Abstract
Background
Sudden death in long QT syndrome type 1 (LQT1), an inherited disease caused by loss-of-function mutations in KCNQ1, is triggered by early afterdepolarizations (EADs) that initiate polymorphic VT (pVT). We investigated ionic mechanisms that underlie pVT in LQT1 using a transgenic rabbit model of LQT1.
Methods and Results
Optical mapping, cellular patch clamping, and computer modeling were used to elucidate the mechanisms of EADs in transgenic LQT1 rabbits. The results showed that shorter APD in the right ventricle (RV) was associated with focal activity during pVT initiation. RV cardiomyocytes demonstrated higher incidence of EADs under 50 nM isoproterenol. Voltage-clamp studies revealed that the transient outward potassium current (Ito) magnitude was 28% greater in RV associated with KChiP2 but with no differences in terms of calcium-cycling kinetics and other sarcolemmal currents. Perfusing with the Ito blocker 4-aminopyridine changed the initial focal sites of pVT from the RV to the LV, corroborating the role of Ito in pVT initiation. Computer modeling showed that EADs occur preferentially in the RV due to the larger conductance of the slow inactivating component of Ito, which repolarizes the membrane potential sufficiently rapidly to allow reactivation of ICa,L before IKr has had sufficient time to activate.
Conclusions
Ito heterogeneity creates both triggers and an arrhythmogenic substrate in LQT1. In the absence of IKs, Ito interactions with ICa,L and IKr promote EADs in the RV while prolonging APD in the LV. This heterogeneity of action potential enhances dispersion of refractoriness and facilitates conduction blocks that initiate pVTs.
Keywords: long QT syndrome, action potential, ventricular tachycardia, early afterdepolarization, transient outward potassium current, APD dispersion
Journal Subject Terms: Arrhythmias, Electrophysiology, Mechanisms, Basic Science Research
Introduction
Long-QT syndrome type 1 (LQT1) is an inherited disease associated with prolongation of QT intervals in ECG recordings and an increased risk for developing polymorphic ventricular tachycardia (pVT) that underlies syncope and SCD.1 The disease is caused by loss-of-function mutations in the KCNQ1 gene, which encodes the pore-forming α subunit of the slowly activating delayed rectifier repolarizing K+ current (IKs). LQT1 is the most common form of long QT syndrome and accounts for almost half of genotyped cases.2 Arrhythmias and SCD in LQT1 are often precipitated by prolonged periods of exercise that sustains sympathetic activity and can in turn trigger early afterdepolarizations (EADs) that initiate pVTs.3
Experimental models using IKs blockers such as chromanol or HMR1556 to mimic LQT1 have been helpful in elucidating the roles of IKs in APD dynamics.4, 5 While chromanol perfusion produces limited APD prolongation, persistent sympathetic stimulation can both prolong APD significantly and enhance APD dispersion.5 Clinical data have also demonstrated that the Tpeak – Tend intervals of surface ECGs were accentuated during exercise tests only in LQT1 patients but not in LQT2 patients,6 supporting the notion that APD dispersion plays significant roles in pVT initiation in LQT1. Although experimental models with chromanol demonstrated enhanced APD dispersion, chromanol often failed to induce EADs.7 In addition, non-specific action of chromanol such as blocking transient outward K+ current (Ito)8 limits the translation of these findings from the experimental models to congenital LQT1-related arrhythmias.
We have generated a transgenic rabbit model for LQT1 syndrome by overexpressing a pore mutant of KCNQ1 (KCNQ1-Y315S) in the heart.9 Our previous studies of LQT1 rabbits show that LQT1 rabbits develop EADs and pVTs following sympathetic stimulation,10, 11 and all LQT1 rabbits die within three weeks after slowing their heart rate with acute ablation of the AV node in vivo. Detailed mapping revealed that triggered activity mostly originated in the right ventricle (RV) despite shorter APD in RV compared to LV.10 Due to the formation of triggered activity in short-APD regions, its propagation often encountered conduction blocks and promoted reentry formation in LQT1 rabbits.10
The present study was designed to elucidate the molecular mechanisms underlying tissue heterogeneity and EAD formation in LQT1 rabbits. Here we combine electrophysiological studies, optical mapping, and computational modeling to delineate the mechanisms underlying paradoxical EAD formation from the RV despite shorter APD than in the LV. The present study demonstrates that the lack of IKs in LQT1 unmasks the critical role of Ito heterogeneity in promoting EADs preferentially in the RV by its effect on AP plateau voltage, ICaL and IKr, and the initiation of arrhythmias.
Methods
The authors declare that all supporting data and computer simulation source code are available within the article and its supplementary files.
Heart Preparation
LQT1 rabbits of either sex, averaging 16.5 months old / 4.2 kg body weight / 9.14 g heart weight, were euthanized with buprenorphene (0.03 mg/kg IM), acepromazine (0.5 mg.kg−1 IM), xylazene (15 mg.kg−1 IM), ketamine (60 mg.kg−1 IM), pentothal (35 mg.kg−1 IV), and heparin (200 U.kg−1). This investigation conformed to the current Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (National Academies Press, revised 2011) and approved by the Lifespan Animal Welfare Committee at Rhode Island Hospital. Detailed methods for optical mapping are available in the supplemental material.
Patch Clamping
Isolation of cardiomyocytes by standard enzymatic techniques and patch-clamp recordings were performed as described previously.9 RV and LV (septal region) myocytes were isolated from hearts (n=5 hearts each from LQT1 and LMC). Whole-cell recordings (11–18 cardiomyocytes per group) were obtained with an Axopatch-200B amplifier (Axon Instruments) with standard patch-clamp techniques (see supplemental material for detail). Our voltage-clamp data on Ito indicates that the inactivation of Ito is well fit by the sum of two exponentials
| (1) |
where Ito,total is the total Ito current, Ito,fi and Ito,si are the amplitudes of the fast-inactivating (fi) and slow-inactivating (si) components, respectively. It should be emphasized that Ito,fi and Ito,si represent the fast and slow inactivating components of Ito,total and in principle contain contributions from both Ito,f, (Kv4.2/Kv4.3) and Ito,s (Kv1.4) that inactivate on similar time scales,12 but recover from inactivation on very different time scales.13 To determine the recovery kinetics from inactivation of Ito, a double-pulse protocol with variable interpulse intervals ranging from 50 ms to 15 seconds was used (see supplemental material for detail). Decay of each Ito current evoked by each 2nd pulse was fit to a double exponential decay to determine the magnitude of the fast and slow inactivating components of the current. The amplitude of each component as a function of inter-pulse interval was fit to a double exponential recovery function to determine the magnitude of the fast and slow recovering components of each decay component.
Confocal Ca2+ recording
Cytosolic and intra-SR Ca2+ changes were monitored using a Leica TCS SP5 II confocal system in line-scan mode, and Vm were simultaneously recorded with the patch-clamp technique.14 Ca2+ transients were recorded in intact cells loaded with Fluo-3-AM at 0.25 Hz field stimulation in the presence of 50 nM isoproterenol. Detailed methods for confocal Ca2+ imaging are available in the supplemental material.
