Abstract
Background:
Epilepsy surgery (ES) can improve seizure outcome. A prolonged duration of presurgical evaluation contributes to epilepsy-related morbidity and mortality. We introduced process changes to decrease evaluation time (ET) and increase ES numbers (excluding vagus nerve stimulation).
Methods:
The University of Colorado Hospital patient database was searched for ESs between January 2009 and May 2016. Measures to reduce ET included (1) increasing patient care conference (PCC) frequency; (2) faster intracarotid amobarbital test (IAT) scheduling; (3) dedicated ES clinic; and (4) adding a nurse navigator. ET from noninvasive video-EEG monitoring (P1) to IAT, PCC, and ES, and ES volume were determined and compared for a baseline group (P1 January 2009–March 2013) and a group exposed to process changes (P1 after March 2013), the postchanges group, to assess the effect of these measures.
Results:
ES number was 61 for the baseline group and 77 for the postchanges group, increasing the annual rate at 3 years after changes from 14.4 to 36.8 (p = 0.0008; 37% yearly increase postchanges). Interventions lowered average ET by 96 days (p ≤ 0.0001), P1 to IAT by 39 days (p = 0.0011), and P1 to PCC by 58 days (p = 0.0002).
Conclusions:
Simple process changes, including more frequent patient care conferences, faster scheduling, a dedicated ES clinic, and a nurse navigator significantly decreased evaluation times and increased ES numbers. Centers could utilize similar strategies to improve process and surgical volume and thereby increase patient seizure control and safety.
Epilepsy is drug-resistant in about a third of patients.1 The majority of adult cases have focal-onset seizures, which are potentially curable by removing the seizure-onset area. Surgery has been shown to be superior to best medical treatment and to reduce patient mortality in temporal lobe epilepsy.2,3 Analyses have demonstrated that the number of epilepsy surgeries (ES) falls short of the estimated demand at the cost of patients' lives.4
The preprocedural workup for elective ES requires a specialized center and consists of multiple tests aimed at defining the epileptogenic zone and determining risk for postoperative deficits. At a minimum, MRI is performed to determine the presence of a lesion, and video-EEG monitoring is done to capture and localize typical seizures. In nonlesional cases, additional functional or metabolic imaging is used to visualize areas of abnormal brain function correlating with a seizure focus, e.g., PET. Neuropsychological testing and intracarotid amobarbital testing (IAT) can identify impaired memory domains that might point to the seizure onset and help stratify risk of surgery. Data are typically discussed in a multidisciplinary patient care conference (PCC), which includes epileptologists, neuropsychologists, neuroradiologists, neurosurgeons, and other specialists, whose recommendations guide the surgical approach.
Surgical volume at our center was low in 2009–2012 (with acceptable record-keeping) when considering a catchment area with approximately 7 million people, including ∼15,000 with drug-resistant seizures,5 prompting process analysis and measures aimed at shortening process time to reduce patient exposure to risks associated with refractory epilepsy.
METHODS
The University of Colorado Hospital patient database was searched for all ES performed between January 2009 and May 2016. We retrospectively determined the number of cranial ES and reviewed the times to different steps in the process, including the procedure itself.
Evaluation time (ET) was defined as the time from noninvasive video-EEG monitoring (P1) to ES. In addition, times to specific milestones within the presurgical work-up, IAT and PCC, were analyzed. IAT was chosen as it requires coordination with another department (interventional radiology or neurosurgery), which creates a barrier to fast processing. The PCC time point was picked as measure of completion of all testing deemed necessary for an individual patient, prior to referral to surgery (e.g., PET and neuropsychological testing). Included were all patients who had cranial surgery and proceeded with testing without apparent delay for personal reasons, called typical patients, in order to capture timelines that represent the process and its effects largely unaffected by individual circumstances. Cranial surgery comprised resection, laser ablation, or invasive monitoring (P2) with or without brain removal. Excluded were patients who postponed testing or ES because of personal reasons (e.g., change of insurance, securing employment, family coverage for children or pets), so-called atypical patients, as they do not reflect process changes, and those with vagus nerve stimulation surgeries, since these were not reliably presented at PCC in the years before our interventions. Patients were categorized into (1) those who had P1 and surgery prior to any process changes between January 2009 and March 2013, constituting a baseline group, and (2) those who had workup and surgery after process changes were introduced between April 2013 and May 2016, the postchanges group.
