Abstract
Background:
Primary CNS vasculitis (PCNSV) can be diagnosed using cerebral angiography or histopathology combined with clinical features. The original diagnostic criteria, which weigh each test equally, have not been validated. Limited sensitivity and specificity for biopsy and angiography are recognized. We systematically reviewed results of diagnostic tests performed in patients with an ultimate diagnosis of PCNSV.
Methods:
We searched the OVID Medline database and bibliographies for original cases of PCNSV. We recorded demographics, diagnostic tests used, and assessed agreement between angiography and biopsy when both tests were performed. We also recorded MRI and CSF results.
Results:
We found 701 original cases with PCNSV diagnosed with angiography or pathology. A total of 269 patients (38.4%) had both cerebral angiography and histopathologic testing (biopsy/postmortem). Classic angiographic features of vasculitis were associated with pathologic confirmation in just 32 patients (4.6%). Seventy-four patients (10.6%) with any abnormality on angiography had a normal biopsy, and 99 patients (14.1%) with abnormal biopsies had normal angiography. Brain MRI was abnormal in 505/541 patients (93.3%) and CSF was abnormal in 360/484 patients (74.4%). Increasing use of angiography and decreasing histopathologic testing were found over time.
Conclusions:
Cerebral angiography and pathologic tissue examination were undertaken in a minority of published cases with a diagnosis of PCNSV. When both diagnostic tests were performed, disagreement between them was more than 5 times more likely than agreement. Diagnostic criteria for PCNSV may require revision to classify the clinical, pathologic, and radiologic features of this condition more accurately.
Primary CNS vasculitis (PCNSV) is a rare inflammatory condition of unknown etiology that may be disabling or potentially fatal.1 It remains a poorly understood disorder and numerous different terms have been used to describe what is regarded, perhaps incorrectly, as a single clinical entity, including cerebral vasculitis, primary angiitis of the CNS, isolated granulomatous angiitis of the CNS, granulomatous angiitis, noninfectious angiitis, and benign angiopathy of the CNS. Diagnostic criteria were proposed in 1988 and diagnosis requires a combination of clinical features of acquired CNS dysfunction, presence of typical pathologic or cerebral angiographic abnormalities, and lack of an alternative unifying explanation.2 Earlier cases were typically diagnosed at postmortem, highlighting the potentially serious nature of PCNSV, but noninvasive testing is now more easily accessible and more benign cases with good outcomes are also recognized. Patients with suspected PCNSV are typically treated aggressively with steroids or other immunosuppressant therapies, which have associated risks,3 but to date no randomized clinical trials of therapies for PCNSV have been undertaken. Proposals for multicenter collaboration to identify predictors of poor outcome have been published, but results from these collaborations are not yet reported.4
Clinical, imaging, and angiographic mimics for PCNSV exist, several of which have been described only in the recent past, or are contingent on recent imaging developments, adding to the difficulty in interpreting historical data. One notable mimic is reversible cerebral vasoconstriction syndrome (RCVS), which may have similar angiographic changes.5 Presentation with thunderclap headache and resolution of angiographic changes over time suggests RCVS rather than PCNSV. However, differentiating between angiographic changes due to different conditions may be difficult early after clinical presentation. In addition, clinical features overlap, leading to potential difficulties in clinical management when considering the need for immunosuppressive therapy. Furthermore, diagnostic criteria for PCNSV remain unvalidated. It is also not clear to what extent angiographic changes reported in cases of PCNSV correlate with pathologically confirmed cases. We hypothesized that if both tests (cerebral angiography and pathologic assessment of brain tissue) contribute to the diagnosis of PCNSV equally, as is suggested in the diagnostic criteria, then when performed together, both angiography and brain pathology from either biopsy or postmortem would often be abnormal in cases of PCNSV. We undertook a systematic review of the published literature on PCNSV. We sought to examine and compare diagnostic test results of pathology and cerebral angiography and to investigate the use of other tests including brain MRI and CSF analysis in published cases on PCNSV.