Western blots of Ito and Ca2+-handling proteins
Western blotting of Kv1.4, Kv4.2, Kv4.3, and KChIP2 were performed as described in 13. Membranes from RV and LV heart (atria dissected away) were isolated by homogenization. The antibodies for Kv1.4, Kv4.2, and KChIP2 were purchased from Alomone Labs (Jerusalem, Israel). Western blot analyses of Ca2+-handling proteins were performed as described in 14. Detailed methods are available in the supplemental material.
Computer modeling of rabbit myocytes
We used the rabbit ventricular myocyte model of Mahajan et al.15 with modified mathematical formulations of Ito, IKr, and ICa,L that quantitatively reproduce voltage-clamp data obtained from LQT1 rabbits under isoproterenol. Following the double-exponential form of Equation (1), Ito was modeled as the sum of Ito,fi and Ito,si with different conductances and kinetics in RV and LV. IKr and ICa,L were taken to be the same in RV and LV consistent with experimental data. We model IKr using the multi-state Markov model of Mazhari et al.16 with parameters fitted to our patch clamp measurements that reproduce the slow-activation kinetics of IKr in the Vm range relevant for EAD formation. In addition, we use a novel formulation of ICa,L gating to quantitatively fit voltage-clamp data under isoproterenol. Details of the ionic model are provided in the supplemental material.
Statistical Analysis
Optical mapping data of intact heart are presented as means ± SD and single cell data are presented as means ± SEM. For normally distributed values, we used Student’s t-test (paired and unpaired) to compare the means of two groups and ANOVA to compare multiple means. A 2-side p value of ≤ 0.05 was considered significant. Origin software (OriginLab, Northampton, MA) was used for Shapiro-Wilk normality test, Student’s t-test, and ANOVA. Fisher’s exact test was used for categorical variables using GraphPad software (San Diego, CA).
Results
Potential role of Ito in pVT initiation in LQT1 rabbits
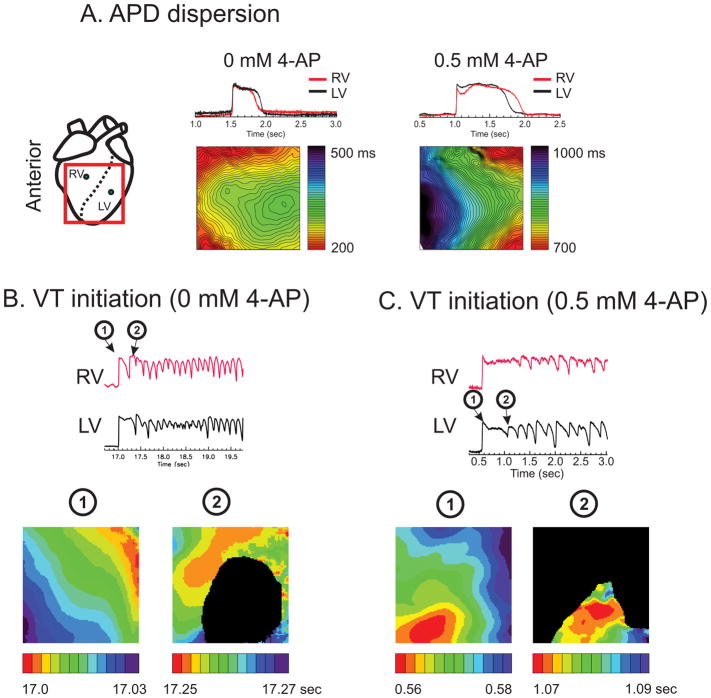
Previous optical mapping study of LQT1 rabbits revealed that APD dispersion became pronounced under slow heart rate (> 1 sec) mostly due to shorter APD in RV than in LV.10 Paradoxically, most pVTs were started from premature ventricular complex originating from RV despite shorter APD in RV.10 We hypothesized that Ito plays a major role in APD gradient and pVT initiation between RV and LV. To test this hypothesis, we investigated the effect of the Ito blocker 4-Aminopyridine (4-AP) on arrhythmia initiation and APD gradient. Figure 1A shows sample traces and APD maps under 0 and 0.5 mM 4-AP. Control APD in RV (red) is shorter than the LV, but 4-AP markedly prolonged APD in RV, causing inversion of the APD gradient between RV and LV (Figure 1A) while LMC hearts did not (see supplementary Figure S2). The addition of isoproterenol induced VT/VF, but the initiation of VT/VF was from the LV rather than the RV (5 out of 7 VTs originated from the LV, n=5 hearts) (Figure 1B&C). These results strongly suggest that higher Ito density in RV plays an important role in shortening APD in the RV and initiating EADs from that region.
Figure 1.
A) APD maps under 4-AP. APD is longer in LV under normal conditions in LQT1 but 4-AP prolonged APDs (135% increase from 312 ± 63 to 729 ± 143 ms at 0.5 Hz) and flipped the APD gradient, resulting in longer APD in RV (n=4/5 hearts, Fisher exact test p < 0.05). B) RV initiation of pVT in LQT1 hearts (RV initiation 83% of pVT events, n=5/5 hearts, p < 0.0510). C) LV initiation of pVT in LV after 0.5 mM 4-AP (n=4/5 hearts, Fisher exact test p < 0.05). The black area indicates failed activation due to conduction block (Movies of activation are provided as supplemental material movie S1 and S2).
RV CMs origin of EADs in LQT1 rabbits
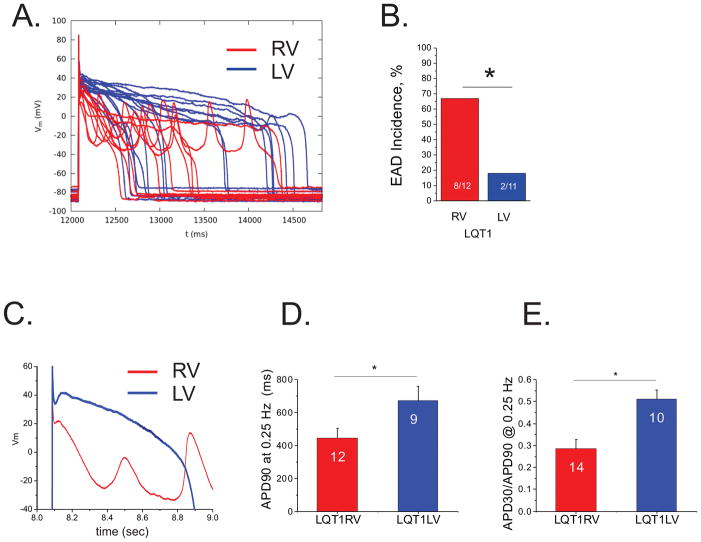
To verify the origin of EADs at the cellular scale, we isolated single myocytes from RV and LV and investigated their propensity to generate EADs under isoproterenol. Figure 2A shows Vm recordings from RV (red) and LV (blue) myocytes (n=12 per group) under 50 nmol/L isoproterenol. In line with the intact heart optical mapping study, current-clamp experiments on single cardiomyocytes (CMs) isolated from the RV of LQT1 rabbits showed frequent EADs, while myocytes from the LV did not show EADs (Figure 2A&B). CMs from LMC rabbits did not show EADs under 50 nmol/L isoproterenol (see Supplementary Figure S3). Note that despite the prolonged APD of LV CMs, Vm at the plateau did not oscillate to form EADs (blue traces in panel A). Detailed examination of Vm traces at expanded time scale (Figure 2C) suggests that the initial plateau Vm is substantially lower than that of LV CMs. Of note, this lower plateau Vm was associated with large-amplitude EADs. EADs were not induced under the same condition in LMC CMs (supplementary Figure S3).
