Abstract
Charge heterogeneity has been broadly studied as a critical quality attribute during monoclonal antibody (mAb) production that may subsequently affect product stability and biopotency. However, the charge variation distribution is poorly controlled, so methods of more effective control need to be explored. In this study, the combined effects of temperature shift (37–34, 37–32, or 37–30 °C) and hydrolysate addition (0.100 g/L) to culture feed on the charge heterogeneity of anti-IgE mAb were investigated. The results showed that the distribution of charge variation was significantly regulated by the combination of hydrolysate addition with a highly sub-physiological temperature (34 °C). In addition, under this condition, the main peak content significantly increased, and the acidic peak content significantly decreased. Furthermore, we explored Lys variant content, which is the major basic variant content, as well as its relationship with temperature shift and hydrolysate addition. Lys variant levels were positively related to the Lys and Arg concentrations in the medium and negatively related to carboxypeptidase B and carboxypeptidase H transcript levels. The combination of temperature shift and hydrolysate addition can thus effectively improve anti-IgE mAb charge heterogeneity and significantly increase main variant levels and decrease acidic variant levels.
Keywords: Carboxypeptidase, Charge heterogeneity, Lysine variants, Temperature
Introduction
Over the past three decades, monoclonal antibodies (mAbs) have undergone a dramatic transformation, from scientific tools to powerful clinical therapeutic agents. And the sales of therapeutic mAbs had exceeded 100 billion US dollars in 2016. Omalizumab, first approved in the USA in 2003 and now used in many other countries, is a humanized mAb that binds serum IgE (Logsdon and Oettgen 2015), thus reducing circulating free IgE levels and blocking both early- and late-phase reactions to allergen challenge.
Charge heterogeneity is a critical quality attribute during mAb production that may subsequently affect mAb pharmacokinetics in vivo (Boswell et al. 2010; Woodside et al. 1998). Post-translational and chemical degradation, such as via deamination (Xie et al. 2016), glycosylation (Yan et al. 2009), glycation, disulfide bond reduction (Khawli et al. 2010), and C-terminal Lys cleavage (Dada et al. 2015), are the main reasons for charge heterogeneity. Particularly, C-terminal Lys cleavage is the most commonly investigated reason for basic charge heterogeneity (Harris et al. 2004). Incomplete C-terminal Lys residue cleavage leads to the presence of Lys variation, which is a major basic variant commonly detected during mAb manufacture (Dorai and Ganguly 2014). C-terminal Lys cleavage is an enzymatic process catalyzed by basic carboxypeptidases (Cps), including CpB and CpH, which are found in Chinese hamster ovary (CHO) cells (Luo et al. 2012; Zhang et al. 2015). Furthermore, C-terminal Lys variation was found to be related to Lys and Arg (Zhang et al. 2015). However, in the mAb production process the charge variant distribution is influenced by a series of culture parameters, such as pH, temperature, and culture mode.
Culture temperature has been extensively studied in the mAb production process; temperature affects not only production but also quality attributes, such as glycosylation (Ahn et al. 2008), charge heterogeneity (Zhang et al. 2015), aggregation and fragmentation (Gomez et al. 2012). In this context, the acidic peak content is significantly decreased, and the basic peak content is significantly increased, but the main peak content remains constant when the culture temperature is decreased (Zhang et al. 2015).
Hydrolysate is usually added to medium and feed, which can effectively increase cell density or production (Buss et al. 2012; Franek et al. 2000; Heidemann et al. 2000). However, the effect of hydrolysate addition on quality attributes, and especially charge heterogeneity, has been seldom studied. Nevertheless, in previous studies, we found that hydrolysate addition to feed could decrease the acidic peak content and enhance mAb production in fed-batch culture.
However, the charge variant distribution is poor and difficult to control using current techniques, so methods of more effective control need to be explored for that charge variants have been shown to affect the in vitro and in vivo binding characteristics of antibodies (Gawlitzek et al. 2000; Mastrangelo et al. 2000; Sauer et al. 2000), in addition, it has been shown with an enriched pool of acidic species from an IgG1 antibody, there was a lower FcRn binding response, despite demonstrating similar in vivo pharmacokinetic (PK) parameters (Riechmann et al. 1988). Therefore, we investigated the combined effect of a series of sub-physiological temperatures (30, 32, and 34 °C) and hydrolysate addition (0.100 g/L) on anti-IgE mAb charge heterogeneity in the present study. Furthermore, we examined the change in Lys variant levels in relation to the temperature shift and hydrolysate addition to the feed.
