Abstract
OBJECTIVE.
To explore the prevalence and drivers of hospital-level variability in antibiotic utilization among hematopoietic cell transplant (HCT) recipients to inform antimicrobial stewardship initiatives.
DESIGN.
Retrospective cohort study using data merged from the Pediatric Health Information System and the Center for International Blood and Marrow Transplant Research.
SETTING.
The study included 27 transplant centers in freestanding children’s hospitals.
METHODS.
The primary outcome was days of broad-spectrum antibiotic use in the interval from day of HCT through neutrophil engraftment. Hospital antibiotic utilization rates were reported as days of therapy (DOTs) per 1,000 neutropenic days. Negative binomial regression was used to estimate hospital utilization rates, adjusting for patient covariates including demographics, transplant characteristics, and severity of illness. To better quantify the magnitude of hospital variation and to explore hospital-level drivers in addition to patient-level drivers of variation, mixed-effects negative binomial models were also constructed.
RESULTS.
Adjusted hospital rates of antipseudomonal antibiotic use varied from 436 to 1121 DOTs per 1,000 neutropenic days, and rates of broad-spectrum, gram-positive antibiotic use varied from 153 to 728 DOTs per 1,000 neutropenic days. We detected variability by hospital in choice of antipseudomonal agent (ie, cephalosporins, penicillins, and carbapenems), but gram-positive coverage was primarily driven by vancomycin use. Considerable center-level variability remained even after controlling for additional hospital-level factors. Antibiotic use was not strongly associated with days of significant illness or mortality.
CONCLUSION.
Among a homogenous population of children undergoing HCT for acute leukemia, both the quantity and spectrum of antibiotic exposure in the immediate posttransplant period varied widely. Antimicrobial stewardship initiatives can apply these data to optimize the use of antibiotics in transplant patients.
Overuse and inappropriate selection of antibiotics have been described in most healthcare settings.1,2 With increasing antimicrobial resistance and limited new antibiotics under development, the identification off high-impact targets for antibiotic stewardship is critical.3 To this end, the Infectious Disease Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) recommend assessing antibiotic utilization across institutions to quantify variability in practice and to provide a metric against which hospitals can benchmark their antibiotic use.4
Evaluating true differences in antibiotic utilization across hospitals requires the analysis of patient and hospital characteristics to standardize comparisons. Previously published analytic approaches reveal considerable variability in antibiotic prescribing across institutions, even after accounting for differences in patient populations.5–8 Alternatively, antibiotic use can be assessed within a more homogeneous population. This approach has been utilized in children with inflammatory bowel disease9 and acute lymphoblastic leukemia,10 demonstrating variability in utilization despite caring for groups with similar disease.
Pediatric hematopoietic cell transplant (HCT) patients are at high risk for infection, particularly during the immuno-suppression and neutropenia immediately posttransplant. Establishing appropriate antibiotic utilization benchmarks will help to optimize days of antibiotic exposure at this critical stage. This effort is particularly important in patients post-transplant. Decreasing antibiotic exposure will not only limit pressure on resistance evolution and curtail healthcare costs but also may reduce adverse clinical outcomes such as mortality,11 graft-versus-host disease (GVHD),11,12 Clostridium difficile infection,13 and acute kidney injury,14 which have been linked to broad-spectrum antibiotic use. While survey-based studies of prophylaxis in HCT patients have identified differences in practice by center,15,16 variability in broad-spectrum antibiotic utilization in this population has not been evaluated.
We aimed to describe and compare antibiotic utilization for children undergoing HCT during the neutropenic period immediately posttransplant. We hypothesized that despite the homogeneity of this patient population and the existence of practice guidelines for antibiotic administration,17 across-hospital variability would exist. We also sought to explore patient-level and hospital-level factors that contribute to this variability.
METHODS
Study Design and Setting
We performed a retrospective cohort study with data merged from 2 distinct data sources: the Pediatric Health Information System (PHIS) and the Center for International Blood and Marrow Transplantation Research (CIBMTR). PHIS is an administrative and clinical database with inpatient data from freestanding children’s hospitals associated with the Child Health Corporation of America. Data elements include demographics, dates of admission and discharge, diagnosis and procedure codes, and adjusted hospital charges. This database also contains billing data corresponding to specific resources utilized, including inpatient pharmaceutical agents with medication name and dates of administration.
The CIBMTR registry represents an international network of > 450 centers that contribute observational data on patients undergoing transplant. The registry captures basic data on all allogeneic transplants in the United States18 and contains information on transplant characteristics, clinical history, and post-HCT outcomes.