Figure 2.
Frequent EADs despite shorter APD in RV myocytes. A) Representative Vm traces recorded from RV (red, n=12 cells) and LV (blue, n=11 cells) myocytes in 50 nM isoproterenol. B) EAD incidence. EADs were frequently in LQT1 RV myocytes (67% in RV vs. 0.18 % LV myocytes, Fisher’s exact test; p < 0.05). C) Detail traces of initial action potential repolarization phase in expanded time scale. Note that the initial rapid repolarization in RV cells is associated with EAD formation. D & E) APD90 and APD30/APD90. APD90 from LV cells showed prolonged APD despite lack of EADs. APD30/APD90 indicates that RV cells have lower Vm during plateau phase, showing an association between rapid initial repolarization phase and EAD formation, suggesting a potential role of Ito in EAD formation (ANOVA, p < 0.05).
Previous studies indicated a general association between prolongation of APD and risk for EADs. In LQT1, APD90 was substantially longer in LV CMs vs. RV CMs (Figure 2D), yet EADs were more frequent in RV CMs than LV CMs. Further analysis of APD30/APD90 (Figure 2E) show that the action potentials of RV cells have rapid repolarization during the early phase of the action potential (phase 1 period). Thus, these data show that in the case of LQT1 rabbits, EADs originate from a region (RV) with a shorter APD, both at the cellular scale and the tissue scale (reference10 and Figure 1).
Ito is larger in RV CMs
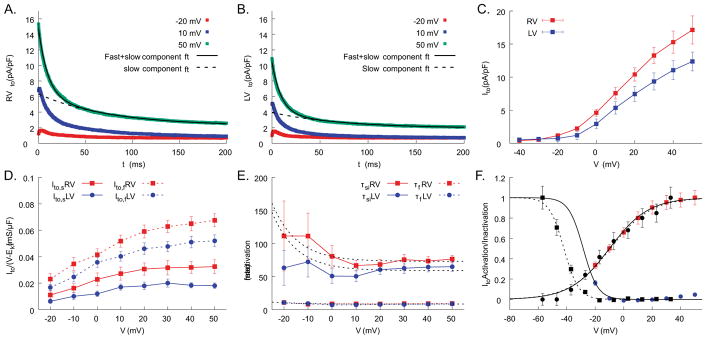
Given the shape of the action potential and the rapid initial repolarization, we hypothesized that the main difference between RV and LV LQT1 CMs is Ito. The ionic currents were measured using voltage clamp under isoproterenol (50 nmol/L). The results show a substantially higher peak Ito amplitude in RV CMs than LV CMs (Figure 3A–C).
Figure 3.
Amplitude and inactivation kinetics of Ito are markedly different between RV and LV cells. Sample voltage clamp traces for Ito from RV (A) and LV cells (B). The inactivation kinetics of Ito showed two-exponential decays, fast-inactivation component (fi) and slow-inactivation component (si), see methods for detail. C) The total amplitude of Ito is 28% larger in RV than LV (17.1 ± 2.1 pA/pF in RV vs. 12.4 ± 1.4 pA/pF in LV at 50 mV, ANOVA p<0.05, n=16 and 15 cells for RV and LV from n=5 hearts). D) Ito,fi and Ito,si from RV (n=16) and LV cells (n=15 from n=5 hearts). Both fast and slow components are greater in RV. E) The fast-inactivation time constants (τfi) are the same in RV and LV cells, whereas the slow-inactivation time constant (τsi) is longer in RV (see supplemental Table S1). F) mean activation and inactivation curves of Ito,total from LQT1 (solid lines) and LMC (dotted lines) myocytes. After normalized to the maximum value, the data were fitted to the Boltzman function and used for computer modeling.
Ito inactivation kinetics from our voltage-clamp measurements could be best fitted with two exponential decays (see Equation (1) and Figure 3A–B). We separated these two exponential decays (see Methods for details): a fast-inactivation component marked as Ito,fi with a time constant of τfi and a slow-inactivating component as Ito,si with a time constant of τsi. Detailed analysis of two components revealed that Ito,fi and Ito,si are 30% and 83% greater in RV (gto,fi = 0.068±0.005 mS/μF and gto,si = 0.033±0.005 mS/μF) than LV CMs (gto,fi = 0.052±0.005 mS/μF and gto,si = 0.018±0.002 mS/μF, see panel D), respectively. The τfi were not significantly different, but the τsi of the slow component was 25% slower in RV CMs (τsi = 73±6.7 ms in RV vs. 59±6.9 ms at high voltage, see panel E).
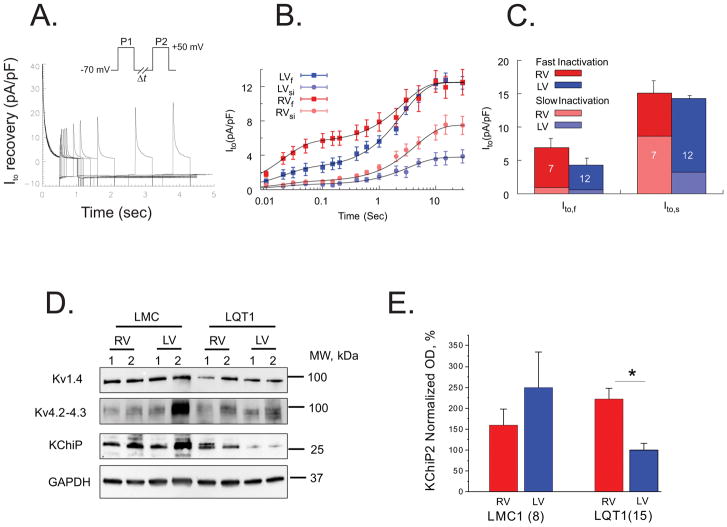
It is generally thought that Ito in rabbits recovers slowly from inactivation (Ito,s) and makes only a limited contribution to APD. We investigated Ito recovery kinetics using a double-pulse protocol (Figure 4A). We found that rabbit CMs have a substantial magnitude of Ito,f (τfast = 19.5 ms), which was larger in RV compared to LV myocytes (Ito,f = 8.9±1.7 and 5.3±1.6 pA/pF for RV and LV respectively, Figure 4B–C). Ito,s (τslow = 3.41 sec) was similar in RV and LV. In addition, the recovery of Ito,si was analyzed by separating fast- and slow-inactivation components of Ito during the double-pulse Ito recovery protocol. The slowly inactivating component of Ito was significantly larger in RV vs. LV (8.6±1.5 and 3.2±0.76 pA/pF for RV and LV respectively, p < 0.05). This results in a significant difference in the amount of available Ito,si when the interpulse interval is >600 ms (Figure 4B).