Materials and methods
Cell culture
Recombinant humanized anti-human IgE antibody was produced in the recombinant CHO-K1 cell line, with the mother cell line originally (Catalogue number CCL-61) purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The recombinant CHO-K1 cell line constructed with pEE12.4 vector (Lonza, Basel, Switzerland) and selected with a glutamine synthetase (GS) system in house at the Cell Culture Bioprocess Engineering (CCBE) laboratory of the Shanghai Jiao Tong University (Shanghai, China). The basic medium used was Dynamis® serum-free basic medium (Thermo Fisher, NH, USA), and the feeds used were in-house feed-A and feed-B [feed-A + 100 g Sheff-CHO Plus PF ACF®L-1 (Kerry, Beloit, WI, USA)]. Sheff-CHO Plus PF ACF, which contains wheat and soy components, was the source of hydrolysate in this study; therefore, the hydrolysate addition group received feed-B, whereas the control group received feed-A. The feed strategy involved feed addition starting on day 4, with 5, 2, 5, 2, and 5% feed (v/v) added sequentially every 2 days. The cells were inoculated in a 500-mL shaker flask (Corning, NY, USA) with 100 mL of medium, and the final cell density was approximately 0.5 × 106 cells/mL. Both groups were cultured at 37 °C until day 4, when the culture temperature was shifted to 30, 32, or 34 °C, which were defined as low, medium, and high sub-physiological temperatures, respectively. Each day, 3-mL samples were removed to determine cell density, cell viability, and off-line pH, and the supernatant were stored at − 80 °C for further determination.
Cell density, cell viability, off-line pH, and mAb titer
The cells were stained with Trypan blue (Sigma, St. Louis, MO, USA), and cell density and viability were measured using a Countstar Automated Cell Counter (Yurui, Shanghai, China). The sample pH was measured immediately after sampling using a pH meter (Mettler Toledo, Zurich, Switzerland). In addition, the titer was measured using a ForteBio Octet® RED96 (Pall, San Diego, CA, USA) system according to the manufacturer’s instructions.
Amino acid concentrations
Lys and Arg concentrations were determined using a high-speed amino acid analyzer (Hitachi, Tokyo, Japan) according to the manufacturer’s instructions.
Weak cation exchange chromatography
Weak cation exchange HPLC (WCX-HPLC) was performed to detect charge variation in the antibody product. Antibody charge variants were separated on a 4.0 mm × 250 mm ProPac WCX-10 column (Thermo Fisher Scientific, Sunnyvale, CA, USA) using an Agilent 1260 HPLC system (Agilent, San Diego, CA, USA) at a flow rate of 1.0 mL/min. Proteins were eluted at ambient temperature using a mobile phase gradient of 0–100 mM NaCl in a buffer consisting of 10 mM MES and 10 mM HEPES at pH 8.0.
Lys variant content
Lys variant content was quantified as the difference in basic variants after CpB treatment of the mAb, which was performed as follows. First, samples underwent ultrafiltration with a 50 mM NH4HCO3 exchange buffer 3 times in a 10-kDa ultrafiltration tube (BD, Franklin Lakes, NJ, USA) and were then diluted to a final concentration of 1.0 mg/mL. Second, 200 μL of the upper solution was transferred to a 1.5-mL EP tube, 1 μL of CpB enzyme was added, and the resulting solution was mixed thoroughly. Third, the samples were incubated in a 37 °C water bath for 2 h. Finally, the samples were cooled to room temperature before analysis.
Real-time PCR
Relative CpB and CpH mRNA levels were quantified by real-time PCR analysis. Total mRNA was extracted with E.Z.N.A.® Total RNA Kit I (Omega Bio-tek, Norcross, GA, USA). The following primers were used: CpB: forward TGAAAAGGAGACCAAGGCCC, reverse CGCAGGCAGCTTATTTGCAT; CpH: forward ACCTGGAGCAGATACACCGA, reverse GGTTGCATTGGCAATTGGGT; and GAPDH: forward GGAGTAAGAAGCCCACCCTG, reverse GGTCTGGGATGGAAACTGTGA. Real-time PCR was performed and monitored using SYBR Green PCR Master Mix and an ABI Prism 7700 Sequence Detection System (Thermo Fisher Scientific, CA, USA).
Statistical analysis
The data are presented as the mean ± standard deviation (S.D.) of three independent experiments and p values were estimated by two tailed Student’s t test. *p < 0.05, **p < 0.01.
