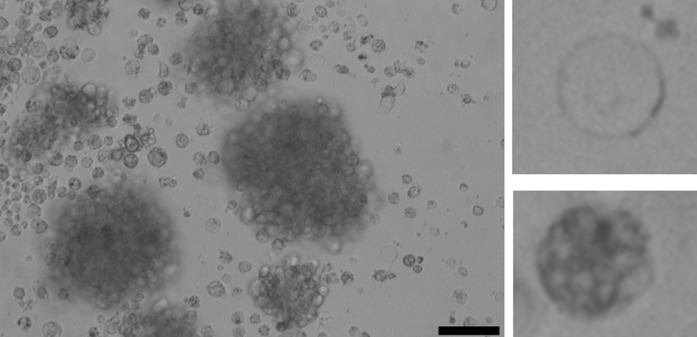
In the present study, two populations of hemocytes were visibly present under a confocal microscope, both averaging approximately 7–10 µm in size but a notable size variation was observed. During the extraction of the hemocytes cell types could be visually differentiated as granulocytes and hyalinocytes using a confocal microscope (Fig. 3). It was observed that most of the attached cells were granulocytes and hyalinocytes were less present (both, approximately 7–10 µm in size). The hyalinocytes that were present displayed a rounded morphology and did not display pseudopodia.
Fig. 3.
Image of extracted hemocytes retained in cell culture demonstrating the mixed cell populations. Hyalinocytes (top right) can be distinguished by the notable lack of granules. Granulocytes (bottom right) can be distinguished by presence of granules. Black bar indicates the scale (20 µm) at 40× magnification
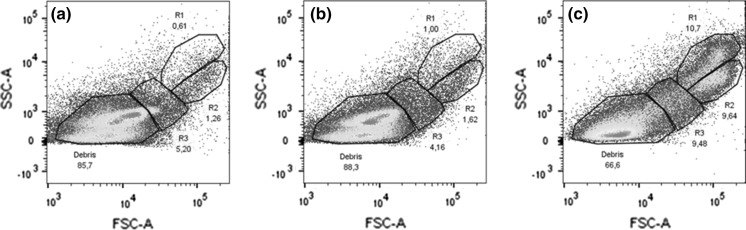
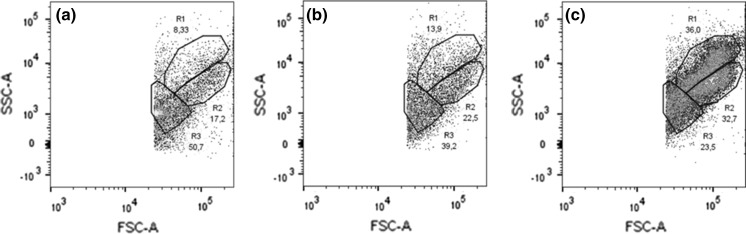
Based on the cytometry results, 3 different cells populations could be distinguished, which were defined as R1–R3 (Fig. 4). R1 displayed the highest SSC values compared to the other two cell types present. R2 cells displayed lower SSC values than R1 but similar FSC values, suggesting similar sizes. R3 showed a high degree of variation in SSC values and lower FSC values compared to the other cells types. The results suggested the R1 and R2 cells may be considered as different types of granulocytes and R3 may correspond to hyalinocytes.
Fig. 4.
Cytometry results for a freshly extracted crude hemolymph, b washed hemolymph and in c hemocytes were maintained in cell culture for 24 h. Retention of different cell types appeared to improve with the cell culture
To determine the change in ratio between cell types and the three test conditions, cytometry results were reanalyzed to focus on counts identified as cells (Fig. 5). Cells defined as R3 were the most prevalent in the crude hemolymph, 50.7%, and washed hemolymph, 39.2%. When cells were maintained in cell culture however, the ratio of R1, 36%, and R2, 32.7%, increased and were more prevalent than R3, 23.5%.
Fig. 5.
Cytometry results for a freshly extracted crude hemolymph, b washed hemolymph and in c hemocytes were maintained in cell culture for 24 h reanalyzed to focus on the ratios between the cell types present
Discussion
Extraction and plating
The aim of this study was to identify the best method to establish a primary cell culture for M. edulis hemocytes as well as identifying the cell types present. The results of the study were able to successful identify the optimal extraction technique, plating method and as well as antibiotic supplements for maintain a primary cell culture. The study also identified a change in the ratio between cell types between hemolymph and cells retained in culture. The study did however identify some considerations that need to be made when establishing a primary cell culture for M. edulis hemocytes.