Figure 4.
Ito recovery from inactivation. A) The recovery kinetics was tested by a double-pulse protocol with interpulse time varying from 50 ms to 15 sec (n=12 RV and 7 LV cells from n=3 hearts). B) The amplitudes of the slow and fast inactivating components of Ito (Ito,si and I Ito,fi) as a function of inter-pulse interval were determined by fitting the time course of Ito decay during the second pulse to a double exponential function. The x-axis of inter-pulse intervals is in a logarithmic scale. C) The amplitudes of Ito,fi and Ito,si from RV and LV. Fast and slow-inactivating components (Ito,fi and Ito,si) of each Ito,f and Ito,s were calculated as described in Methods and represented as a stacked column plot. D) Western blots of Kv4.2, Kv1.4, and KChIP2 from LQT1 hearts. E). The accessory unit of Ito, KChIP2, known to affect inactivation and recovery kinetics, was twofold higher in RV (ANOVA, p < 0.05). The currents were measured under 50 nM isoproterenol. Additional Kv4.2 and Kv1.4 gel analyses are provided in the supplemental material.
The expression of K+ channel interacting protein (KChIP2) was significantly higher in RV (Figure 4D–E) but the western blots of Kv4.2 and Kv1.4 of RV and LV CMs (panel D and Figure S6) were not significantly different. Since KChIP2 modulates Ito,f channel expression and kinetics, higher KChIP2 expression in RV likely provides molecular mechanisms underlying differences of Ito,f magnitude and kinetics between RV and LV myocytes.
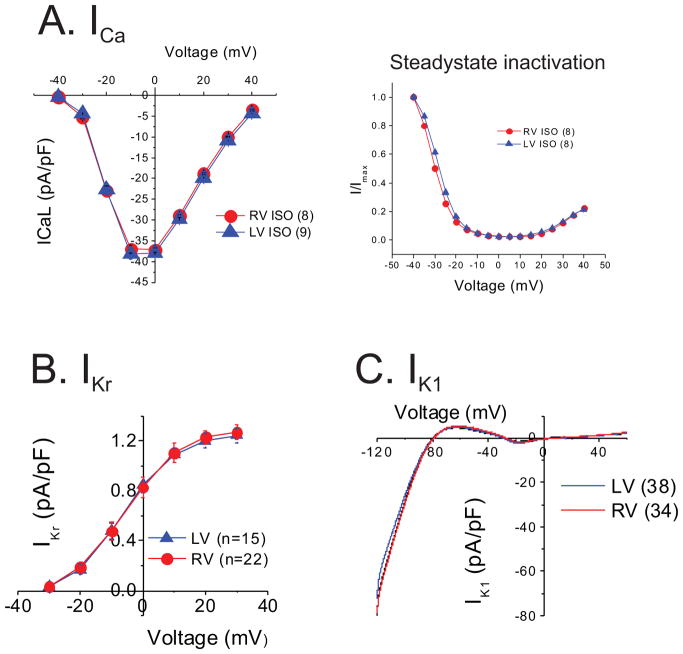
Of note, there were no significant differences in ICa,L or its inactivation properties as well as IKr and IK1 between RV and LV myocytes under 50 nmol/L isoproterenol (Figure 5A–C). These results strongly suggest that Ito is responsible for APD gradient and RV-specific EAD formation in LQT1 rabbits.
Figure 5.
ICa, (A), IKr (B), and IK1 (C) did not differ between RV and LV cells. All currents were measured under 50 nM isoproterenol.
Ca2+ handling between RV and LV CMs
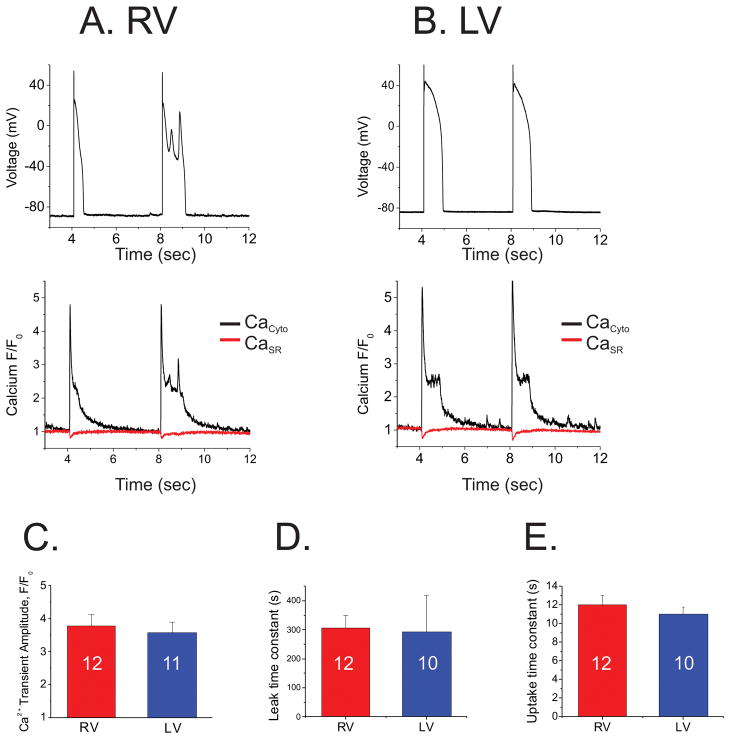
Ca2+ handling and its coupling with voltage have frequently been suspected of promoting EADs.17 Our previous studies of EAD mechanisms in LQT2 rabbits also demonstrated that abnormal Ca2+ dynamics and leaky ryanodine receptors increased the forward mode of the electrogenic Na+/Ca2+ exchanger (INCX), slowing down repolarization and allowing reactivation of the ICa,L window current to produce EADs.14 We therefore investigated whether differences in Ca2+ handling between RV and LV myocytes in LQT1 rabbits underlie preferential EAD formation in RV using confocal Ca2+ imaging. Figure 6 shows an example of simultaneous recordings of Vm, cytosolic and SR Ca2+ during action potentials. Panels A&B show typical examples of Vm and Ca2+ from RV and LV CMs. The amplitude and the fractional release of Ca2+ from SR did not differ between RV and LV myocytes. We found no significant difference in Ca2+ handling between RV and LV (panel C–E), suggesting that Ca2+ handling does not underlie preferential EAD formation in RV myocytes. Western blots of Ca2+-handling proteins from RV and LV were not significantly different (see supplementary Figure S7).
Figure 6.
