Abstract
Melanoma is a predominant cause of skin cancer-related deaths. It was reported that, the methanolic extract of Pouzolzia Indica (P. indica) on chromatography gave five compounds (1-hentriacontanyl palmitate, myricyl alcohol, 6,7-dimethoxycoumarin, trichadonic acid and friedelane), which inhibited the acute promyelocytic leukemia cell lines, NB4, and HT93A. Friedelane was extracted as active compound from methanolic extract of P. indica. In this study, friedelane was tested on murine metastatic B16F10 and B16BL6 melanoma cell lines. To achieve the target, the cell viability using trypan blue exclusion, acridine orange/EtBr staining and cell cytotoxicity were tested using MTT assay. Caspase-3, caspase-9, Cyt-c, BAD and Bax protein were assayed to evidence the apoptosis induction. The compound friedelane shows potent cytotoxic effect against metastatic melanoma mouse cell lines in 10 µg/ml concentration.
Electronic supplementary material
The online version of this article (10.1007/s10616-017-0174-4) contains supplementary material, which is available to authorized users.
Keywords: Pauzolzia indica Gaud., Friedelane (Fr), Melanoma, Cell viability, Cytotoxicity, Apoptosis
Introduction
Now a days, cancer emerges as the most challenging threat towards the human being. Natural products derived from especially higher plants, have long been considered to be potential sources for developing potential anticancer drugs. Out of several therapies, chemotherapy has become the preferred choice for treatment of cancer and transplantation is an option for the treatment in some cases. Besides this, a major problem in the treatment of cancer is the development of resistance to chemotherapy and subsequent relapse, therefore, the search for novel anticancer agents from natural sources is currently receiving great attention.
Cell viability and cytotoxicity assays are used for drug screening and cytotoxicity tests of drugs before going into in vivo treatment. Different compounds might have different functions depending on their structures. They might alter the enzyme activity, cell membrane permeability, cell adherence, ATP production, co-enzyme production, nucleotide uptake activity and so on. Apoptosis or programmed cell death is a specific form of cell death which plays a crucial role to maintain the balance and integrity of multi cellular organisms. Apoptosis also plays a role in preventing cancer; if a cell is unable to undergo apoptosis, due to different biochemical inhibition, or genetic alteration, it can continue dividing and develop into a tumor cells. Some compounds may act on those pathways to induce apoptosis or inhibit the biochemical pathways leading either to cell death or to survival.
P. indica, a medicinal plant belongs to Urticaceae family (Sangsuwon et al. 2013). This plants species are wildly distributed throughout India and Bangladesh. It contains steroids, flavonoids, tannins, alkaloids, carotenoids, ascorbic, tartaric, malic and pectic acids, gum, minerals, carbohydrates, terpenoids, coumarins and their salts (Ghani 2003; Ahmed et al. 2010; Sangsuwon et al. 2013). P. indica, distributed in the tropical parts of the world, has several medicinal properties including treatment of menstrual disorders. It helps to pass urine and helps treating infections with pus (Trakulsomboon et al. 2006). It was reported that, the methanolic crude extract of P. indica via chromatographic isolation into five compounds, namely friedeline, 28-hydroxy-3-friedelanone, 7-methoxycoumarin, 6,7-dimethoxycoumarin and scopoletin, which inhibited growth of the acute promyelocytic leukemia cell lines, NB4, and HT93A (U-Pratya et al. 2008). It was also reported that, the plant is used in traditional medicine as anti-snake venom by some communities in southern India (Ahmed et al. 2010). The antibacterial activity of the methanol extract and killing properties against Acanthamoeba cysts have also been reported previously (Roongruangchai et al. 2010). In traditional medicine, P. indica used for different purposes like remedy for the ailments in female infertility, cancer, inflammation, emmenagogue and insecticide (Srisapoomi et al. 2008).
A new compound friedelane (triterpenoid ester, 28-dodecyl-7β-hydroxy-3-oxo-friedelan-28-oate) was isolated from P. indica (Sil Sarma and Dinda 2013). Naturally occurring friedelane triterpenoids are used as insulin sensitizers in the treatment of type 2 diabetes mellitus (Ardilesa et al. 2012). Friedelane is also isolated from mangrove plant Hibiscus tiliaceus (Li et al. 2006). In this study, friedelane is tested for murine melanoma cell apoptosis.
