Abstract
Although hepatocyte transplantation and bioartificial liver support system provide new promising opportunities for those patients waiting for liver transplantation, hepatocytes are easily losing liver-specific functions by using the common in vitro cultured methods. The co-culture strategies with mimicking the in vivo microenvironment would facilitate the maintenance of liver-specific functions of hepatocytes. Considering that hepatocytes and endothelial cells (ECs) account for 80–90% of total cell populations in the liver, hepatocytes and ECs were directly co-cultured with hepatic stellate cells (HSCs) or adipose tissue-derived stem cells (ADSCs) at a ratio of 700:150:3 or 14:3:3 in the present study, and the liver-specific functions were carefully analyzed. Our results showed that the two co-culture systems presented the enhanced liver-specific functions through promoting secretion of urea and ALB and increasing the expressions of ALB, CYP3A4 and HNF4α, and the vessel-like structure in the co-culture system consisted of hepatocytes, ECs and ADSCs. Hence, our results suggested that the directly co-culture of hepatocytes and ECs with HSCs or ADSCs could significantly improve liver-specific functions of hepatocytes, and the co-culture system could further promote angiogenesis of ECs at a later stage. Therefore, this study provides potential interesting in vitro strategies for enhancing liver-specific functions of hepatocytes.
Keywords: Co-culture system, Hepatocytes, Endothelial cells, Adipose tissue-derived stem cells, Liver, Hepatic stellate cells
Introduction
Although liver transplantation is considered as an ultimate treatment for various liver diseases, such as acute liver failure and end-stage liver disease (Boudechiche et al. 2015), it is actually limited in the clinical practice owing to the severe shortage of donor liver organs. At present, several novel approaches, including hepatocyte transplantation and bioartificial liver support system, are being developed to replace the non-functioning liver of those patients waiting for liver transplantation with the aim of improving survival and preventing severe complications (Chen et al. 2012; Hughes et al. 2008; Lee et al. 2016; Rohn et al. 2016; Shi et al. 2016; Struecker et al. 2014). However, the primary hepatocytes easily lose their phenotype and liver-specific functions in vitro if cells are not cultured under the appropriate conditions (Watanabe et al. 2016). Several methods for hepatocyte culture have been proposed to maintain liver-specific functions through optimization of medium components, cell sources and cultured methods (Liu et al. 2014). Among them, establishment of an appropriate cell-culture system that can reflect the native environment of in vivo plays a prerequisite role in maintaining liver-specific functions of hepatocytes, since hepatocytes are rapidly deprived of their original growth condition during the process of cell isolation.
Currently it is well recognized that co-culture systems using a mixture of Kupffer cells or NIH 3T3 cells in co-culture with hepatocytes could facilitate to improve their in vitro viability (Chia et al. 2005; Matis et al. 2017). However, these co-culture systems using only one of the non-parenchymal cells or non-liver cells could not precisely mimic the growth microenvironment of hepatocytes since it is different from the native environment of liver cells. Therefore, it is necessary to develop a co-culture system with multiple cells that could closely mimic the intrinsic environment of hepatocytes, since liver is a complex unit that consists of parenchymal cells (hepatocytes) and a variety of non-parenchymal cells, such endothelial cells (ECs), hepatic stellate cells (HSCs) and Kupffer cells (Sakai et al. 2012).
Considering that hepatocytes and ECs account for 80–90% of the total cell populations in the liver (Van As et al. 2002), it is essential to develop a co-culture system that is similar to the native structure of liver, by using the communications between hepatocytes and ECs. Recently, some reports have also suggested that HSCs could actively support the cell viability and function of hepatocytes by secreting a number of cytokines, growth factors and extracellular matrix components (Ahmed et al. 2017). Hence, the co-culture of ECs, HSCs and hepatocytes that learned from the cell proportion of liver, can closely simulate the growth environment of hepatocytes.
Additionally, a main obstacle for liver regenerative medicine is the difficulty in vascularization of engineered tissue-constructs (Chen et al. 2015). One possible salutation is to integrate pre-vascularization into engineered tissue constructs in vitro by using the co-cultivation of ECs with mesenchymal stem cells (MSCs), such as bone marrow-derived stem cells (BMSCs) and adipose tissue-derived stem cells (ADSCs) (Ma et al. 2014; Takebe et al. 2013), since MSCs can express and release several important angiogenic growth factors and cytokines, including the vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) (Matsuda et al. 2013). In view of the promotion of angiogenesis of MSCs, it is significant to create a co-culture system consisting of hepatocytes, MSCs and ECs to mimic the visualized microenvironment of liver.
Herein, in the current study, we developed two co-culture systems that are consisting of hepatocytes, ECs and HSCs with a ratio of 700:150:3, and hepatocytes, ECs and ADSCs with a ratio of 14:3:3 to investigate the enhancing of liver-specific functions of hepatocytes. Our results showed that these two in vitro co-culture methods could well maintain the long-term viability and excellent functions of hepatocytes, including albumin production and cytochrome P450 activity. Therefore, this study have provided two potential interesting in vitro approaches for enhancing liver-specific functions of hepatocytes, and might be applied in the bioartificial liver support system or liver tissue regeneration in the future.
Materials and methods
Regents and cells
Human liver cell line (LO2) was purchased from Institute of Zoology, Chinese Academy of Sciences (Kunming, China). Human ADSCs and HEK-293T cell lines were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Human umbilical vein endothelial cell line (HUVECs) was purchased from National Institutes for Food and Drug Control (Beijing, China), and human hepatic stellate cell line (LX2) was purchased from Bogu Biotech Co., Ltd (Shanghai, China). Lentivirus vectors (LV-GFP and LV-DsRed) were constructed by Jikai Gene Chemistry Technology Co., Ltd (Shanghai, China). A-MEM, DMEM, RPMI 1640, fetal bovine serum (FBS) and 0.25% trypsin-0.02% EDTA were from Gibco (Carlsbad, CA, USA).