Ca2+ handling is not responsible for preferential EAD formation from RV cells. A & B) Simultaneous recordings of Vm, Ca2+ transients (CaCyto), and SR Ca2+ contents (CaSR) from single RV and LV cells. Note that higher Ca2+ level during action potential plateau in LV did not trigger EADs. C) Amplitudes of Ca2+ transients. D) Ca2+ leak from RyR. E) Ca2+ uptake. Ca2+ handling was not markedly different between RV and LV cells.
Ito facilitates EAD formation: Computer-modeling studies
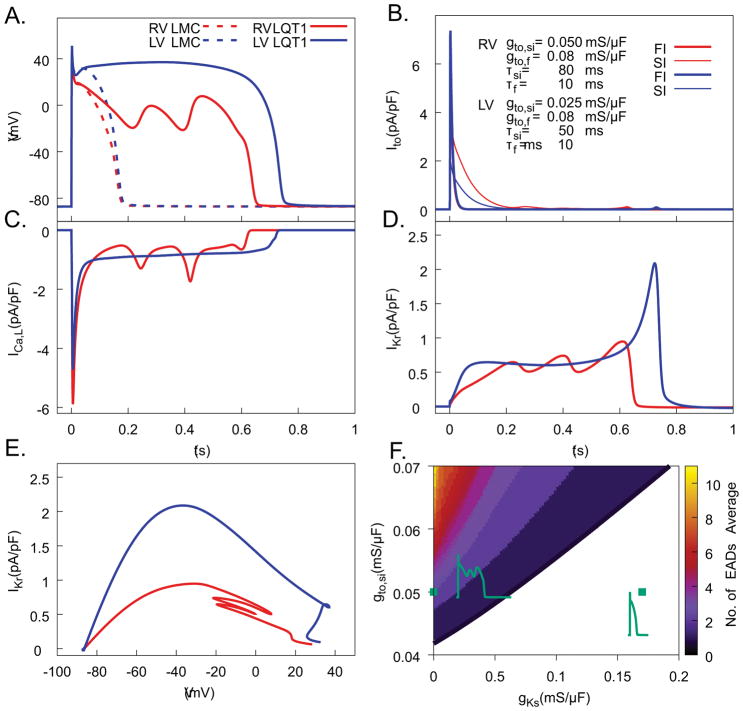
We examined whether computer modeling could reproduce the AP waveforms in RV and LV under isoproterenol, only through varying Ito. Figure 7A shows that when Ito conductance and inactivation kinetics are comparable to those measured in LV myocytes (gto,si = 0.025 mS/μF, gto,fi = 0.08 mS/μF, τ si = 50 ms), the AP produced a long plateau at high Vm (solid blue curve) with long APDs (> 700ms). In contrast, when Ito conductance and inactivation kinetics are comparable to those measured in RV myocytes (gto,si = 0.050 mS/μF, gto,fi = 0.08 mS/μF, τsi = 80 ms), we see an AP waveform similar to that found in experiments, with long APD due to many EADs (Figure 7A solid red curve). When parameters are altered to include the IKs current to model LMC1 RV and LV, AP traces show much shorter APD without EAD formation in both cell types (blue and red dashed lines in LV and RV, respectively). To further elucidate the role of Ito in EAD formation, we distinguish in Figure 7B the contributions of the fast- and slow-inactivating components of Ito, Ito,fi (FI) and Ito,si (SI), respectively. Two main differences of Ito properties between the LV and RV can be distinguished. Firstly, the larger peak Ito in the RV produces a faster initial repolarization during phase 1 of the AP (i.e. a larger notch). Secondly, the larger amplitude of the slow-inactivating component of Ito (Ito,si) in the RV makes a dominant contribution to the subsequent repolarization during phase 2 of the AP (t<200 msec), thereby bringing the voltage into the window range for reactivation of ICa,L and promoting EADs. Figure 7C shows that re-activation of ICa,L coincides with EADs in RV, while in LV repolarization is complete before ICa,L sufficiently recovers from inactivation to initiate an EAD during late phase 3 of the AP. The faster rate of repolarization during late phase 3 in LV is due to IKr (Figure 7D), which activates significantly more in LV than RV due to the long AP plateau at high Vm. Figure 7E directly compares IKr at the same Vm and shows that IKr contributes twice as much to repolarization in LV than RV in the −20 to +10 mV range relevant to EAD onset.
Figure 7.
Computer modeling study of EADs reproduces Ito-dependent EADs in LQT1 myocytes under isoproterenol stimulation. A) Computed Vm traces for different Ito model parameters fitted to voltage-clamp measurements of Ito in RV and LV cells of LQT1 and LMC rabbits. Only RV (red) from LQT1 demonstrated EADs. B) Ito traces broken into fast-inactivating component (red in RV, dark blue in LV) and slowly inactivating component (orange in RV, light blue in LV) for action potentials in panel A. C) ICaL from RV (red) and LV (blue) during action potentials in panel A. D) IKr during action potentials in panel A. E) Dynamic I–V curves showing IKr during repolarization as a function of Vm. Note that in the range of Vm oscillations during EADs, IKr from RV (red) is markedly lower than LV (blue). F) EAD formation in the parameter space of Ito,si conductance (gto,si, Y axis) vs. IKs conductance (gKs, X axis) with and other parameters gto,fi = 0.074 mS/μF, τsi = 69 ms, and τfi = 8.5 ms. Representative AP traces within each region are shown, with parameters indicated by green diamonds. The right-most green diamond corresponds to parameters representative of LMC cells.
Figure 7F shows that, with addition of IKs into the model, EADs become fewer with increasing IKs conductance and vanish for a normal value of gKs corresponding to LMC myocytes. Figure 7F also shows the importance of varying Ito conductance in absence of IKs. When Ito conductance is small (gto,si<0.04 ms/μF), as in LQT1 LV CMs, the high Vm AP plateau prevents EAD formation, despite APD prolongation. For greater Ito conductance, as in LQT1 RV CMs, multiple EADs occur (green square on the y axis for gKs = 0). In this case, Ito conductance is large enough for Vm to traverse the critical window for reactivation of ICa,L before sufficient activation of IKr, but small enough to avoid complete repolarization, allowing EADs.
To further demonstrate the important role of slow IKr activation kinetics in EADs formation, we repeated the simulations represented in Figure 7F using a Hodgkin-Huxley (HH) formulation of IKr with parameters fitted to our voltage-clamp data (Figure S10). The results shown in Figure S10 reproduce the RV and LV AP phenotypes obtained with the multi-state Markov IKr model (compare Figures 7A–D with Figures S10A–D). Furthermore, in Figures S10E–F, we compare the steady-state activation/inactivation curves and activation-time constant for the new IKr HH formulation fitted to our voltage-clamp data and the Zeng et al HH IKr formulation18 commonly used in rabbit ventricular myocyte ionic models. This comparison underscores the importance of accurate modeling of IKr activation kinetics to elucidate arrhythmogenic mechanisms in the setting of LQT1 where IKs is absent.