Results
The combined effects of temperature and hydrolysate on cell growth and mAb production
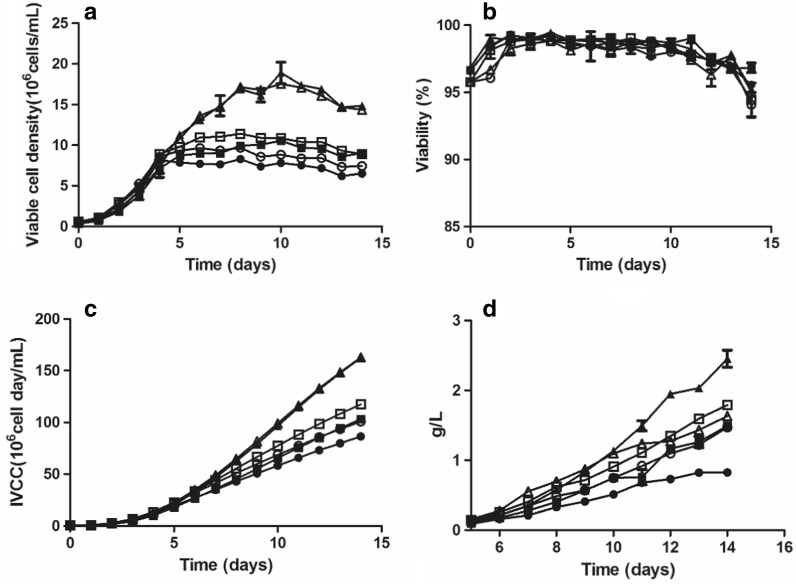
In both the hydrolysate addition and the control groups, the viable cell density was significantly higher at the high sub-physiological temperature than at the medium or low sub-physiological temperature, and there was no significant difference in density between the medium and the low sub-physiological temperatures (Fig. 1a). Specifically, the peak viable cell densities were approximately 18.2 × 106, 10.1 × 106 and 8.5 × 106 cells/mL at the high, medium, and low sub-physiological temperatures, respectively. Cell viability was similar under all conditions and remained high (> 90%) during the whole culture phase (Fig. 1b). The integral of viable cell concentration (IVCC) yielded a similar result as for viable cell density (Fig. 1c).
Fig. 1.
Profiles of a cell growth, b cell viability, c the integral of viable cell concentration (IVCC = ∫t0 Cxdt, Cx: Viable cell density), and d mAb production during fed-batch culture at different temperatures and with or without hydrolysate addition to the feed. Control group at 30 °C (open circles); control group at 32 °C (open squares); control group at 34 °C (open triangles); hydrolysate addition group at 30 °C (filled circles); hydrolysate addition group at 32 °C (filled squares); hydrolysate addition group at 34 °C (filled triangles). The error bars indicate the standard deviation of three independent experiments
However, the titer was significantly higher in the hydrolysate addition group at the high sub-physiological temperature compared with the other group, even though the IVCC and cell density were comparable between this group and the control group at the high sub-physiological temperature. In particular, the titer in the hydrolysate addition group at the high sub-physiological temperature was approximately 2.6 g/L, whereas in the control group, the titer was only approximately 1.3 g/L. Meanwhile, the titer in the hydrolysate addition group was similar to that in the control group at the medium sub-physiological temperature but was significantly lower at the low sub-physiological temperature.
