Abstract
Currently, orthotopic liver transplantation is the gold standard therapy for liver failure. However, it is limited by the insufficient organ donor and risk of immune rejection. Stem cell therapy is a promising alternative treatment for liver failure. One of the most ideal sources of stem cells for regenerative medicine is adipose-derived stem cells (ADSCs). In this study, primary ADSCs seeded on cell culture insert were indirectly co-cultured with injured HepG2 to elucidate the role of ADSCs in promoting the recovery of injured HepG2 in non-contact manner. HepG2 recovery was determined by the surface area covered by cells and growth factor concentration was measured to identify the factors involved in regeneration. Besides, HepG2 were collected for q-PCR analysis of injury, hepatocyte functional and regenerative markers expression. For the ADSCs, expression of hepatogenic differentiation genes was analyzed. Results showed that non-contact co-culture with ADSCs helped the recovery of injured HepG2. ELISA quantification revealed that ADSCs secreted higher amount of HGF and VEGF to help the recovery of injured HepG2. Furthermore, HepG2 co-cultured with ADSCs expressed significantly lower injury markers as well as significantly higher regenerative and functional markers compared to the control HepG2. ADSCs co-cultured with injured HepG2 expressed significantly higher hepatic related genes compared to the control ADSCs. In conclusion, ADSCs promote recovery of injured HepG2 via secretion of HGF and VEGF. In addition, co-cultured ADSCs showed early sign of hepatogenic differentiation in response to the factors released or secreted by the injured HepG2.
Keywords: Non-contact culture, Adipose-derived stem cells, Hepatocytes, Hepatogenic differentiation, Liver, Regeneration
Introduction
Liver is a unique organ with the capacity to restore itself after partial hepatectomy or acute liver injury. However, certain debilitating diseases tend to compromise the regenerating ability of liver resulting in insufficient functional mass and lead to liver failure. Current gold standard therapy for liver failure is orthotopic liver transplantation. Nonetheless, limitations of orthotopic liver transplantation such as insufficient organ donor and risk of immune rejection signaled the importance of developing novel therapies. Advances in regenerative medicine and stem cell research lead to the introduction of hepatocyte transplantation (Hughes et al. 2012) and stem cell therapy (Shiota and Itaba 2017) as alternative therapeutic options for acute liver failure.
Among various types of stem cell, mesenchymal stem cell (MSC) is widely regarded as one of the most promising cell source in regenerative medicine (Fitzpatrick et al. 2015; Hayato et al. 2014). MSCs are multipotent adult stem cells that can differentiate into various cell types. MSCs can be isolated from many tissues such as cord blood, umbilical cord, bone marrow and adipose tissue (Lim et al. 2016; Lim et al. 2018; Secunda et al. 2015). MSCs had been found to promote liver regeneration either by differentiation into hepatocytes or by secretion of trophic factors that facilitate liver recovery (Duncan et al. 2009). Bone marrow-derived MSCs (BM-MSCs) had been successfully induced into hepatocyte-like cells and infusion of these hepatocyte-like cells improved the liver function of rodents with liver injury (Banas et al. 2009).
Recently, adipose tissue has been proposed as the ideal cell source of MSCs as it is abundant and easy to access. Adipose-derived mesenchymal stem cells (ADSCs) had been successful differentiated into hepatic lineage in vitro (Sgodda et al. 2007). Banas et al. (2008) found that human ADSCs transplanted into immunodeficient mice with CCl4-induced liver injury integrated into the tissue and improved the liver function. Other studies found that ADSCs secrete cytokines and growth factors that promote liver regeneration. These factors include hepatocyte growth factor (HGF) (Ramadori and Armbrust 2001), vascular endothelial growth factor (VEGF) (Taniguchi et al. 2001) and basic fibroblast growth factor (bFGF) (Bönninghoff et al. 2012).
The aim of this study is to investigate the role of ADSCs in the promotion of HepG2 recovery via the measurement of growth factors in the co-culture medium. The expression of injury, regenerative and hepatocyte functional genes in injured HepG2 were also studied. In addition, early hepatogenic differentiation of co-cultured ADSCs was also evaluated by measuring the expression of hepatic-related genes. We hypothesized that ADSCs exposed to injured HepG2 will secrete factors that promote liver regeneration. Furthermore, co-culture of ADSCs with injured HepG2 will create a microenvironment that stimulates the early hepatogenic differentiation of ADSCs.
Materials and methods
Isolation and culture of human ADSCs
This research was conducted with ethical approval from the Universiti Kebangsaan Malaysia Research Ethics Committee (Reference number: UKM 1.5.3.5/244/UKM-FF-FRGS0165-2010). Human ADSCs were isolated from adipose tissue of donors undergoing caesarean at Universiti Kebangsaan Malaysia Medical Centre with written informed consent.
To isolate ADSCs, adipose tissue was minced into very fine pieces and digested with 0.3% type I collagenase (Worthington, Lakewood, NJ, USA) for 45 min at 37 °C. Digested tissue was centrifuged to harvest the cell pellet that was subsequently washed with phosphate-buffered saline (PBS). The isolated cells were cultured in Dulbecco’s Modified Eagle Medium/Ham’s F12 medium (DMEM/F12; 1:1, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 1% antibiotic–antimycotic (Invitrogen), 1% glutamax (Invitrogen) and 1% vitamin C (Sigma Aldrich, St. Louis, MO, USA). ADSCs were maintained at 37 °C and 5% carbon dioxide with medium changed every 3 days.
Flow cytometry analysis of ADSCs
Briefly, ADSCs at passage 3 were trypsinized and suspended in 0.5% bovine serum albumin in PBS. The cells were filtered and centrifuged at 1000 rpm for 10 min. The supernatants were discarded and resuspended in 100 μL sheath fluid. Then, the cells were incubated with the following fluorescein isothiocynate (FITC)- or phycoerythrin (PE)-conjugated antibodies: CD90, CD34, CD45, CD73, CD166, CD31, CD105, HLA-ABC and HLA-DR DP DQ (all from BD Biosciences, Lakewood, NJ, USA). After 15 min of incubation, 2 mL of sheath fluid was added into each tube and the cells were washed before resuspension in 500 μL BD CellFix (BD Bioscience). The cells were analyzed using a FACSCanto II flow cytometer (BD Bioscience). A total of 10,000 cells were acquired for each sample.