Materials and methods
Reagents
MEM, FBS, vitamin solution, non-essential amino acid, glutamine, antibiotic–antimycotic solution, trypsin, trypan blue and Enzcheck caspase-3 assay kit, MTT from Life Technologies (Grand Island, NY, USA), monoclonal antibodies were purchased from Santa Cruz Biotechnologies (Dallas, TX, USA), HRP-conjugated secondary antibody obtained from AbCam (Cambrigde, UK), ECL clarity kit from Bio-Rad (Hercules, CA). Tissue culture plastic wares were obtained from BD falcon (Corning, NY, USA), Acridine orange and other chemicals were procured from Merck Millipore (Billerica, MA) and SRL (Mumbai, India).
Cell culture
Murine metastatic melanoma cell lines B16BL6 and B16F10 were used in this study and were procured from National Centre for Cell Science (Pune, India). These cell lines were cultured in MEM supplemented with vitamin, non-essential amino acids (MEM-NEAA, 1X), glutamine (1X), sodium pyruvate (1 mM), 10% FBS and penicillin streptomycin (100 U/ml–100 µg/ml) solution (all purchased from Life Technologies). Cells were cultured at 37 °C in a humidified atmosphere with 5% CO2. After regular monitoring of culture achieving 75–80% confluence, cells were seeded at desired density in culture plate with serum free media after proper wash.
Preparation of plant extract and isolation of friedelane
The aerial parts of P. indica were collected locally. The plant was identified by Dr. B. K. Datta, Professor, Plant Taxonomy, Dept. of Botany, Tripura University. Air-dried whole plants (2.5 kg) of P. indica were extracted with MeOH (8L × 2, 6 d each time) by percolation at room temperature and the combined MeOH extract was evaporated under reduced pressure in a rotavapor to get a semisolid residue (250 g). The major part (240 g) of the residue was suspended in H2O (60 ml) and extracted successively with CH2Cl2, EtOAc and n-BuOH (3 × 100 ml with each solvent). The residue (35 g) obtained from CH2Cl2 extract was subjected to Silica gel (60–100 mesh, Merck, Mumbai, India) column chromatography and the column was eluted with a gradient of petroleum ether (PE)-CHCl3 in the ratio of 8:2, 7:3, 5:5, 4:6, and 3:7. Elution of the column with PE-CHCl3 (4:6) afforded friedelin (= friedelane) (150 mg) in colourless needles, mp 262 °C. It was identified by comparison of its spectral data (NMR and MS) (Akihisa et al. 1992; Sil Sarma and Dinda 2013).
Determination of cell viability
Trypan blue exclusion assay was performed for cytotoxicity determination (Kwok et al. 2004). Briefly, the cells were plated at a density of 5 × 105 in 12-well flat-bottomed tissue culture (TC) plates and either treated with friedelane at concentrations ranging from 5 and 10 µg/well or left untreated as control. The drug cisplatin was treated as positive control. Cells were then harvested, washed twice with PBS, concentrated to 200 µl and stained with 0.4% trypan blue. Approximately 100 cells were counted with a hemocytometer for each experiment. The percentage of cytotoxicity was calculated as follows:
Toxicity of friedelane on murine normal spleen cell
Male Swiss albino mice, 3 weeks old, were obtained from the National Institute of Nutrition (Hyderabad, India). These mice were kept and maintained specific pathogen free condition with proper humidity (60–65%), and temperature (25–28 °C) in the Tripura University Animal House. Food, dietary supplements and water were provided ad libitum. All treatments were according to the animal use protocol approved by the Institutional Animal Ethics Committee, Tripura University.
Three normal mice were used for the experiment. At the day of experiment all mice were sacrificed and collected the spleens were collected under aseptic condition. Then the spleenic cell suspension was made from the smashed spleen in PBS by filtering with BD Falcon cell strainer (BD Biosciences, Singapore). RBCs remaining in the smashed cell suspension were lysed using the RBC lysis buffer. Then the cells were cultured in RPMI-1640 media for overnight.
Finally the cells are treated with friedelane and cisplatin checked for its cytotoxicity assay.
Assessment of cell morphology
Cells (5 × 105/well) were grown in 6-well TC plates and treated with or without friedelane at concentrations ranging from 5 and 10 µg/well. The drug cisplatin (10 µg/ml) was used as positive control. Morphological changes were observed with an inverted phase contrast microscope (Model: Carl Zeiss, Oberkochen, Germany) and photographs were taken with the help of a digital camera.
Fluorescence microscopy
To detect nuclear damage or chromatin condensation, treated and untreated cells were washed twice with PBS. The conventional acridine orange/ethidium bromide (AO/EtBr) staining procedure was followed to differentiate the live, apoptotic and necrotic cells (Dey et al. 2013). Briefly, treated or untreated cells were stained with acridine orange (0.03 mg/ml), ethidium bromide (0.03 mg/ml) and analyzed under a fluorescence microscope with LASER beam excitation at 488 and 550 nm (Leica DM400 with DFC45C CCD model fluorescence microscope, Wetzlar, Germany). Photographs were acquired.
In vitro caspase-3 activity assay
Approximately 5 × 105 cells were treated with friedelane (5 and 10 µg/ml, final concentration), for 24 h. Caspase 3 activity (Solowey et al. 2014) within the cells was assessed by using Enzcheck caspase-3 assay kit (Molecular Probe, Eugene, OR, USA). Experiments were carried out in parallel with cell viability assays.
In vitro proliferation assay
For cell proliferation assay, 5 × 105 cells were incubated with 5 and 10 μg/ml of friedelane in a final volume of 100 μl for 24 h. Positive controls were carried out with cisplatin, and negative controls with 1% DMSO supplemented MEM medium. Cell viability was quantified using the MTT test (Figueiredo et al. 2014). In brief: 1 × 105 cells were incubated with 5 and 10 μg/ml of friedelane in a final volume of 100 μl for 24 h. Positive controls were carried out with cisplatin at 10 µg/ml, and negative controls with 1% DMSO supplemented MEM medium. After 24 h treatment, 10 µl of 12 mM MTT (Vibrant MTT cell proliferation assay kit, Thermo Fisher Scientific, Waltham, MA, USA) solution was added to each well. The plate was incubated for 4 h at 37 °C. After removing all but 25 µl of medium from each well, 50 µl of DMSO was added in each well. The plate was incubated at 37 °C for 10 min after proper mixing. Readings were performed in a plate reader (BioTek, Winooski, VT, USA) at 570 nm. All experiments were performed in triplicate.
Western blot analysis
To determine proteins expression levels, Western blot analysis was performed by the technique described earlier with minor modifications (Towbin et al. 1979). Cells were rinsed with cold PBS and lysed with 0.25 ml of lysis buffer. Concentration of soluble protein from total cell lysate was determined using Lowry’s method (Lowry et al. 1951). Approximately 5–10 μg of proteins of total cell lysate per sample was separated on 10% SDS-PAGE, transferred to nitrocellulose membrane, blotted with anti-p53 polyclonal, anti-Bax monoclonal and IgG anti mouse secondary antibody HRP-conjugated using an enhanced chemiluminescence (ECL) detection system. Membranes were stripped and re-blotted with anti-GAPDH antibody to ensure an equal amount of proteins loaded on gel. The signals were captured using X-ray films.
Statistical analysis
All readings were taken 3–5 times repeats of same experiments. Data were expressed as mean ± SEM. The bar diagrams in Figs. 1, 3, 5, and 6 were designed using Sigma Plot and data were analyzed using ANOVA for test of significant using GraphPad Prism Software. All statistical test were considered as ***p < 0.001, **p < 0.01, *p < 0.05.
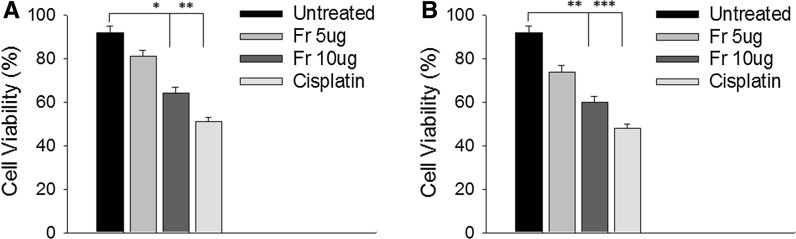
Fig. 1.