Study Population and Cohort Assembly
The cohort assembly process is depicted in Figure 1. The PHIS database was screened for patients with acute leukemia who underwent HCT based on an admission with the following characteristics: (1) ICD-9 discharge diagnosis denoting acute leukemia (204.xx or 205.xx); (2) code suggesting HCT, including procedure code (41.xx), clinical service code (531537, 531527, 531533, 531531), or pharmaceutical codes (busulfan or cyclophosphamide plus tacrolimus or cyclosporine); (3) admission between 2004 and 2011; and (4) age <21 years at admission. Next, the CIBMTR database was queried for similar criteria: (1) children <21 years of age undergoing first allogeneic HCT for acute leukemia, (2) year of transplant between 2004 and 2011, and (3) consent for participation in the registry with research level data through 100 days posttransplant. Lastly, patients identified from the 2 data sources were merged using the following common data elements: sex, underlying disease, date of birth, date of transplant, and location of transplant.19
FIGURE 1.
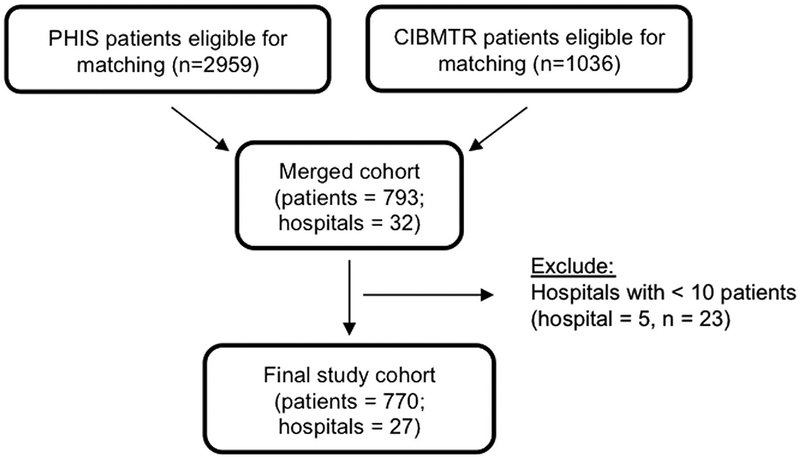
Flow diagram depicting cohort creation.
Patients common to both datasets were included in subsequent data analyses. Hospitals with <10 patients in the merged dataset were excluded because we considered it too difficult to generalize practice patterns based on such limited patient numbers.
Outcome: Antimicrobial Use
The primary outcome was antibiotic utilization rate defined as days of antibiotic therapy (DOTs) per 1,000 neutropenic days. Utilization rates were calculated for each hospital for specific antibiotic groups. The DOTs can be > 1,000 if patients receive multiple agents in the class of interest on the same day. We focused on the following groups of antibiotics: (1) anti-pseudomonal antibiotics recommended for empiric treatment of febrile neutropenia including cephalosporins (cefepime, ceftazidime), penicillins (piperacillin-tazobactam and ticarcillinclauvulanate), and carbapenems (meropenem, imipenemcilastin, ertapenem, and doripenem)17 and (2) broad-spectrum gram-positive antibiotics (vancomycin, linezolid, and daptomycin). We also analyzed carbapenem utilization separately because this class is a mainstay of therapy for drug-resistant infections and is also a Centers for Disease Control and Prevention target for antibiotic stewardship.20
The primary analysis evaluated antibiotic exposures between day of transplant and time of neutrophil engraftment or death (if the patient died prior to engraftment). Time to engraftment is defined as the first of 3 laboratory values in which the absolute neutrophil count is ≥ 500 cells/mm3. If the patient failed to engraft, follow-up time was limited to 30 days after transplant to capture a uniform period of posttransplant care before additional interventions.
Covariate Definitions
Patient-level demographics and transplant characteristics.
Patient-level demographic variables and transplant characteristics were captured from CIBMTR data. Demographic variables, including age, sex, and race (white or nonwhite), were summarized by hospital. Similarly, transplant characteristics including underlying disease (acute myeloid leukemia, acute lymphoblastic leukemia), disease status at transplant (first clinical remission (CR1), ≥ CR2, induction failure), donor (matched related, matched unrelated, or mismatched unrelated), graft source (bone marrow, cord blood, or peripheral stem cells), conditioning regimen (chemotherapy or total body irradiation), and recipient CMV status were summarized by hospital. Age was included as a continuous variable and all others were categorical variables.
Hospital-level variables.
Hospital-level variables were captured from the PHIS database. We hypothesized that hospital-level contributors to variation in antibiotic use would span departments. Therefore, hospital-level variables were based on total hospital admissions in 2011. Volume was defined as total inpatients, 1–19 years old; female gender, non-white race, and public insurance were reported as respective proportions of all inpatients at each hospital. All hospital-level variables were utilized as continuous variables.
Days of significant illness.