Trilineage differentiation of ADSCs
Osteogenic differentiation was induced by cultivation of confluent cells in DMEM/F12 medium supplemented with 10% FBS, 0.1 mM dexamethasone (Sigma Aldrich), 10 mM β-glycerophosphate (Sigma Aldrich) and 50 μg/mL ascorbate-2-phosphates (Sigma Aldrich). The culture medium was changed every 3 days for a total of 21 days before fixation and staining with Alizarin Red staining.
For adipogenic induction, the confluent cells were cultivated in DMEM/F12 medium supplemented with 10% FBS, 1 μM dexamethasone, 10 μM human insulin solution (Sigma Aldrich), 200 μM indomethacin (Sigma Aldrich), and 0.5 mM 3-isobutyl-1-methylxanthine (Sigma Aldrich). The culture medium was replaced every 3 days. After 21 days, the cultures were fixed with 10% formalin and stained with Oil Red O staining to detect the lipid deposition.
Chondrogenic differentiation induction was performed on human ADSCs cultured in pellet form. The pellets was cultured in chondrogenic induction medium which consisted of DMEM/F12 medium supplemented with 1% antibiotic antimycotic, 1% glutamax, 1% vitamin C, 40 μg/mL l-proline (Sigma Aldrich), 1% Insulin-Transferrin-Selenium (ITS-G, Invitrogen), 1% FBS, 100 nM dexamethasone, 10 ng/mL TGFβ3 (Peprotech, Rocky Hill, NJ, USA), 50 ng/mL IGF (Peprotech), and 50 μg/mL ascorbate-2 phosphate.
HepG2
HepG2 (American Type Tissue Culture Collection, ATCC), a human hepatocellular carcinoma cell line, was grown in DMEM/F12 medium supplemented with 10% FBS, 1% antibiotic–antimycotic, 1% glutamax and 1% vitamin C. The cells were maintained at 37 °C and 5% carbon dioxide. Cells were trypsinized and expanded every 48 to 72 h.
Indirect co-culture system
HepG2 was seeded in a 6-well plate at density of 7.5 × 105 cells/well. The cells were left to attach for 4 h in DMEM/F12 medium supplemented with 10% FBS before exposed to serum-free medium with 2.4 mM hydrogen peroxide (H2O2) to induce injury as described previously (Liau et al. 2016). Control HepG2 was cultured with serum-free medium. After 2 h, H2O2-containing medium was removed and culture surface was rinsed with PBS. Then, the serum-free medium was added for both control and injured HepG2 group. For ADSC-injured HepG2 co-culture, 8 × 104 ADSCs were cultured on cell culture insert (BD Bioscience) with DMEM/F12 medium supplemented with 10% FBS for overnight before co-cultured with injured HepG2.
Recovery rate of injured HepG2
Photos of control HepG2, injured HepG2 and ADSC-injured HepG2 co-culture groups were captured at time point 24, 48 and 72 h. The area occupied by cells was measured using software Image-Pro Express (MediaCybernetics, Rockville, MD, USA).
Quantification of HGF, VEGF and bFGF
Culture medium was collected at time points 24, 48 and 72 h. Quantification of HGF, VEGF and bFGF presence within the culture medium was performed via ELISA (R&D System, Minneapolis, MN, USA) according to the manufacturer’s recommended protocol.
Total RNA extraction
Total RNA was extracted from the control HepG2, injured HepG2 and ADSC-injured HepG2 co-culture groups as well as the ADSC of ADSC control and ADSC-injured HepG2 co-culture groups at time point 72 h using TRI reagent (Molecular Research Center, Cincinnati, OH, USA). The procedure was carried out according to the manufacturer’s recommended protocol which includes homogenization, phase separation, RNA precipitation, RNA wash and RNA solubilization. RNA precipitation was increased by adding polyacryl carrier (Molecular Research Centre).
cDNA synthesis
Extracted RNA was converted to cDNA using SuperscriptTM III First Strand Synthesis SuperMix (Invitrogen). Reaction was carried out according to the protocol recommended by the manufacturer. The protocol conditions were 10 min at 25 °C, 30 min at 50 °C, 5 min at 85 °C and 20 min at 37 °C. The synthesized cDNA was stored at − 20 °C and was later used as template for quantitative PCR to determine the gene expression.
Quantitative polymerase chain reaction (q-PCR)
The control HepG2, injured HepG2 and HepG2 from the ADSC-injured HepG2 co-culture groups were analyzed for injury, regenerative and hepatocyte functional markers whilst the control ADSCs and ADSCs from the ADSC-injured HepG2 co-culture group were analyzed for hepatogenic differentiation markers. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. Primers for each gene were designed using Primer 3 software based on published Gene Bank database sequences. The sequences of the primers used are listed in Table 1.
Table 1.