Literature on hemolymph extraction has described significant differences in the technique used to extract the hemolymph (Caza et al. 2015; Chen and Bayne 1995; Höher et al. 2015; Gustafson et al. 2005; Conrad et al. 2005; Coles et al. 1995). With respect to the techniques used in the study, the insertion of the scalpel in the anterior-dorsal section of the organism was the least invasive technique and allows for the repeated sampling from the same organism. However, access to the posterior adductor muscle was not reliable and resulted in the extraction of retained seawater along with hemolymph, often resulting in visible extraction of aquatic parasites along with the hemolymph making results unsuitable for cell culture. Cutting open the organism allowed for direct insertion of the syringe into the posterior adductor muscle and a reliably clean extraction. The application of this method of hemocyte extraction is however slow and results in a low cell yielding, making the technique unrealistic for use in in vitro screening. Inserting the scalpel in the ventral-anterior side of the animal allowed for relatively easy draining of retained seawater and easy access to the posterior muscle. Cell concentrations were relatively high and clean compared to the insertion of the syringe from the anterior-dorsal section of the organism, making it the most ideal extraction technique for in vitro studies. This is also the technique recommended by the International Council for Exploration of the Sea (ICES) as ideal for the withdrawal of hemolymph (Martínez-Gómez et al. 2015).
The results for cell attachment efficiency with the different types of plating showed differences between the plate types. The glass cover slip improved attachment efficiency of over the 35 mm dish without the glass slip, which is consistent with previous publications suggesting the hemocytes can have difficulty in attaching to plastic (Canesi et al. 2010; Chen and Bayne 1995). However, previous research has also shown the hemocytes can effectively attach to polystyrene, which was not observed in the present study (Chen 1992). Comparing the 24 multiwell plate to the 96 multiwell plate showed notably improved cellular attachment in the 96-well plate. Both plates are TC-treated and of similar plastic composition, suggesting that total surface area may influence the attachment efficiency of the cells. The use of the 96-well plate with TC-treatment may be ideal for establishing a primary cell culture for toxicity studies and could be an initial starting point for investigating an HTS testing strategy. The 96-well plate is also ideal as it reduces the number of cells required per well and is effectively increasing the number of doses or chemicals that can be tested from a single extraction.
Tests with antibiotics
Testing with the different antibiotic cocktails demonstrated the need for antibiotic supplements in culture medium. Of previously published papers on Mytilus hemocyte primary cell cultures, only Cao et al. (2003) reported the maintenance of the cell culture past 3 days. In the present study, this technique was identified as the most effective for maintaining the sterility of the cell culture without notably changing the cell attachment behavior. In the present study however, contamination by mycoplasma and yeast was visibly present in the culture after 7 days. To our best knowledge this is the first time that mycoplasma contamination has been reported when establishing a primary cell culture for Mytilus hemocytes. Presence of mycoplasma like organisms has however been previously reported in mussels (Comps and Tigé 1999; Villalba et al. 1997). Mycoplasma are often a cryptic contamination that can go undetected until serious contamination occurs (Freshney 2016). In the case of Mytilus hemocytes, this contamination appeared much latter than the cell culture would normally be used for testing, suggesting that caution may be necessary to identify naturally occurring cryptic contamination prior to starting ecotoxicological testing. It is also important to note that previous studies have established clean primary cell cultures for marine bivalves with fewer types of antibiotics than were used in this study, which may be due to intersite variations in microbes inherently present within Mytilus species. As a result, the antibiotic cocktail required to establish a clean culture for Mytilus hemocytes may be dynamic and warrants further investigation to establish a suitable and replicable testing strategy for ecotoxicity testing.