The combined effects of temperature and hydrolysate on charge variant distribution
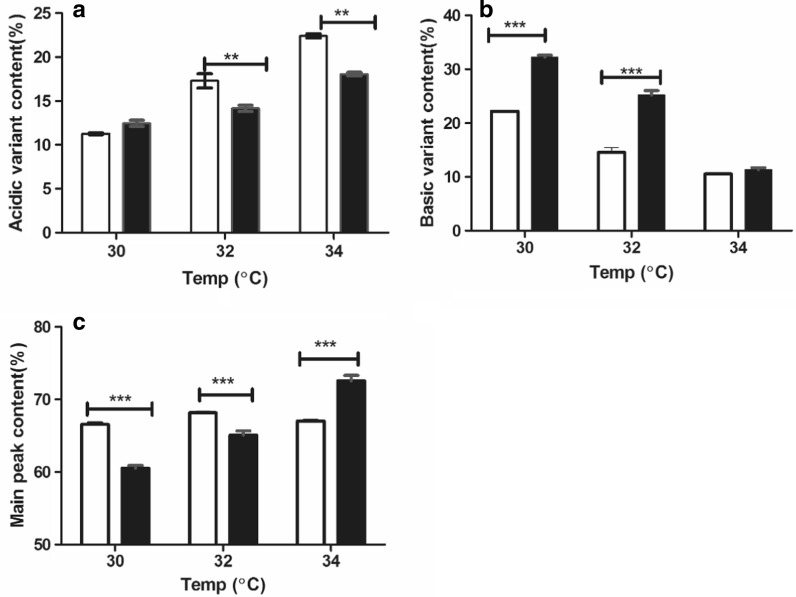
The acidic variant content increased with sub-physiological temperature elevation in both the hydrolysate addition and the control groups. The acidic variant content specifically increased from 12.0 to 16.8% in the hydrolysate addition group and from 11.2 to 23.1% in the control group. However, the acidic variant peak content was lower in the hydrolysate addition group than in the control group at all sub-physiological temperatures, with significantly lower contents at the medium (13.8 vs. 17.2%) and high (16.8 vs. 23.1%) sub-physiological temperatures (Fig. 2a).
Fig. 2.
Profiles of mAb charge variant content during fed-batch culture at different temperatures and with or without hydrolysate addition to the feed. a Acidic variant content; b basic variant content; c main peak content. White represents the control group, and black represents the hydrolysate addition group. The error bars indicate the standard deviation of three independent experiments; p values were calculated using the t test. **p < 0.01; ***p < 0.001
The basic variant content decreased with elevation of sub-physiological temperature in both the hydrolysate addition and the control groups. The basic variant content specifically increased from 32.3 to 9.8% in the hydrolysate addition group and from 22.1 to 10.2% in the control group. In particular, the content was significantly higher in the hydrolysate addition group than in the control group at the low (22.1 vs. 32.3%) and medium (16.0 vs. 25.1%) sub-physiological temperatures but was comparable between the groups at the high sub-physiological temperature (10.2 vs. 9.8%) (Fig. 2b).
The main peak content varied between the hydrolysate addition and the control groups with elevation of sub-physiological temperature. Specifically, the content in the control group remained steady, whereas that in the hydrolysate addition group gradually increased (from 60.1 to 73.4%). The main peak content in the hydrolysate addition group was significantly lower than that in the control group at the low and medium sub-physiological temperatures but significantly higher at the high sub-physiological temperature (73.4 vs. 68.4%) (Fig. 2c).
The combined effects of temperature and hydrolysate on Lys variants
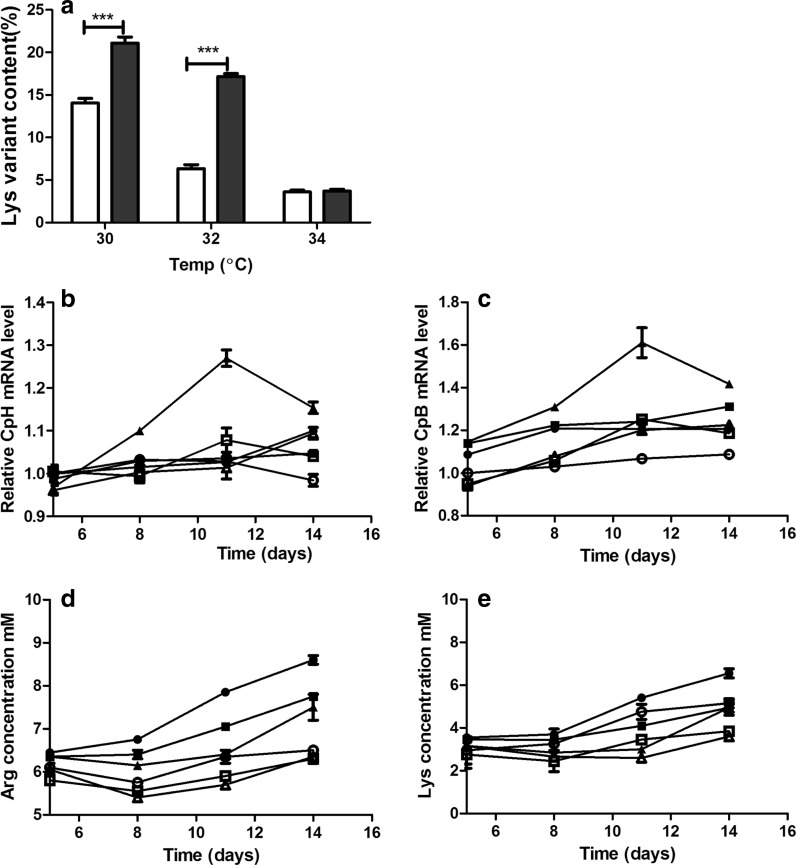
The Lys variant content decreased with elevation of sub-physiological temperature in both the hydrolysate addition and the control groups. In particular, the content of Lys variants in the hydrolysate addition group was significantly higher than that in the control group at the low (22.1 vs. 14.8%) and the medium (7.4 vs. 17.5%) sub-physiological temperatures but was comparable between the groups at the high sub-physiological temperature (Fig. 3a).