Cell culture
HEK-293T cells were cultured in medium containing DMEM and 10% FBS. LX2 and HUVECs cells were cultured in the complete medium containing RPMI 1640 and 10% FBS. LO2 cells were cultured in complete medium containing RPMI 1640, 10% FBS, 50 μM dexamethasone (Sigma-Aldrich, St. Louis, MO, USA), 10 ng/mL HGF (BD Bioscience, San Jose, CA, USA) and 20 ng/mL EGF (BD Bioscience). Human ADSCs were cultured in α-MEM containing 10% FBS, and ADSCs from the passage 3 or 4 were used in this study. All cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Recombinant lentivirus production
HEK-293T cells were cultured at a density of 1 × 106 cells/mL in a 10 cm plate for 24 h, and lipofectamine 3000 (Life technologies, Carlsbad, CA, USA) was used to transfect the cells following the manufacture’s instruction, and 10 μg of plasmids was used for one 10 cm plate. After that, transfection medium was replaced with fresh culture medium after 16 h of transfection, and the viruses was subsequently harvested after 48 and 72 h of transfection and centrifugated at 3000g for 30 min, followed by filtering through a 0.45 μm filter before used for transduction.
Establishment of EGFP-LO2 and DsRed-HUVECs cell lines
For establishment of EGFP-LO2, LO2 cells were transduced with the supernatant of LV-GFP; for establishment of DsRed-HUVECs, HUVECs cells were transduced with the supernatant of LV-DsRed. All target cells were transduced in presence of 5 μg/mL polybrene (Santa Cruz Biotechnology, Dallas, TX, USA), and the transduced cells were selected for stable integration by culturing in complete medium containing 4 μg/mL puromycin (Sigma-Aldrich).
Cell co-culture
Two co-culture systems were established in the current study. For establishment of LO2-HUVECs-LX2 system, LO2, HUVECs and LX2 were directly co-cultured at a ratio of 700:150:3 (about 4 × 105 total cells/plate) in the 6-well plate. For establishment of LO2-HUVECs-ADSCs system, LO2, HUVECs and ADSCs were directly co-cultured at a ratio of 14:3:3 (about 4 × 105 total cells/plate) in the 6-well plate. All cultured media were changed every 2 days. The cell supernatants were centrifuged at 3000g for 10 min, and collected at − 80 °C for further investigation.
LDH activity assay
Lactate dehydrogenase (LDH) activity in the co-culture medium was measured using LDH assay kit (Dojindo Molecular Technologies, Tokyo, Japan) following the manufacturer’s instructions.
Albumin secretion assay
Albumin (ALB) concentration in the co-culture medium was measured using human ALB enzyme-linked immunosorbent assay (ELISA) quantification kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Urea production assay
Urea concentration in the co-culture medium was measured using quantichrome urea assay kit (BioAssay Systems, Cambridge, UK) according to the manufacturer’s instructions.
Immunofluorescent assay
The expression of liver-specific biomarkers of hepatocytes was analyzed by immunofluorescence. Briefly, two co-culture systems including the co-culture system of EGFP-LO2, DsRed-HUVECs and LX2, and the co-culture system of EGFP-LO2, DsRed-HUVECs and ADSCs, were firstly cultured in the confocal dish (NEST Biotechnology Co., Ltd, Wuxi, China) for 7 days, and the adherent cells were fixed with 4% paraformaldehyde (PFA) at room temperature for 15 min. Afterwards, the cells were incubated in PBS containing 5% bovine serum albumin (BSA; Sigma-Aldrich) for 20 min at room temperature, followed by incubating with different primary antibodies at 4 °C for overnight, including mouse anti human CYP3A4 (monoclonal, 1:100) (Santa Cruz Biotechnology, Inc.; Cat. No. SC-53850), mouse anti human ALB (monoclonal, 1:50) (Santa Cruz Biotechnology, Inc., Dallas, TX, USA; CA; Cat. No. SC-69873), and mouse anti human HNF4α (monoclonal, 1:50) (Santa Cruz Biotechnology, Inc.; Cat. No. SC-101059), respectively. Then, the cells were washed twice with PBS, and further incubated with the fluorescent conjugated secondary antibodies including donkey anti mouse IgG-Alexa Fluor 647 (polyclonal, 1:1000) (Life Technologies; Cat. No. A-31571) for 30 min at room temperature. Finally, the cells were washed twice with PBS and observed using LSCM (Zeiss, LSM780, Oberkochen, Germany). The fluorescence intensity was further analyzed by ZEN 2012 Blue Edition imaging analysis system (Zeiss, Germany).
Western blot analysis
The expression of liver-specific biomarkers of hepatocytes was further analyzed by western blot analysis. Briefly, two co-culture systems including the co-culture system of EGFP-LO2, HUVECs and LX2, and the co-culture system of EGFP-LO2, HUVECs and ADSCs, were co-cultured for 7 days, and then the cells were selected for EGFP-LO2 by culturing in complete medium containing 4 μg/mL puromycin (Sigma-Aldrich) for another 12 h. After that, the cells were lysed in ice-cold RIPA buffer (TransGen Biotech Co., Ltd., Beijing, China) with protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Protein lysate (40 μg) were separated by 12% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Afterwards, the membranes were blocked for 2 h in the TBST buffer with 5% BSA and probed with the anti-ALB, CYP3A4, HNF4α antibodies (all from Santa Cruz Biotechnology, Inc.; 1:500 dilution) and anti-β-actin antibody (TransGen Biotech Co., Ltd.; 1:5000 dilution) overnight at 4 °C. The membranes were washed with TBST buffer for three times, followed by incubation with HRP-conjugated secondary antibody (1:5000 dilution; TransGen Biotech Co., Ltd.) for 1 h at room temperature. Finally, the protein expression levels were detected by enhanced chemiluminescence and visualized by autoradiography.