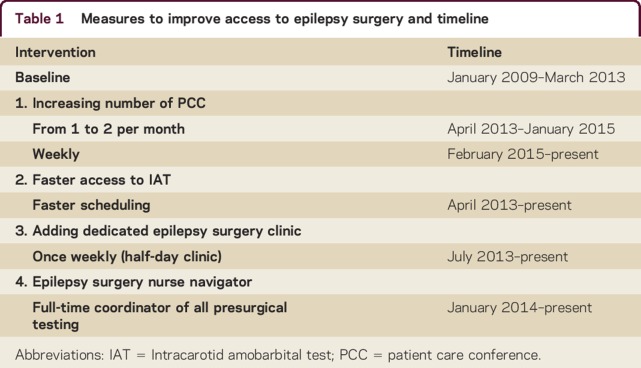
Measures to improve access to surgery are listed in table 1, and included (1) increasing PCC frequency; (2) faster scheduling of presurgical patients for tests with longer wait times, such as IAT, by creating appointment slots specifically for ES patients; (3) adding a dedicated ES clinic for review of results, education of patients, planning of further testing, and referral to neurosurgery; and (4) hiring of a nurse navigator (NN) to coordinate the evaluation process.
Table 1.
Measures to improve access to epilepsy surgery and timeline
Surgery numbers and ET from P1 to resection or P2, and to essential steps within the evaluation, i.e., IAT and PCC, were recorded and compared between the baseline group and the postchanges group. Process changes were not all introduced at once, but were staggered, with the NN added last since the position had to be approved and financed by administration. For the purpose of determining the effect of different factors introduced sequentially, we further divided the postchanges group into an intermediate subgroup that was exposed to the abovementioned improvement measures 1, 2, and 3, between April 2013 and December 2013, and a navigator subgroup, which underwent the presurgical workup with all process changes in place, including the NN, starting in January 2014. Time to IAT, PCC, and ES were compared for baseline and both subgroups.
Regarding statistical analysis, only patients who actually received a test or met a milestone and ultimately had surgery were considered. A p value of 0.05 was used as a cutoff for significance. Summary statistics and confidence intervals were prepared by status (baseline group vs postchanges group), and boxplots, histograms, and QQ plots were inspected. Log transforms, geometric means, ratios, and nonparametric tests were considered as remedies for severe non-normality, right skewness, and large outliers. Two-sample Satterthwaite t tests were performed and confidence intervals were constructed for the mean differences between groups. Wilcoxon tests were also considered. Poisson and Piecewise models were assumed for ES rates.
Standard protocol approvals, registrations, and patient consents
Our project received approval from our ethical standards committee on human experimentation (Colorado Multiple Institutional Review Board associated with the University of Colorado School of Medicine) for any experiments using human subjects. The protocol and interventions were reviewed and the project was determined not to be human subject research.
RESULTS
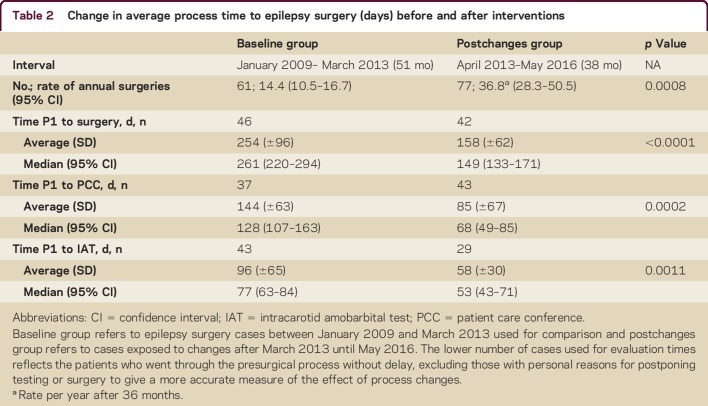
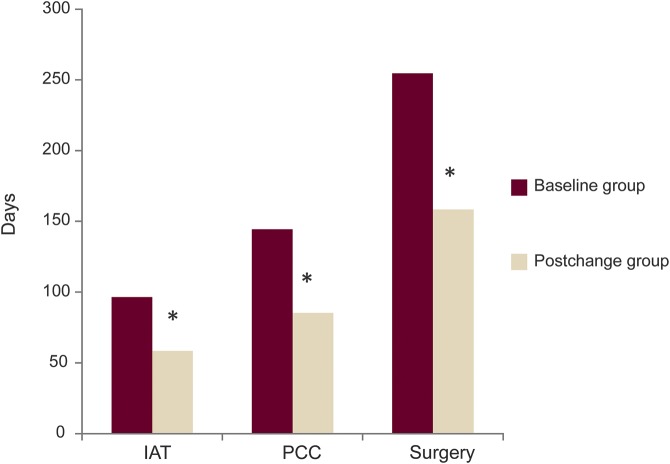
Results of our analysis comparing baseline and postchanges groups with respect to time to surgery, and to critical milestones within the preoperative evaluation process, are described in table 2 and graphically depicted in figure 1. Note that the designated interventions—increased frequency of PCC, preferred scheduling for IAT, formation of a dedicated ES clinic, and implementation of a NN—led to decreases in time to surgery (p < 0.0001) and preoperative evaluation milestones. Interventions lowered average ET by 96 days (p ≤ 0.0001), time from P1 to IAT by 39 days (p = 0.0011), and time from P1 to PCC by 58 days (p = 0.0002).