METHODS
Protocol
The study was performed in line with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.6
Search strategy and selection criteria
We used a comprehensive search strategy of the OVID Medline database on September 29, 2015, for articles that met our predefined eligibility criteria. We searched the reference lists of published cases and review articles on PCNSV for additional articles not detected by the original search strategy. The search strategy is shown in appendix e-1 at Neurology.org/cp. Articles were included if they reported on any case of PCNSV in an adult population (defined as age ≥16 years) and were published in English. Diagnostic terms considered synonymous with PCNSV included cerebral angiitis, cerebral vasculitis, CNS vasculitis, granulomatous angiitis of the nervous system, primary angiitis of the CNS, isolated angiitis of the CNS, cerebral arteritis, intracranial vasculitis, idiopathic angiitis of the CNS, or noninfectious angiitis of the CNS. If multiple publications from the same patient cohort were found, we reviewed the most recent or largest cohort publication to prevent duplication of results from individual patients. Exclusion criteria were cases with systemic forms of vasculitis (i.e., vasculitis affecting any organ beyond the CNS), temporal arteritis, secondary cause for CNS vasculitis (e.g., sarcoidosis, lymphoma, Behçet disease, infection associated arteritis), and pediatric cases (<16 years). If articles described a mixed population of vasculitis, only those patients defined as having PCNSV (or other synonymous term) were analyzed.
Data collection
Data collection was performed independently by 2 reviewers (F.M. and M.O.M.). Recorded data included number of cases of PCNSV, mean age, sex, use of proposed diagnostic criteria, use of tissue biopsy, presence/absence of a pathologically confirmed diagnosis including postmortem, use of cerebral angiography, presence/absence of any angiographic abnormality, presence/absence of classical angiographic appearances for CNS vasculitis (multifocal arterial stenosis/ectasia in multiple vessels, sometimes referred to as a string of beads sign),7,8 use of brain MRI, and brain MRI results (including presence of infarction, hemorrhage, T2/fluid-attenuated inversion recovery–based hyperintensities, gadolinium-enhancing lesions, abnormalities in multiple territories). Use of lumbar puncture and presence/absence of CSF abnormalities were documented when available. Abnormal CSF was defined as CSF protein concentration >0.4 g/L, CSF leucocyte count >5 per mm3, or CSF reported as abnormal by the author. Presence of spinal involvement and venous abnormalities in pathologically confirmed cases were also recorded. Clinical outcomes and treatments administered were not included. Any differences in recorded data from reviewers were resolved by consensus after further review of the relevant publications.
Analysis
Published cases of PCNSV were collected and amalgamated for descriptive purposes. Frequency of investigations and diagnostic yields were measured.
RESULTS
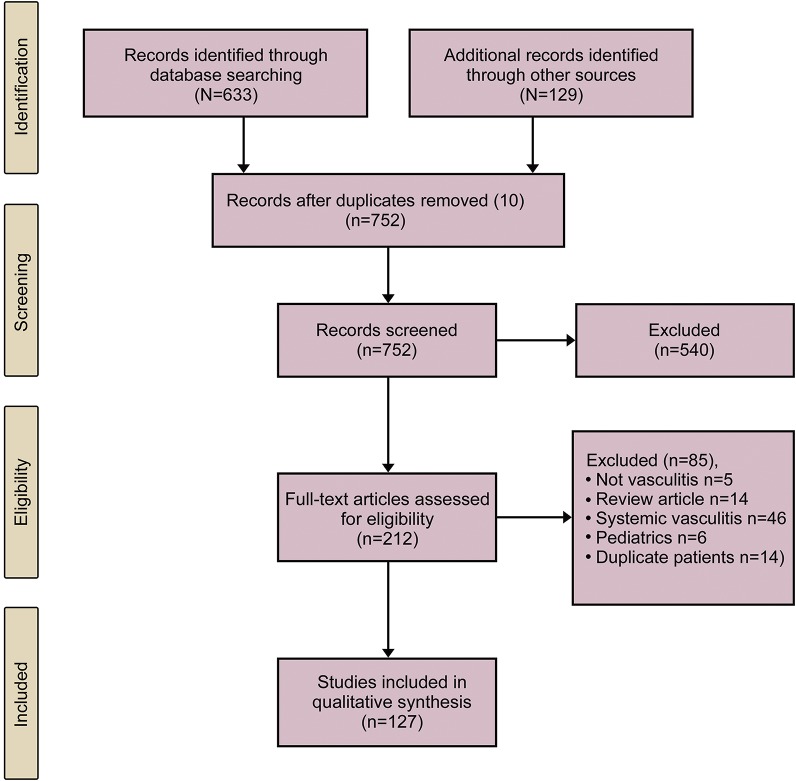
A total of 633 articles were generated from the Medline literature search and an additional 129 articles were reviewed after searching bibliographies of published cases. After screening and removal of articles not meeting the specified diagnostic criteria, a total of 127 original articles were included.2,7–40,e1–e92 A flowchart of inclusion and exclusion of articles is shown in figure 1.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
A total of 914 patients were reported overall in these studies. A total of 213 individual cases from these articles were excluded as they related to secondary CNS vasculitis or other diagnoses, leaving a final cohort of 701 patients with PCNSV. The median number of patients included per publication was 1 (interquartile range 1–5, range 1–163). The mean age from those publications where demographics were reported was 45 (SD 14) years with a range from 19 to 96 years.e11,e38 Fifty-five percent of patients were female.