Primers sequences used in quantitative PCR
| Gene | Accession number | PRIMER 5′–3′ | Product size |
|---|---|---|---|
| GAPDH | NM_002046 | F: 5′-TCC CTG AGC TGA ACG GGA AG-3′ R: 5′-GGA GGA GTG GGT GTC GTC GCT GT-3′ |
217 |
| IL-1β | NM_000576 | F: 5′-GGA CAA GCT GAG GAA GAT GC-3′ R: 5′-TCG TTA TCC CAT GTG TCG AA-3′ |
120 |
| MMP-1 | NM_002421 | F: 5′-AGG GTT GAA AAG CAT GAG CA-3′ R: 5′-CTG GTT GAA AAG CAT GAG CA-3′ |
111 |
| ALT | NM_005309.2 | F: 5′-GCC TCA TTG AAG ACC TGC TC-3′ R: 5′-GCA GAT GCT GAA GCT GAT GA-3′ |
186 |
| CYP7A1 | NM_000780.3 | F: 5′-TGC CTT CCA AGC TGA CTT TT-3′ R: 5′-TCC AGC GAC TTT CTG GAG TT-3′ |
119 |
| FoxA3 | NM_004497.2 | F: 5′-GGT GCT GGT GTC TGT TCT GA-3′ R: 5′-CCT ATT TCA CTG GCC TGG AG-3′ |
109 |
| FoxM1 | NM_202002.2 | F: 5′-GGC TTA AAC ACC TGG TCC AA-3′ R: 5′-CGT GGA TTG AGG ACC ACT TT-3′ |
187 |
| IL-6 | NM_000600 | F: 5′-TAC CCC CAG GAG AAG ATT CC-3′ R: 5′-TTT TCT GCC AGT GCC TCT TT-3′ |
175 |
| IL-8 | NM_000584 | F: 5′-GTG CAG TTT TGC CAA GGA GT-3′ R: 5′-CTC TGC ACC CAG TTT TCC TT-3′ |
196 |
| ALB | NM_000477.5 | F: 5′-TGG CAT AGC ATT CAT GAG GA-3′ R: 5′-CTT CCT GGG CAT GTT TTT GT-3′ |
140 |
| AFP | NM_001134.1 | F: 5′-GCC ACA GGC CAA TAG TTT GT-3′ R: 5′-AAA TGC GTT TCT CGT TGC TT-3′ |
136 |
| CK18 | NM_000224 | F: 5′-TTG ACC GTG GAG GTA GAT GC -3′ R: 5′-GTC GTC TCA GCA GCT CCA AC-3′ |
188 |
| TDO2 | NM_005651.2 | F: 5′-CAC CAG CAG TTT CAG GAT CA-3′ R: 5′-CAA ATC CTC TGG GAG TTG GA-3′ |
126 |
The PCR reaction was carried out with Bio-Rad (Hercules, CA, USA) iCycler PCR machine using SYBR green as the indicator. The reaction mixture contained SYBR Select Master Mix (Applied Biosystem, Carlsbad, CA, USA), forward and reverse primers (5 µM each), DNase/RNase-free water and 1 µl of cDNA. The reaction condition was cycle 1 (1×): Step 1 50.0 °C for 2 min; cycle 2 (1×): Step 1 95.0 °C for 2 min; cycle 3 (50×): step 1 95.0 °C for 10 s, step 2 58 °C for 30 s; cycle 4 (1×): step 1 95.0 °C for 1 min; cycle 5 (1×): step 1 55.0 °C for 1 min; and cycle 6 (70×): step 1 60.0 °C to 94.5 °C for 10 s each. The specificity of the primers and PCR protocol were confirmed by melting curve analysis. The expression level of each gene was then normalized to GAPDH.
Periodic acid-Schiff (PAS) staining
The glycogen deposition in ADSCs from control and indirect co-culture groups was determined using the PAS staining kit (Merck, Darmstadt, Germany). Briefly, ADSCs were fixed with 10% formalin at time point 72 h. The cells were rinsed with the distilled water before staining with periodic acid solution for 5 min. Then, the cells were rinsed with running tap water and distilled water before staining with Schiff’s reagent for 15 min. Subsequently, the cells were counterstained with hematoxylin for 2 min and further washed. The cells were coated with a thin layer of 70% glycerin prepared in PBS before viewing under microscope.
Statistical analysis
All results were reported as mean ± S.E.M. Paired T test was performed for statistical analysis using IBM® SPSS® Statistics version 20.0 with p < 0.05 considered significant.
Results
Characterization of ADSCs
Flow cytometry analysis showed that more than 97% of human ADSCs were positive for mesenchymal stem cell CD markers (CD90, CD73, and CD105) and less than 0.5% of population were positive for hematopoietic stem cell CD markers (CD31, CD34 and CD45). Expression of MHC class I and class II were low among the isolated ADSCs as less than 5% of the population were positive for HLA-ABC and less than 0.2% were positive for HLA-DR-DP-DQ (Table 2).
Table 2.
Flow cytometry analysis of ADSC surface markers
| CD marker | Percentage of positive cells (Mean ± SEM) |
|---|---|
| CD90 | 97.18 ± 1.45 |
| CD73 | 97.56 ± 1.30 |
| CD105 | 98.68 ± 0.43 |
| CD106 | 64.78 ± 16.89 |
| CD31 | 0.26 ± 0.10 |
| CD34 | 0.26 ± 0.15 |
| CD45 | 0.14 ± 0.04 |
| HLA-DR-DP-DQ | 0.16 ± 0.05 |
| HLA-ABC | 4.66 ± 2.49 |
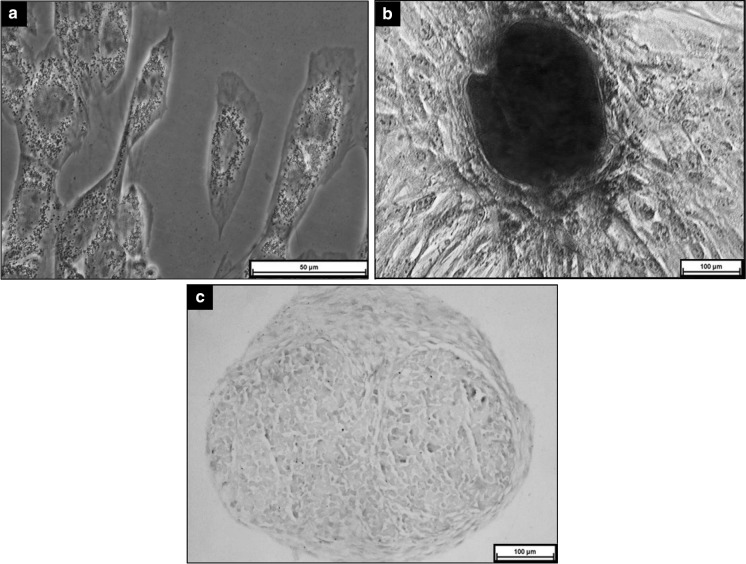
The isolated human ADSCs were able to differentiate into adipogenic, osteogenic and chondrogenic lineages. Induction of ADSCs with adipogenic medium led to formation of lipid droplet in cells as shown in positive staining of Oil Red O (Fig. 1a). Calcium deposition was detected on ADSCs after 21 days of induction (Fig. 1b). Cell pellet induced with chondrogenic medium showed positive staining for Alcian blue which indicated proteoglycan deposition (Fig. 1c).