Stability of cell culture
Alamar Blue is a technique that has been used over the past 50 years to measure cell viability and cytotoxicity and has been applied to a wide range of biological and environmental systems (Rampersad 2012). Alamar Blue contains a cell permeable nonfluorescent blue dye, resazuruin, which is transformed to fluorescent, pink (resorufin), in the presence of cellular metabolic reduction. The rate of metabolic reduction can be used to extrapolate cell viability as cell concentration will impact the rate of dye reduction. Alamar blue is a low-cost assay that does not require cell lysis and is non-toxic to the cell, allowing for multiple measurements to be conducted on the same samples (Rampersad 2012). This makes Alamar Blue a useful technique for future use in an HTS screening strategy for ecotoxicology. Alamar Blue has also been used with a variety of different cell types, allowing for comparison with cell cultures from other organisms.
To our best knowledge this is the first study of the use of Alamar Blue with Mytilus edulis hemocytes. Based on the observation of the experiment, there were no significant changes in cellular metabolism over the duration that the hemocytes were maintained in cell culture with antibiotics. This suggests that the cocktail of antibiotics used in the present study did not adversely impact retention of cells in culture over the duration of the test. Consequently, it is also important to note that the results demonstrate that cellular metabolism did not increase, indicating concurrently that cell densities did not change. Currently one of the major limitations in establishing a long term culture with mussel hemocytes is the lack of understanding in parameters that influence hemocyte cell division, with previous results suggesting that while hemocytes do undergo cell division it is in not at a high enough rate to result in a significant change in cell concentration (Renwrantz et al. 2013). It has also been determined that as hemocyte cells are terminally differentiated, they only survive 2–3 days in culture with some studies reporting up to 4 days in culture (Rioult et al. 2013; Yoshino et al. 2013). The aim of the current study was not to establish a long term cell culture but was focused on identifying if the antibiotic supplements were adapted for an acute exposure assay.
The duration of the cell culture on mussel hemocytes using this antibiotic supplement is sufficient for a preliminary prescreening of chemicals but more longer-term cultures will be required for testing the effects of contaminants at lower concentrations over an increased duration. Until the mechanisms regarding cell division for hemocytes are fully understood current cell techniques will continue to focus on the retention rather than growth of hemocytes, leading to many studies to focus on screening chemicals using short term exposure scenarios at high concentrations. It is also important to highlight that while these results showed no impact through the use of Alamar Blue, this does not mean the antibiotic cocktail caused no effects to the hemocyte cells. There may be sublethal effects that need to be further investigated in order to determine if this cocktail of antibiotics is suitable for ecotoxicity testing in the long term.
Cytometry
Characterization using flow cytometry to characterize cell types present in hemocytes has demonstrated some variation with previous research characterizing 3–4 cell types (García-García et al. 2008; Rioult et al. 2013). In the current study, three cell types could be clearly distinguished using cytometry for the freshly extracted hemolymph, washed hemolymph and cells retained in cell culture. The cell types observed in the present study are consistent with the cell types defined in Moore and Lowe (1977). In the present study two types of granulocyte cells were identified which may corresponded to basophilic and eosinophilic granulocytes previously described (Carballal et al. 1997). The ratio of cell types in the cell culture were notably different when compared to the other two cell suspensions, with both granulocyte cells (R1 and R2) being more prevalent. This suggests that these cells are more effectively retained in the cell culture than the hyalinocytes.
To our best knowledge this is the first time this type of M. edulis cells maintained in primary cell culture have been characterized. This can have potential implications for ecotoxicity studies as hemocyte types have been demonstrated to vary in the phagocytic abilities and by extension, how they may respond in toxicity assays (García-García et al. 2008; Moore and Lowe 1977). In addition to this, it has also been suggested that the ratio between cell types can vary depending on the season as well as the organisms stress level (Renwrantz et al. 2013). As a result, ecotoxicity studies may need to investigate the cell types being maintained in order to more accurately characterize toxicity profiles of contaminants.
Conclusions
There are external forces, like societal pressures to reduce the need for whole organism testing as well as a shift in environmental policies to prevention rather than mitigation, that are driving a need for in vitro testing on aquatic invertebrates. To adequately address the growing demand for industrial products to be approved for market use, in vitro techniques could potentially be used to prescreen products and identify materials or chemicals that require more in-depth analysis using in vivo studies. Thus, there is a need for techniques that can be adapted to high throughput screening platforms that quickly screen a large number of chemicals simultaneously as well as reduce the number of animals used in testing. Due to the relative ease that a mixed primary cell culture can be quickly established for M. edulis hemocytes they present an opportunity to investigate the suitability of HTS screening strategies for novel products and investigating the suitability of in vitro testing strategies to predict in vivo responses as well as potential exploration of adverse outcome pathways.