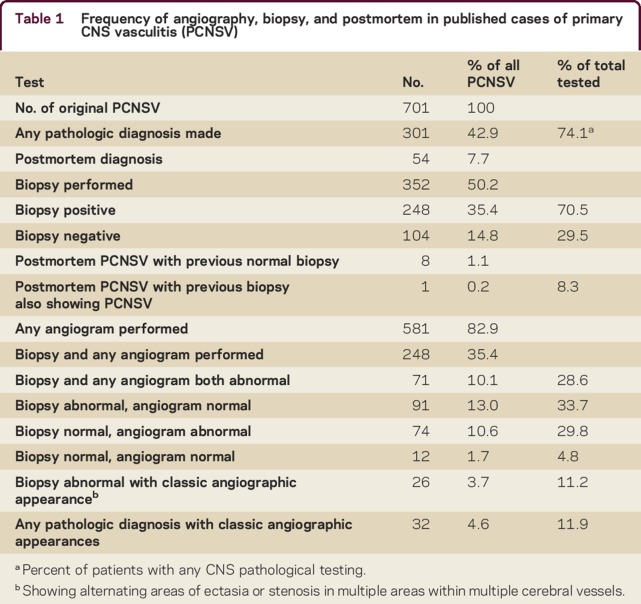
Biopsy and angiographic results are summarized in table 1. CNS biopsy was performed for 352 patients (50.2%) and confirmed PCNSV in 248 (35.4%). A total of 54 patients were diagnosed at postmortem.2,8,9,40,e13,e20,e28,e32,e35,e38,e43–e46,e48,e50–e53,e55,e57,e58,e62–e65,e68,e69,e71,e73–e75,e85,e91,e93 Of those with PCNSV confirmed at postmortem, 8 cases had a previously performed brain biopsy that had not shown evidence for CNS vasculitis8,e35,e38,e48,e59,e71,e74,e93; however, only one of these patients had brain MRI performed as part of a diagnostic workup prior to cerebral biopsye35 and the biopsy procedures were usually blind or needle biopsies.
Table 1.
Frequency of angiography, biopsy, and postmortem in published cases of primary CNS vasculitis (PCNSV)
Angiography was performed in 581/701 cases (82.9%), including catheter angiography in 538/701 (76.7%) and magnetic resonance angiography/CT angiography in 211/701 (30.1%). Catheter angiography revealed the presence of any abnormality in 417/538 cases (77.5% of group, 59.5% of total cohort), and 322/538 (59.8% of group, 45.9% of total cohort) patients had a classic angiographic appearance7,8 with multiple areas of stenosis or ectasia in multiple vessels.
Pathologic examination of CNS tissue combined with any form of angiography was performed in 268/701 patients (38.2%) and CNS biopsy with catheter angiography was performed in 232/701patients (33.1%). A total of 71/302 patients (23.5% of group, 10.1% of total cohort) with PCNSV confirmed by pathologic examination also had an abnormal angiogram but only 32/302 patients (10.6% of group, 4.6% of total cohort) with pathologically confirmed PCNSV were reported with an angiogram that showed classical appearances associated with PCNSV.7,8
In 99 patients with pathologically confirmed vasculitis, cerebral angiography was normal (14% of total cohort). In 74 patients (10.6% of total cohort), CNS pathology was normal but angiography was classical for PCNSV. PCNSV was diagnosed despite both CNS pathology examination and angiography being normal for 12 patients (1.7%).