We assumed a priori that patients requiring intensive care unit (ICU)–level care would receive more broad-spectrum antibiotics5,21 and, thus, that an increased prevalence of significant illness days at an institution would confound the comparison of antibiotic utilization between hospitals. Therefore, like DOTs, days of significant illness (DSIs) were indexed to total neutropenic days at the hospital level (for correlation testing) and at the patient level (for multivariable modeling). A DSI was defined using PHIS healthcare utilization data as follows: (1) administration of a vasopressor or cardiac support medication; (2) resource code indicating respiratory support; (3) procedure code denoting advanced cardiovascular monitoring or resuscitation; (4) procedure code for extracorporeal membrane oxygenation; or (5) resource or procedure code indicating dialysis. This metric has been employed previously to identify ICU–level care.10,22–25
Statistical Analysis
Patient demographic and transplant characteristics were summarized for the entire cohort and within each institution. We estimated 30-mortality with 95% confidence intervals. The Spearman correlation coefficient was used to measure the correlation of hospitals’ unadjusted DOTs with mortality and hospital-level DSIs.
To compare hospital-level antibiotic utilization we employed a multivariable negative binomial regression analysis to establish rates of DOTs per 1,000 neutropenic days, adjusting for patient-level demographic and transplant characteristics and DSIs. In calculating hospital-adjusted utilization rates from the fitted negative binomial models, average values of demographics, transplant characteristics, and DSIs of the study cohort were used. To account for secular changes in antibiotic use or stewardship, we performed a sensitivity analysis considering utilization rates in only the latter years of the cohort (2008–2011). Additionally, we assessed the impact of ICU-level care on antibiotic utilization by repeating the aforementioned models agnostic to DSIs.
To better quantify the magnitude of between-hospital variation in utilization rates and to explore factors that might account for variation, we constructed mixed-effects (ME) negative binomial models. The base ME model included only a hospital-level random effect without fixed effects. The estimated variance of the random effect reflects the magnitude of antibiotic variation across hospitals, and a test of variance greater than zero suggests that the between-hospital variation is statistically significant. We then included patient-level and additional hospital-level factors as defined above to the base model as fixed effects to explore whether the variation remained significant after adjusting for these factors.
Excel version 14.7 software (Microsoft, Seattle, WA) and Stata version 14.0 software (StataCorp, College Station, TX) were used for all analyses.
Human Subjects Oversight
The merger of PHIS and CIBMTR data occurred under the guidance of the CIBMTR via the National Marrow Donor Program institutional review board. Analysis was performed on a limited dataset; therefore informed consent was waived.
RESULTS
We identified 793 patients common to both the PHIS and CIBMTR databases (Figure 1). Among them, 5 hospitals (23 patients) were excluded for low patient numbers. The final cohort included 770 patients representing 27 hospitals, ranging from 13 to 65 patients per hospital. Table 1 shows the demographic characteristics for the cohort by institution. Additional transplant characteristics and hospital-level characteristics are provided in Supplemental Tables 1 and 2.
TABLE 1.
Descriptive Characteristics of 27 Hospitals Included in the Analysis
| Hospital No. | No. of Patients | Time to Engraftment (median, d) | Age (median, y) | Sex (female, %) | Race (nonwhite, %) | DSI/1,000 Neutropenic Days |
|---|---|---|---|---|---|---|
| 21 | 13 | 21.7 | 12.4 | 41 | 18 | 89 |
| 16 | 14 | 23.7 | 10.4 | 44 | 35 | 97 |
| 23 | 15 | 23.7 | 8.5 | 31 | 0 | 0 |
| 6 | 16 | 19.3 | 13.2 | 29 | 18 | 20 |
| 27 | 16 | 21.2 | 11.5 | 47 | 0 | 83 |
| 7 | 17 | 16.8 | 10.7 | 48 | 38 | 74 |
| 26 | 17 | 20.7 | 8.8 | 43 | 30 | 27 |
| 12 | 18 | 18.2 | 10.1 | 43 | 9.2 | 0 |
| 15 | 19 | 15.8 | 10.4 | 50 | 36 | 6 |
| 25 | 19 | 22.7 | 10.8 | 33 | 14 | 0 |
| 17 | 21 | 26.7 | 9.6 | 36 | 30 | 14 |
| 19 | 21 | 19.7 | 7.3 | 37 | 11 | 72 |
| 8 | 22 | 19.2 | 12.0 | 44 | 13 | 0 |
| 13 | 22 | 22.7 | 9.6 | 38 | 21 | 115 |
| 22 | 23 | 18.3 | 9.2 | 29 | 29 | 94 |
| 4 | 24 | 13.8 | 8.6 | 48 | 8.7 | 22 |
| 2 | 28 | 24.7 | 9.1 | 56 | 22 | 51 |
| 20 | 28 | 15.8 | 6.8 | 29 | 64 | 55 |
| 1 | 34 | 16.8 | 10.7 | 41 | 41 | 39 |
| 5 | 39 | 24.7 | 6.6 | 33 | 25 | 10 |
| 3 | 40 | 20.7 | 9.7 | 31 | 56 | 11 |
| 11 | 40 | 24.7 | 8.5 | 34 | 18 | 63 |
| 18 | 40 | 23.7 | 7.7 | 50 | 18 | 24 |
| 9 | 48 | 15.8 | 8.7 | 44 | 10 | 55 |
| 24 | 50 | 19.2 | 11.4 | 43 | 15 | 6 |
| 14 | 61 | 21.7 | 10.0 | 37 | 0 | 48 |
| 10 | 65 | 17.8 | 8.6 | 43 | 36 | 33 |
| Full cohort | 770 | 19.7 | 9.6 | 41 | 22 | 39 |
Note. DSI, days of significant illness.