Statistical analyses
All quantitative data were expressed as the mean ± standard deviation (SD). All statistical analyses were performed with Graph Pad Prism version 6.0, and statistical significance among different groups was performed using Student T Test. The P < 0.05 was considered as statistically significant.
Results
Viability of co-cultured cells
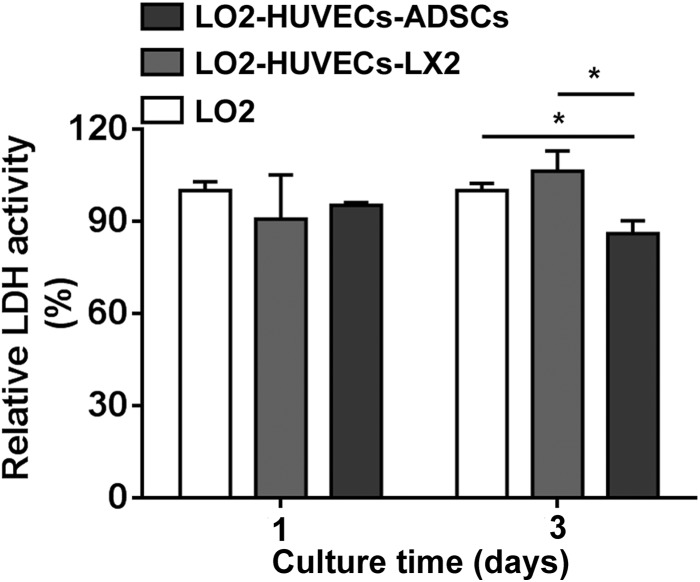
To investigate the effects of co-culture systems on cell viability of co-cultured cells, two co-culture systems including LO2-HUVECs-LX2 and LO2-HUVECs-ADSCs were performed for 3 days, and the cell viability was evaluated using LDH assay. As shown in Fig. 1, compared with the monoculture of LO2, the increased cell number of the two co-culture systems including LO2-HUVECs-LX2 and LO2-HUVECs-ADSCs did not increase the LDH activity of cell supernatants after culturing for 3 days, suggesting that these two co-culture systems did not interfere in the cell viability of co-cultured cells. Especially, compared with the monoculture of LO2 or the co-culture system of LO2-HUVECs-LX2, the LDH activity was significantly decreased in the co-culture system of LO2-HUVECs-ADSCs after culturing for 3 days, which indicated that co-culture system of LO2-HUVECs-ADSCs promotes cell viability of co-cultured cells.
Fig. 1.
LDH activity of co-culture systems. After culturing for 3 days, the LDH activity was significantly decreased in the co-culture system of LO2-HUVECs-ADSCs compared with those in the monoculture of LO2 or co-culture system of LO2-HUVECs-LX2. For all groups, n = 3. *P < 0.05
Urea secretion of hepatocytes
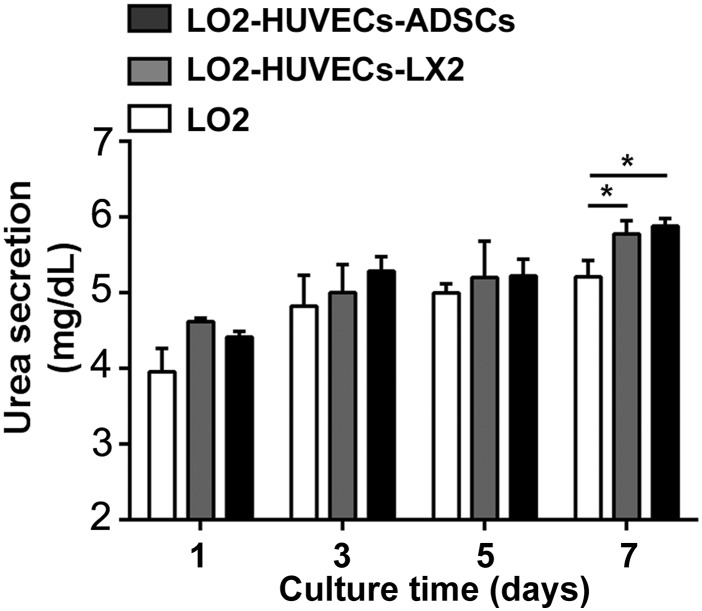
In order to evaluate the effects of co-culture systems on urea secretion of hepatocytes, we analyzed the urea concentration of cell supernatants of the co-cultures after culturing for 7 days. Although a slight increase of urea concentration was observed in the two co-culture systems as compared with the monoculture of LO2, there was no significant difference in all groups after culturing for 1, 3 and 5 days; however, compared with the monoculture of LO2, an increased urea concentration was clearly observed in the two co-culture systems including LO2-HUVECs-LX2 and LO2-HUVECs-ADSCs after culturing for 7 days (Fig. 2), suggesting that these two co-culture systems could enhance urea secretion of hepatocytes.
Fig. 2.
Urea secretion of co-culture systems. After culturing for 7 days, the urea secretion was significantly increased in the two co-culture systems including LO2-HUVECs-LX2 and LO2-HUVECs-ADSCs compared with that in the monoculture of LO2. For all groups, n = 3. *P < 0.05
Co-culture systems promote ALB expression of hepatocytes
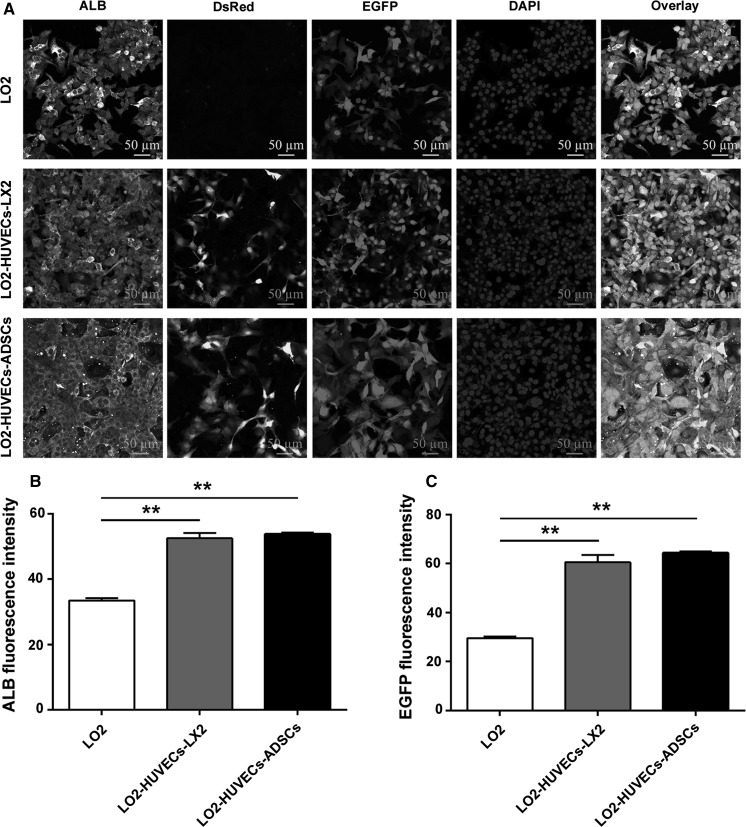
We further evaluated ALB expression of hepatocytes after co-culturing for 7 days. Compared with the monoculture of hepatocytes, an increased ALB expression was observed in the two co-culture systems, including LO2-HUVECs-LX2 and LO2-HUVECs-ADSCs (Fig. 3a, b), which suggesting that these two co-culture systems could promote ALB expression of hepatocytes; additionally, the number and the fluorescence intensity of hepatocytes (EGFP) was also increased in the two co-culture systems compared with the monoculture of hepatocytes (Fig. 3a, c), which indicates that these two co-culture systems could also facilitate hepatocyte proliferation.
Fig. 3.
Co-culture systems promote ALB expression of hepatocytes. a Representative confocal immunofluorescence images of ALB expression in hepatocytes after co-culturing for 7 days (magnification, ×40; scale bar, 50 μm). HUVECs are stained with DsRed; LO2 cells are stained with EGFP. Co-culture systems significantly enhanced ALB expression in hepatocytes compared with those in the monoculture. b Fluorescence intensity of ALB expression. c Fluorescence intensity of EGFP (LO2). For all groups, n = 3. *P < 0.05; **P < 0.01
Co-culture systems promote CYP3A4 expression of hepatocytes
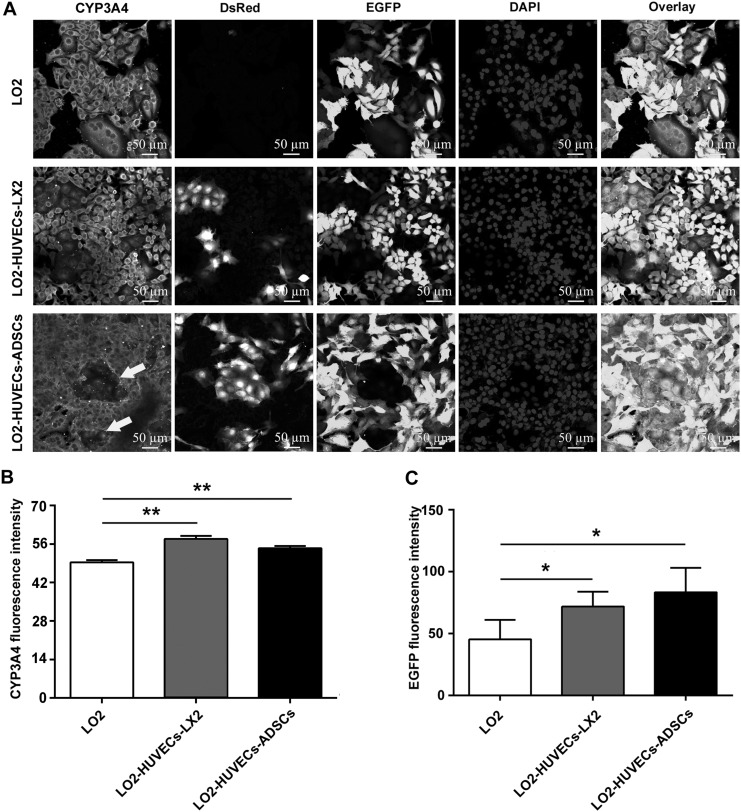
Cytochrome P450 enzymes (CYPs) are a major elimination pathway through which many drugs are metabolized in the liver (Zuo et al. 2017). In particular, CYP3A4 is involved in the oxidation of approximately 50–60% of drugs metabolized by CYPs (Ueyama et al. 2017). To evaluate the effects of co-culture systems on the xenobiotic metabolism of hepatocytes, we analyzed CYP3A4 expression of hepatocytes after co-culturing for 7 days. Compared with the monoculture of hepatocytes, an increased CYP3A4 expression was observed in the two co-culture systems (Fig. 4a, b), which suggestes that these two co-culture systems could promote CYP3A4 expression of hepatocytes; meanwhile, the number and the fluorescence intensity of hepatocytes (EGFP) was also increased in the two co-culture systems compared with the monoculture of hepatocytes (Fig. 4a, c), which is in accordance with the results shown in Fig. 3c, indicating the promotion of hepatocyte proliferation.