Discussion
LQT1 is the most common form of long QT syndrome and accounts for almost half of genotyped cases.2 Pore missense mutations that confer loss of function with dominant negative effect are associated with a more severe form of the disease with a higher frequency of cardiac events.19 This study highlights the crucial role of Ito in repolarization when IKs is absent and elucidates the molecular underpinning for the malignant phenotype of LQT1. At the organ level, we found that the heterogeneity of Ito underlies pVT initiation by creating APD dispersion and EAD formation that propagates unidirectionally to the RV region to form a reentry. Consistent with those observations, we found that cellular EADs are present in myocytes isolated from RV, but absent from LV myocytes despite much prolonged APD in LV. Computer modeling, combined with detailed voltage-clamp and confocal Ca2+ imaging, revealed that heterogeneity in Ito, both in terms of amplitude and inactivation kinetics, causes membrane voltage to traverse the window for reactivation of ICa,L sooner, before IKr has had sufficient time to activate and prevent the depolarization that underlies EAD formation and pVTs.
EADs formation has been traditionally associated with an instability of Vm dynamics driven by reactivation of ICa,L during the plateau phase of the AP.20, 21 While ICa,L is a necessary depolarizing current to induce EADs, several other membrane currents have been shown experimentally or computationally to modulate EAD formation, including INCX14, 22, IKs23, and Ito.23 The present study highlights heterogeneity in Ito amplitude and inactivation kinetics as major factors in the initiation of EADs and reentry in LQT1 rabbits.
The role of Ito in the genesis of EADs has also been highlighted in previous experimental and computational studies in isolated rabbit ventricular myocytes exposed to oxidative stress23, 24 or hypokalemia.24. A main finding of those studies is that, even though Ito helps repolarization, blocking this current can (counter-intuitively) suppress EADs. This paradoxical effect is linked mechanistically to the fact that Ito can lower the AP plateau voltage into a range where activation of IKs is slowed sufficiently to allow reactivation of ICa,L and induction of EADs.23, 24 Importantly, Ito is also implicated in arrhythmogenesis in Brugada syndrome. By contrast, deletions of Ito in several models in mice were not associated with sudden cardiac death.25 Our results highlight a similar mechanism of Ito in LQT1 rabbits, but with the slow activation of IKr allowing reactivation of ICa,L when the lower AP plateau voltage is reached rapidly.
Two factors may precipitate pVT initiation in LQT1 rabbits: i) APD gradient between RV and LV and ii) EAD formation from the short-APD region. Enhanced APD dispersion has been well recognized as a substrate for reentry in LQTS.4, 26 However, in order to initiate reentry, the propagating AP must encounter a conduction block. In LQT1, because EADs form in RV where APD is shorter, the propagation of EADs eventually reaches the LV region where APD is longer, causing conduction block and initiation of reentry (Figure 1). Therefore, this study emphasizes the importance of Ito heterogeneity, which allows EAD formation in the short-APD region, and forming unidirectional conduction and reentry in LQT1 rabbits.
Ito heterogeneity between RV and LV27, 28, and also epi- to endocardium13, 29, has been well recognized. In our transgenic rabbit model of LQT1, Ito heterogeneity between RV and LV may have greater impact on arrhythmogenesis but in large animals including human, transmural heterogeneity of Ito can also have significant impact on EADs and reentry formation due to sufficient transmural dimension to form reentry. Two major transient outward (Ito) currents are likely responsible for this current in large mammals: Ito,s (Kv1.4), and Ito,f (Kv4.2 and Kv4.3), which differ in their recovery-time constants12, 30 but both of which have multi-exponential inactivation kinetics12. Even though previous work suggests that the main component in rabbits is Ito,s, our double-pulse voltage-clamp study demonstrated that Ito,f in rabbit RV constitutes ~28% of total Ito and is the major component that underlies a large Ito in RV (Figure 4). Our western blot study demonstrated that Ito heterogeneity is associated with KChIP2 expression level (Figure 4E). KChIP2 has been reported to increase trafficking and expression of Kv4.2 and Kv4.3 that underlie Ito,f.31 KChIP2 slows Ito inactivation kinetics32, which may partly explain the molecular mechanisms underlying greater Ito in RV. However, further studies are needed to clarify the origin of the greater amplitude of the slow-inactivating component of Ito (Ito,si) in RV (Figure 3 and 4) and its potential transmural heterogeneity, which is potentially linked to different phosphorylated states of Kv1.4 and Kv4.2/4.3 channels in RV and LV12, 30
Our computer modeling study showed that Ito,si plays a crucial role in determining the plateau Vm and initiating EADs (Figure 7 and Figure S12). Ito,si recovers to a larger amplitude in rabbit RV than LV at moderately slow heart rate > 600 ms (Figure 4). This may explain why LQT1 rabbits show dramatically more SCDs after bradycardia caused by AV node ablation.33
Ito heterogeneity is no greater in LQT1 than in LMC (supplemental figure S4). However, this heterogeneity has a greater impact on pVT initiation in LQT1 than in LMC (supplementary Figure S2–3), where IKs abolishes the arrhythmogenic effect of Ito at a cellular level (Figure 7F). Previous computer modeling studies of LQT1 found critical roles of IKs on APD prolongation34 and EAD formation under reduced IKr condition.35 In particular, the study by O’Hara and Rudy35 showed that, in addition to isoproterenol, partial block of IKr was needed to evoke EADs when IKs kinetics is altered by the Q357R KCNQ1 mutation while in the present study isoproterenol suffices to evoke EADs without block of IKr in the absence IKs. Since Ito is heterogeneously expressed even in normal myocardium between RV and LV as well as epi- and endocardium,12, 13, 36 our study suggests that IKs has an important protective role beyond repolarization reserve by acting as a safety mechanism that counters the arrhythmogenic effect of Ito heterogeneity revealed by the present study. Hence, the main consequence of the loss of function of IKs is to unmask the arrhythmogenic role of Ito heterogeneity, which is masked by IKs in LMC hearts.
Regarding Ca2+-handling proteins, the hyperphosphorylation of RyR was recently shown to play an important role in the genesis of EADs in myocytes isolated from LQT2 transgenic rabbits carrying a loss-of-function mutation of the HERG potassium channel encoding the IKr channels.14 Experiments and computational modeling demonstrated that EADs are promoted by aberrant late Ca2+ releases by hyperactivity of RyR Ca2+ channels. The Ca2+-handling machinery under isoproterenol in LQT1, however, is not markedly different between myocytes forming EADs (RV) vs. myocytes not forming EADs (LV), indicating that EAD formation in RV cannot be ascribed to differences in Ca2+ homeostasis in RV vs. LV (Figure 6). However, it is still possible that sustained Ca2+ release during AP plateau can influence APDs and further studies are needed for potential roles of Ca2+ handling in LQT1.
Our voltage-clamp data from both LMC and LQT1 rabbits demonstrated the existence of both fast and slow components of IKr activation, with the slow component being dominant for Vm up to 10 mV. Unless Vm is high for a long duration (such as in LQT1 LV CMs), this dominant slow component will prevent IKr from significantly activating, which is key for EAD formation (supplemental Figure S9 and S10). Since IKr activation and deactivation kinetics are similar in rabbits and humans,37 our results suggest that IKr kinetics is likely to play an important role in LQT1 EAD formation.
Limitations
This study focuses on the role of Ito in the initiation of EADs through determining the trajectory of Vm during phase 1 of early repolarization, but did not study the potential effects of sustained Ito that may also have contributed to the maintenance of multiple EADs. Ito,s is the major component of Ito in rabbits, and EADs in different species (including humans) may occur at faster heart rates. In optical mapping, 4-AP may have non-specific action on IKr and further prolong APD. Chromanol is known to alter Ito at higher concentrations, and Ito measurements in LMC myocytes may have been underestimated.
Conclusion
Using a transgenic rabbit model of LQT1 and computer modeling, we demonstrated that Ito plays a key role in arrhythmogenesis, creating both triggers and an arrhythmogenic substrate for reentry. When IKs is absent, complex interactions between Ito, ICaL, and IKr transduce the marked heterogeneity of Ito properties into a marked heterogeneity of the AP phenotype leading to pVT initiation. This study suggests that Ito is a major contributor to LQT-related arrhythmias and that its heterogeneity may underlie regional EAD formation and initiation of pVTs.
Supplementary Material
WHAT IS KNOWN?
Long QT syndrome type 1 (LQT1), an inherited disease caused by loss-of-function mutations in KCNQ1, is associated with sudden cardiac arrest triggered by early afterdepolarizations (EADs).
EADs have been traditionally associated with an instability of Vm dynamics driven by reactivation of the L-type Ca2+ current (ICa,L) during the plateau phase of the action potential that initiates polymorphic VT (pVT).
WHAT THE STUDY ADDS?
It uses a transgenic rabbit model of LQT1 to shed light on the mechanisms of EADs formation and pVT initiation at the cellular and organ scales.
It finds that the transient outward K+ current (Ito) is both significantly larger and inactivates more slowly in the right ventricle (RV) than the left ventricle (LV), and links mechanistically this regional variation of Ito properties to the observed regional variation of EADs frequency, abundant in the RV but absent in the LV.
It shows that larger Ito in the RV causes Vm to traverse the critical window range for reactivation of ICa,L earlier during the AP plateau before sufficient activation of IKr promoting EADs formation in the short-APD region. These EADs propagate uni-directionally to form reentry and polymorphic ventricular tachycardia (VT).
Acknowledgments
Sources of Funding: This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health R01HL110791 to G.K., R01HL096669 to B.C, and R01HL121796 to D.T.
Footnotes
Disclosures: none
References
- 1.Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 2.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102:1178–1185. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Robinson JL, Gessman L, Gillespie R, Zareba W, Schwartz PJ, Vincent GM, Benhorin J, Heilbron EL, Towbin JA, Priori SG, Napolitano C, Zhang L, Medina A, Andrews ML, Timothy K. Comparison of clinical and genetic variables of cardiac events associated with loud noise versus swimming among subjects with the long QT syndrome. Am J Cardiol. 1999;84:876–879. doi: 10.1016/s0002-9149(99)00458-0. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu W, Antzelevitch C. Cellular basis for long QT, transmural dispersion of repolarization, and torsade de pointes in the long QT syndrome. J Electrocardiol. 1999;32:177–184. doi: 10.1016/s0022-0736(99)90077-8. [DOI] [PubMed] [Google Scholar]
- 5.Volders PG, Stengl M, van Opstal JM, Gerlach U, Spatjens RL, Beekman JD, Sipido KR, Vos MA. Probing the contribution of IKs to canine ventricular repolarization: key role for beta-adrenergic receptor stimulation. Circulation. 2003;107:2753–2760. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- 6.Takenaka K, Ai T, Shimizu W, Kobori A, Ninomiya T, Otani H, Kubota T, Takaki H, Kamakura S, Horie M. Exercise stress test amplifies genotype-phenotype correlation in the LQT1 and LQT2 forms of the long-QT syndrome. Circulation. 2003;107:838–844. doi: 10.1161/01.cir.0000048142.85076.a2. [DOI] [PubMed] [Google Scholar]
- 7.Burashnikov A, Antzelevitch C. Block of I(Ks) does not induce early afterdepolarization activity but promotes beta-adrenergic agonist-induced delayed afterdepolarization activity. J Cardiovasc Electrophysiol. 2000;11:458–465. doi: 10.1111/j.1540-8167.2000.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 8.Virag L, Jost N, Papp R, Koncz I, Kristof A, Kohajda Z, Harmati G, Carbonell-Pascual B, Ferrero JM, Jr, Gy Papp J, Nanasi PP, Varro A. Analysis of the contribution of Ito to repolarization in canine ventricular myocardium. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunner M, Peng X, Liu GX, Ren XQ, Ziv O, Choi BR, Mathur R, Hajjiri M, Odening KE, Steinberg E, Folco EJ, Pringa E, Centracchio J, Macharzina RR, Donahay T, Schofield L, Rana N, Kirk M, Mitchell GF, Poppas A, Zehender M, Koren G. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest. 2008;118:2246–2259. doi: 10.1172/JCI33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim TY, Kunitomo Y, Pfeiffer Z, Patel D, Hwang J, Harrison K, Patel B, Jeng P, Ziv O, Lu Y, Peng X, Qu Z, Koren G, Choi BR. Complex excitation dynamics underlie polymorphic ventricular tachycardia in a transgenic rabbit model of long QT syndrome type 1. Heart Rhythm. 2015;12:220–228. doi: 10.1016/j.hrthm.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu GX, Choi BR, Ziv O, Li W, de Lange E, Qu Z, Koren G. Differential conditions for early after-depolarizations and triggered activity in cardiomyocytes derived from transgenic LQT1 and LQT2 rabbits. J Physiol. 2012;590:1171–1180. doi: 10.1113/jphysiol.2011.218164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SP, Campbell DL. Transient outward potassium current, ‘Ito’, phenotypes in the mammalian left ventricle: underlying molecular, cellular and biophysical mechanisms. J Physiol. 2005;569:7–39. doi: 10.1113/jphysiol.2005.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmajothi MV, Campbell DL, Rasmusson RL, Morales MJ, Trimmer JS, Nerbonne JM, Strauss HC. Distinct transient outward potassium current (Ito) phenotypes and distribution of fast-inactivating potassium channel alpha subunits in ferret left ventricular myocytes. J Gen Physiol. 1999;113:581–600. doi: 10.1085/jgp.113.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terentyev D, Rees CM, Li W, Cooper LL, Jindal HK, Peng X, Lu Y, Terentyeva R, Odening KE, Daley J, Bist K, Choi BR, Karma A, Koren G. Hyperphosphorylation of RyRs underlies triggered activity in transgenic rabbit model of LQT2 syndrome. Circ Res. 2014;115:919–928. doi: 10.1161/CIRCRESAHA.115.305146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahajan A, Shiferaw Y, Sato D, Baher A, Olcese R, Xie LH, Yang MJ, Chen PS, Restrepo JG, Karma A, Garfinkel A, Qu Z, Weiss JN. A rabbit ventricular action potential model replicating cardiac dynamics at rapid heart rates. Biophys J. 2008;94:392–410. doi: 10.1529/biophysj.106.98160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazhari R, Greenstein JL, Winslow RL, Marban E, Nuss HB. Molecular interactions between two long-QT syndrome gene products, HERG and KCNE2, rationalized by in vitro and in silico analysis. Circ Res. 2001;89:33–38. doi: 10.1161/hh1301.093633. [DOI] [PubMed] [Google Scholar]
- 17.Nemec J, Kim JJ, Salama G. The link between abnormal calcium handling and electrical instability in acquired long QT syndrome - Does calcium precipitate arrhythmic storms? Prog Biophys Mol Biol. 2016;120:210–221. doi: 10.1016/j.pbiomolbio.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng J, Laurita KR, Rosenbaum DS, Rudy Y. Two components of the delayed rectifier K+ current in ventricular myocytes of the guinea pig type. Theoretical formulation and their role in repolarization. Circ Res. 1995;77:140–152. doi: 10.1161/01.res.77.1.140. [DOI] [PubMed] [Google Scholar]
- 19.Moss AJ, Shimizu W, Wilde AA, Towbin JA, Zareba W, Robinson JL, Qi M, Vincent GM, Ackerman MJ, Kaufman ES, Hofman N, Seth R, Kamakura S, Miyamoto Y, Goldenberg I, Andrews ML, McNitt S. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation. 2007;115:2481–2489. doi: 10.1161/CIRCULATIONAHA.106.665406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu Z, Xie LH, Olcese R, Karagueuzian HS, Chen PS, Garfinkel A, Weiss JN. Early afterdepolarizations in cardiac myocytes: beyond reduced repolarization reserve. Cardiovasc Res. 2013;99:6–15. doi: 10.1093/cvr/cvt104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- 22.Xie Y, Grandi E, Puglisi JL, Sato D, Bers DM. beta-adrenergic stimulation activates early afterdepolarizations transiently via kinetic mismatch of PKA targets. J Mol Cell Cardiol. 2013;58:153–161. doi: 10.1016/j.yjmcc.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Z, Xie Y, Wen H, Xiao D, Allen C, Fefelova N, Dun W, Boyden PA, Qu Z, Xie LH. Role of the transient outward potassium current in the genesis of early afterdepolarizations in cardiac cells. Cardiovasc Res. 2012;95:308–316. doi: 10.1093/cvr/cvs183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen TP, Singh N, Xie Y, Qu Z, Weiss JN. Repolarization reserve evolves dynamically during the cardiac action potential: effects of transient outward currents on early afterdepolarizations. Circ Arrhythm Electrophysiol. 2015;8:694–702. doi: 10.1161/CIRCEP.114.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunner M, Guo W, Mitchell GF, Buckett PD, Nerbonne JM, Koren G. Characterization of mice with a combined suppression of I(to) and I(K,slow) Am J Physiol Heart Circ Physiol. 2001;281:H1201–1209. doi: 10.1152/ajpheart.2001.281.3.H1201. [DOI] [PubMed] [Google Scholar]
- 26.Napolitano C, Priori SG, Schwartz PJ. Significance of QT dispersion in the long QT syndrome. Prog Cardiovasc Dis. 2000;42:345–350. doi: 10.1053/pcad.2000.0420345. [DOI] [PubMed] [Google Scholar]
- 27.Di Diego JM, Sun ZQ, Antzelevitch C. I(to) and action potential notch are smaller in left vs. right canine ventricular epicardium. Am J Physiol. 1996;271:H548–561. doi: 10.1152/ajpheart.1996.271.2.H548. [DOI] [PubMed] [Google Scholar]
- 28.Volders PG, Sipido KR, Carmeliet E, Spatjens RL, Wellens HJ, Vos MA. Repolarizing K+ currents ITO1 and IKs are larger in right than left canine ventricular midmyocardium. Circulation. 1999;99:206–210. doi: 10.1161/01.cir.99.2.206. [DOI] [PubMed] [Google Scholar]
- 29.Nabauer M, Beuckelmann DJ, Uberfuhr P, Steinbeck G. Regional differences in current density and rate-dependent properties of the transient outward current in subepicardial and subendocardial myocytes of human left ventricle. Circulation. 1996;93:168–177. doi: 10.1161/01.cir.93.1.168. [DOI] [PubMed] [Google Scholar]
- 30.Guo W, Li H, London B, Nerbonne JM. Functional consequences of elimination of i(to,f) and i(to,s): early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking Kv1. 4 and expressing a dominant-negative Kv4 alpha subunit. Circ Res. 2000;87:73–79. doi: 10.1161/01.res.87.1.73. [DOI] [PubMed] [Google Scholar]
- 31.Rosati B, Grau F, Rodriguez S, Li H, Nerbonne JM, McKinnon D. Concordant expression of KChIP2 mRNA, protein and transient outward current throughout the canine ventricle. J Physiol. 2003;548:815–822. doi: 10.1113/jphysiol.2002.033704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel SP, Parai R, Parai R, Campbell DL. Regulation of Kv4. 3 voltage-dependent gating kinetics by KChIP2 isoforms. J Physiol. 2004;557:19–41. doi: 10.1113/jphysiol.2003.058172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gravelin L, Ziv O, Liu G, Hartmann K, Patel D, Schofield L, Chaves L, Shearer M, Koren G, Choi B. Heart Rhythm. Denver, Colorado: 2012. Transgenic LQT1 Animal Model Reveals EADs and Multifocal Activity as Mechanism for polymorphic Ventricular Tachycardia (pVT) pp. PO3–104. [Google Scholar]
- 34.Hoefen R, Reumann M, Goldenberg I, Moss AJ, JOU, Gu Y, McNitt S, Zareba W, Jons C, Kanters JK, Platonov PG, Shimizu W, Wilde AA, Rice JJ, Lopes CM. In silico cardiac risk assessment in patients with long QT syndrome: type 1: clinical predictability of cardiac models. J Am Coll Cardiol. 2012;60:2182–2191. doi: 10.1016/j.jacc.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 35.O’Hara T, Rudy Y. Arrhythmia formation in subclinical (“silent”) long QT syndrome requires multiple insults: quantitative mechanistic study using the KCNQ1 mutation Q357R as example. Heart Rhythm. 2012;9:275–282. doi: 10.1016/j.hrthm.2011.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antzelevitch C, Fish J. Electrical heterogeneity within the ventricular wall. Basic Res Cardiol. 2001;96:517–527. doi: 10.1007/s003950170002. [DOI] [PubMed] [Google Scholar]
- 37.Cheng JH, Kodama I. Two components of delayed rectifier K+ current in heart: molecular basis, functional diversity, and contribution to repolarization. Acta Pharmacol Sin. 2004;25:137–145. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.