Fig. 3.
Profiles of mAb Lys variant content, the relative mRNA levels of basic Cps, and Lys and Arg concentrations during fed-batch culture at different temperatures and with or without hydrolysate addition to the feed. a Lys variant content; b relative CpH mRNA content; c relative CpB mRNA content; d Arg concentration; and e Lys concentration. The relative levels of basic carboxypeptidases mRNAs were analyzed using the 2−ΔΔCt method with GAPDH as an internal control and normalized to 37 °C on day 3. White represents the control group, and black represents the hydrolysate addition group. Control group at 30 °C (open circles); control group at 32 °C (open squares); control group at 34 °C (open triangles); hydrolysate addition group at 30 °C (filled circles); hydrolysate addition group at 32 °C (filled squares); and hydrolysate addition group at 34 °C (filled triangles). The error bars indicate the standard deviation of three independent experiments; p values were calculated using the t test. ***p < 0.001
The lowest CpB mRNA levels were observed in the control group at the low sub-physiological temperature, and the highest levels were observed in the hydrolysate addition group at the high sub-physiological temperature, whereas the other levels were comparable. In addition, CpH mRNA levels were comparable, except in the hydrolysate addition group at the high sub-physiological temperature, which showed significantly higher levels. Additionally, the CpB and CpH mRNA levels were highest on day 11 at the high sub-physiological temperature in the hydrolysate addition group (Fig. 3b, c).
The Lys and Arg concentrations increased with elevation of sub-physiological temperature in both the hydrolysate addition and the control groups. The Lys and Arg concentrations were higher in the hydrolysate addition group than in the control group at all times and sub-physiological temperatures. Furthermore, the Lys and Arg concentrations increased after day 8 and achieved the highest concentrations, namely, 8.5 mM and 6.2 mM, respectively, at day 14 in the hydrolysate addition group at the high sub-physiological temperature.
Discussion
Temperature shifts affect mAb charge heterogeneity, glycosylation, and production (Ahn et al. 2008; Gomez et al. 2012; Marchant et al. 2008). In the present study, the change in the charge heterogeneity of anti-IgE mAb was observed following temperature shift. Some known modifications have been reported to contribute to the formation of acidic variants such as the sialylation (Cruz et al. 1999; Eaton 1995; Riechmann et al. 1988), deamidation (Zanghi et al. 2000) and glycation (Riechmann et al. 1988). On the other hand, the C-terminal Lysine (Lys) clipping (Riechmann et al. 1988; Zhang et al. 2015), pyro-glutamate cyclization (Eaton 1995), C-terminal amidation, and the formation of succinimide from isomerization of aspartate (Asp) residues (Zanghi et al. 2000) are commonly observed modifications that form basic variants. Moreover, the addition of hydrolysate to culture medium or feed has been reported to significantly enhance mAb production and glycosylation (Ho et al. 2016; Kim and Lee 2009), and in the current study, the production of anti-IgE mAb was also enhanced in the presence of hydrolysate. However, the effect of the addition of hydrolysate on charge heterogeneity during anti-IgE mAb production has been relatively unknown, so further investigation of how charge heterogeneity can be regulated based on temperature shift was needed. Thus, we studied the combined effects of temperature shift and hydrolysate addition on the charge heterogeneity of anti-IgE mAb. In addition, we explored the reason for the change in Lys variant levels, which were the major source of basic variants under the study conditions.
The data suggest that cell growth is mainly influenced by temperature, with cell growth being inhibited at a low sub-physiological temperature (Oguchi et al. 2006). The same phenomenon was observed for mAb production. However, at the high sub-physiological temperature, production was primarily related to the levels of nutrients due to the effective utilization of raw materials for mAb synthesis.