Fig. 1.
Tri-lineage differentiation of ADSCS after induction for 21 days. a Adipogenic differentiation- positive Oil Red O staining (x200 magnification); b Osteogenic differentiation- positive Alizarin Red staining (x100 magnification); c Chondrogenic differentiation—positive Alcian blue staining (x100 magnification)
Morphological assessment
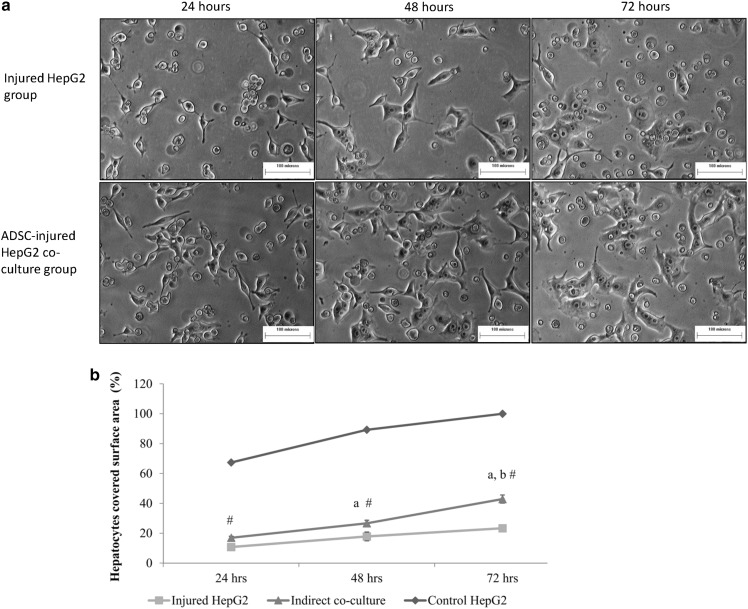
Under the microscopic, the ADSC-injured HepG2 co-culture group demonstrated higher HepG2 survival compared to the injured HepG2 group (Fig. 2a). The HepG2 cells in both groups showed the elongated cell process at time point 24 h which indicated that the cells were under stress. At time point 48 h, HepG2 in ADSC-injured HepG2 co-culture group proliferated faster compared to the injured HepG2 group as more cells have been observed in ADSC-injured HepG2 co-culture group. The cells in ADSC-injured HepG2 co-culture group also became more epithelial like whereby the cell processes got shorter compared to time point 24 h. At time point 72 h, all HepG2 cells have spread membrane and epithelial-like shape. ADSC-injured HepG2 co-culture group contained more cells compared to the injured HepG2 group at time point 72 h.
Fig. 2.
a Morphological assessment of HepG2 cells in injured HepG2 and ADSC-injured HepG2 co-culture groups after 24, 48 and 72 h (100× magnification). b Recovery rate of hepatocytes. “#”: Significant difference (p < 0.05) compared to control HepG2 and injured HepG2 groups at that particular time point. “a”: Significant difference (p < 0.05) compared to time point 24 h. “b”: Significant difference (p < 0.05) compared to time point 48 h
Co-culture improved hepatocyte recovery
The percentage of hepatocyte covered surface area for all three groups increased proportionally with time (Fig. 2b). At time point 24 h, the percentage of hepatocyte covered surface area for injured HepG2 was only 10.74 ± 0.96%, whereas the ADSC-injured HepG2 co-culture group achieved coverage of 17.02 ± 1.34%. Hepatocyte covered surface area of injured HepG2 increased to 17.82 ± 2.05% at time point 48 h and 23.34 ± 2.72% at time point 72 h. For the ADSC-injured HepG2 co-culture group, the percentage of hepatocyte covered surface area increased significantly reaching 26.68 ± 2.96% at time point 48 h and 42.68 ± 1.05% at time point 72 h. The percentage of hepatocyte covered surface area for control HepG2 also increased with time, from 67.36 ± 4.31% at time point 24 h to 100% at time point 72 h.
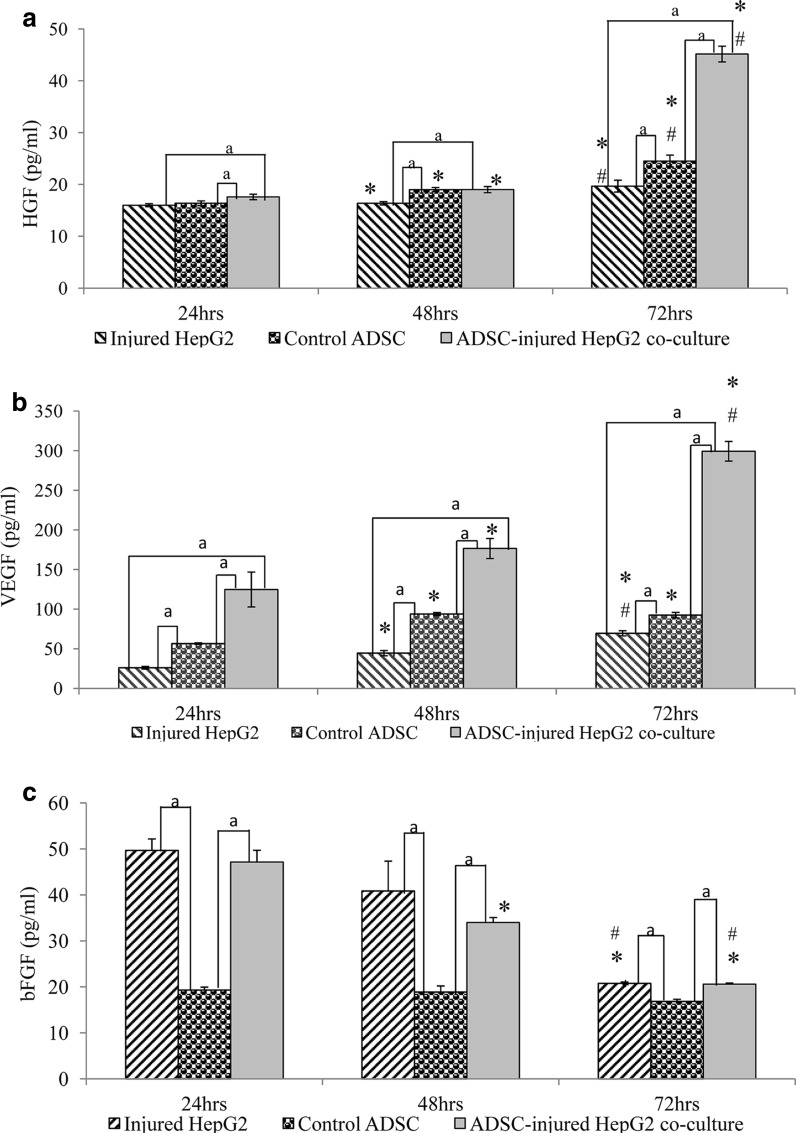
ADSCs secreted VEGF and HGF to promote hepatocyte proliferation
HGF secretion of all three groups increased with time (Fig. 3a). At time point 24 h, ADSC-injured HepG2 co-culture group scored the highest level of HGF, followed by the ADSC control and injured HepG2 groups. At time point 48 h, HGF concentration of ADSC-injured HepG2 co-culture group was 1.16-fold higher compared to the injured HepG2 group. At time point 72 h, HGF concentration of the ADSC-injured HepG2 co-culture group increased drastically compared to the previous time point whereby the secretion was 1.29-fold higher than the injured HepG2 group and 1.84-fold higher than the ADSC control group.
Fig. 3.
HGF (a), VEGF (b) and bFGF (c) secretion by injured HepG2, ADSC control and ADSC-injured HepG2 co-culture groups after 24, 48 and 72 h. Data are denoted as mean ± S.E.M of n = 6. “*”: Significant difference (p < 0.05) compared to time point 24 h. “#”: Significant difference (p < 0.05) compared to time point 48 h. “a”: Significant difference (p < 0.05) between two groups
Figure 3b shows that VEGF secretion of the injured HepG2 and ADSC-injured HepG2 groups increased significantly throughout the time. VEGF secretion of the control ADSCs at time point 48 h increased significantly compared to time point 24 h and was maintained at time point 72 h. Comparison of VEGF concentration between the ADSC-injured HepG2 co-culture group with injured HepG2 group showed that the secretion of VEGF was higher in the co-culture group throughout the time (24 h: 4.7-fold higher, 48 h: 3.9-fold higher and 72 h: 4.3-fold higher).
At time point 24 h, bFGF secretion was significantly higher in the injured HepG2 and ADSC-injured HepG2 co-culture groups compared to the ADSC control group (Fig. 3c). Injured HepG2 has the highest concentration, approximately 5% higher than the ADSCs-injured HepG2 co-culture group. The bFGF concentration decreased significantly with time for the injured HepG2 and ADSCs-injured HepG2 co-culture groups. The concentration of bFGF of the ADSC control group was very low throughout the experiments.
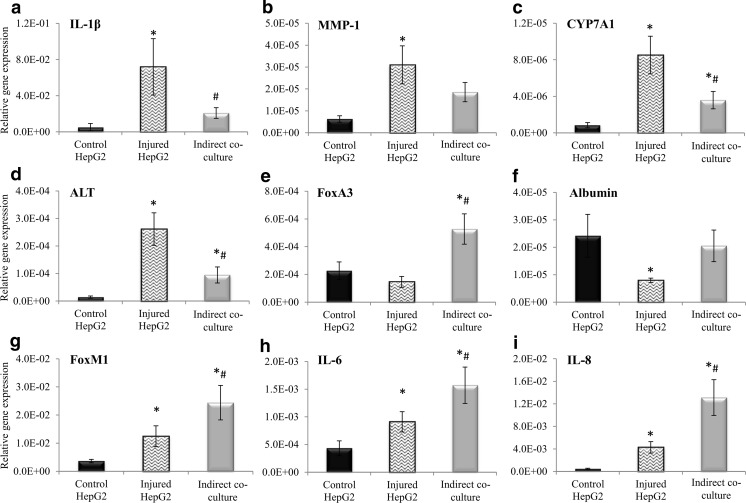
Co-culture HepG2 has lower expression of inflammatory markers and higher expression of regeneration and functional markers
Expression of injury markers, IL-1β (Fig. 4a) and MMP1 (Fig. 4b) increased significantly in the injured HepG2 group by 14.5-fold and 4.9-fold, respectively, compared to the control HepG2 group. Nonetheless, co-culture with ADSCs reduced the expression of these markers whereby the expression of IL-1β and MMP-1 decreased by 3.46-fold and 1.67-fold, respectively, compared to the injured HepG2 group. Expression of CYP7A1 (Fig. 4c) and ALT (Fig. 4d) increased by 10.20-fold and 18.55-fold, respectively, in the injured HepG2 group compared to the control HepG2 group. However, the expression of ALT and CYP7A1 was reduced by 2.76-fold and 2.38-fold, respectively, when the injured HepG2 were co-cultured with ADSCs.
Fig. 4.
Injury markers: a IL-1β, b MMP-1, c CYP7A1, and d ALT; Functional markers: e FoxA3, and f ALB; Regeneration markers: g FoxM1, h IL-6 and i IL-8 mRNA relative gene expression by control HepG2, injured HepG2 and HepG2 in ADSC-injured HepG2 co-culture group. Data are denoted as mean ± S.E.M. of n = 6. “*”: Significant difference (p < 0.05) compared to control HepG2 group. “#”: Significant difference (p < 0.05) compared to injured HepG2 group
The expression of hepatocyte regeneration markers, FoxM1 (Fig. 4g), IL-6 (Fig. 4h) and IL-8 (Fig. 4i) increased by 3.43-fold, 2.10-fold and 9.59-fold, respectively, in the injured HepG2 group compared to the control HepG2 group. Co-culture with ADSCs elevated the expression of FoxM1 by 1.96-fold, IL-6 by 1.73-fold and IL-8 by 3.07-fold compared to the injured HepG2 group.
FoxA3 and albumin are hepatocyte function genes. Expression of FoxA3 (Fig. 4e) and albumin (Fig. 4f) were highest in the ADSC-injured HepG2 co-culture group, followed by the control HepG2 and injured HepG2 groups. Co-cultured with ADSCs elevated the expression of FoxA3 by 3.60-fold and albumin by 2.60-fold compared to the injured-HepG2 group.
Co-culture ADSCs expressed higher hepatogenic differentiation markers and positive for glycogen deposition
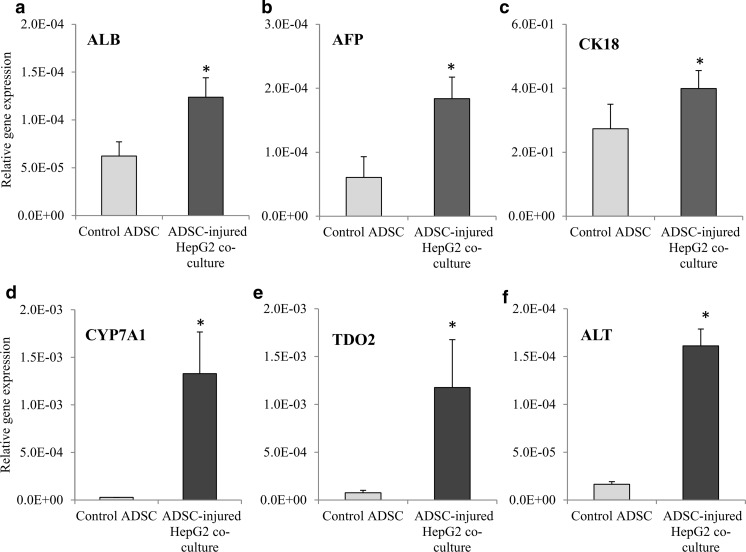
Three early liver markers, i.e. albumin, alpha-fetoprotein and CK18, were measured in this study. The expression of albumin (Fig. 5a), alpha-fetoprotein (Fig. 5b) and CK18 (Fig. 5c) increased significantly by 1.99-fold, 3.04-fold and 1.46-fold, respectively, in the ADSC-injured HepG2 co-culture group compared to the ADSC control group. In addition, the expression of hepatocyte functional genes, CYP7A1 (Fig. 5d), TDO2 (Fig. 5e) and ALT (Fig. 5f) also increased significantly in the ADSC-injured HepG2 co-culture group compared to the ADSC control group whereby CYP7A1 expression increased by 48.05-fold, TDO2 expression increased by 15.50-fold and ALT expression increased by 9.83-fold.
Fig. 5.
Hepatogenic trans-differentiation markers expression in ADSCs from different experiment groups. a ALB, b AFP, c CK18, d CYP7A1, e TDO2 and f ALT mRNA relative gene expression of ADSC control and ADSC-Injured HepG2 co-culture group. Data are denoted as mean ± S.E.M. of n = 6. “*”: Significant difference (p < 0.05) compared to ADSC control group
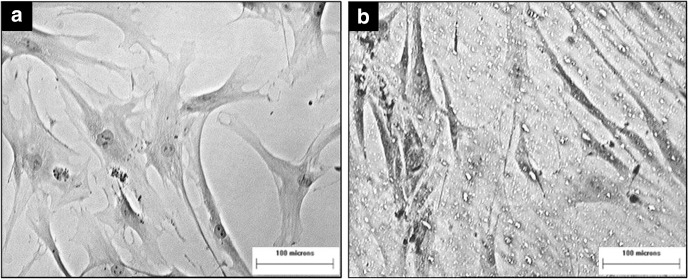
The PAS staining showed that the ADSCs in the indirect co-culture group were positively stained for glycogen deposition as the nuclei of the cells appeared blue and the membranes of the cells were purple coloured (Fig. 6b). The membrane of the ADSCs from control group did not pick up the stain and appeared colourless (Fig. 6a).
Fig. 6.
Periodic acid-Schiff staining of ADSCs from different experiment groups. a ADSC control group, b ADSC- Injured HepG2 co-culture group. (100× magnification)
Discussion
MSCs can be derived from various tissues and are capable to differentiate into many cell types in vitro (Ullah et al. 2015). MSCs can reduce oxidative stress in injured liver and promote proliferation of hepatocytes (Li et al. 2011). Among many sources, ADSCs derived from adipose tissue present a good source of MSC which can be obtained in large quantity using a less invasive method and can be expanded for more passages without significant phenotypic and genotypic changes, and possess higher proliferation capacity compared to BM-MSCs (Puglisi et al. 2011).
To create the indirect co-culture model, ADSCs were seeded on the cell culture insert whilst HepG2 was cultured on the tissue culture plate. The in vitro model of injured HepG2 induced by hydrogen peroxide has been established in our previous study (Liau et al. 2016). The exposure of HepG2 cells to 2.4 mM hydrogen peroxide had slowly increased the aspartate aminotransferase (AST) activity in the culture medium which indicated the amino acid metabolic enzyme found in the cytosol and mitochondria was leaked out from HepG2. Leakage of AST showed the cellular injury of HepG2 and slowly elevation of AST showed the cell died via apoptosis as the necrotic cells were losing the membrane integrity rapidly. Besides, the apoptotic HepG2 demonstrated the features of cell shrinkage, membrane blebbing and chromatin condensation which are the hallmarks of apoptosis (Merrill et al. 2002). This finding similiar with finding by Li et al. (2008) which found hydrogen peroxide induced apoptosis in HepG2. Li et al. (2008) found that the apoptosis event was activated through the caspases-9 and caspases-3. Quantitative PCR analysis has shown that the hydrogen peroxide induction increased the expression of inflammatory genes, such as TGFβ-1, MMP-3, NF-κβ, IL-8 and IL-6. Cell culture insert permits the crosstalk between ADSCs and HepG2 via the secretion of cytokines and growth factors. Our results revealed that HepG2 of the co-culture group regenerated faster compared to the control group, indicating direct contact is not exclusively necessary to promote the healing process and improve the functionality of injured HepG2. We postulated that this could be due to the paracrine effect of ADSCs towards the injured HepG2. To prove this, ELISA was performed to determine the concentration of 3 important growth factors within the culture medium.
HGF is a growth factor involved in the initiation of hepatic regeneration. It is the most potent liver mitogen which exhibits direct stimulation effect on hepatocytes (Bansal 2016; Tanaka and Miyajima 2016). VEGF is a growth factor involved in angiogenesis and healing of injured tissue. It has indirect effect on hepatocyte proliferation and has been suggested to be induced by IL-6 and IL-1 (Gnecchi et al. 2008). In 2001, Taniguchi et al. (2001) showed that VEGF has important role in rat liver regeneration whereby the injection of exogenous VEGF in partially hepatectomized rats promoted the proliferation of hepatocytes. ELISA results from this study showed that both ADSCs and injured HepG2 secreted HGF and VEGF. However, co-culture of these cells has no effect on the secretion of HGF and VEGF at time points 24 and 48 h as the concentration of the co-culture group was approximately equal to the concentration of ADSC control and injured HepG2 groups. This could be due to injured HepG2 did stimulate ADSCs to produce more HGF and VEGF but not much increase in concentration was observed probably due to the higher uptake of these growth factors by injured HepG2 to initiate the repair/regeneration process. Nonetheless, the concentration of HGF and VEGF increased drastically at time point 72 h. This drastic increment coincided with the higher HepG2 covered surface area during the same period of time indicating a more efficient healing process of the injured HepG2. According to Hioki et al. (1996), bFGF was secreted upon liver injury to stimulate regeneration. In this study, bFGF was detected at higher concentration at time points 24 and 48 h in the injured HepG2 and ADSCs-injured HepG2 co-culture groups. Nevertheless, bFGF concentration gradually decreased with time in all groups. Injured HepG2 is the major producer of bFGF and co-culture with ADSC did not stimulate the secretion of this factor. These results suggested that ADSCs neither directly secrete bFGF nor indirectly stimulate bFGF secretion from injured HepG2 to promote regeneration.
Quantitative PCR revealed that injured HepG2 indirectly co-cultured with ADSC has lower expression of inflammation genes, i.e. IL-1β and MMP-1. FoxM1, also known as Forkhead box M1, is a transcriptional factor regulating the expression of cell cycle genes. FoxM1 plays an important role in DNA replication and mitosis during organ repair (Song et al. 2010; Tan et al. 2007). After injury, gene expression of FoxM1 elevated significantly compared to the control HepG2. The gene expression of FoxM1 was found to further escalate upon co-culture with ADSCs indicating that ADSCs promote the proliferation of injured HepG2.
IL-6 is a pro-inflammatory cytokine that increased in liver disease and after liver dissection (Selzner et al. 2003). IL-6 is also a crucial cytokine in liver regeneration. Cressman et al. (1996) showed that IL-6 knockout mice have impaired liver regeneration capacity. IL-8 is a cytokine of CXC chemokines with chemotactic, angiogenic and mitogenic properties (Osawa et al. 2002; Turner et al. 2014). The expression of both IL-6 and IL-8 increased in injured HepG2. Higher expression of these markers was detected in injured HepG2 of co-culture group indicating that ADSCs can reduce inflammation and help in hepatocyte regeneration.
Injured HepG2 showed reduction in the expression of functional genes, i.e. FoxA3 and albumin. Co-culture with ADSCs increased the expression of both genes. FoxA3, also known as hepatocyte nuclear factor-3 gamma, encoded the transcriptional activators of liver specific transcripts such as albumin (Cereghini 1996). Elevation of FoxA3 led to the increment of albumin expression as shown in the result.
In this study, we also found that ADSCs exposed to the injured HepG2 demonstrated higher expression of hepatic-related genes. These findings demonstrated that ADSCs not only help in the proliferation and healing of injured HepG2 but also demonstrated signs of early hepatogenic differentiation. Many studies found that mesenchymal stem cells such as BM-MSCs and ADSCs can be differentiated into hepatic lineage using a cocktail of cytokines (Banas et al. 2009; Seo et al. 2005). A previous study showed that induction of ADSCs into hepatocytes-like cells required 6 days to elevate the expression of hepatic-related genes, i.e. AFP, albumin, TDO2 and CYP7A1 (Banas et al. 2007). In this study, we found that indirect co-culture with injured HepG2 for only 3 days was sufficient to stimulate the ADSCs to express early hepatogenic markers such as AFP, albumin, and CK18. Furthermore, the expression of mature hepatocyte markers such as CYP7A1 and TDO2 was also increased. PAS staining showed glycogen deposition in ADSCs of the indirect co-culture group while there was no glycogen deposition in the control ADSCs. These findings suggested that ADSCs showed early sign of hepatogenic differentiation. More experiments such as long-term co-culture and functional testing of cells should be conducted in the future to support the findings in this study.
Conclusion
Indirect co-culture of ADSCs with injured HepG2 enhances the recovery of the cells through the secretion of HGF and VEGF. In addition, injured HepG2 created a microenvironment that might stimulate the early hepatogenic differentiation of ADSCs, evidenced by the increment in the expression of hepatic-related markers. This is a very important finding as it might lead to the development of a new culture condition to prepare hepatogenic differentiation of ADSCs for clinical applications.
Acknowledgement
This work was supported by FRGS grant (Grant Number: UKM-FF-03-FRGS0165-2010) from Ministry of Higher Education (MOHE) of Malaysia.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Banas A, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- Banas A, et al. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells. 2008;26:2705–2712. doi: 10.1634/stemcells.2008-0034. [DOI] [PubMed] [Google Scholar]
- Banas A, et al. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24:70–77. doi: 10.1111/j.1440-1746.2008.05496.x. [DOI] [PubMed] [Google Scholar]
- Bansal MB. Hepatic stellate cells: fibrogenic, regenerative or both? Heterogen Context Key Hepatol Int. 2016;10:902–908. doi: 10.1007/s12072-016-9758-x. [DOI] [PubMed] [Google Scholar]
- Bönninghoff R, et al. Effect of different liver resection methods on liver damage and regeneration factors VEGF and FGF-2 in mice. Can J Surg. 2012;55:389. doi: 10.1503/cjs.007911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghini S. Liver-enriched transcription factors and hepatocyte differentiation. FASEB J. 1996;10:267–282. doi: 10.1096/fasebj.10.2.8641560. [DOI] [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science (New York, NY) 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137:466–481. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick E, et al. Coculture with mesenchymal stem cells results in improved viability and function of human hepatocytes. Cell Transpl. 2015;24:73–83. doi: 10.3727/096368913X674080. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayato K, Rie T, Takeshi K, Shumpei I, Tsuyoshi I, et al. Adipose tissue-derived mesenchymal stem cells in regenerative medicine treatment for liver cirrhosis—focused on efficacy and safety in preclinical and clinical studies. JSM Regen Med Bio Eng. 2015;3(1):1012. [Google Scholar]
- Hioki O, et al. Expression and localization of basic fibroblast growth factor (bFGF) in the repair process of rat liver injury. J Hepatol. 1996;24:217–224. doi: 10.1016/S0168-8278(96)80032-8. [DOI] [PubMed] [Google Scholar]
- Hughes RD, Mitry RR, Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012;93:342–347. doi: 10.1097/TP.0b013e31823b72d6. [DOI] [PubMed] [Google Scholar]
- Li GP, Wu LF, Pu ZJ. Oxidative stress induces apoptosis in HepG2 cells. Chin J Pathophysiol. 2008;24:105–111. [Google Scholar]
- Li J, Li M, Niu B, Gong J. Therapeutic potential of stem cell in liver regeneration. Front Med. 2011;5:26–32. doi: 10.1007/s11684-011-0107-0. [DOI] [PubMed] [Google Scholar]
- Liau LL, Hui CK, Makpol S, Azurah AGN. Hydrogen peroxide induces acute injury and up-regulates inflammatory gene expression in hepatocytes. Sains Malays. 2016;45:451–458. [Google Scholar]
- Lim J, Razi ZRM, Law J, Nawi AM, Idrus RBH, Ng MH. MSCs can be differentially isolated from maternal, middle and fetal segments of the human umbilical cord. Cytotherapy. 2016;18:1493–1502. doi: 10.1016/j.jcyt.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Lim J, et al. Mesenchymal stromal cells from the maternal segment of human umbilical cord is ideal for bone regeneration in allogenic setting. Tissue Eng Regen Med. 2018;15:75–87. doi: 10.1007/s13770-017-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill CL, et al. Etomoxir-induced oxidative stress in HepG2 cells detected by differential gene expression is confirmed biochemically. Toxicol Sci. 2002;68:93–101. doi: 10.1093/toxsci/68.1.93. [DOI] [PubMed] [Google Scholar]
- Osawa Y, et al. Tumor necrosis factor alpha-induced interleukin-8 production via NF-κB and phosphatidylinositol 3-kinase/Akt pathways inhibits cell apoptosis in human hepatocytes. Infect Immun. 2002;70:6294–6301. doi: 10.1128/IAI.70.11.6294-6301.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi MA, Saulnier N, Piscaglia A, Tondi P, Agnes S, Gasbarrini A. Adipose tissue-derived mesenchymal stem cells and hepatic differentiation: old concepts and future perspectives. Eur Rev Med Pharmacol Sci. 2011;15:355–364. [PubMed] [Google Scholar]
- Ramadori G, Armbrust T. Cytokines in the liver. Eur J Gastroenterol Hepatol. 2001;13:777–784. doi: 10.1097/00042737-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Secunda R, Vennila R, Mohanashankar A, Rajasundari M, Jeswanth S, Surendran R. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology. 2015;67:793–807. doi: 10.1007/s10616-014-9718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzner M, Graf R, Clavien P-A. IL-6: A magic potion for liver transplantation? Gastroenterology. 2003;125:256–259. doi: 10.1016/S0016-5085(03)00811-4. [DOI] [PubMed] [Google Scholar]
- Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258–264. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- Sgodda M, et al. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp Cell Res. 2007;313:2875–2886. doi: 10.1016/j.yexcr.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Shiota G, Itaba N. Progress in stem cell-based therapy for liver disease. Hepatol Res. 2017;47:127–141. doi: 10.1111/hepr.12747. [DOI] [PubMed] [Google Scholar]
- Song G, et al. MicroRNAs control hepatocyte proliferation during liver regeneration. Hepatology. 2010;51:1735–1743. doi: 10.1002/hep.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Raychaudhuri P, Costa RH. Chk2 mediates stabilization of the FoxM1 transcription factor to stimulate expression of DNA repair genes. Mol Cell Biol. 2007;27:1007–1016. doi: 10.1128/MCB.01068-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Miyajima A. Liver regeneration and fibrosis after inflammation. Inflamm Regen. 2016;36:19. doi: 10.1186/s41232-016-0025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi E, Sakisaka S, Matsuo K, Tanikawa K, Sata M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem. 2001;49:121–129. doi: 10.1177/002215540104900112. [DOI] [PubMed] [Google Scholar]
- Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta Mol Cell Res. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells-current trends and future prospective. Biosci Rep. 2015;35:e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]