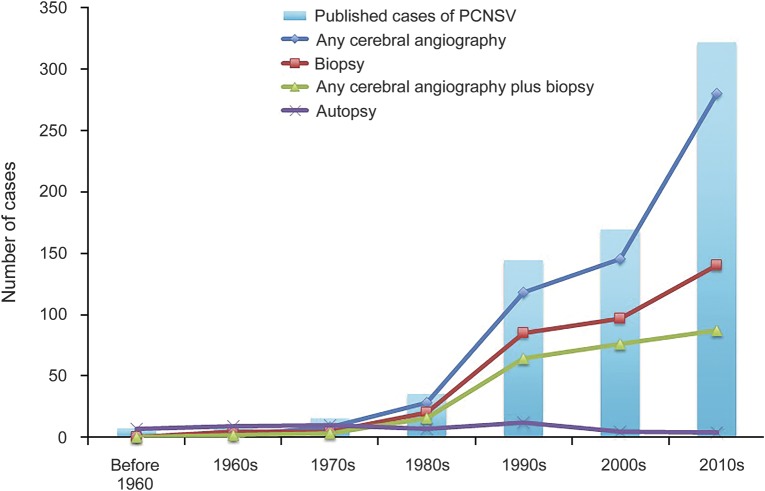
The overall number of new cases published per decade has increased from 7 prior to the 1960s to 322 in the decade from 2010 onward. The proportion of cases diagnosed at postmortem declined, over time, while the use of less invasive testing with angiography has increased over the same time period. Changes in the frequency for use of different diagnostic tests for PCNSV over time are shown in figure 2.
Figure 2. Frequency of use of angiographic and tissue pathology testing in published cases of primary CNS vasculitis (PCNSV) per decade.
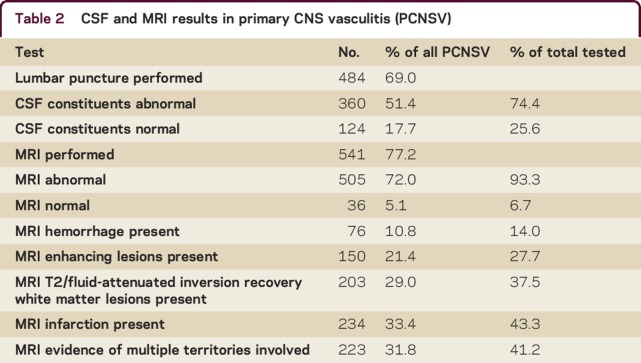
MRI brain and CSF findings are summarized in table 2. A total of 541 patients (77.2%) had brain MRI performed, which was abnormal in 505 (93.3%) and normal in 36 (6.7%). Abnormalities reported on MRI brain included infarction (n = 234 [43.3%]), white matter hyperintensities (n = 203 [37.5%]), gadolinium-enhancing lesions (n = 150 [27.7%]), hemorrhagic lesions (n = 76 [14.0%]), and multiple territory involvement (n = 223 [41.2%]). A total of 484 patients (69% of total cases) had lumbar puncture performed. CSF results were abnormal in 360 (74.4%) and normal in 124 (25.6%). Ancillary test results are shown in table 2.
Table 2.
CSF and MRI results in primary CNS vasculitis (PCNSV)
PCNSV with spinal cord involvement was reported in 9 patients.22,23,e29,e39,e43,e57,e64,e75,e81
Venous involvement in pathologically confirmed cases was reported in 6 patients.e31,e50,e58,e63,e69,e91
DISCUSSION
This systematic review reveals substantial heterogeneity in the use of diagnostic tests performed in patients given a final diagnosis of PCNSV. Our analysis shows that a wide range of diagnostic tests are used when diagnosing PCNSV, but both definitive tests (cerebral angiography and tissue histopathologic examination) were performed together in only a minority of patients (34%). Furthermore, when cerebral angiography and brain biopsy/postmortem are undertaken in the same patient, they give conflicting results more frequently than agreement. Only 32 patients (4.6%) had a classic angiographic appearance reported in combination with a pathologically confirmed vasculitis.7,8 When PCNSV was confirmed pathologically, cerebral angiography was approximately 3 times more likely to be normal than abnormal. Conversely, when angiography did suggest PCNSV, a cerebral biopsy was more than twice as likely to be normal than to show changes in keeping with vasculitis.