Results of trypan blue cell viability assay using a B16F10 and b B16BL6 cells (from left to right: negative control, after treatment with 5 and 10 μg/ml of friedelane and cisplatin (10 µg/ml). Cell viability decreased with increasing concentration of friedelane. ***p < 0.001; **p < 0.01; *p < 0.05. Fr Friedelane
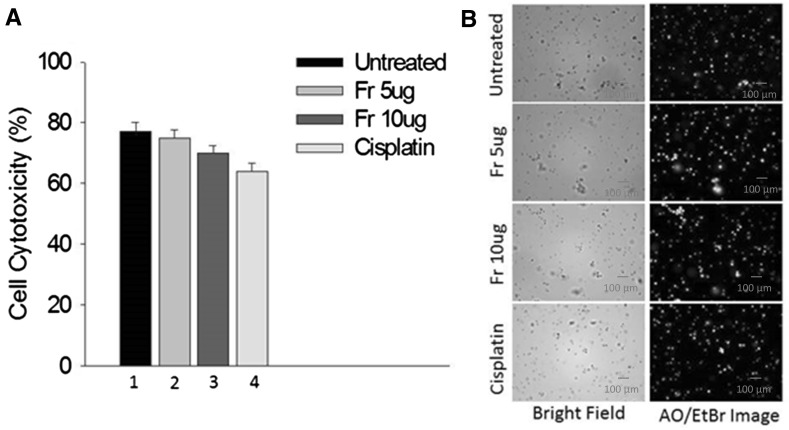
Fig. 3.
Cytotoxic effect of friedelane and cisplatin on mouse splenocytes, a MTT assay (from left to right: negative control (1), after treatment with 5 (2) and 10 μg/ml (3) of friedelane and cisplatin (10 µg/ml) (4)), b fluorescent imaging of AO/EtBr staining. Friedelane did not show significant effects on splenocytes
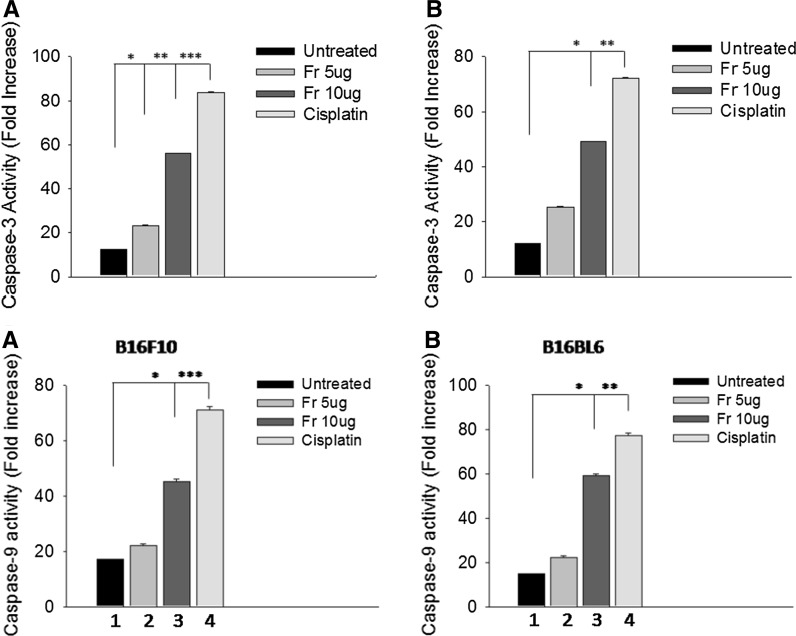
Fig. 5.
Caspase-3 activity (in the upper panel) and caspase 9 activity (in lower panel) changes in a B16F10 and b B16BL6 cells (from left to right: control (1), treatment with 5 (2) and 10 μg (3) of friedelane or cisplatin (10 µg/ml) (4) for 24 h). The results are expressed as the mean ± standard error of the mean of three independent experiments. ***p < 0.001; **p < 0.01; *p < 0.05. Fr Friedelane
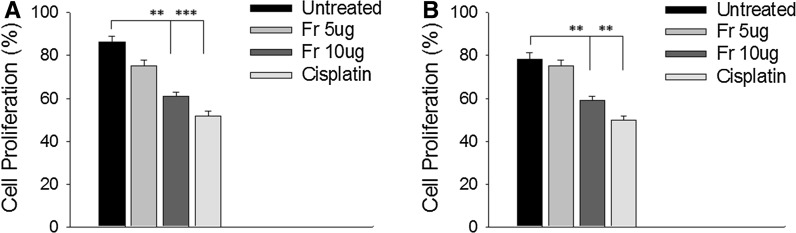
Fig. 6.
Inhibitory activity of friedelane on proliferation of (a) B16F10 and (b) B16BL6 cells. 105 viable cells were not incubated, incubated with friedelane at concentrations of 5 and 10 μg/ml for 24 h. Cell proliferation was determined by the MTT assay. ***p < 0.001; **p < 0.01; *p < 0.05. Fr Friedelane
Results
Cell viability analysis
Measurements of cell viability in a B16F10 and B16BL6 cells were determined using trypan blue assay after exposure to 5 and 10 μg of friedelane and cisplatin for 24 h. After treatment with 10 µg/ml of friedelane for 24 h 40% of both cell lines B16F10 and B16BL6 died, however, after treatment with cisplatin at a dose of 10 µg/ml, 50% of the cells died (Fig. 1).
Assessment of cell morphology
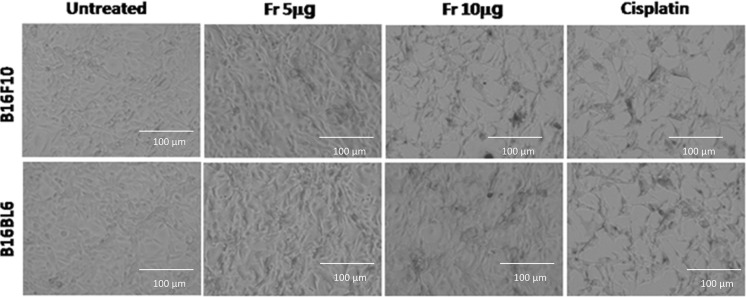
Following treatment with friedelane, the morphological changes in the cells were observed with and inverted microscopy. In the friedelane treated well, both cells became irregular and exhibited shrinkage. Detachment of the cells from the surface of the well was also observed (Fig. 2). These changes were characteristic of apoptotic cell death. In the control groups, cell morphology did not change significantly.
Fig. 2.
Morphological alterations of B16F10 and B16BL6 cells observed by inverted microscopy. 1st panel: control cells; 2nd panel: friedelane at a 5 μg treatment; 3rd panel: friedelane at a 10 μg treatment; 4th panel: cisplatin treatment. Magnification, ×200. Fr Friedelane
Toxicity on normal murine splenocyte cells
To investigate the toxicity of friedelane, 5 and 10 µg/ml concentrations were used to treat normal mouse splenocytes. The cells did not show significant apoptosis with both concentrations as well as the positive cisplatin control (10 µg/ml) after 24 h of treatment. This result confirmed the low toxicity level of the compound for the normal cell (Fig. 3) in the above mentioned doses.
Cell viability by acridine orange and ethidium bromide staining
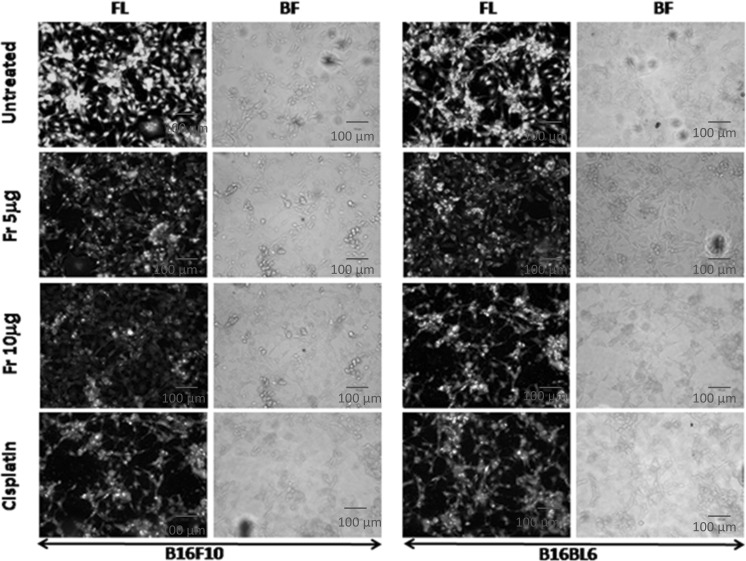
With the microscopic study using the nuclear staining dye acridine orange and ethidium bromide (AO/EtBr), 5-10 μg/ml of friedelane treated cells showed characteristic apoptotic changes after 24 h. Cells treated with 10 μg/ml showed more nuclear fragmentation and dead (red color) cells in both cell lines compared to the control well (green) (Fig. 4). In the positive control well the number of dead and nuclear fragmented cells were also higher than those of the untreated cells. Thus, the microscopic study indicated that death of B16F10 and B16BL6 cells induced by friedelane was probably due to apoptosis.
Fig. 4.
Morphological and nuclear changes seen in B16F10 and B16BL6 cells after treatment with friedelane. Cells were cultured in coverslips and treated with 5 and 10 μg of freidelane or cisplatin (10 µg/ml) for 24 h. Cells were observed under a light microscope and/or under a fluorescence microscope following nuclear staining with acridine orange and ethidium bromide (AO & EtBr). FL fluorescence image; BR bright field image. Magnification: X 200. Fr Friedelane
In vitro caspase-3 and caspase 9 activities assay
Molecular mechanism of apoptosis was evaluated using caspase-3 protein activity. The results demonstrated that the caspase-3 activity of B16F10 and B16BL6 cells was markedly increased following treatment with friedelin at different concentrations for 24 h compared to control (Fig. 5).
In vitro proliferation assay
The effects of friedelane at different concentrations on murine melanoma cells proliferation were determined by MTT assay. The results show that friedelane at 10 μg/ml concentration inhibited proliferation maximally in B16F10 and B16BL6 cells (Fig. 6). The positive control was run with cisplatin (10 µg/ml) on both cells. We observed that friedelane mediated proliferation inhibition at 10 µg/ml concentration was close to the positive cisplatin control which mediated inhibition in melanoma cell.
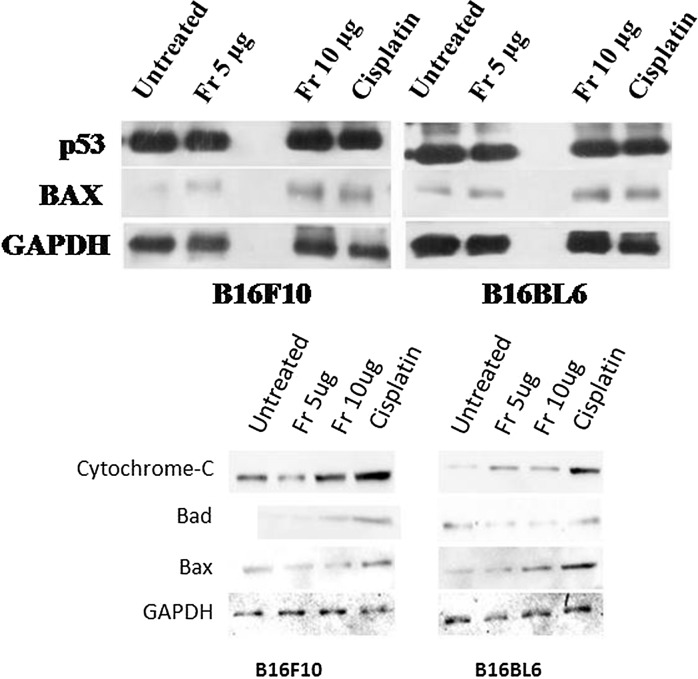
Expression of p53 and Bax proteins after friedelane treatment
To investigate whether, friedelane regulates the pro-apoptotic proteins like p53 and Bax expression, we treated B16F10 and B16BL6 cells with friedelane for overnight. Figure 7 shows that the p53 protein level was very similar in treated and untreated cells but Bax expression showed increased levels in treated cells. Bax expression indicated that apoptosis occured through the mitochondrial pathway. Cytochrome c, Bad, and Bax proteins are involved in apoptosis through mitochondrial pathway. Figure 7 also shows the higher expression of Cytochrome c, Bad and Bax proteins in the 10 µg/ml friedelane treatment condition.
Fig. 7.