Temperature shift during the stationary phase has been reported as changing the charge variant distribution (Zhang et al. 2015). A similar result was also observed in the present study. In particular, with sub-physiological temperature elevation, the acidic variant content was increased, the basic variant was decreased, and the main variant content remained constant.
However, hydrolysate addition to the feed at the three different sub-physiological temperatures resulted in a significant change in the charge distribution. Specifically, the acidic variant content was significantly decreased at the medium and high sub-physiological temperatures and remained constant at the low sub-physiological temperature. Meanwhile, the basic variant content was significantly increased at the low and medium sub-physiological temperatures and remained constant at the high sub-physiological temperature. Finally, the main variant content significantly decreased at the low and medium sub-physiological temperatures but significantly increased at the high sub-physiological temperature.
Obviously, hydrolysate addition to the feed base along with temperature shift could significantly change the charge variant distribution, and especially hydrolysate addition to the feed at the high sub-physiological temperature. In the present study, under these conditions, the acidic variant levels significantly decreased, the main variant levels significantly increased, and the basic variant levels remained steady. The reason for the change in acidic variant levels deserves further investigation, whereas the change in the basic variant levels has been elucidated.
Lys variants are commonly considered the major basic variants due to incomplete C-terminal Lys residue cleavage (Dorai and Ganguly 2014). Here, it was also observed that the Lys variants were the major basic variants, and the major change in basic variant levels originated from the Lys variants in our study.
Lys variants have been evaluated by WCX-HPLC to indirectly monitor the C-terminal Lys variants after chemical or enzymatic digestion of the antibody (Dick et al. 2008). This method can be used to directly determine the relative percentage of C-terminal Lys contained in the original antibody.
In the present study, the basic variants were predominantly Lys variants, so the trends for Lys and basic variants were similar: the content decreased with elevation of the sub-physiological temperature in both the hydrolysate addition and the control groups.
It is widely assumed that Lys variants of mAbs are created by Cps in complex cell culture inside bioreactors (Dick et al. 2008; Zhang et al. 2015). The two main factors impacting Cps are the Cp level and Cp activity. The relative CpB and CpH mRNA levels were considered as indicators of the Cp level in the present study, and both the relative CpB and CpH mRNA levels were significantly higher in the hydrolysate addition group at the high sub-physiological temperature.
Cp activity is influenced by pH, temperature and amino acid concentrations in the medium (Bradley et al. 1996; Folk et al. 1962; Zhang et al. 2015). In the present study, the sample pH was measured immediately after sampling using a pH meter, the hydrolysate addition and the control groups’ pH values were not significantly different at each sampling time (data not shown), suggesting that pH should not be responsible for the charge variants distribution of mAbs. However, sub-physiological temperature elevation might increase Cp activity (Bradley et al. 1996). Additionally, the increases in the Lys and Arg concentrations in the medium might be a factor that decreased Cp activity, which was inhibited in prior studies (Folk et al. 1962; Zhang et al. 2015).
Conclusions
The combined effects of temperature shift and hydrolysate addition on anti-IgE mAb charge variant distribution were investigated. Hydrolysate addition could further change the charge variant distribution along with temperature shift. At the high sub-physiological temperature, the acidic variant content was significant decreased, and the main variant content was increased. Lys variant levels were positively related to the Lys and Arg concentrations in the medium and negatively related to CpB and CpH transcript levels. Therefore, the combination of temperature shift and hydrolysate addition could effectively regulate charge heterogeneity in CHO fed-batch culture.
Acknowledgements
This work was supported by the Science and Technology Commission of Shanghai Municipality (Nos. 14431903100, 17431905800).
Contributor Information
Chen Zheng, Phone: +86-021-34204745.
Chao Zhuang, Phone: +86-021-34204745.
Yantian Chen, Phone: +86-021-34204745.
Tong Wu, Phone: +86-021-34204745.
Yanchao Wang, Phone: +86-021-34204745.
Nianmin Qi, Phone: +86-021-34204745, Email: Biotech@sjtu.edu.cn.