The reasons for conflicting results between diagnostic tests in PCNSV are likely to be multiple. One reason for negative biopsy results in PCNSV confirmed angiographically is sampling error due to the patchy anatomical nature of the condition itself with skip lesions.e15,e48 The fact that biopsy results were negative in some patients who went on to have PCNSV confirmed at postmortem confirms that false-negative biopsies occur. However, these cases were reported predominantly in patients who did not have other imaging such as brain MRI as part of their clinical investigations prior to biopsy. Neuroimaging would now be considered routine and could help guide sampling of affected tissue to increase diagnostic yield from biopsy. When no accessible tissue is available for biopsy, biopsy of nondominant temporal lobe leptomeninges may be considered. The false-negative rate for cerebral biopsy after obtaining brain MRI when postmortem is used as a gold standard is not known. Negative biopsy results in the context of abnormal cerebral angiography have usually been considered as false-negative biopsies in patients who went on to be treated as having PCNSV.11 However, the very low agreement between angiography and histopathology in PCNSV is worthy of further attention and may reflect different vessel size involvement in angiogram-positive and angiogram-negative cases. Lack of autopsy reporting following a positive biopsy is an inevitable publication bias compounded by declines in autopsy rates.e94
The original diagnostic criteria for PCNSV give equal weight to angiography and histopathologic examination.2 Some authors have suggested more recently that pathologic examination should be a gold standard for diagnosing definite cases of PCNSV3 while others have highlighted the importance of biopsy to look for an alternative diagnosis in patients initially suspected of having PCNSV.e15 If pathologic confirmation is considered the reference standard for PCNSV,3 the data presented here suggest that angiography may be prone to giving false-positive results. This is particularly important given the presence of known disorders such as RCVS, which can have similar angiographic imaging appearances to PCNSV and for which radiologic features to reliably distinguish the disorders have yet to be confirmed.26 Angiographically diagnosed PCNSV with good outcomes (often after administration of corticosteroids)13,14 could potentially represent other more benign conditions like RCVSe95 or posterior reversible encephalopathy syndrome in which the pathologic changes are not known, but diagnostic criteria permit labeling such cases as PCNSV. Furthermore, negative biopsy results in patients investigated for PCNSV have not, to our knowledge, been reported as true negatives, with sampling error or inherent low sensitivity typically offered as the explanation for a normal biopsy.
Tests of diagnostic accuracy may not always be compared to a reference standard for several reasons, as missing reference data can be due to study design, clinical practice, and unfeasibility, all of which may be relevant when considering a relatively uncommon condition of diverse clinical presentations such as PCNSV.e96 However, our analysis shows that a proposed reference standard such as pathologic examination is available in a proportion of patients and even then angiography gives conflicting results when compared to this reference standard, possibly due to limited specificity. Furthermore, when frequencies of diagnostic tests are shown over time (figure 2), there is a shift towards less invasive testing with biopsy and in particular postmortem, and an increased use of angiography, which is more accessible. This is appropriate to reduce unnecessary morbidity associated with biopsy and is consistent with similar neurologic conditions such as cerebral amyloid angiopathy.e97 Our analyses suggest that biopsy and angiography should not be considered as equivalent tests given the lack of agreement and that further validation of imaging criteria for PCNSV is required against biopsy/postmortem confirmed cases as well as defining features to distinguish PCNSV from RCVS.e95 Concerns over safety of CNS biopsy are common and although complication rates from biopsy can vary from low to moderate, the importance of biopsy is highlighted by the detection of PCNSV and its mimics using histopathologic testing.e55,e98
Although MRI and CSF examination were not part of the proposed diagnostic criteria, they are used frequently in the assessment of patients with PCNSV.9 Vessel wall contrast enhancement on MRI has recently been reported as a potential imaging biomarker for PCNSV,e11 but most parenchymal abnormalities seen on brain MRI are not specific for vasculitis and may be seen in many other disorders. Our analysis shows that MRI brain is frequently abnormal in patients with PCNSV, but that the reported changes vary among cases, and multiple different imaging abnormalities are likely to be seen in various combinations. Apart from vessel wall contrast enhancement, no other MRI feature has been proposed to show specific signs for PCNSV. In addition, CSF results when available were usually abnormal in patients with reported PCNSV, but as with brain MRI are not specific for PCNSV. The fact that spinal involvement has been pathologically confirmed on several occasions in PCNSV highlights the possible anatomical extent of this condition, and suggests that other terms such as cerebral vasculitis are misleading and should be avoided. Furthermore, the presence of venous abnormalities on biopsy and postmortem samples may point towards different forms of vasculitis, which may inherently not have an arterial abnormality present on imaging or even on pathology. The range of pathologic and anatomical changes reported emphasizes that PCNSV remains poorly understood and heterogeneous, and international collaboration to form registries as a means of further study is overdue.