Protein expression (p53, Bax in the upper panel and Bad, cytochrome C in the lower panel) by B16F10 and B16BL6 cell in untreated condition, and after treatment with friedelane at 5, 10 μg and cisplatin (AT 10 µg/ml). Western blot images showed that proapoptotic protein levels increased after friedelane and cisplatin treatment
Discussion
Melanoma are increasing incidents of cancer in last 30 years. In the USA, it is uncommon in African American as well as in Asian people, however, the survival rate decreases in white people. The mortality rate increases when it occurs in the palm of the hand or the sole of the foot or nail bed. Different types of surgery, photodynamic therapy, radiation therapy or chemotherapy is being implemented currently to fight against this disease. Still there is no significant cure rate of the disease. Complete remission of the disease depends on stages of detection. At advance stages the mortality rate is higher. This motivates to find out naturally occurring or synthesized compounds which have considerably reduced or no side effects in the patients.
Friedelane was reported to exhibit anticancer activity against MCF7, SF268 and H460 cells (Olmedo et al. 2008). In this study we have investigated its effects on murine metastatic melanoma cells. One of the easiest way to show cell death is to stain with trypan blue. After friedelane treatment for 24 h, at 10 μg/ml, apoptosis was significantly induced in both B16F10 and B16BL6 cells as observed under the inverted microscope and this effect was very similar to the positive control cisplatin. Further study with fluorescent microscopy, staining with AO/EtBr supposed to show green, when they are alive. After treatment, cell lines showed yellowish red staining under the fluorescence microscope which is the symptom of apoptosis. Yellowish red dots in the nuclei represent the consequence of chromatin condensation and nuclear fragmentation (Ribble et al. 2005). Cells after staining with AO/EtBr revealed the morphological features of apoptosis such as membrane blebbing, cell shrinkage and chromatin condensation after 48 h of treatment. The percentage of apoptosis increased in dose dependent manner (Farha et al. 2013). Thus, this study based on fluorescent microscopy indicated that friedelane extracted from P. indica induced death of B16F10 and B16BL6 melanoma cells.
A number of pro- and anti-apoptotic protein families like the anti-apoptotic Bcl-2 subfamily and the pro-apoptotic Bax subfamily proteins (Ku et al. 2011) regulate cellular apoptosis via mitochondrial pathways. Bax of the Bcl-2 family members proteins regulate the release of cytochrome-c from the mitochondria to the cytosol. This cytochrome-c released from the mitochondria in response to apoptotic stimuli activates procaspase-9 at the early stage of apoptosis. The tumor suppressor and pro-apoptotic gene p53, is responsible for triggering the apoptosis process within the cells (Fridman and Lowe 2003). Western blot analysis revealed that the treatment of melanoma cells with friedelane increased the levels of Bax proteins, but the p53 level remained unchanged. Cytochrome-c and Bad are mitochondrial apoptosis markers and are increased after treatment with 10 µg/ml friedelane. Increased expression of Bax, may initiate apoptosis from mitochondria of the both melanoma cells upon friedelane treatment. This induces the release of cytochrome-c from the mitochondria, which induces caspase cascade pathway. The increased caspase-3 activity with the friedelane treatment also supports the apoptosis hypothesis. Altogether, Friedelane inhibits proliferation of murine melanoma cells and induces apoptosis of the same. This may hint to use plant extract friedelane as for therapeutic purposes against melanoma disease.
Conclusion
Friedelane, an active compound isolated from the P. indica showed cytotoxic effects on both metastatic melanoma cells. The drugs which are used currently have some side effects. The study showed that friedelane blocks the proliferation and also induces apoptosis through the mitochondrial pathway as evidenced by higher expression of Bax, cytochrome C and Bad proteins and finally the caspase 3 proteins. Our target was to investigate the effect of friedelane on melanoma cell lines. As this compound is a natural product so that the side effects can be minimized in the patient. Further studies are required to test this drug in an in vivo model of melanoma disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are thankful to DBT for funding and State Biotech Hub, Tripura University for providing the instrumental facilities. We are also thankful to ICMR for providing fellowship to AKS to complete this work.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest for this study.