Overall, 38 (4.9%) patients failed to engraft. Of those, 32 patients (84.2%) died before engraftment at a median of 42 days posttransplant. Among those who did engraft, the median time to engraftment was 20 days (range, 8–84 days). The remaining patients received additional therapy but were censored at 30 days for DOT and DSI assessments.
Correlation of Antibiotic Utilization With Mortality and Days of Significant Illness
Hospital 30-day mortality rates ranged from 0 to 9.5%, with a median of 2.1% overall. Days of significant illness ranged from 0 to 115 days per 1,000 neutropenic days (Table 1). The Spearman correlation coefficients between specific antibiotic groups and mortality rates and DSIs are shown in Table 2. Mortality was not correlated with any antibiotic group. Days of significant illness were moderately positively correlated with gram-positive antibiotic use and moderately negatively correlated with antipseudomonal antibiotic use; no correlation with carbapenems was identified.
TABLE 2.
Spearman Correlation Coefficient Between Hospital Antibiotic Use, Days of Significant Illness, and Mortality
| Variable | Antipseudomonal Antibioticsa | P Value | Gram-Positive Antibioticsb | P Value | Carbapenems | P Value |
|---|---|---|---|---|---|---|
| 30-day mortality | 0.167 | .410 | −0.095 | .640 | 0.149 | .460 |
| Days of significant illness | −0.396 | .040 | 0.467 | .014 | −0.097 | .630 |
Anti-pseudomonal antibiotics: cephalosporins (cefepime, ceftazidime), penicillins (piperacillin-tazobactam, ticarcillin-clauvulanate), and carbapenems (meropenem, imipenem-cilastin, ertapenem, and doripenem).
Vancomycin, linezolid, and daptomycin.
Hospital Rates of Antibiotic Utilization
Overall, 90.1% of patients received at least 1 day of a broad-spectrum antipseudomonal or gram-positive antibiotic; the proportion by hospital ranged from 64.7% to 100% for anti-pseudomonal antibiotic receipt and from 50% to 100% for gram-positive antibiotic receipt. Composite unadjusted anti-pseudomonal DOTs per 1,000 neutropenic days ranged from 424 to 1012 (median, 731); for carbapenems specifically, DOTs ranged from 415 to 987 (median, 719); and for gram-positive antibiotics, DOTs varied from 148 to 824 (median, 399). In the negative binomial models, adjusted hospital DOTs varied minimally from unadjusted estimates, and the scope of the variability across hospitals was similar (Figures 2 and 3). As demonstrated in Figure 2a, the choice of antipseudomonal agents varied across hospitals. In contrast, gram-positive antibiotic utilization was driven primarily by vancomycin prescribing (Figure 3). The sensitivity analysis restricting the evaluation to only the later years did not meaningfully change the rates of utilization between hospitals, nor did the exclusion of DSIs from the adjusted models.
FIGURE 2.