Our study has strengths and weaknesses. We collected information from all known cases of PCNSV published in the English language from as early as 1922e91 to capture information about diagnostic tests in the largest possible number of patients. PCNSV cases by their nature are inevitably prone to publication bias, and the fact that only a minority had both angiography and CNS pathologic testing performed in the same patient may reflect a preference for less invasive testing in clinical practice, which could further contribute to publication bias. In addition, determining abnormalities in angiography from case reports rather than review of original images has inherent drawbacks, but each publication was reviewed with prespecified criteria by 2 reviewers independently to minimize bias. Lack of consistently detailed clinical, radiologic, and treatment follow-up data are also a limitation. Measuring the diagnostic accuracy of one test compared to another test using a validated tool such as QUADASe99 was not undertaken because no agreed reference standard test for PCNSV exists, a finding emphasized within the results. In addition, postmortem findings were not systematically reported in such cases.
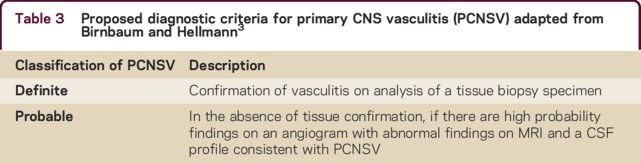
In order to determine more information about PCNSV and its mimics, use of more specific diagnostic criteria are required in order to prevent misclassification of other conditions as PCNSV. Involvement of vessels with different calibers in different PCNSV subtypes could be an explanation for poor agreement between tests, but it is unclear how to accurately define these based on current criteria. In addition to devising new and more specific diagnostic criteria, international collaboration using registries is required to classify this condition more accurately and to compare specific clinical, laboratory, and radiologic features against biopsy and postmortem.4 Although a rare condition, PCNSV is often considered in clinical practice but mimics are relatively more common. In order to tailor treatment appropriately, use of diagnostic criteria that define PCNSV more specifically should be used in the first instance3 (table 3).
Table 3.
Proposed diagnostic criteria for primary CNS vasculitis (PCNSV) adapted from Birnbaum and Hellmann3
Supplementary Material
Footnotes
Supplemental data at Neurology.org/cp
AUTHOR CONTRIBUTIONS
F. McVerry: study concept and design, data acquisition, drafting and revision of manuscript. G. McCuskey: analysis and interpretation of data. P. McCarron: analysis and interpretation of data, revision of manuscript. K.W. Muir: analysis and interpretation of data, revision of manuscript. M.O. McCarron: data acquisition, analysis and interpretation of data, revision of manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
F. McVerry, G. McCluskey, and P. McCarron report no disclosures. K.W. Muir serves on a scientific advisory board for ReNeuron Ltd.; has received speaker honoraria from Boehringer Ingelheim and Bayer; serves on the editorial boards of Stroke and International Journal of Stroke; and receives research support from Covidien (Medtronic), Codman, Boehringer Ingelheim, Stroke Association, NIH, and British Heart Foundation. M.O. McCarron has received publishing royalties from UpToDate. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Salvarani C, Brown RD Jr, Hunder GG. Adult primary central nervous system vasculitis. Lancet 2012;380:767–777. [DOI] [PubMed] [Google Scholar]
- 2.Calabrese LH, Mallek JA. Primary angiitis of the central nervous system: report of 8 new cases, review of the literature, and proposal for diagnostic criteria. Medicine 1988;67:20–39. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaum J, Hellmann DB. Primary angiitis of the central nervous system. Arch Neurol 2009;66:704–709. [DOI] [PubMed] [Google Scholar]