References
- Ahn WS, Jeon JJ, Jeong YR, Lee SJ, Yoon SK. Effect of culture temperature on erythropoietin production and glycosylation in a perfusion culture of recombinant CHO cells. Biotechnol Bioeng. 2008;101:1234–1244. doi: 10.1002/bit.22006. [DOI] [PubMed] [Google Scholar]
- Boswell CA, Tesar DB, Mukhyala K, Theil FP, Fielder PJ, Khawli LA. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjugate Chem. 2010;21:2153–2163. doi: 10.1021/bc100261d. [DOI] [PubMed] [Google Scholar]
- Bradley G, Naudé RJ, Muramoto K, Yamauchi F, Oelofsen W. Ostrich (Struthio camelus) carboxypeptidase B: purification, kinetic properties and characterization of the pancreatic enzyme. Int J Biochem Cell Biol. 1996;28:521. doi: 10.1016/1357-2725(95)00166-2. [DOI] [PubMed] [Google Scholar]
- Buss N, Henderson SJ, McFarlane M, Shenton JM, de Haan L. Monoclonal antibody therapeutics: history and future. Curr Opin Pharmacol. 2012;12:615–622. doi: 10.1016/j.coph.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Cruz HJ, Moreira JL, Carrondo MJ. Metabolic shifts by nutrient manipulation in continuous cultures of BHK cells. Biotechnol Bioeng. 1999;66:104–113. doi: 10.1002/(SICI)1097-0290(1999)66:2<104::AID-BIT3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Dada OO, Jaya N, Valliere-Douglass J, Salas-Solano O. Characterization of acidic and basic variants of IgG1 therapeutic monoclonal antibodies based on non-denaturing IEF fractionation. Electrophoresis. 2015;36:2695–2702. doi: 10.1002/elps.201500219. [DOI] [PubMed] [Google Scholar]
- Dick LW, Qiu DF, Mahon D, Adamo M, Cheng KC. C-terminal lysine variants in fully human monoclonal antibodies: investigation of test methods and possible causes. Biotechnol Bioeng. 2008;100:1132–1143. doi: 10.1002/bit.21855. [DOI] [PubMed] [Google Scholar]
- Dorai H, Ganguly S. Mammalian cell-produced therapeutic proteins: heterogeneity derived from protein degradation. Curr Opin Biotechnol. 2014;30:198–204. doi: 10.1016/j.copbio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Eaton LC. Host cell contaminant protein assay development for recombinant biopharmaceuticals. J Chromatogr A. 1995;705:105–114. doi: 10.1016/0021-9673(94)01249-E. [DOI] [PubMed] [Google Scholar]
- Folk JE, Wolff EC, Schirmer EW. The kinetics of carboxypeptidase B activity. II. Kinetic parameters of the cobalt and cadmium enzymes. J Biol Chem. 1962;237:3100–3104. [PubMed] [Google Scholar]
- Franek F, Hohenwarter O, Katinger H. Plant protein hydrolysates: preparation of defined peptide fractions promoting growth and production in animal cells cultures. Biotechnol Prog. 2000;16:688–692. doi: 10.1021/bp0001011. [DOI] [PubMed] [Google Scholar]
- Gawlitzek M, Ryll T, Lofgren J, Sliwkowski MB. Ammonium alters N-glycan structures of recombinant TNFR-IgG: degradative versus biosynthetic mechanisms. Biotechnol Bioeng. 2000;68:637–646. doi: 10.1002/(SICI)1097-0290(20000620)68:6<637::AID-BIT6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Gomez N, Subramanian J, Ouyang J, Nguyen MDH, Hutchinson M, Sharma VK, Lin AA, Yuk IH. Culture temperature modulates aggregation of recombinant antibody in cho cells. Biotechnol Bioeng. 2012;109:125–136. doi: 10.1002/bit.23288. [DOI] [PubMed] [Google Scholar]
- Harris RJ, Shire SJ, Winter C. Commercial manufacturing scale formulation and analytical characterization of therapeutic recombinant antibodies. Drug Dev Res. 2004;61:137–154. doi: 10.1002/ddr.10344. [DOI] [Google Scholar]
- Heidemann R, Zhang C, Qi HS, Larrick Rule J, Rozales C, Park S, Chuppa S, Ray M, Michaels J, Konstantinov K, Naveh D. The use of peptones as medium additives for the production of a recombinant therapeutic protein in high density perfusion cultures of mammalian cells. Cytotechnology. 2000;32:157–167. doi: 10.1023/A:1008196521213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SC, Nian R, Woen S, Chng J, Zhang P, Yang Y. Impact of hydrolysates on monoclonal antibody productivity, purification and quality in Chinese hamster ovary cells. J Biosci Bioeng. 2016;122:499–506. doi: 10.1016/j.jbiosc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Khawli LA, Goswami S, Hutchinson R, Kwong ZW, Yang JH, Wang XD, Yao ZL, Sreedhara A, Cano T, Tesar D, Nijem I, Allison DE, Wong PY, Kao YH, Quan C, Joshi A, Harris RJ, Motchnik P. Charge variants in IgG1 Isolation, characterization, in vitro binding properties and pharmacokinetics in rats. MAbs. 2010;2:613–624. doi: 10.4161/mabs.2.6.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee GM. Development of serum-free medium supplemented with hydrolysates for the production of therapeutic antibodies in CHO cell cultures using design of experiments. Appl Microbiol Biotechnol. 2009;83:639–648. doi: 10.1007/s00253-009-1903-1. [DOI] [PubMed] [Google Scholar]
- Logsdon SL, Oettgen HC. Anti-IgE therapy: clinical utility and mechanistic insights. In: Lafaille JJ, DeLafaille MAC, editors. Ige antibodies: generation and function. Current topics in microbiology and immunology. Berlin: Springer; 2015. pp. 39–61. [DOI] [PubMed] [Google Scholar]
- Luo J, Zhang J, Ren D, Tsai WL, Li F, Amanullah A, Hudson T. Probing of C-terminal lysine variation in a recombinant monoclonal antibody production using Chinese hamster ovary cells with chemically defined media. Biotechnol Bioeng. 2012;109:2306–2315. doi: 10.1002/bit.24510. [DOI] [PubMed] [Google Scholar]
- Marchant R, Al-Fageeh M, Underhill M, Racher A, Smales C. Metabolic rates, growth Phase, and mRNA levels influence cell-specific antibody production levels from in vitro-cultured mammalian cells at sub-physiological temperatures. Mol Biotechnol. 2008;39:69–77. doi: 10.1007/s12033-008-9032-0. [DOI] [PubMed] [Google Scholar]
- Mastrangelo AJ, Hardwick JM, Zou S, Betenbaugh MJ. Part II. Overexpression of bcl-2 family members enhances survival of mammalian cells in response to various culture insults. Biotechnol Bioeng. 2000;67:555–564. doi: 10.1002/(SICI)1097-0290(20000305)67:5<555::AID-BIT6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Oguchi S, Saito H, Tsukahara M, Tsumura H. pH condition in temperature shift cultivation enhances cell longevity and specific hMab productivity in CHO culture. Cytotechnology. 2006;52:199–207. doi: 10.1007/s10616-007-9059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988;332:323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- Sauer PW, Burky JE, Wesson MC, Sternard HD, Qu L. A high-yielding, generic fed-batch cell culture process for production of recombinant antibodies. Biotechnol Bioeng. 2000;67:585–597. doi: 10.1002/(SICI)1097-0290(20000305)67:5<585::AID-BIT9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Woodside SM, Bowen BD, Piret JM. Mammalian cell retention devices for stirred perfusion bioreactors. Cytotechnology. 1998;28:163–175. doi: 10.1023/A:1008050202561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie PP, Niu HJ, Chen XN, Zhang XT, Miao SW, Deng XC, Liu XP, Tan WS, Zhou Y, Fan L. Elucidating the effects of pH shift on IgG1 monoclonal antibody acidic charge variant levels in Chinese hamster ovary cell cultures. Appl Microbiol Biotechnol. 2016;100:10343–10353. doi: 10.1007/s00253-016-7749-4. [DOI] [PubMed] [Google Scholar]
- Yan BX, Steen S, Hambly D, Valliere-Douglass J, Bos TV, Smallwood S, Yates Z, Arroll T, Han YH, Gadgil H, Latypov RF, Wallace A, Lim A, Kleemann GR, Wang WC, Balland A. Succinimide formation at Asn 55 in the complementarity determining region of a recombinant monoclonal antibody IgG1 heavy chain. J Pharm Sci. 2009;98:3509–3521. doi: 10.1002/jps.21655. [DOI] [PubMed] [Google Scholar]
- Zanghi JA, Renner WA, Bailey JE, Fussenegger M. The growth factor inhibitor suramin reduces apoptosis and cell aggregation in protein-free CHO cell batch cultures. Biotechnol Prog. 2000;16:319–325. doi: 10.1021/bp0000353. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tang H, Sun YT, Liu X, Tan WS, Fan L. Elucidating the effects of arginine and lysine on a monoclonal antibody C-terminal lysine variation in CHO cell cultures. Appl Microbiol Biotechnol. 2015;99:6643–6652. doi: 10.1007/s00253-015-6617-y. [DOI] [PubMed] [Google Scholar]