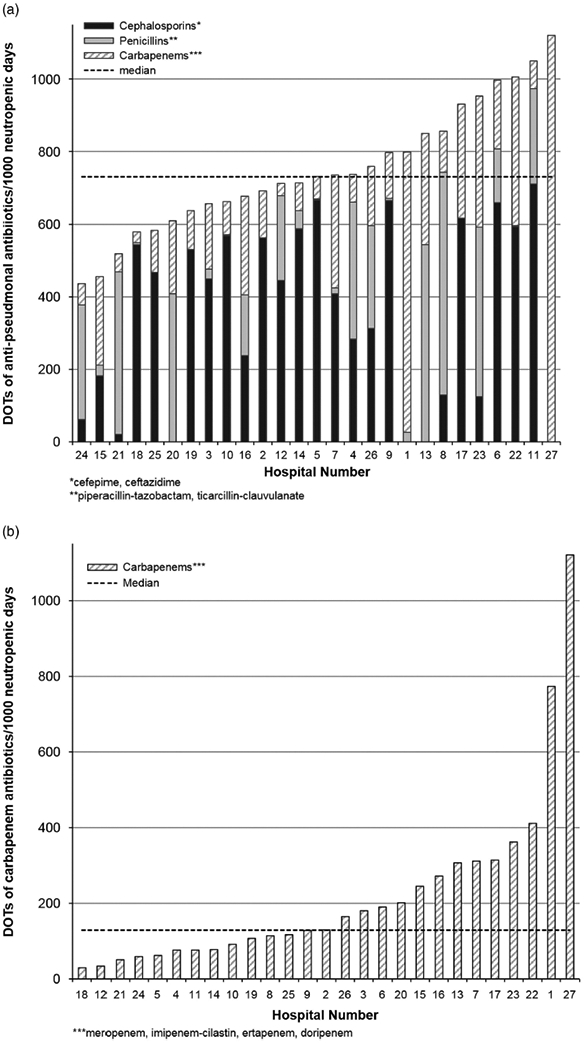
Variation in (A) anti-pseudomonal antibiotic use and (B) carbapenem use, adjusted for age, sex, race, diagnosis, disease status, donor type, graft source, conditioning regimen, cytomegalovirus (CMV) status and days of significant illness (DSIs) per 1,000 neutropenic days.
FIGURE 3.
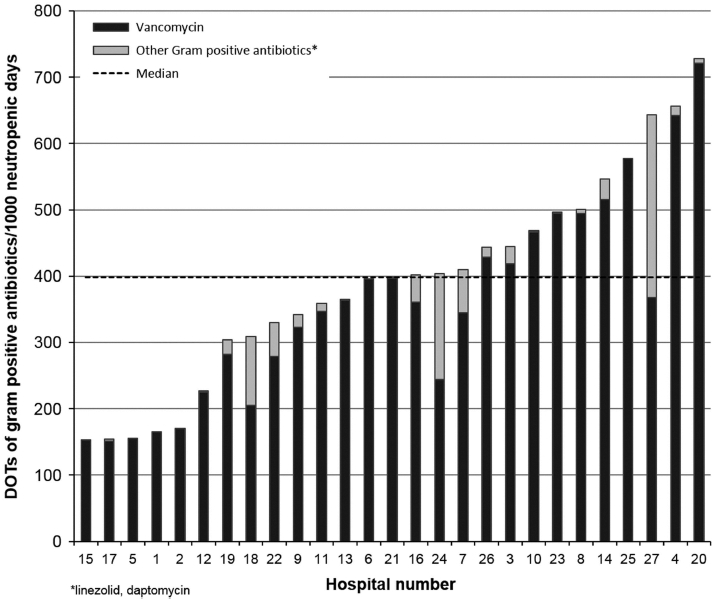
Variation in gram-positive antibiotic use, adjusted for age, sex, race, diagnosis, disease status, donor type, graft source, conditioning regimen, recipient cytomegalovirus (CMV) status and days of significant illness (DSIs) per 1,000 neutropenic days.
Drivers of Variation in Antibiotic Utilization
In the base ME models that included only hospital-level random effects, the estimated variance is statistically significantly different from zero, suggesting significant variation in antibiotic utilization across hospitals. As indicated by a higher point estimate of variance, this variability is more pronounced for gram-positive antibiotics and carbapenems compared to antipseudomonal antibiotics (Table 3). In each of the 3 models, the point estimate of variance decreased with the addition of patient- and hospital-level factors. However, despite the inclusion of these factors, statistically significant between-hospital variation remained for all ME models (Table 3).
TABLE 3.
Mixed-Effect Models Demonstrating Variance of Hospital Effect After Controlling for Patient-Levela and Hospital-Levelb Factors
| Variable | Variance of Hospital Level Random Effectc | 95% CI of Variance |
|---|---|---|
| Antipseudomonal antibiotics | ||
| Hospital alone | 0.030 | 0.014–0.064 |
| Hospital plus patient factors | 0.029 | 0.014–0.062 |
| Hospital plus hospital factors | 0.021 | 0.009–0.050 |
| Hospital plus patient and hospital factors | 0.022 | 0.010–0.051 |
| Gram-positive antibiotics | ||
| Hospital alone | 0.148 | 0.070–0.314 |
| Hospital plus patient factors | 0.141 | 0.065–0.305 |
| Hospital plus hospital factors | 0.136 | 0.063–0.294 |
| Hospital plus patient and hospital factors | 0.129 | 0.060–0.290 |
| Carbapenems | ||
| Hospital alone | 0.560 | 0.266–1.180 |
| Hospital plus patient factors | 0.534 | 0.247–1.157 |
| Hospital plus hospital-level factors | 0.438 | 0.198–0.966 |
| Hospital plus patient and hospital factors | 0.451 | 0.202–1.007 |
Patient-level factors include age, sex, race, diagnosis, disease status, donor type, graft source, recipient CMV status and days of severe illness.