- 4.Lanthier S, Calabrese LH, Ferro JM, et al. The international study on primary angiitis of the central nervous system: a call to the world. Int J Stroke 2014;9:E23. [DOI] [PubMed] [Google Scholar]
- 5.Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol 2012;11:906–917. [DOI] [PubMed] [Google Scholar]
- 6.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- 7.Woolfenden AR, Tong DC, Marks MP, Ali AO, Albers GW. Angiographically defined primary angiitis of the CNS: is it really benign? Neurology 1998;51:183–188. [DOI] [PubMed] [Google Scholar]
- 8.Hinck VC, Carter CC, Rippey JG. Giant cell (cranial) arteritis: a case with angiographic abnormalities. Am J Roentgenol Radium Ther Nucl Med 1964;92:769–775. [PubMed] [Google Scholar]
- 9.Salvarani C, Brown RD, Christianson T, et al. An update of the Mayo Clinic cohort of patients with adult primary central nervous system vasculitis: description of 163 patients. Medicine 2015;94:e738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceccarelli A, De Blasi R, Pavone I, et al. Primary angiitis of the central nervous system: a misinterpreted clinical onset of CNS vasculitis. Eur Neurol 2005;53:40–42. [DOI] [PubMed] [Google Scholar]
- 11.de Boysson H, Zuber M, Naggara O, et al. Primary angiitis of the central nervous system: description of the first fifty-two adults enrolled in the French cohort of patients with primary vasculitis of the central nervous system. Arthritis Rheumatol 2014;66:1315–1326. [DOI] [PubMed] [Google Scholar]
- 12.Montefort M, Dashora U, Chowdhury M. Cerebral vasculitis presenting as a stroke. BMJ Case Rep 2012:2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdulqawi R, Ashawesh K, Ahmad S. Medical image: Anton's syndrome secondary to cerebral vasculitis. NZ Med J 2008;121:89–90. [PubMed] [Google Scholar]
- 14.Amagasaki K, Takeuchi N, Kakizawa T, Shimizu T. Bilateral cortical blindness associated with cerebral angiitis. Acta Neurochir 2002;144:749. [DOI] [PubMed] [Google Scholar]
- 15.Berlit P. Isolated angiitis of the CNS and bacterial endocarditis: similarities and differences. J Neurol 2009;256:792–795. [DOI] [PubMed] [Google Scholar]
- 16.Herath SS, Law DB, Stride PJO, Heazlewood VJ, Gaffney LS. A case of primary cerebral vasculitis. Med J Aust 2008;188:547–549. [DOI] [PubMed] [Google Scholar]
- 17.Hajj-Ali RA, Furlan A, Abou-Chebel A, Calabrese LH. Benign angiopathy of the central nervous system: cohort of 16 patients with clinical course and long-term followup. Arthritis Rheum 2002;47:662–669. [DOI] [PubMed] [Google Scholar]
- 18.Guo R, Liu H, Li M, et al. Cerebral arteriostenosis associated with elevated serum-immunoglobulin E level in young adults without risk factors for ischemic stroke: a possible manifestation of cerebral vasculitis? J Clin Neurosci 2014;21:95–99. [DOI] [PubMed] [Google Scholar]
- 19.Geri G, Saadoun D, Guillevin R, et al. Central nervous system angiitis: a series of 31 patients. Clin Rheumatol 2014;33:105–110. [DOI] [PubMed] [Google Scholar]
- 20.Cosottini M, Canovetti S, Pesaresi I, et al. 3-T magnetic resonance angiography in primary angiitis of the central nervous system. J Comput Assist Tomogr 2013;37:493–498. [DOI] [PubMed] [Google Scholar]
- 21.Beppu T, Inoue T, Nishimoto H, et al. Primary granulomatous angiitis of the central nervous system: findings of magnetic resonance spectroscopy and fractional anisotropy in diffusion tensor imaging prior to surgery: case report. J Neurosurg 2007;107:873–877. [DOI] [PubMed] [Google Scholar]