This research investigates techniques that could be used to establish a primary cell culture of Mytilus edulis hemocytes that can potentially be adapted to an HTS strategy. However, the current study identified some considerations that need be made when establishing a primary cell culture, in particular the antibiotic supplements used and the cell types present in cell culture.
Another consideration that needs to be made is that hemocyte cell concentrations in Mytilus species display a high degree of inter-site variation and are heavily influenced by the overall stress of the organisms (Renwrantz et al. 2013).This may suggest as well that there is seasonal variation in microbial contamination present as well, which may influence the antibiotic supplements required to establish a clean cell culture. As a result, this provides a challenge for the use of in vitro testing for chemicals as this suggests that in order to maintain a level of comparison between chemicals, assays needed to be conducted simultaneously in order to reduce this risk of inter-site or inter-seasonal variation. It would also be recommended to test chemicals multiple times, and at different seasons, to guarantee that the results are not an artifact of the hemocyte condition.
Taking these limitations into account, a primary cell culture of hemocytes can be a useful tool for a preliminary investigation into emerging contaminants like engineered nanomaterials as in vitro techniques may be the only way to adequately screen these products in a time effective manner for environmental risk while meeting regulatory needs (Barrick et al. 2017). Compared to in vivo testing, primary cell culture can rapidly test many chemicals, a wide range of doses and at a comparatively lower cost. However, despite the advantages of using in vitro testing strategies for marine invertebrates there needs to be a demonstration that in vitro testing can adequately predict in vivo responses. Until this has been fully characterized, it would be recommended to conduct in vitro and in vivo testing on M. edulis hemocytes in parallel to fully characterize the relationship.
References
- Barrick A, Châtel A, Bruneau M, Mouneyrac C. The role of high-throughput screening in ecotoxicology and engineered nanomaterials. Environ Toxicol Chem. 2017;9999:1–11. doi: 10.1002/etc.3811. [DOI] [PubMed] [Google Scholar]
- Bouki E, Dimitriadis VK, Kaloyianni M, Dailianis S. Antioxidant and pro-oxidant challenge of tannic acid in mussel hemocytes exposed to cadmium. Mar Environ Res. 2013;85:13–20. doi: 10.1016/j.marenvres.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Buffet PE, Zalouk-Vergnoux A, Châtel A, Berthet B, Métais I, Perrein-Ettajani H, Poirier L, Luna-Acosta A, Thomas-Guyon H, Risso-de Faverney C, Guibbolini M, Gilliland D, Valsami-Jones E, Mouneyrac C. A marine mesocosm study on the environmental fate of silver nanoparticles and toxicity effects on two endobenthic species: the ragworm Hediste diversicolor and the bivalve mollusc Scrobicularia plana. Sci Total Environ. 2014;470–471:1151–1159. doi: 10.1016/j.scitotenv.2013.10.114. [DOI] [PubMed] [Google Scholar]
- Canesi L, Ciacci C, Lorusso LC, Betti M, Gallo G, Pojana G, Marcomini A. Effects of Triclosan on Mytilus galloprovincialis hemocyte function and digestive gland enzyme activities: possible modes of action on non target organisms. Comp Biochem Physiol C Toxicol Pharmacol. 2007;145:464–472. doi: 10.1016/j.cbpc.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Canesi L, Ciacci C, Vallotto D, Gallo G, Marcomini A, Pojana G. In vitro effects of suspensions of selected nanoparticles (C60 fullerene, TiO2, SiO2) on Mytilus hemocytes. Aquat Toxicol. 2010;96:151–158. doi: 10.1016/j.aquatox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Canesi L, Ciacci C, Fabbri R, Marcomini A, Pojana G, Gallo G. Bivalve molluscs as a unique target group for nanoparticle toxicity. Mar Environ Res. 2012;76:16–21. doi: 10.1016/j.marenvres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Cao A, Mercado L, Ramos-Martinez JI, Barcia R. Primary cultures of hemocytes from Mytilus galloprovincialis Lmk.: expression of IL-2Rα subunit. Aquaculture. 2003;216:1–8. doi: 10.1016/S0044-8486(02)00140-0. [DOI] [Google Scholar]
- Carballal M, López M, Azevedo C, Villalba A. Hemolymph cell types of the mussel Mytilus galloprovincialis. Dis Aquat Organ. 1997;29:127–135. doi: 10.3354/dao029127. [DOI] [Google Scholar]
- Caza F, Betoulle S, Auffret M, Brousseau P, Fournier M, St-Pierre Y. Comparative analysis of hemocyte properties from Mytilus edulis desolationis and Aulacomya ater in the Kerguelen Islands. Mar Environ Res. 2015;110:174–182. doi: 10.1016/j.marenvres.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Chatziargyriou V, Dailianis S. The role of selenium-dependent glutathione peroxidase (Se-GPx) against oxidative and genotoxic effects of mercury in haemocytes of mussel Mytilus galloprovincialis (Lmk.) Toxicol Vitr. 2010;24:1363–1372. doi: 10.1016/j.tiv.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Chen J-H. The cell activation model of hemocyte aggregation and adhesion in the California mussel, Mytilus Californianus. College Town: Oregon State University; 1992. [Google Scholar]
- Chen J-H, Bayne CJ. Bivalve mollusc hemocyte behaviors: characterization of hemocyte aggregation and adhesion and their inhibition in the California mussel (Mytilus californianus) Biol Bull. 1995;188:255–266. doi: 10.2307/1542303. [DOI] [PubMed] [Google Scholar]
- Chevé J, Bernard G, Passelergue S, Prigent J-L (2014) Suivi bactériologique des gisements naturels de coquillages de l’Ille-et-Vilaine et des Côtes- d’Armor fréquentés en pêche à pied 1–99
- Coles JA, Farley SR, Pipe RK. Alteration of the immune response of the common marine mussel Mytilus edulis resulting from exposure to cadmium. Dis Aquat Org. 1995;22:59–65. doi: 10.3354/dao022059. [DOI] [Google Scholar]
- Comps M, Tigé G. Procaryotic infections in the mussel Mytilus galloprovinciallis and in its parasite the turbellarian Urastoma cyprinae. Dis Aquat Org. 1999;38:211–217. doi: 10.3354/dao038211. [DOI] [Google Scholar]
- Conrad P, Atwill E, Garner I, Miller M, Leutenegger C, Arkush K, Jesuup D (2005) Cryptosporidium in bivalves as indicators of fecal pollution in the California coastal ecosystem. UC Berkeley Tech. Complet. Reports
- Daugavet MA, Blinova MI. Culture of mussel (Mytiuls edulis L.) mantle cells. Cell Tissue Biol. 2015;9:233–243. doi: 10.1134/S1990519X15030037. [DOI] [PubMed] [Google Scholar]
- Domart-Coulon I, Auzoux-Bordenave S, Doumenc D, Khalanski M. Cytotoxicity assessment of antibiofouling compounds and by-products in marine bivalve cell cultures. Toxicol Vitr. 2000;14:245–251. doi: 10.1016/S0887-2333(00)00011-4. [DOI] [PubMed] [Google Scholar]
- Droguet M (2006) Etude des caracteristiques fonctionnelles des cardiomyocete d’huitre en culture. Universite de Bretagne Occidentale
- Droguet M, Devauchelle N, Pennec J-P, Quinn B, Dorange G. Cultured heart cells from oyster: an experimental approach for evaluation of the toxicity of the marine pollutant tributyltin. Aquat Living Resour. 2012;25:185–194. doi: 10.1051/alr/2012017. [DOI] [Google Scholar]
- Faucet J, Maurice M, Gagnaire B, Renault T, Burgeot T. Isolation and primary culture of gill and digestive gland cells from the common mussel Mytilus edulis. Methods Cell Sci. 2004;25:177–184. doi: 10.1007/s11022-004-8227-4. [DOI] [PubMed] [Google Scholar]
- Fenwick N, Griffin G, Gauthier C. The welfare of animals used in science: how the “Three Rs” ethic guides improvements. Can Vet J. 2009;50:523–530. [PMC free article] [PubMed] [Google Scholar]
- Freshney IR. Culture of animal cells: a manual of basic technique and specialized applications. 7. London: Wiley; 2016. [Google Scholar]
- García-García E, Prado-Álvarez M, Novoa B, Figueras A, Rosales C. Immune responses of mussel hemocyte subpopulations are differentially regulated by enzymes of the PI 3-K, PKC, and ERK kinase families. Dev Comp Immunol. 2008;32:637–653. doi: 10.1016/j.dci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Gómez-Mendikute A, Cajaraville MP. Comparative effects of cadmium, copper, paraquat and benzo[a]pyrene on the actin cytoskeleton and production of reactive oxygen species (ROS) in mussel haemocytes. Toxicol Vitr. 2003;17:539–546. doi: 10.1016/S0887-2333(03)00093-6. [DOI] [PubMed] [Google Scholar]
- Gomez-Mendikute A, Etxeberria A, Olabarrieta I, Cajaraville MP. Oxygen radicals production and actin filament disruption in bivalve haemocytes treated with benzo(a)pyrene. Mar Environ Res. 2002;54:431–436. doi: 10.1016/S0141-1136(02)00177-0. [DOI] [PubMed] [Google Scholar]
- Gustafson LL, Stoskopf MK, Bogan AE, Showers W, Kwak TJ, Hanlon S, Levine JF. Evaluation of a nonlethal technique for hemolymph collection in Elliptio complanata, a freshwater bivalve (Mollusca: Unionidae) Dis Aquat Org. 2005;65:159–165. doi: 10.3354/dao065159. [DOI] [PubMed] [Google Scholar]
- Hanana H, Talarmin H, Pennec JP, Droguet M, Gobin E, Marcorelle P, Dorange G. Establishment of functional primary cultures of heart cells from the clam Ruditapes decussatus. Cytotechnology. 2011;63:295–305. doi: 10.1007/s10616-011-9347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höher N, Turja R, Köhler A, Lehtonen KK, Broeg K. Immunological responses in the mussel Mytilus trossulus transplanted at the coastline of the northern Baltic Sea. Mar Environ Res. 2015;112:113–121. doi: 10.1016/j.marenvres.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Jimeno-Romero A, Bilbao E, Izagirre U, Cajaraville MP, Marigómez I, Soto M. Digestive cell lysosomes as main targets for Ag accumulation and toxicity in marine mussels, Mytilus galloprovincialis, exposed to maltose-stabilised Ag nanoparticles of different sizes. Nanotoxicology. 2017 doi: 10.1080/17435390.2017.1279358. [DOI] [PubMed] [Google Scholar]
- Judson R, Kavlock R, Martin M, Reif D, Houck K. Perspectives on validation of high-throughput assays supporting 21st century toxicity testing. ALTEX. 2013;30:51–66. doi: 10.14573/altex.2013.1.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumiti A, Berhanu D, Howard KT, Arostegui I, Oron M, Reip P, Valsami-Jones E, Cajaraville MP. Cytotoxicity of TiO2 nanoparticles to mussel hemocytes and gill cells in vitro: influence of synthesis method, crystalline structure, size and additive. Nanotoxicology. 2014;5390:1–11. doi: 10.3109/17435390.2014.952362. [DOI] [PubMed] [Google Scholar]
- Katsumiti A, Gilliland D, Arostegui I, Cajaraville MP. Cytotoxicity and cellular mechanisms involved in the toxicity of CdS quantum dots in hemocytes and gill cells of the mussel Mytilus galloprovincialis. Aquat Toxicol. 2014;153:39–52. doi: 10.1016/j.aquatox.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Katsumiti A, Arostegui I, Oron M, Gilliland D, Valsami-Jones E, Cajaraville MP. Cytotoxicity of Au, ZnO and SiO2 NPs using in vitro assays with mussel hemocytes and gill cells: relevance of size, shape and additives. Nanotoxicology. 2016;10:185–193. doi: 10.3109/17435390.2015.1039092. [DOI] [PubMed] [Google Scholar]
- Le Marrec-Croq F, Glaise D, Guguen-Guillouzo C, Chesne C, Guillouzo A, Boulo V, Dorange G. Primary cultures of heart cells from the scallop Pecten maximus (mollusca-bivalvia) In Vitro Cell Dev Biol Anim. 1999;35:289–295. doi: 10.1007/s11626-999-0073-x. [DOI] [PubMed] [Google Scholar]