Hospital-level factors include hospital volume, proportions female, nonwhite race, and public insurance.
P value for all reported variances < .001.
The full details of the ME models are provided in Supplemental Table 3. Hospital volume was negatively associated with antipseudomonal antibiotic use (incident rate ratio, 0.973 per 1,000 patients; P = .04). No other statistically significant associations were identified.
DISCUSSION
The study results demonstrate that HCT patients at free-standing children’s hospitals are frequently exposed to broad-spectrum antibiotics during the immediate posttransplant period. However, significant between-hospital variability in rates of broad-spectrum antibiotic prescribing exists, even after adjusting for patient characteristics. These analyses did not identify any specific patient-level or hospital-level drivers of variability.
Overall, 90% of patients received at least 1 antipseudomonal antibiotic, and >65% received a gram-positive antibiotic. Similar rates of antibiotic utilization have been reported among adult transplant patients.6 There was substantial variability in DOTs across hospitals. A 2.2-fold increase existed between the hospitals with the lowest and highest utilization for antipseudomonal antibiotics, and there was a 5.7-fold increase for gram-positive antibiotic use. Although this range of antibiotic utilization across institutions is consistent with reports for patients with other diseases,2,5–8,13 this degree of variability in a patient population where infection risk should be consistent from one hospital to the next is striking. While these analyses cannot determine appropriate antibiotic prescribing, the range suggests that some hospitals are either undertreating patients or, conversely, are exposing patients to antibiotics unnecessarily. We have shown here (1) a low mortality rate and (2) no association between antibiotic utilization and mortality. Both findings argue against under-treatment. Our analyses did not identify any statistically significant predictors antibiotic utilization, including patient age or ICU-level care, which are predictors of use in other studies.2,8
The persistence in statistically significant utilization variability even after adjusting for patient and hospital covariates suggests that patient need does not explain this variance. Instead, we hypothesize that individual physician preference or practice standards developed by local transplant groups drive this differential antibiotic utilization. Physician or hospital practices may reflect local antibiograms, institutional stew-ardship initiatives, hospital formulary choices, or the reliance of transplant groups on historical practice outside the influence of a stewardship program.26 Data regarding physician- or hospital-specific practice patterns were not available in this dataset so this hypothesis could not be evaluated. However, it should be considered in future investigations.
The inverse correlation between antibiotic class and DSI was notable. It is possible that starting antipseudomonal agents early and continuing them through the duration of febrile neutropenia aborts downstream days of severe illness, leading to the negative correlation between antipseudomonal antibiotics and DSI. Conversely, vancomycin use was positively associated with DSI and likely indicates that some hospitals reserve vancomycin for critically ill patients. However, carbapenems are often employed for critical illness, so we might have expected a similar association between carbapenems and DSI, yet no correlation was identified. These relationships deserve further exploration.
Ultimately, these findings demonstrate that HCT recipients have a differential exposure to antibiotics that is not explained by patient- or hospital-level characteristics. This variability presents an opportunity for standardization and education on appropriate use via antimicrobial stewardship interventions. No specific recommendations exist for stewardship in transplant units.26 Options for intervention recommended by the 2016 IDSA guidelines include prospective audit of antibiotic prescriptions with feedback to individual prescribers and formulary restriction.27 Both strategies have been successfully implemented in an adult hematology/oncology and transplant units.28–30 An alternative intervention would provide iterative feedback to institutions based on hospital-level data like ours, enabling stewardship programs to benchmark how their quantity and composition of antibiotic utilization compare to peer institutions for similar patients. The absence of correlation between antibiotic utilization and outcomes, such as mortality, suggests that initial targets for utilization should be at or below the median across institutions.
Publication of guidelines is another mechanism that may help ameliorate variation in antibiotic utilization for a specific patient population. The initial IDSA guidelines for antibiotic utilization during neutropenia were available prior to the start of our cohort,31 but disappointingly, they did not seem effective in harmonizing antibiotic prescribing practices. Notably, these guidelines were updated in 2011.17 Most of our cohort predates this update; thus, it is possible that the revised guidelines will more effectively reduce variation. Ultimately, prospective assessment of antibiotic utilization by hospital is necessary to inform more time-relevant benchmarks and to provide data to assess the impact of any intervention.