- 22.Manara R, Schiavon F, Carraro V, Cagnin A, Briani C. Angiographic diagnosis of primary central nervous system vasculitis with spinal cord involvement. Arch Neurol 2009;66:532–533. [DOI] [PubMed] [Google Scholar]
- 23.Campi A, Benndorf G, Filippi M, Reganati P, Martinelli V, Terreni MR. Primary angiitis of the central nervous system: serial MRI of brain and spinal cord. Neuroradiology 2001;43:599–607. [DOI] [PubMed] [Google Scholar]
- 24.Woolfenden AR, Wade NK, Tang P, Chalmers A, Reid G, Teal PA. Uveitis associated with primary angiitis of the central nervous system. Can J Neurol Sci 2007;34:81–83. [DOI] [PubMed] [Google Scholar]
- 25.Rucker JC, Biousse V, Newman NJ. Leptomeningeal enhancement and venous abnormalities in granulomatous angiitis of the central nervous system. J Neuroophthalmol 2003;23:148–150. [DOI] [PubMed] [Google Scholar]
- 26.Néel A, Auffray-Calvier E, Guillon B, et al. Challenging the diagnosis of primary angiitis of the central nervous system: a single-center retrospective study. J Rheumatol 2012;39:1026–1034. [DOI] [PubMed] [Google Scholar]
- 27.Bakhru A, Atlas RO. A case of postpartum cerebral angiitis and review of the literature. Arch Gynecol Obstet 2011;283:663–668. [DOI] [PubMed] [Google Scholar]
- 28.Imbesi SG. Diffuse cerebral vasculitis with normal results on brain MR imaging. AJR Am J Roentgenol 1999;173:1494–1496. [DOI] [PubMed] [Google Scholar]
- 29.Kadkhodayan Y, Alreshaid A, Moran CJ, Cross DT, Powers WJ, Derdeyn CP. Primary angiitis of the central nervous system at conventional angiography. Radiology 2004;233:878–882. [DOI] [PubMed] [Google Scholar]
- 30.Kinsella JA, O'Brien W, Mullins GM, Brewer J, Whyte S. Primary angiitis of the central nervous system with diffuse cerebral mass effect and giant cells. J Clin Neurosci 2010;17:674–676. [DOI] [PubMed] [Google Scholar]
- 31.Kraemer M, Berlit P. Primary central nervous system vasculitis: clinical experiences with 21 new European cases. Rheumatol Int 2011;31:463–472. [DOI] [PubMed] [Google Scholar]
- 32.Krasnianski M, Schlüter A, Neudecker S, Spielmann RP, Stock K. Serial magnet resonance angiography in patients with vasculitis and vasculitis-like angiopathy of the central nervous system. Eur J Med Res 2004;9:247–255. [PubMed] [Google Scholar]
- 33.Krummert B, Scherer K, Whitley J, Sirelkhatim A, Panda M. A rare cause of seizure masquerading as neoplasm. BMJ Case Rep 2010:2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Küker W, Gaertner S, Nagele T, et al. Vessel wall contrast enhancement: a diagnostic sign of cerebral vasculitis. Cerebrovasc Dis 2008;26:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y, Kim J, Kim E, et al. Tumor-mimicking primary angiitis of the central nervous system: initial and follow-up MR features. Neuroradiology 2009;51:651–659. [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim WN, Paroski MW. Communicating hydrocephalus as a complication of isolated CNS angiitis–a case report. Angiology 1987;38:635–641. [DOI] [PubMed] [Google Scholar]
- 37.Razumovsky AY, Wityk RJ, Geocadin RG, Bhardwaj A, Ulatowski JA. Cerebral vasculitis: diagnosis and follow-up with transcranial Doppler ultrasonography. J Neuroimaging 2001;11:333–335. [DOI] [PubMed] [Google Scholar]
- 38.Yin Z, Li X, Fang Y, Luo B, Zhang A. Primary angiitis of the central nervous system: report of eight cases from southern China. Eur J Neurol 2009;16:63–69. [DOI] [PubMed] [Google Scholar]
- 39.Wijdicks EFM, Manno EM, Fulgham JR, Giannini C. Cerebral angiitis mimicking posterior leukoencephalopathy. J Neurol 2003;250:444–448. [DOI] [PubMed] [Google Scholar]
- 40.White ML, Zhang Y. Primary angiitis of the central nervous system: apparent diffusion coefficient lesion analysis. Clin Imaging 2010;34:1–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.