Fig. 4.
Co-culture systems promote CYP3A4 expression of hepatocytes. a Representative confocal immunofluorescence images of CYP3A4 expression in hepatocytes after co-culturing for 7 days (magnification, ×40; scale bar, 50 μm). HUVECs are stained with DsRed; LO2 cells are stained with EGFP. Co-culture systems significantly enhanced CYP3A4 expression in hepatocytes compared with that in the monoculture, and vessel-like structure formation was clearly present in the co-culture system of LO2-HUVECs-ADSCs (indicated by arrows). b Fluorescence intensity of ALB expression. c Fluorescence intensity of EGFP (LO2). For all groups, n = 3. *P < 0.05; **P < 0.01
It has been proven that the ADSCs play an active role in the formation, stabilization and maturation of vessel-like structure after co-culturing with the endothelial cells (Ma et al. 2014). Consistent with the previous report, we also found the vessel-like structure in the co-culture system of LO2-HUVECs-ADSCs (Fig. 4a), which oints to the potential angiogenesis in this co-culture system. Considering that the difficulty in vascularization of engineered tissue-constructs is the main obstacle for regenerative medicine, this co-culture system may provide a new interesting strategy for establishing engineered liver tissue constructs.
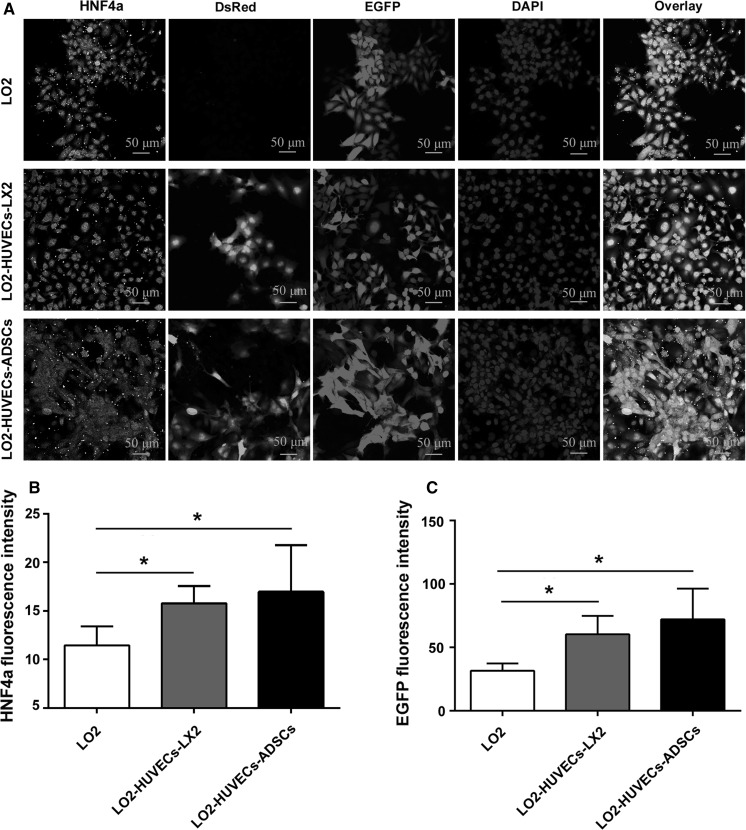
Co-culture systems promote HNF4α expression of hepatocytes
HNF4α, a key transcription factor in the liver, plays a central role in regulating lipid, glucose, bile acid and drug metabolism (Xu et al. 2016). We further evaluated HNF4α expression of hepatocytes after co-culturing for 7 days. As shown in Fig. 5, co-culture systems, including LO2-HUVECs-LX2 and LO2-HUVECs-ADSCs, significantly increased the HNF4α expression of hepatocytes and promoted hepatocyte proliferation compared with those in the monoculture of hepatocytes, which indicates that these two co-culture systems could enhance the liver-specific functions of hepatocytes.
Fig. 5.
Co-culture systems promote HNF4α expression of hepatocytes. a Representative confocal immunofluorescence images of HNF4α expression in hepatocytes after co-culturing for 7 days (magnification, ×40; scale bar, 50 μm). HUVECs are stained with DsRed; LO2 cells are stained with EGFP. Co-culture systems significantly enhanced HNF4α expression in hepatocytes compared with those in the monoculture. b Fluorescence intensity of ALB expression. c Fluorescence intensity of EGFP (LO2). For all groups, n = 3. *P < 0.05; **P < 0.01
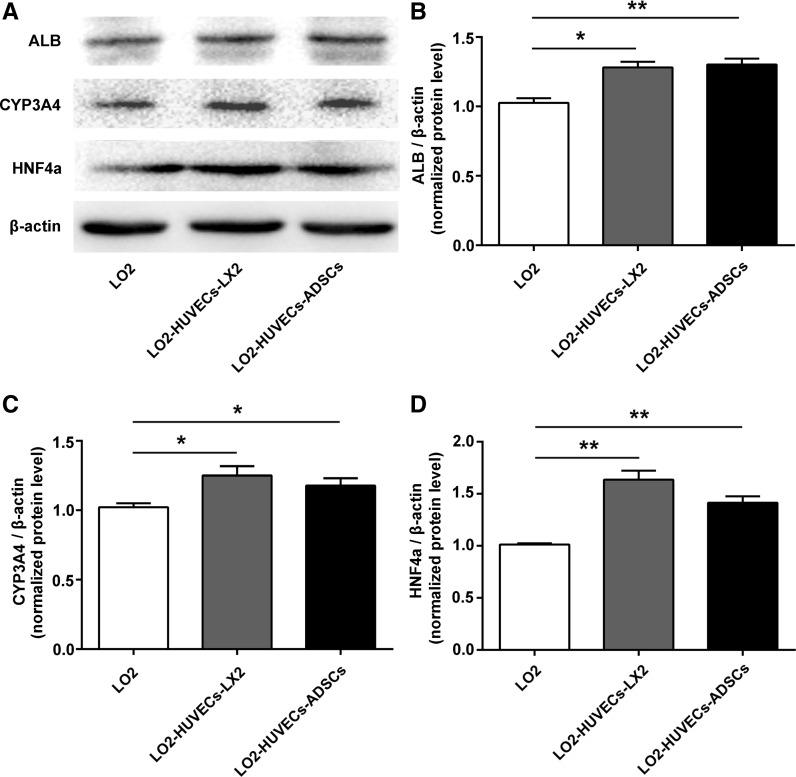
Co-culture systems promote protein expression of ALB, CYP3A4 and HNF4α in hepatocytes
To further determine the enhanced liver specific functions of co-culture systems in hepatocytes. We further evaluated the protein level of ALB, CYP3A4 and HNF4α in hepatocytes. As shown in Fig. 6, the protein level of ALB, CYP3A4 and HNF4α was significantly up-regulated in the co-culture systems compared with those in the monoculture of hepatocytes, suggesting that enhanced hepatic functions of hepatocytes could be achieved by these two co-culture systems.
Fig. 6.
Co-culture systems promote protein expression of ALB, CYP3A4 and HNF4α in hepatocytes. a Western blot analysis for ALB, CYP3A4, HNF4α and β-actin in hepatocytes after 7 days. Relative expression of ALB (b), CYP3A4 (c), and HNF4α (d) in hepatocytes. For all groups, n = 3. *P < 0.05; **P < 0.01
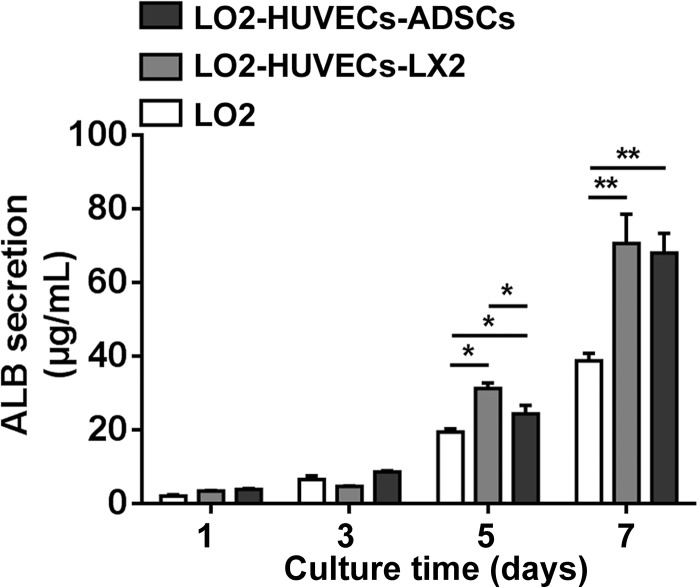
ALB secretion of hepatocytes
We next investigated the ALB secretion of hepatocytes in the cell supernatants of the co-cultures. After culturing for 1 and 3 days, the ALB concentration was not significantly different between the monoculture and co-culture systems; however, after culturing for 5 and 7 days, the ALB concentration was significantly increased in the two co-culture systems including LO2-HUVECs-LX2 and LO2-HUVECs-ADSCs compared with monoculture of LO2 (Fig. 7), which suggested that these two co-culture systems promoted ALB secretion of hepatocytes.
Fig. 7.
ALB secretion of co-culture systems. After culturing for 5 and 7 days, the ALB secretion was significantly increased in the two co-culture systems including LO2-HUVECs-LX2 and LO2-HUVECs-ADSCs compared with the monoculture of LO2; compared with the co-culture system of LO2-HUVECs-ADSCs, the ALB secretion was significantly increased in the co-culture system of LO2-HUVECs-LX2 after culturing for 5 days. For all groups, n = 3. *P < 0.05
Discussion
Since hepatocyte functions are known to lack in the conventional in vitro conditions, it is necessary to establish and make available and functional cultured systems for maintaining liver-specific functions of hepatocytes. It widely accepted that improved microenvironment of cell growth by co-culturing with non-parenchymal cells can lead to stabilization of the hepatocyte phenotype and functions (Ahmed et al. 2017; Matis et al. 2017; Xiao et al. 2015). Significantly, in view of the fact that the endothelial cells account for about 50% of the total cell population in the non-parenchymal cells, and that endothelical cells might be used to enhance hepatic functions of hepatocytes, we established two co-culture systems of hepatocytes and endothelial cells in the current study. We demonstrated that these two co-culture systems could be successfully used to improve secretion of ALB and urea, to increase the expressions of ALB, CYP3A4 and HNF4α, and to promote cell proliferation of hepatocytes, which provide two in vitro strategies for maintaining liver-specific functions of hepatocytes.
There are closed relationships between the hepatocytes and hepatic stellate cells. On one hand, the hepatic stellate cells lie in the perisinusoidal space of the liver to produce hepatocyte growth factor (HGF), transforming growth factor-α (TGF-α) and epidermal growth factor (EGF), promoting hepatocyte proliferation during liver regeneration; on the other hand, hepatocytes influence stellate cells by expressing cytokines, such as insulin-like growth factor 1 (IGF-1), to activate the cell proliferation of hepatic stellate cells (Ahmed et al. 2017). Additionally, hepatic stellate cells are the main producers of the extracellular matrix in the liver. Hence, it is necessary to establish a co-culture system containing hepatic stellate cells to maintain liver-specific functions of hepatocytes, considering that hepatic stellate cells play an important role in maintenance of hepatocyte phenotype and functions. Although the co-culture systems consisting of hepatocytes, endothelial cells and hepatic stellate cells have been used to create in vitro liver models (Ahmed et al. 2017; Bale et al. 2016), these co-culture systems could not mimic the real cell proportion in vivo. To mimic the cell composition of native liver tissue (Racanelli and Rehermann 2006), in this work, we established a co-culture system consisting of hepatocytes, endothelial cells and hepatic stellate cells, by reproducing a more in vivo-like cell proportion of liver with a LO2:HUVECs:LX2 ratio at 700:150:3 cells. We found that this co-culture method could effectively promote the maintenance of typical hepatic functions, including albumin and urea production, as well as the expression of ALB, CYP3A4 and HNF4α. Therefore, this physiologically-relevant co-cultured method could be used to enhance liver-specific functions of hepatocytes.
It is widely acknowledged that vascularization is one of the major difficulty lying in establishing an engineered liver tissue construct. To overcome this difficulty, the co-culture of endothelial cells with MSCs including ADSCs and BMSCs have been recently used to establish a vessel-like structure for tissue engineering (Ma et al. 2014; Takebe et al. 2013). Since ADSCs have many advantages, such as being abundant, easy acquisition and more suitable for autologous transplantation than BMSCs (Pan et al. 2015), we further established a co-culture system of LO2-HUVECs-ADSCs. As predicted, a vessel-like structure was clearly observed in this co-culture system (Fig. 4); the enhanced liver-specific functions including synthesis of ALB and urea, as well as the expressions of ALB, CYP3A4 and HNF4α were also achieved by this co-culture system. Hence, our data suggested that this co-culture system not only has the ability to enhance liver-specific functions of hepatocytes, but also provides a potential for integrating pre-vascularization into liver tissue engineering.
Although these two co-culture systems could enhance liver-specific functions of hepatocytes, the two in vitro approaches presented their own characteristics and advantages. On one hand, the enhanced ALB secretion of the co-culture system of LO2-HUVECs-LX2 (after culturing for 5 days) was achieved when compared with the co-culture system of LO2-HUVECs-ADSCs (Fig. 7). On the other hand, the co-culture system of LO2-HUVECs-ADSCs could promote cell viability of co-cultured cells compared with those culture in the system of LO2-HUVECs-LX2, and this co-culture system could further promote angiogenesis of endothelial cells.
Conclusion
In summary, on the base of cell proportion of liver, direct co-culture with endothelial cells and hepatic stellate cells/adipose tissue-derived stem cells could improve liver-specific functions of hepatocytes, and direct co-culture with endothelial cells and adipose tissue-derived stem cells could further promote angiogenesis of endothelial cells. Therefore, this study provides potentially interesting in vitro strategies for enhancing liver-specific functions of hepatocytes.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81472830); the Natural Science Foundation of Fujian (Grant No. 2017J01266); the Science and Technology Infrastructure Construction Program of Fujian Province (Grant No. 2014Y2005); the Youth Scientific Research Project of Fujian Provincial Health and Family Planning Commission (Grant No. 2017-1-85); the Startup Fund for scientific research, Fujian Medical University (Grant No. 2016QH081); the Project of Fuzhou Science and Technology Department (Grant Nos. 2016-s-124-9 and 2016-s-124-4); the Scientific Foundation of Fuzhou Health Department (Grant No. 2015-S-wq13); and the Project of Quanzhou Science and Technology Department (Grant No. Z [2014] 0098).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Naishun Liao, Email: liaons046@163.com.
Wenmin Zhang, Email: wminz@163.com.