Importantly, not only did utilization of antipseudomonal antibiotics vary across hospitals, so, too, did the choice of antipseudomonal class. This finding is particularly relevant because recent data from adult transplant cohorts have suggested that the risk of noninfectious outcomes, such as mortality,12,32 GVHD,12,33 and relapse,34,35 may be mediated by antibiotic class via the gut microbiota.36 In addition, specific classes of antibiotics have been associated with an increased risk of C. difficile infection13 and acute kidney injury14 in children. If additional research confirms a differential impact of antipseudomonal antibiotics on these secondary outcomes, it will also be important to incorporate this evidence into guidelines for appropriate antibiotic choice.
This analysis is limited by the absence of microbiology data needed to distinguish antibiotics used for treatment versus those used empirically. The inclusion of DSIs in the model serves as a proxy for severe infection but does not account for all infections. Because we would not expect rates of infection to be substantially different across institutions, we suspect that, as demonstrated in other settings,37 empiric antimicrobial therapy, rather than definitive therapy for documented infections, is driving the variability. The merged dataset also does not reliably capture central catheter utilization, prophylaxis practices or resistance patterns that could vary by institution and thus explain some of the detected variability. Although misclassification is a limitation when using administrative data, the process of merging an administrative dataset with data from CIBMTR ensures that all patients included in this cohort did undergo transplant on the date identified. Additionally, the use of billing data rather than actual drug administration potentially overestimates DOTs.38 Finally, the study population included in this analysis is only generalizable to freestanding academic children’s hospitals. However, most pediatric HCTs occur in this setting.
In summary, we found substantial variation in antibiotic use in children undergoing HCT for acute leukemia that was not explained by severity of illness, demographics, transplant characteristics, or hospital-level factors. Variation of antibiotic utilization in this homogenous patient population represents a unique opportunity to implement interventions that optimize use. Contemporary and prospective assessment of antibiotic utilization practices by hospital is necessary to inform targeted benchmarks for appropriate utilization and to provide feedback to sites aiming to achieve these benchmarks. Additionally, further study is needed to determine the clinical implications of antibiotic choice on noninfectious adverse outcomes after pediatric HCT.
Supplementary Material
ACKNOLEDGMENTS
Financial support: This study was supported by a training grant from the National Institutes of Health, Clinical Pharmacoepidemiology training grant (grant no. T32-GM075766 to C.W.E.).
Footnotes
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2018.96
REFERENCES
- 1.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015;60:1308–1316. [DOI] [PubMed] [Google Scholar]
- 2.MacDougall C, Polk RE. Variability in rates of use of antibacterials among 130 US hospitals and risk-adjustment models for interhospital comparison. Infect Control Hosp Epidemiol 2008;29:203–211. [DOI] [PubMed] [Google Scholar]
- 3.Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis 2008;46:155–164. [DOI] [PubMed] [Google Scholar]
- 4.Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44: 159–177. [DOI] [PubMed] [Google Scholar]
- 5.Gerber JS, Newland JG, Coffin SE, et al. Variability in antibiotic use at children’s hospitals. Pediatrics 2010;126:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polk RE, Hohmann SF, Medvedev S, Ibrahim O. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis 2011;53:1100–1110. [DOI] [PubMed] [Google Scholar]
- 7.Kanerva M, Ollgren J, Lyytikainen O, Finnish Prevalence Survey Study Group. Benchmarking antibiotic use in Finnish acute care hospitals using patient case-mix adjustment. J Antimicrob Chemother 2011;66:2651–2654. [DOI] [PubMed] [Google Scholar]
- 8.Tan C, Vermeulen M, Wang X, Zvonar R, Garber G, Daneman N. Variability in antibiotic use across Ontario acute care hospitals. J Antimicrob Chemother 2017;72:554–563. [DOI] [PubMed] [Google Scholar]
- 9.Kronman MP, Gerber JS, Prasad PA, et al. Variation in antibiotic use for children hospitalized with inflammatory bowel disease exacerbation: a multicenter validation study. J Pediatr Infect Dis Soc 2012;1:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher BT, Gerber JS, Leckerman KH, et al. Variation in hospital antibiotic prescribing practices for children with acute lymphoblastic leukemia. Leukemia Lymphoma 2013;54:1633–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peled JU, Jenq RR, Holler E, van den Brink MR. Role of gut flora after bone marrow transplantation. Nat Microbiol 2016;1:16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber D, Jenq RR, Peled JU, et al. Microbiota disruption induced by early use of broad-spectrum antibiotics is an independent risk factor of outcome after allogeneic stem cell transplantation. Biol Blood Marrow Transpl 2017;23:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher BT, Sammons JS, Li Y, et al. Variation in risk of hospital-onset clostridium difficile infection across beta-lactam antibiotics in children with new-onset acute lymphoblastic leukemia. J Pediatr Infect Dis Soc 2014;3:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downes KJ, Cowden C, Laskin BL, et al. Association of acute kidney injury with concomitant vancomycin and piperacillin/tazobactam treatment among hospitalized children. JAMA Pediatr 2017;171:e173219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trifilio S, Verma A, Mehta J. Antimicrobial prophylaxis in hematopoietic stem cell transplant recipients: heterogeneity of current clinical practice. Bone Marrow Transpl 2004;33: 735–739. [DOI] [PubMed] [Google Scholar]
- 16.Whangbo J, Ritz J, Bhatt A. Antibiotic-mediated modification of the intestinal microbiome in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl 2017;52:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis 2011;52:e56–e93. [DOI] [PubMed] [Google Scholar]
- 18.Current uses and outcomes of hematopoietic stem cell transplantation: CIBMTR summary slides. Center for International Blood and Marrow Transplant Research website https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx. Published 2015 Accessed April 10, 2018.