- Le Pennec G, Le Pennec M. Acinar primary cell culture from the digestive gland of Pecten maximus (L.): an original model for ecotoxicological purposes. J Exp Mar Bio Ecol. 2001;259:171–187. doi: 10.1016/S0022-0981(01)00232-5. [DOI] [PubMed] [Google Scholar]
- Leverett D, Thain J (2013) ICES techniques in marine environmental sciences: oyster embryo-larval bioassay (revised). ICES Tech Mar Environ Sci 54. 10.13140/RG.2.1.3484.6165
- Martínez-Gómez C, Bignell J, Lowe D. Lysosomal membrane stability in mussels. ICES Tech Mar Environ Sci. 2015;56:41. [Google Scholar]
- Moore MN, Lowe DM. The cytology and cytochemistry of the hemocytes of Mytilus edulis and their responses to experimentally injected carbon particles. J Invertebr Pathol. 1977;29:18–30. doi: 10.1016/0022-2011(77)90167-7. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Austin B. Aquatic invertebrate cell culture. Berlin: Springer; 2000. [Google Scholar]
- National Institute of Environmental and Health Sciences (2001) Guidance document on using In vitro data to estimate in vivo starting doses for acute toxicity. Natl Toxicol Progr Interag Cent Eval Altern Toxicol Methods 1–102
- OECD Environment Directorate (2017) Alternative testing strategies in risk assessment of manufactured nanomaterials: current state of knowledge and research needs to advance their use. OECD Environment, Health and Safety Publications Series on Safety of Manufactured Nanomaterials. 80:JT03408320
- Quinn B, Costello MJ, Dorange G, Wilson JG, Mothersill C. Development of an in vitro culture method for cells and tissues from the zebra mussel (Dreissena polymorpha) Cytotechnology. 2009;59:121–134. doi: 10.1007/s10616-009-9202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersad SN. Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Switzerland) 2012;12:12347–12360. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renwrantz L, Siegmund E, Woldmann M. Variations in hemocyte counts in the mussel, Mytilus edulis: similar reaction patterns occur in disappearance and return of molluscan hemocytes and vertebrate leukocytes. Comp. Biochem Physiol A Mol Integr Physiol. 2013;164:629–637. doi: 10.1016/j.cbpa.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Rioult D, Lebel JM, Le Foll F. Cell tracking and velocimetric parameters analysis as an approach to assess activity of mussel (Mytilus edulis) hemocytes in vitro. Cytotechnology. 2013;65:749–758. doi: 10.1007/s10616-013-9558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Jean SD, Courtenay SC, Parker RW. Immunomodulation in Blue Mussels (Mytilus edulis) exposed to a pulp and paper mill effluent in eastern Canada. Water Qual Res. 2003;38:647–666. doi: 10.2166/wqrj.2003.041. [DOI] [Google Scholar]
- Stone V, Pozzi-Mucelli S, Tran L, Aschberger K, Sabella S, Vogel U, Poland C, Balharry D, Fernandes T, Gottardo S, Hankin S, Hartl MG, Hartmann N, Hristozov D, Hund-Rinke K, Johnston H, Marcomini A, Panzer O, Roncato D, Saber AT, Wallin H, Scott-Fordsmand JJ. ITS-NANO—prioritising nanosafety research to develop a stakeholder driven intelligent testing strategy. Part Fibre Toxicol. 2014;11:9. doi: 10.1186/1743-8977-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguy M, McKenna P, Gauthier-Clerc S, Pellerin J, Danger JM, Siah A. Sequence analysis of a normalized cDNA library of Mytilus edulis hemocytes exposed to Vibrio splendidus LGP32 strain. Results Immunol. 2013;3:40–50. doi: 10.1016/j.rinim.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba A, Mourelle SG, Carballal MJ, López C. Symbionts and diseases of farmed mussels Mytilus galloprovincialis throughout the culture process in the Rias of Galicia (NW Spain) Dis Aquat Org. 1997;31:127–139. doi: 10.3354/dao031127. [DOI] [Google Scholar]
- Yoshino TP, Bickham U, Bayne CJ. Molluscan cells in culture: primary cell cultures and cell lines. Can J Zool. 2013;91:391–404. doi: 10.1139/cjz-2012-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]