References
- Ahmed A, Rajendaran JD, Singh HP, Mishra A, Chandra D, Yadav IK, Jain DA. Anti-snake venom activity of different extracts of Pouzolzia indica against russel viper venom. Int J ChemTech Res. 2010;2:744–751. [Google Scholar]
- Akihisa T, Yamamoto K, Tamura T, Kimura Y, Iida T, Nambara T, Chang FC. Triterpenoid ketones from Lingnania chungii McClure: arborinone, friedelin and glutinone. Chem Pharm Bull. 1992;40:789–791. doi: 10.1248/cpb.40.789. [DOI] [Google Scholar]
- Ardilesa AE, González-Rodríguezb A, Núñeza MJ, Peresteloa NR, et al. Studies of naturally occurring friedelane triterpenoids as insulin sensitizers in the treatment type 2 diabetes mellitus. Phytochem. 2012;84:116–124. doi: 10.1016/j.phytochem.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Dey SK, Bose D, Hazra A, Naskar S, Nandy A, Munda RN, et al. Cytotoxic activity and apoptosis-inducing potential of di-spiropyrrolidino and di-spiropyrrolizidino oxindole andrographolide derivatives. PLoS ONE. 2013;8:e58055. doi: 10.1371/journal.pone.0058055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farha AK, Geetha BS, Nair MS, Dhanya SR, Latha PG, Andremani P. Apoptosis mediated cytotoxicity induced by isodeoxyelephantopin on nasopharyngeal carcinoma cells. Asian J Pharm Clin Res. 2013;6:51–56. [Google Scholar]
- Figueiredo CR, Matsuo AL, Massaoka MH, Girola N, Azevedo RA, et al. Antitumor activity of Kielmeyera coriacea leaf constituents in experimental melanoma, tested in vitro and in vivo in syngeneic mice. Adv Pharm Bull. 2014;4:429–436. doi: 10.5681/apb.2014.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- Ghani A. Medicinal plants of Bangladesh with chemical constituents and uses. 2. Dhaka: Asiatic Society of Bangladesh; 2003. [Google Scholar]
- Ku B, Liang C, Jung JU, Oh BH. Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res. 2011;21:627–641. doi: 10.1038/cr.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok AKH, Yeung C-K, Lai TYY, Chan K-P, Pang CP. Effects of trypan blue on cell viability and gene expression in human retinal pigment epithelial cells. Br J Ophthalmol. 2004;88:1590–1594. doi: 10.1136/bjo.2004.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Huang X, Sattler I, Fu H, Grabley S, Lin W. Structure elucidation of a new friedelane triterpene from the mangrove plant Hibiscus tiliaceus. Magn Reson Chem. 2006;44:624–628. doi: 10.1002/mrc.1802. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265. [PubMed] [Google Scholar]
- Olmedo DA, López-Pérez JL, del Olmo E, Vásquez Y, San Feliciano A, Gupta MP. A new cytotoxic friedelane acid–pluricostatic acid–and other compounds from the leaves of Marila pluricostata. Molecules. 2008;13:2915–2924. doi: 10.3390/molecules13112915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribble D, Goldstein NB, Norris DA, Shellman YG. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005;5:12. doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roongruangchai K, Kummalue T, Sookkua T, Roongruangchai J. Comparison of Pouzolzia indica methanolic extract and Virkon against cysts of Acanthamoeba spp. Southeast Asian J Trop Med Public Health. 2010;41:776–784. [PubMed] [Google Scholar]
- Sangsuwon C, Jiratchariyakul W, U-pratya Y, Kummalue T. Antiproliferative effect and the isolated compounds of Pouzolzia indica. Evid Based Complement Altern Med. 2013;2013:342352. doi: 10.1155/2013/342352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil Sarma I, Dinda B. A new friedelane triterpene ester from Pouzolzia indica. Indian J Chem. 2013;52B:1527–1530. [Google Scholar]
- Solowey E, Lichtenstein M, Sallon S, Paavilainen H, Solowey E, Lorberboum-Galski H. Evaluating medicinal plants for anticancer activity. Sci World J. 2014;2014:721402. doi: 10.1155/2014/721402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisapoomi T, Jiratchariyakul W, Partkaittikul N, Kummalue T. Effect of two Thai herbal remedies on the sensitivity of chemotherapeutic agents in human cancer cells. Asian J Tradit Med. 2008;3:108–111. [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakulsomboon S, Kummalue T, Jiratchariyakul W. Antibacterial activities of four Thai medicinal plants. J Med Assoc Thai. 2006;89:1466–1471. [PubMed] [Google Scholar]
- U-pratya Y, Jiratchariyakul W, Kummalue T. Anti-proliferative effects of Pouzolzia indica on acute promyelocytic cell lines: NB4 and HT93A. Asian J Tradit Med. 2008;3:124–133. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.