- 19.Arnold SD, Brazauskas R, He N, et al. Role of donor source on clinical outcomes and inpatient resource utilization for hematopoietic cell transplantation in children with acute leukemia. Blood 2016;128:1.27389536 [Google Scholar]
- 20.Centers for Disease Control and Prevention. Core Elements of Hospital Antibiotic Stewardship Programs. Atlanta, GA: US Department of Health and Human Services, CDC; 2014. [Google Scholar]
- 21.Fridkin SK, Steward CD, Edwards JR, et al. Surveillance of antimicrobial use and antimicrobial resistance in United States hospitals: project ICARE phase 2. Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) hospitals. Clin Infect Dis 1999;29:245–252. [DOI] [PubMed] [Google Scholar]
- 22.Winestone LE, Getz KD, Miller TP, et al. The role of acuity of illness at presentation in early mortality in black children with acute myeloid leukemia. Am J Hematol 2017;92:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkes JJ, Hennessy S, Xiao R, et al. Volume-outcome relationships in pediatric acute lymphoblastic leukemia: association between hospital pediatric and pediatric oncology volume with mortality and intensive care resources during initial therapy. Clin Lymphoma Myeloma Leuk 2016;16:404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Getz KD, Miller TP, Seif AE, et al. Early discharge as a mediator of greater ICU-level care requirements in patients not enrolled on the AAML0531 clinical trial: a Children’s Oncology Group report. Cancer Med 2016;5:2412–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maude SL, Fitzgerald JC, Fisher BT, et al. Outcome of pediatric acute myeloid leukemia patients receiving intensive care in the United States. Pediatr Crit Care Med 2014;15:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf J, Sun Y, Tang L, et al. Antimicrobial stewardship barriers and goals in pediatric oncology and bone marrow transplantation: a survey of antimicrobial stewardship practitioners. Infect Control Hosp Epidemiol 2016;37:343–347. [DOI] [PubMed] [Google Scholar]
- 27.Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016;62:e51–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeo CL, Chan DS, Earnest A, et al. Prospective audit and feedback on antibiotic prescription in an adult hematologyoncology unit in Singapore. Eur J Clin Microbiol Infect Dis 2012;31:583–590. [DOI] [PubMed] [Google Scholar]
- 29.Azap A, Topcuoglu P, Yesilkaya A, et al. The effect of a nationwide antibiotic restriction policy on antibiotic usage in a stem cell transplantation unit. Turk J Haematol 2005;22:87–90. [PubMed] [Google Scholar]
- 30.Saito T, Yoshioka S, Iinuma Y, et al. Effects on spectrum and susceptibility patterns of isolates causing bloodstream infection by restriction of fluoroquinolone prophylaxis in a hematologyoncology unit. Eur J Clin Microbiol Infect Dis 2008;27:209–216. [DOI] [PubMed] [Google Scholar]
- 31.Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 2002;34:730–751. [DOI] [PubMed] [Google Scholar]
- 32.Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014;124:1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shono Y, Docampo MD, Peled JU, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Translat Med 2016;8:339ra371–339ra371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peled JU, Devlin SM, Staffas A, et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. J Clin Oncology 2017;35:1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antin JH. Relationship between intestinal bacteria and the anticancer effect of hematopoietic stem-cell transplantation. J Clin Oncology 2017;35:1636–1637. [DOI] [PubMed] [Google Scholar]
- 36.Staffas A, Burgos da Silva M, van den Brink MR. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood 2017;129:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grohskopf LA, Huskins WC, Sinkowitz-Cochran RL, et al. Use of antimicrobial agents in United States neonatal and pediatric intensive care patients. Pediatr Infect Dis J 2005;24:766–773. [DOI] [PubMed] [Google Scholar]
- 38.Courter JD, Parker SK, Thurm C, et al. Accuracy of administrative data for antimicrobial administration in hospitalized children. J Pediatric Infect Dis Soc 2017. doi: 10.1093/jpids/pix064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