References
- Ahmed HMM, Salerno S, Morelli S, Giorno L, De Bartolo L. 3D liver membrane system by co-culturing human hepatocytes, sinusoidal endothelial and stellate cells. Biofabrication. 2017;9:025022. doi: 10.1088/1758-5090/aa70c7. [DOI] [PubMed] [Google Scholar]
- Bale SS, Geerts S, Jindal R, Yarmush ML. Isolation and co-culture of rat parenchymal and non-parenchymal liver cells to evaluate cellular interactions and response. Sci Rep. 2016;6:25329. doi: 10.1038/srep25329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudechiche L, Tranchart H, Branchereau S, Davit-Spraul A, Lainas P, Groyer-Picard MT, Weber A, Hadchouel M, Dagher I. Improvement of hepatocyte transplantation efficiency in the mdr2-/- mouse model by glyceryl trinitrate. Transplantation. 2015;99:36–40. doi: 10.1097/TP.0000000000000463. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang S, Liu T, Liu Y, Wang Y. Maintenance of rat hepatocytes under inflammation by coculture with human orbital fat-derived stem cells. Cell Mol Biol Lett. 2012;17:182–195. doi: 10.2478/s11658-012-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QH, Liu AR, Qiu HB, Yang Y. Interaction between mesenchymal stem cells and endothelial cells restores endothelial permeability via paracrine hepatocyte growth factor in vitro. Stem Cell Res Ther. 2015;6:44. doi: 10.1186/s13287-015-0025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia SM, Lin PC, Yu H. TGF-beta1 regulation in hepatocyte-NIH3T3 co-culture is important for the enhanced hepatocyte function in 3D microenvironment. Biotechnol Bioeng. 2005;89:565–573. doi: 10.1002/bit.20372. [DOI] [PubMed] [Google Scholar]
- Hughes RD, Mitry RR, Dhawan A. Hepatocyte transplantation in the treatment of liver diseases—future seems bright after all. Pediatr Transplant. 2008;12:4–5. doi: 10.1111/j.1399-3046.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- Lee CA, Dhawan A, Smith RA, Mitry RR, Fitzpatrick E. Instant blood-mediated inflammatory reaction in hepatocyte transplantation: current status and future perspectives. Cell Transplant. 2016;25:1227–1236. doi: 10.3727/096368916X691286. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li H, Yan S, Wei J, Li X. Hepatocyte cocultures with endothelial cells and fibroblasts on micropatterned fibrous mats to promote liver-specific functions and capillary formation capabilities. Biomacromolecules. 2014;15:1044–1054. doi: 10.1021/bm401926k. [DOI] [PubMed] [Google Scholar]
- Ma J, Yang F, Both SK, Prins HJ, Helder MN, Pan J, Cui FZ, Jansen JA, van den Beucken JJ. In vitro and in vivo angiogenic capacity of BM-MSCs/HUVECs and AT-MSCs/HUVECs cocultures. Biofabrication. 2014;6:015005. doi: 10.1088/1758-5082/6/1/015005. [DOI] [PubMed] [Google Scholar]
- Matis G, Kulcsar A, Petrilla J, Talapka P, Neogrady Z. Porcine hepatocyte–Kupffer cell co-culture as an in vitro model for testing the efficacy of anti-inflammatory substances. J Anim Physiol Anim Nutr (Berl) 2017;101:201–207. doi: 10.1111/jpn.12547. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Falkenberg KJ, Woods AA, Choi YS, Morrison WA, Dilley RJ. Adipose-derived stem cells promote angiogenesis and tissue formation for in vivo tissue engineering. Tissue Eng Part A. 2013;19:1327–1335. doi: 10.1089/ten.tea.2012.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Liao N, Zheng Y, Wang Y, Gao Y, Wang S, Jiang Y, Liu X. Intrahepatic transplantation of adipose-derived stem cells attenuates the progression of non-alcoholic fatty liver disease in rats. Mol Med Rep. 2015;12:3725–3733. doi: 10.3892/mmr.2015.3847. [DOI] [PubMed] [Google Scholar]
- Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- Rohn S, Schroeder J, Riedel H, Polenz D, Stanko K, Reutzel-Selke A, Tang P, Brusendorf L, Raschzok N, Neuhaus P, Pratschke J, Sawitzki B, Sauer IM, Mogl MT. Allogeneic liver transplantation and subsequent syngeneic hepatocyte transplantation in a rat model: proof of concept for in vivo tissue engineering. Cells Tissues Organs. 2016 doi: 10.1159/000445792. [DOI] [PubMed] [Google Scholar]
- Sakai N, Van Sweringen HL, Belizaire RM, Quillin RC, Schuster R, Blanchard J, Burns JM, Tevar AD, Edwards MJ, Lentsch AB. Interleukin-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J Gastroenterol Hepatol. 2012;27:1609–1616. doi: 10.1111/j.1440-1746.2012.07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XL, Gao Y, Yan Y, Ma H, Sun L, Huang P, Ni X, Zhang L, Zhao X, Ren H, Hu D, Zhou Y, Tian F, Ji Y, Cheng X, Pan G, Ding YT, Hui L. Improved survival of porcine acute liver failure by a bioartificial liver device implanted with induced human functional hepatocytes. Cell Res. 2016;26:206–216. doi: 10.1038/cr.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struecker B, Raschzok N, Sauer IM. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol. 2014;11:166–176. doi: 10.1038/nrgastro.2013.204. [DOI] [PubMed] [Google Scholar]
- Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Tsuji S, Sugiyama T, Tada M. Fluorometric evaluation of CYP3A4 expression using improved transgenic HepaRG cells carrying a dual-colour reporter for CYP3A4 and CYP3A7. Sci Rep. 2017;7:2874. doi: 10.1038/s41598-017-03146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van As AB, Lotz Z, Tyler M, Adams S, Ryffel B, Kahn D. Histological assessment after different methods of reperfusion following liver transplantation. S Afr J Surg. 2002;40:95–98. [PubMed] [Google Scholar]
- Watanabe M, Zemack H, Johansson H, Hagbard L, Jorns C, Li M, Ellis E. Maintenance of hepatic functions in primary human hepatocytes cultured on xeno-free and chemical defined human recombinant laminins. PLoS ONE. 2016;11:e0161383. doi: 10.1371/journal.pone.0161383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Perry G, Komori K, Sakai Y. New physiologically-relevant liver tissue model based on hierarchically cocultured primary rat hepatocytes with liver endothelial cells. Integr Biol (Camb) 2015;7:1412–1422. doi: 10.1039/C5IB00170F. [DOI] [PubMed] [Google Scholar]
- Xu J, Xu Y, Li Y, Jadhav K, You M, Yin L, Zhang Y. Carboxylesterase 1 is regulated by hepatocyte nuclear factor 4alpha and protects against alcohol- and MCD diet-induced liver injury. Sci Rep. 2016;6:24277. doi: 10.1038/srep24277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo R, Li F, Parikh S, Cao L, Cooper KL, Hong Y, Liu J, Faris RA, Li D, Wang H. Evaluation of a novel renewable hepatic cell model for prediction of clinical CYP3A4 induction using a correlation-based relative induction score approach. Drug Metab Dispos. 2017;45:198–207. doi: 10.1124/dmd.116.072124. [DOI] [PMC free article] [PubMed] [Google Scholar]