Table 2.
Change in average process time to epilepsy surgery (days) before and after interventions
Figure 1. Change in average process time to epilepsy surgery (days) before and after interventions.
Baseline group refers to epilepsy surgery cases between January 2009 and March 2013, and postchanges group (exposed to process changes) to cases after March 2013 until May 2016. IAT = intracarotid amobarbitol test; PCC = patient care conference. *Significant decrease compared to baseline.
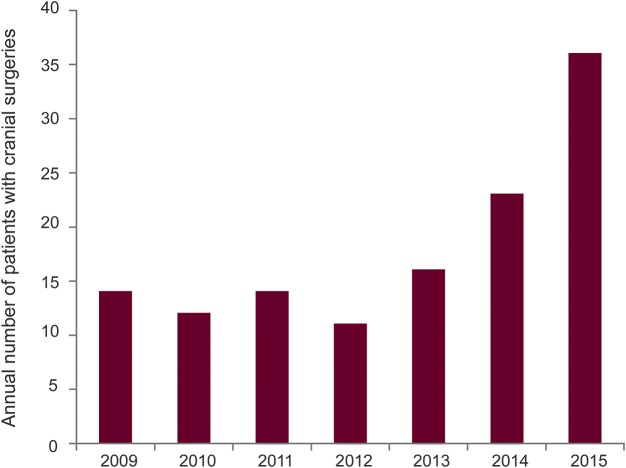
The number of cranial surgeries was 61 in the baseline group and 77 in the postchanges group, representing an increase of the annual surgery rate at 3 years after introducing changes from 14.4 to 36.8 (p = 0.0008), equivalent to a 37% yearly increase after changes (figure 2). It should be emphasized that the estimate of the annual rate reflects the rate at the time point of 3 years postchanges. The rate at earlier postchanges times and the average over the whole 3-year period postchanges were less (77 cases averaged over 3 years is ∼26/y). However, those numbers do not reflect the dynamic change over time, with an increasing annual surgery volume as demonstrated in figure 2.
Figure 2. Annual number of patients with cranial epilepsy surgery (2009–2015).
Process changes were introduced starting in April 2013. At 3 years after process changes were introduced, the annual surgery rate increased from 14.4 to 36.8 (p = 0.0008), equivalent to a 37% yearly increase.
We determined that the increase of procedures was not simply due to an increase in referrals. The surgery candidates were all taken from the pool of patients referred to the video-EEG monitoring unit (EMU). Between 2009 and 2012, there was an average of 334 annual EMU admissions (range 325–343/y), and between 2013 and 2015 an average of 349 admissions (range 303–386/y). Of all patients admitted to the EMU, before 2013, 3%–4% had surgery, and since 2013, 5%–10% underwent a cranial procedure (higher percentage in more recent years).
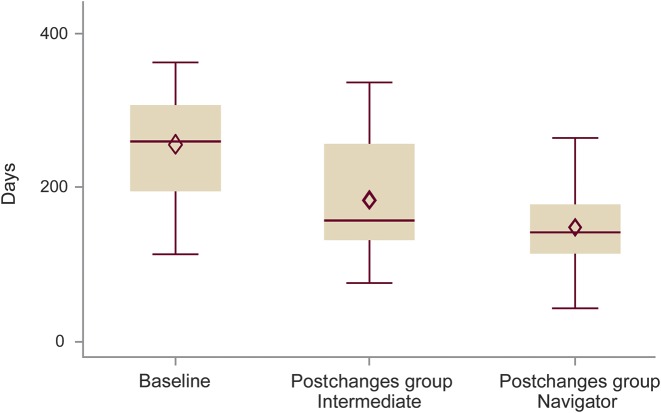
How much exactly each individual intervention influenced ET could not be determined. However, an analysis of the time to surgery for subcategories, i.e., the intermediate subgroup (patients exposed to improvement measures 1, 2, and 3 between April 2013 and December 2013) and the navigator subgroup (patients who underwent the presurgical workup with all process changes in place, including implementation of a NN), revealed that initial changes already improved time to surgery, with the addition of a NN conferring extra benefit in terms of reducing process time (figure 3). Specifically, statistical analysis showed a reduced ET comparing the baseline group with the navigator subgroup (p < 0.0211). ET was faster between the baseline group and the intermediate subgroup, and the intermediate and navigator subgroups, but those differences were not significant. For patients who did not proceed directly with surgery (atypical patients), the time to PCC was shortened by 156 days (p = 0.0112), but times to IAT and ES were not different compared to before and after process changes.
Figure 3. