Abstract
Growth factors are the key elements in wound healing signaling for cell migration, differentiation and proliferation. Platelet-rich plasma (PRP), one of the most studied sources of growth factors, has demonstrated to promote wound healing in vitro and in vivo. Adipose tissue is an alternative source of growth factors. Through a simple lipoaspirate method, adipose derived growth factor-rich preparation (adipose tissue extract; ATE) can be obtained. The authors set out to compare the effects of these two growth factor sources in cell proliferation and migration (scratch) assays of keratinocyte, fibroblast, endothelial and adipose derived stem cells. Growth factors involved in wound healing were measured: keratinocyte growth factor, epidermal growth factor, insulin-like growth factor, interleukin 6, platelet-derived growth factor beta, tumor necrosis factor alfa, transforming growth factor beta and vascular endothelial growth factor. PRP showed higher growth factor concentrations, except for keratinocyte growth factor, that was present in adipose tissue in greater quantities. This was reflected in vitro, where ATE significantly induced proliferation of keratinocytes at day 6 (p < 0.001), compared to plasma and control. Similarly, ATE-treated fibroblast and adipose stem cell cultures showed accelerated migration in scratch assays. Moreover, both sources showed accelerated keratinocyte migration. Adipose tissue preparation has an inductive effect in wound healing by proliferation and migration of cells involved in wound closure. Adipose tissue preparation appears to offer the distinct advantage of containing the adequate quantities of growth factors that induce cell activation, proliferation and migration, particularly in the early phase of wound healing.
Keywords: Platelet-rich plasma, Adipose tissue extract, Growth factors, Cell proliferation, Biomedical engineering
Introduction
Chronic wounds affect more than 6 million people in the US with costs estimated at 9.7 billion dollars a year (Bickers et al. 2006). To date, a myriad of tissue regeneration products are available in the market, but are either too costly or ineffective in promoting accelerated wound healing (Tamama and Kerpedjieva 2012). Wound healing is a complex signaling process where keratinocyte, endothelial cell and fibroblast migration and proliferation are the key players. Whilst keratinocytes promote ultimate wound coverage, fibroblasts play an active role in inducing keratinocyte proliferation and migration providing a stable vascularized bed of granulating tissue. In the latter, endothelial cells form a rich network of blood vessels that bestows an oxygen and nutrient-rich foundation through which cells migrate for wound closure and scar formation (Broughton et al. 2006; Guo and DiPietro 2010). Mesenchymal and adipose derived stem cells are known to participate in all phases of wound healing by proliferating and differentiating into different cell lineages thus promoting and accelerating repair (Lee et al. 2016; Hassan et al. 2014).
The role of growth factors in the complex and overlapping phases of wound healing has been studied consistently over time (Lee et al. 2016; Hassan et al. 2014; Demidova-Rice et al. 2012). In response to injury, platelets degranulate releasing epidermal growth factor (EGF), platelet derived growth factor beta (PDGF-B), keratinocyte growth factor (KGF), basic fibroblast growth factor (bFGF), and a series of interleukins (such as IL-8), insulin-like growth factor 1 (IGF-1), transforming growth factor beta (TGF-β) and tumor necrosis factor alpha (TNF-α), among others (Cohen 2008, Nolte et al. 2008). These chemoattract neutrophils, which decontaminate the wound bed and stimulate macrophages to remove detritus through phagocytosis (Werner and Grose 2003). Subsequently, the proliferative phase (3–5 days of injury) is composed of fibroplasia, granulation and epithelization (Demidova-Rice et al. 2012). Fibroblast proliferation, migration and extracellular matrix formation is stimulated by TGF-β, bFGF and PDGF-B and the following wound epithelialization is induced by EGF, KGF, TGF-α and bFGF (Wright et al. 2009; Demidova-Rice et al. 2012). Simultaneously, VEGF and bFGF promote angiogenesis. The last phase of wound healing, where mature scar tissue is formed (remodelation), is mediated by bFGF, TGF-β and PDGF-B (Nolte et al. 2008; Wright et al. 2009).
It has been suggested that there is a deficit of growth factors in chronic wounds and that the use of exogenous growth factors brings benefits to the healing process (Demidova-Rice et al. 2012). In order to mimic what occurs physiologically in wounds, researchers have isolated growth factors from whole blood (platelet-rich plasma; PRP) or therapeutically used single recombinant factors, such as PDGF-B (Fonder et al. 2008; Balfour and Noble 1999). PRP is an abundant source of platelet-derived growth factors that has demonstrated positive wound healing effects in vivo and in vitro (Demidova-Rice et al. 2012). Nonetheless, several randomized control trials have failed to find benefit in its use (Montalvan et al. 2016; Foster et al. 2009). One of the reasons for this is that there are many variations in the methodology of PRP preparation, such as the amount of blood extracted, centrifugation speed, number of spins, activation agents, and final layer of plasma included, resulting in a lack in consistency in growth factor yield (Leitner et al. 2006; Rutkowski et al. 2008) and variations in clinical results (Weibrich et al. 2002; Salamanna et al. 2015). Thus, there is an imperative need for a new reliable source of growth factors to promote wound healing.
Subcutaneous adipose tissue, easily available through lipoaspiration, is a rich source of growth factors and cytokines (Zhao et al. 2013). Research has demonstrated the presence of cytokines such as leptin, adiponectin, angiotensin, adipsin, resistin, prostaglandins, glucocorticoids, interleukins (IL-1B, -6,-8,-10), hepatocyte growth factor (HGF), VEGF, bFGF, IGF-1, TGFα and β and angiopoietins 1 and 2 (Ang-1 and Ang-2) in adipose tissue, particularly in its stromal vascular fraction and mesenchymal stem cells (Bunnell et al. 2008; Salgado et al. 2010). Although current attention has deviated towards stem cell research, the whole adipose tissue secretome contains a plethora of cytokines (Li et al. 2014; Li et al. 2015; Lee et al. 2014).
We have previously extracted proteins and growth factors from whole adipose tissue and demonstrated that adipose tissue derived preparation (adipose tissue extract, ATE) contains cytokines with adipogenic and angiogenic properties (Sarkanen et al. 2012a; Sarkanen et al. 2012b). Furthermore, we have developed a simple standardized method to prepare autologous ATE in an operating theater setting for clinical use, with minimal sample variability regardless of patient age, gender or body mass index (Lopez et al. 2016). The aim of our present study was to investigate the wound healing properties of our novel agent, ATE and clinically used wound treatment PRP in in vitro cell migration and proliferation assays and to characterize their growth factor content while comparing their wound healing properties.
Materials and methods
Ethical considerations
The study was approved by the Ethics Committee of the Pirkanmaa District (R15034). 13 lipoaspirate (ATE) and 11 blood (PRP) samples were obtained with patient informed consent.
Platelet rich plasma collection
The PRP was prepared with GLO Pro kit (Glofinn Oy, Salo, Finland) according to the manufacturer’s instructions. Briefly, 9 ml of whole blood was combined with 1 ml of sodium citrate in a syringe and centrifuged at 1200×g for 5 min. The excess red blood cells in the bottom of the syringe were removed and the syringe centrifuged at 1200×g for 10 min. PRP was then obtained and activated with 10% calcium gluconate and autologous thrombin according to manufacturer’s instructions. Platelet activation was observed as a formation of a gel. The supernatant of the activated PRP was stored at − 20 °C prior to analysis.
Adipose tissue derived preparation (adipose tissue extract, ATE)
Preparation of ATE followed previously published procedures (Lopez et al. 2016). Briefly, subcutaneous infiltration of tumescent solution composed of 1000 ml of pre-warmed Ringer lactate + 1 ml of adrenaline was performed in the lower abdomen with the water assisted liposuction technique (Bodyjet, HumanMed, Schwerin, Germany). 100–200 ml of adipose tissue and lipoaspirate liquid were collected in a sterile canister (LipoCollector, Human Med) and processed under sterile conditions. The fat was incubated at room temperature for 30 min. The decanted liquid was sterile filtered (Acrodisc®, Pall Corporation, Port Washington, NY, USA) prior to use. The adipose tissue preparation ATE, samples were stored at − 20 °C until analysis.
Measurement of protein concentration
Using Pierce™ BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA) according to manufacturer’s instructions, total protein content was quantified using BSA as a standard. Measurements were performed at 562 nm with Varioskan™ Flash Multimode Reader (Thermo Scientific).
Measurement of growth factor concentration
Colorimetric sandwich ELISA Custom human cytokine and Angiogenesis strips (both from Signosis®, Santa Clara, CA, USA) were used for EGF, IGF-I, IL-6, PDGF-B, TNF-α, TGF-β, FGF-β and VEGF, and KGF Kit (Boster Biological Technology Co, Ltd, Pleasanton, CA, USA) for KGF to measure the amount of individual growth factors. Assays was performed according to manufacturer instructions and measured at 450 nm with Varioskan Flash multimode reader (Thermo Scientific).
Cell culture
Human keratinocytes (HaCaT; ATCC, Manassas, VA, USA) were cultured in DMEM (Lonza, Basel, Switzerland) supplemented with 1% l-glutamine (Gibco, Grand Islands, NY, USA), 1% antibiotics and 10% FBS (Gibco). Human foreskin fibroblasts (BJ; ATCC CRL-2522) were cultured in MEM, 10% FBS and 1% l-glutamine. Human adipose stem cells (hASC) were isolated as stromal cell fraction from adipose tissue obtained from surgical samples of healthy volunteers with informed consent (ethics approval no. R03058) from the Tampere University Hospital as described previously (Sarkanen et al. 2012a, b, c). hASC medium included DMEM/F12, 10% Human Serum AB (Biowest, Nuaillé, France) and 1% l-glutamine. Human umbilical vein endothelial cells (HUVEC) were isolated from umbilical cords obtained from scheduled caesarean sections from healthy volunteers with informed consent (ethics approval no. R08028) as described previously (Sarkanen et al. 2012a, b, c) and cultured in EGM-2 (Endothelial Medium Bullet Kit, Lonza).
Cell proliferation assay
Cell proliferation reagent Presto blue was employed to detect the growth of adipose stem cells, fibroblasts, endothelial cells and keratinocytes. Before the study, the cell number for each cell type was optimized so that the control would reach confluency within 6 days. In brief, cells were plated as monocultures in 96-well plates at following densities: keratinocytes 7500 cells/cm2, fibroblasts 5000 cells/cm2, adipose stem cells 5000 cells/cm2 and endothelial cells 7500 cells/cm2. A maximum of 50% of the culture medium volume (corresponding to 450 µg/ml) of ATE (n = 10) and 10% (n = 7) PRP were added to cells in their respective culture media. Presto blue (Life Technologies, Invitrogen Corporation, Carlsbad, CA, USA) cell viability reagent was added to each well and incubated until a visible change in color was observed. Fluorometric analysis was performed at excitation wave length of 570 nm and emission wave length of 600 nm with Varioskan Flash Multimode Reader (Thermo Scientific) at 0, 1, 3 and 6 days of culture. The results derived from eight (ATE) or seven (PRP) independent experiments were performed in duplicate or triplicate.
Scratch wound assay
For scratch wound assay, prior to the study, a cell number optimization study was performed for each cell type (data not shown). The cell number was optimized to reach confluent cell monolayer at 24 h. For the scratch wound assay, 48-well plates were then plated with the following cell counts: keratinocytes 300,000 cells/cm2, fibroblasts 100,000 cells/cm2, adipose stem cells 100,000 cells/cm2 and endothelial cells 150,000 cells/cm2. The day after plating, the confluent monolayer in each well was scraped with a pipette tip to create a vertical scratch in each plate. In order to precisely find the same position for measurement, the center of each well was labeled with a horizontal line. In order to remove the cell debris after the scratch, the cells were washed once with growth medium after which ATE or PRP samples were added. Of the total culture medium volume, a maximum of 50% of ATE and 10% of PRP were applied on the cells in their respective culture media. The PRP concentration was optimized prior to the assay (data not shown). Subsequently, photography and digital measurements were done at distinct time points depending on the cell line (adipose stem cells 0, 4, 8, 11, 24, 30, 48, 54 and 72 h; keratinocytes 0, 24, 48, 72, 96 and 120 h; endothelial cells 0, 4, 8, 12, 24, 30, 48, 54 and 72 h; fibroblasts 0, 5, 8, 17, 24, 30 and 44 h), using a digital camera (Nikon TS-100, Tokyo, Japan) connected to an inverted microscope (Nikon). Measurements were made until complete wound gap closure was reached in all study wells. The results derived from independent experiments ATE (n = 8) and PRP (n = 7) independent experiments were performed in duplicate or triplicate.
Statistical analysis
Statistical analysis and graph illustrations were performed with GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA). The data in this study is presented in mean and standard deviations. Differences were considered significant when p < 0.05. Two-way ANOVA was employed to analyze cell proliferation and migration and two-tailed paired t test for analyzing the growth factors.
Results
Adipose tissue extract contains higher concentrations of KGF
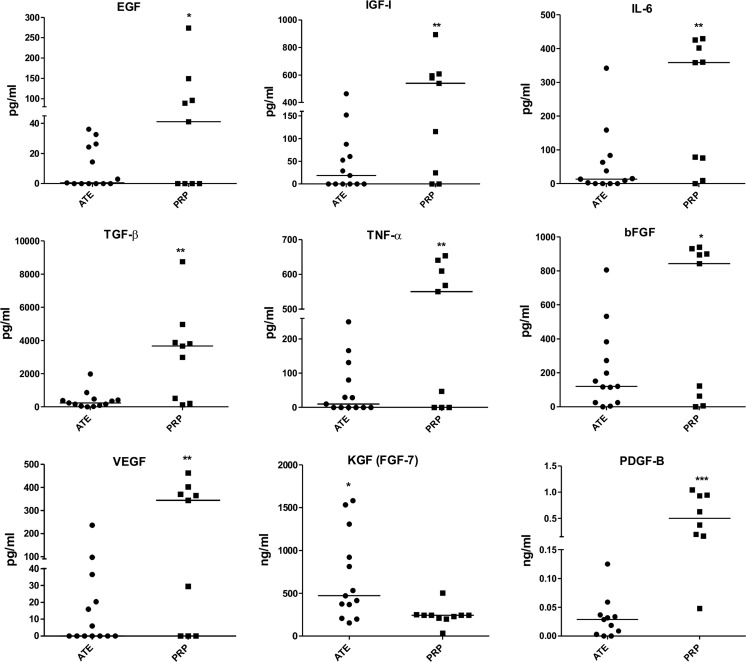
Mean protein concentrations were 0.4953 mg/ml (± 0.155) for ATE donor samples with a range from 0.2450 to 0.8500 mg/ml. PRP had significantly overall higher (19 fold) growth factor concentrations. KGF was the only growth factor that was significantly higher in ATE (p < 0.05). Growth factors that were found to be significantly lower in ATE were: bFGF (p < 0,05), VEGF (p < 0.01), EGF (p < 0.0505), IGF-1 (p < 0.01), IL-6 (p < 0.01), for PDGF-B (p < 0.001), TGF-β (p < 0.01) and TNF-α (p < 0.01). Figure 1 represents the obtained growth factor values and their medians for both ATE and PRP samples.
Fig. 1.
Medians of the growth factors measured with ELISA for ATE and PRP patient samples. Number of samples: for PRP n = 9 except for KGF n = 10, and for PDGF-B n = 8; for ATE n = 13 except for PDGF-B n = 11. The statistical analysis was performed with two-tailed unpaired t-test. Results were considered significant when p < 0.05*, p < 0.01**, p < 0.001***
ATE promotes keratinocyte proliferation
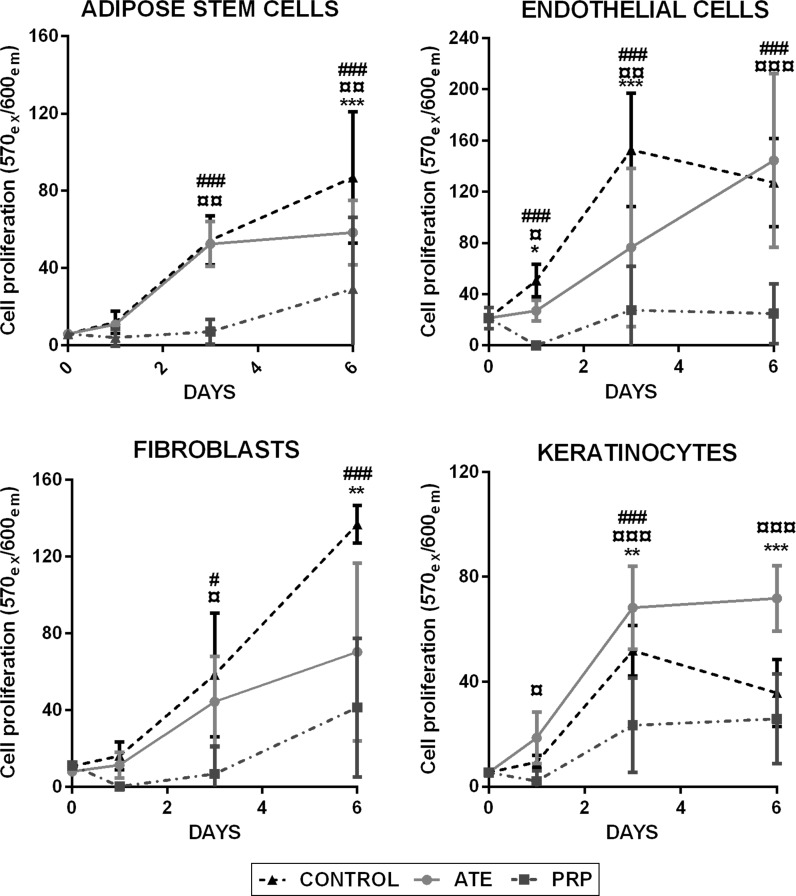
Cell proliferation assays were employed for keratinocytes, fibroblasts, adipose stem cells and endothelial cells to detect the growth of the studied cell populations in the presence of ATE or PRP at 1, 3 and 6 days of culture. Compared to the control cell culture media, results showed that ATE and PRP did not promote the proliferation of adipose stem cells, fibroblasts and endothelial cells during culture. Instead, both ATE and PRP seemed to proliferate slower during culture, especially PRP. However, ATE significantly induced (p < 0.001) the proliferation of keratinocytes. The addition of 10% activated PRP supernatant did not promote keratinocyte proliferation either. Figure 2 illustrates cell proliferation of the four different cell lines during exposure to ATE and PRP.
Fig. 2.
Cell proliferation of ATE and PRP-treated cultures of adipose stem cells, endothelial cells, fibroblasts and keratinocytes when compared to control. Proliferation was measured with Presto Blue from live cultures at days 0, 1, 3 and 6. Results are depicted as mean ± SD. The statistical analysis was performed with two-way ANOVA with Tukey’s post-test. Differences were considered significant when p < 0.05*, p < 0.01** and p < 0.001***. Significances between ATE and control group are shown with (*), significances between PRP and control group with (#) and significances between ATE and PRP with (¤). (Color figure online)
ATE stimulates fibroblast and adipose stem cell migration
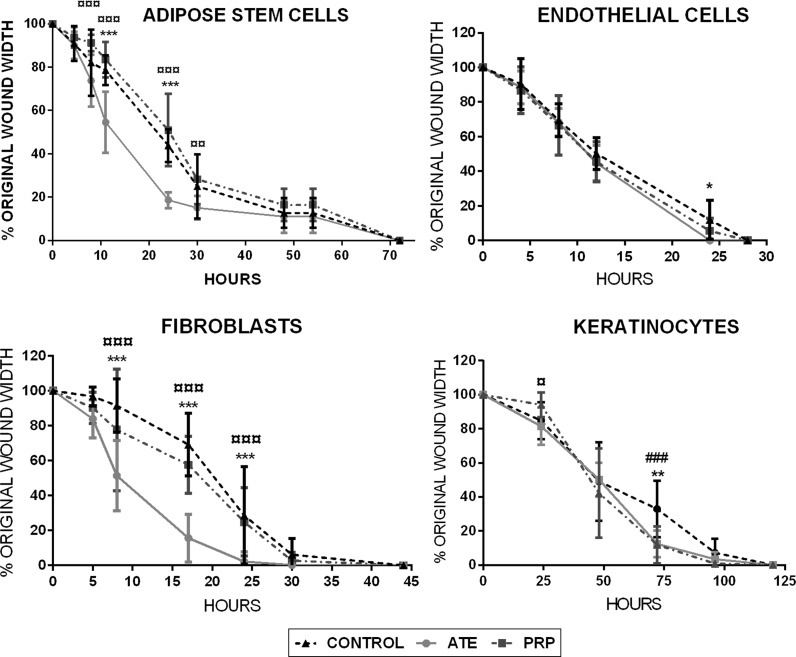
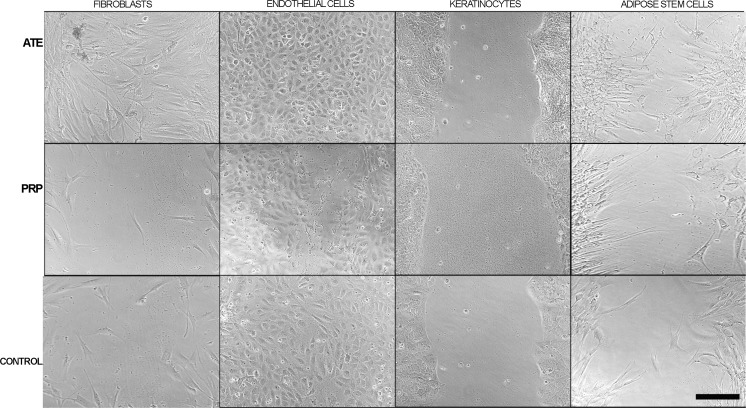
The addition of ATE to fibroblast cultures significantly induced their migration rate throughout the experiment, when compared both to the control group and to PRP group (both, p < 0.001). Moreover, ATE significantly induced adipose stem cell migration during the culture (11 and 24 h) when compared to the PRP group and to the control group (both p < 0.001 and p < 0.001 respectively). PRP seemed to share migration properties of the control group with fibroblast and adipose stem cells. For endothelial cells both treatment groups presented similar growth rates to controls, with the ATE group with significant difference to control at 24 h (p < 0.05). However, for keratinocytes, ATE and PRP both led to significantly faster wound closure with significant difference to control at the 75 h time point (p < 0.01 and p < 0.001 respectively). Figure 3 illustrates the percentage of wound closure of the four studied cell lines at distinct time points in terms of percentage of original wound width in vitro. Figure 4 illustrates the phase contrast microscopic digital images of scratch wound assay of the four cell types at 24 h.
Fig. 3.
In vitro scratch wound assays presented in percentages of original wound width. Cell migration of the four studied cell types was measured under microscopy at determined time points until complete wound closure was achieved. In all treatment groups, adipose stem cells reached wound closure within 74 h, endothelial cells within 25 h, fibroblasts within 30 h and keratinocytes within 125 h. Results are depicted as mean ± SD. The statistical analysis was performed with two-way ANOVA with Tukey’s post-test. Differences were considered significant when p < 0.05*, p < 0.01** and p < 0.001***. Significances between ATE and control group are shown with (*), significances between PRP and control group with (#) and significances between ATE and PRP with (¤)
Fig. 4.
Phase contrast microscopic images of in vitro cell migration after scratch wound assays of fibroblasts, endothelial cells, keratinocytes and adipose stem cells at the 24 h timepoint. Cells were plated with the following counts: keratinocytes 300,000 cells/cm2, fibroblasts 100,000 cells/cm2, adipose stem cells 100,000 cells/cm2 and endothelial cells 150,000 cells/cm2. These cultures were treated with ATE, PRP or with their optimal cell culture medium, as control. Scale bar 100 µm
Discussion
Growth factors are paramount in cell signaling to promote complex, yet orchestrated events such as cell activation, proliferation, differentiation and migration leading to ultimate wound healing. Wound treatment with exogenous sources of growth factors has thus become increasingly popular (Salamanna et al. 2015; Zhao et al. 2013). PRP has long been known as a source of these growth factors, however, individual variations in platelet count as well as differences in methods to prepare, activate and deliver PRP, make growth factor yields as well as clinical results variable (Leitner et al. 2006; Rutkowski et al. 2008; Weibrich et al. 2004; Salamanna et al. 2015). Adipose tissue and its stromal vascular fraction arise as an alternative source of growth factors (Tamama and Kerpedjieva 2012; Sarkanen et al. 2012a, b). Many authors have previously suggested that adipose derived stem cell conditioned media and advanced adipose derived stem cell protein extract contains growth factors that accelerate and improve in vitro wound closure and cell proliferation (Kilroy et al. 2007; Walter et al. 2010). In this study, the authors tested the in vitro wound healing potential of adipose tissue preparation compared to platelet rich plasma in cell migration and proliferation assays. The adipose preparation contains soluble extracellular matrix components like growth factors, cytokines and extracellular matrix components (Sarkanen et al. 2012a, b, c; Lopez et al. 2016). Our aim was to study how cells respond to these growth factor sources and their mechanism.
When studying the concentrations of the principal growth factors affecting wound healing in PRP and ATE, we found that all growth factors, with the exception of KGF (FGF-7) were significantly higher in PRP specimens. KGF, present at a significantly higher concentration in ATE than in PRP, is a potent epithelial cell-specific growth factor secreted by stromal fibroblasts and acts as one of the main inducers of keratinocyte proliferation, maturation and migration in wound healing (Seeger and Paller 2015; Frank et al. 2000) and its activity is exhibited in keratinocytes but not in fibroblasts and endothelial cells. Additionally KGF plays a role in stimulating the production of other growth factors that promote wound healing (Lucarelli et al. 2003). The finding that ATE contains enhanced quantities of KGF compared to PRP can be explained by the nature of mature adipose tissue, that is a combination of several cells, among these fibroblasts, included in a rich network of stromal cells, responsible for secreting KGF. The growth factor concentrations found in ATE correspond to our previous studies (Sarkanen et al. 2012a, b, c; Lopez et al. 2016). Similarly, PRP growth factor concentrations, although variable, correlate with published experiments (Leitner et al. 2006; Rutkowski et al. 2008; Weibrich et al. 2002).
Walter et al. showed that mesenchymal stem cell conditioned medium accelerated fibroblast and keratinocyte migration in vitro, and fibroblasts migrated faster than keratinocytes (Walter et al. 2010). This result is in line with our study, where fibroblast and adipose stem cell migration were significantly accelerated, followed by a slower, although induced migration of keratinocytes, when compared to control. In contrast to Walter’s experiments that tested the proliferation in the presence of bone derived mesenchymal stem cell conditioned medium, we also observed a significant acceleration of cell proliferation with keratinocytes with the addition of ATE. ATE also seemed to stimulate cell proliferation of adipose stem cells, fibroblasts, keratinocytes and endothelial cells compared to PRP-treated cells.
The significantly accelerated keratinocyte proliferation is in line with our results of high concentration of KGF in ATE. Once activated by KGF, keratinocytes proliferate and form a structured epithelial protective barrier that withstands potential chemical, mechanical and microbial noxa (Nolte et al. 2008; Wright et al. 2009; Lee et al. 2016). Moreover, we found that in addition to KGF, ATE yielded appreciable quantities of PDGF-B, TGF-β, EGF, IGF-1 and bFGF that stimulate fibroblast migration and keratinocyte proliferation, detected in our studies. In addition, several other growth factors present in ATE, e.g. leptin (Sarkanen et al. 2012a, b, c), have been shown to promote keratinocyte proliferation and wound re-epithelialization (Frank et al. 2000). Fibroblasts are crucial cells during the proliferative and remodeling phases of wound healing (Nolte et al. 2008; Wright et al. 2009). The influx of fibroblasts forms a matrix scaffold that is not only the foundation for the primitive wound scar, but also a stable highway over which keratinocytes migrate and endothelial cells proliferate to form healthy, vascularized regenerated tissue (Lee et al. 2016). Accelerated keratinocyte proliferation then translates clinically as a quicker formation of a stable and stronger wound bed with the ability to scar in an orderly fashion, in quicker wound closure, which results in greater patient comfort, less pain and decreased dressing-related costs.
Wound treatment with autologous and recombinant PRP has demonstrated satisfactory wound healing outcomes in vivo (Demidova-Rice et al. 2012). However, contradictory in vitro results have also been found. The growth-stimulating effect of PRP has been reported to show dose-dependent behavior (Lucarelli et al. 2003; Gruber et al. 2004; Kilian et al. 2004; Soffer et al. 2004). Barsotti et al. studied the effect of platelet lysate at 10 and 20% in four different cell lines and noticed that low concentrations induced greater cell viability, proliferation and migration while the highest concentration seemed to provoke angiogenesis (2013). We also optimized the PRP concentration in our preliminary studies (data not shown) and found that lower concentrations of PRP (10 vs. 20%) resulted in greater cell proliferation. However, in contrast to e.g. Barsotti et al. (2013), our experiments demonstrated slow and stagnant in vitro cell proliferation for PRP treated cultures. We did not find any proliferative effect with PRP in vitro, only slight acceleration in keratinocyte migration. Our findings are supported by published experiments that have failed to find a direct relationship of PRP with cell proliferation (Graziani; Liu et al. 2002; Xian et al. 2015). A possible reason for this negative effect of PRP on cell growth may be related to the excessively high concentrations of growth factors, among these, interferon gamma (IFN-γ) that inhibits fibroblast proliferation (Nolte et al. 2008; Wright et al. 2009; Lee et al. 2016), which was seen in our study. Additionally, PRP contains regulators found in platelet α-granules named thrombospondins (TBSP) (Hsu et al. 2009). In our previous studies, ATE has been found to contain only low amounts or not at all of IFN-γ and TBSP (Sarkanen et al. 2012a, b, c). In vitro, this family of proteins, in particular TBSP 1 and 2, have demonstrated anti-angiogenic effects, as well as negative effects on cell adhesion, proliferation and migration (Armstrong and Siadak 2002; Iruela-Arispe et al. 1999). Even though cell differentiation and proliferation are essential for wound healing, both do not occur simultaneously. PRP, with its high concentration of platelet-derived growth factors, may support cell differentiation more than proliferation (Graziani; Weibrich et al. 2004). The concentration of leukocytes in PRP has proven to induce pro-inflammatory states, resulting in deleterious effects on cell proliferation in vitro (Anitua et al. 2015). According to the growth factors that ATE and PRP contain, e.g. TGF-β, bFGF and PDGF-B reported here and previously (Sarkanen et al. 2012a, b, c), they also contribute to the formation of extracellular matrix. This may also explain the differential results obtained for PRP in vitro and in vivo.
It therefore seems, also according to our study, that very high concentrations of growth factors are not a prerequisite for optimal cell proliferation and migration in vitro. High concentrations of growth factors, as observed in PRP, insinuated to be more a hindrance than an advantage. Healing is, in fact, known to be disturbed by both under and over production of growth factors (Nolte et al. 2008; Wright et al. 2009; Lee et al. 2016). When growth factor concentrations are below normal there is a delay in healing (Nolte et al. 2008; Wright et al. 2009). On the other hand, an excess of certain regulatory growth factors can prevent the necessary steps for wound healing (Salamanna et al. 2015). Understanding the relationship between growth factors and their cellular environment could aid in predicting the clinical outcomes in the treatment with ATE or PRP.
Adipose derived stem cells reflect mesenchymal cells that have the ability to differentiate towards a variety of cell lines. They have shown in vitro and in vivo advantages in cell regeneration and wound repair (Tamama and Kerpedjieva 2012; Cohen 2008; Balfour and Noble 1999). The addition of ATE to these cells resulted in stimulated proliferation and migration, which could also be in itself a therapeutic alternative. Our previous results, where also adipose stem cell differentiation was induced, support this (Sarkanen et al. 2012a, b, c). Although our results are very promising for the use of ATE clinically in wound healing, in vitro assays do not reflect the complete complex microenvironment found in in vivo wound repair, and therefore can be used only for predicting and estimating the possible clinical outcomes. Another shortcoming with the use of ATE is that the current extraction procedure requires liposuction from the patient in order to obtain ATE. Therefore, the extraction procedure is unsuitable for very lean patients. Furthermore, ATE requires correct handling and preservation for optimal biological effect. Currently, further in vivo studies are being performed to support our findings and to further develop the procedures.
Conclusions
ATE is an easy to obtain, alternative source of growth factors derived from adipose tissue with the ability to promote wound healing in vitro. Compared to PRP, ATE seems to provide more optimal amounts of growth factors involved in wound healing. Especially the high concentration of KGF, responsible for keratinocyte growth and maturation as well as inducing the secretion of other growth factors, could be a key feature in ATE. As epithelization is an important step in optimal wound healing with minimal scar formation, ATE could be considered for treatment of wounds in the clinical setting. This unique advantage of ATE, containing the adequate measurements of growth factors at ideal concentrations, may hold the key to future therapeutics in tissue healing and regeneration.
Acknowledgements
We would like to thank Ms. Hilkka Mäkinen for her invaluable work with laboratory assistance. Our thanks for volunteer patients and the health staff at the Tampere University Hospital.
Abbreviations
- Ang-1
Angiopoietin 1
- Ang-2
Angiopoietin 2
- ATE
Adipose tissue extract
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- bFGF
Basic fibroblast growth factor
- IFN-γ
Interferon-gamma
- IGF-1
Insulin-like growth factor
- IL-6
Interleukin 6
- HGF
Hepatocyte growth factor
- KGF (FGF-7)
Keratinocyte growth factor (fibroblast growth factor 7)
- PDGF-B
Platelet-derived growth factor beta
- PRP
Platelet-rich plasma
- TGF-α
Transforming growth factor alfa
- TGF-β
Transforming growth factor beta
- TNFα
Tumor necrosis factor alfa
- TSBP
Thrombospondins
- VEGF
Vascular endothelial growth factor
Funding
This study was supported by the Finnish Technology and Innovation Agency TEKES, and Competitive State Research Financing of the Expert Responsibility area of Tampere University Hospital, Grant number 9S025.
Compliance with ethical standards
Conflict of interest
Patent issued in The USA (9056084 B2) and pending (WO2010026299A1) in other countries.
References
- Anitua E, Zalduendo M, Troya M, Padilla S, Orive G. Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS ONE. 2015;10:e0121713. doi: 10.1371/journal.pone.0121713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong LC, Siadak AW. Thrombospondin 2 inhibits microvascular endothelial cell proliferation by a caspase-independent mechanism. Mol Biol Cell. 2002;13:1893–1905. doi: 10.1091/mbc.e01-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour JA, Noble S. Becaplermin BioDrugs: clinical immunotherapeutics. Biopharm Gene Ther. 1999;11:359–364. doi: 10.2165/00063030-199911050-00007. [DOI] [PubMed] [Google Scholar]
- Barsotti MC, Losi P, Briganti E, Sanguinetti E, Magera A, Kayal TA, Feriani R, Stefano RD, Soldani G. Effect of platelet lysate on human cells involved in different phases of wound healing. PLoS ONE. 2013;8:e84753. doi: 10.1371/journal.pone.0084753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickers D, Lim H, Margolis D, Weinstock M, Goodman C, Faulkner E, Gould C, Gemmen E, Dall T. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490–500. doi: 10.1016/j.jaad.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Broughton G, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. Origins of growth factors: NGF and EGF. J Biol Chem. 2008;283:33793–33797. doi: 10.1074/jbc.X800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidova-Rice T, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: Biology, causes, and approaches to care. Adv Skin Wound Care. 2012;25:304–314. doi: 10.1097/01.ASW.0000416006.55218.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58:185–206. doi: 10.1016/j.jaad.2007.08.048. [DOI] [PubMed] [Google Scholar]
- Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- Frank S, Stallmeyer B, Kämpfer H, Kolb N, Pfeilschifter J. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J Clin Invest. 2000;106:501–509. doi: 10.1172/JCI9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Karreth F, Kandler B, Fuerst G, Rot A, Fischer MB, Watzek G. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets. 2004;15:29–35. doi: 10.1080/09537100310001643999. [DOI] [PubMed] [Google Scholar]
- Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan W, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014;22:313–325. doi: 10.1111/wrr.12173. [DOI] [PubMed] [Google Scholar]
- Hsu C, Yuan K, Tseng C. The negative effect of platelet-rich plasma on the growth of human cells is associated with secreted thrombospondin-1. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:185–192. doi: 10.1016/j.tripleo.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe M, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999;100:1423–1431. doi: 10.1161/01.CIR.100.13.1423. [DOI] [PubMed] [Google Scholar]
- Kilian O, Flesch I, Wenisch S, Taborski B, Jork A, Schnettler R, Jonuleit T. Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro. Eur J Med Res. 2004;9:337–344. [PubMed] [Google Scholar]
- Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, et al. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- Lee SM, Lee SC, Kim S. Contribution of human adipose tissue–derived stem cells and the secretome to the skin allograft survival in mice. J Surg Res. 2014;188:280–289. doi: 10.1016/j.jss.2013.10.063. [DOI] [PubMed] [Google Scholar]
- Lee D, Ayoub N, Agrawal D. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Research & Therapy. 2016;7:37. doi: 10.1186/s13287-016-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner GC, Gruber R, Neumüller J, Wagner A, Kloimstein P, Höcker P, Körmöczi GF, Buchta C. Platelet content and growth factor release in platelet-rich plasma: a comparison of four different systems. Vox Sang. 2006;91:135–139. doi: 10.1111/j.1423-0410.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- Li J, Qiao X, Yu M, Li F, Wang H, Guo W, Tian W. Secretory factors from rat adipose tissue explants promote adipogenesis and angiogenesis. Artif Organs. 2014;38:E33–E45. doi: 10.1111/aor.12162. [DOI] [PubMed] [Google Scholar]
- Li M, Luan F, Zhao Y, Hao H, Liu J, Dong L, Fu X, Han W. Mesenchymal stem cell-conditioned medium accelerates wound healing with fewer scars: Mesenchymal stem cell-conditioned medium enhance wound scarless healing. Int Wound J. 2015;14:64–73. doi: 10.1111/iwj.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kalén A, Risto O, Wahlström O. Fibroblast proliferation due to exposure to a platelet concentrate in vitro is pH dependent. Wound Rep Reg. 2002;10:336–340. doi: 10.1046/j.1524-475X.2002.10510.x. [DOI] [PubMed] [Google Scholar]
- Lopez J, Huttala O, Sarkanen J-R, Kaartinen I, Kuokkanen H, Ylikomi T. Cytokine-rich adipose tissue extract production from water-assisted lipoaspirate: methodology for clinical use. BioRes Open Access. 2016;5:269–278. doi: 10.1089/biores.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, Del Vento AM, Meotti C, Bertoja AZ, Giardino R, Fornasari PM, et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24:3095–3100. doi: 10.1016/S0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- Montalvan B, Goux PL, Klouche S, Borgel D, Hardy P, Breban M. Inefficacy of ultrasound-guided local injections of autologous conditioned plasma for recent epicondylitis: results of a double-blind placebo-controlled randomized clinical trial with one-year follow-up. Rheumatology. 2016;55:279–285. doi: 10.1093/rheumatology/kev326. [DOI] [PubMed] [Google Scholar]
- Nolte SV, Xu W, Rennekampff H, Rodemann HP. Diversity of fibroblasts–a review on implications for skin tissue engineering. Cells Tissues Organs. 2008;187:165–176. doi: 10.1159/000111805. [DOI] [PubMed] [Google Scholar]
- Rutkowski JL, Thomas JM, Bering CL, Speicher JL, Radio NM, Smith DM, Johnson DA. An analysis of a rapid, simple, and inexpensive technique used to obtain platelet-rich plasma for use in clinical practice. J Oral Implantol. 2008;34:25–33. doi: 10.1563/1548-1336(2008)34[25:AAOARS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Salamanna F, Veronesi F, Maglio M, Della Bella E, Sartori M, Fini M. New and emerging strategies in platelet-rich plasma application in musculoskeletal regenerative procedures: general overview on still open questions and outlook. Biomed Res Int. 2015;2015:846045. doi: 10.1155/2015/846045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado AJ, Reis RL, Sousa N, Gimble JM. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- Sarkanen JR, Vuorenpää H, Huttala O, Mannerström B, Kuokkanen H, Miettinen S, Heinonen T, Ylikomi T. Adipose stromal cell tubule network model provides a versatile tool for vascular research and tissue engineering. Cells Tissues Organs. 2012;196:385–397. doi: 10.1159/000336679. [DOI] [PubMed] [Google Scholar]
- Sarkanen J, Ruusuvuori P, Kuokkanen H, Paavonen T, Ylikomi T. Bioactive acellular implant induces angiogenesis and adipogenesis and sustained soft tissue restoration in vivo. Tissue Eng Part A. 2012;18:2568–2580. doi: 10.1089/ten.tea.2011.0724. [DOI] [PubMed] [Google Scholar]
- Sarkanen J, Kaila V, Mannerström B, Räty S, Kuokkanen H, Miettinen S, Ylikomi T. Human adipose tissue extract induces angiogenesis and adipogenesis in vitro. Tissue Eng Part A. 2012;18:17–25. doi: 10.1089/ten.tea.2010.0712. [DOI] [PubMed] [Google Scholar]
- Seeger M, Paller A. The roles of growth factors in keratinocyte migration. Adv Wound Care. 2015;4:213–224. doi: 10.1089/wound.2014.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffer E, Ouhayoun J, Dosquet C, Meunier A, Anagnostou F. Effects of platelet lysates on select bone cell functions. Clin Oral Implants Res. 2004;15:581–588. doi: 10.1111/j.1600-0501.2004.01063.x. [DOI] [PubMed] [Google Scholar]
- Tamama K, Kerpedjieva SS. Acceleration of wound healing by multiple growth factors and cytokines secreted from multipotential stromal cells/mesenchymal stem cells. Adv Wound Care. 2012;1:177–182. doi: 10.1089/wound.2011.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter MNM, Wright KT, Fuller HR, MacNeil S, Johnson WEB. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res. 2010;316:1271–1281. doi: 10.1016/j.yexcr.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Weibrich G, Kleis WKG, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofacial Surg. 2002;30:97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34:665–671. doi: 10.1016/j.bone.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Wright CS, van Steensel Maurice AM, Hodgins MB, Martin PEM. Connexin mimetic peptides improve cell migration rates of human epidermal keratinocytes and dermal fibroblasts in vitro. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2009;17:240–249. doi: 10.1111/j.1524-475X.2009.00471.x. [DOI] [PubMed] [Google Scholar]
- Xian LJ, Roy Chowdhury S, Bin Saim A, Idrus BH. Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing. Cytotherapy. 2015;17:293–300. doi: 10.1016/j.jcyt.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Graziani F. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17:212–219. doi: 10.1111/j.1600-0501.2005.01203.x. [DOI] [PubMed] [Google Scholar]
- Zhao J, Hu L, Liu J, Gong N, Chen L. The effects of cytokines in adipose stem cell-conditioned medium on the migration and proliferation of skin fibroblasts in vitro. Biomed Res Int. 2013;2013:1–11. doi: 10.1155/2013/578479. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]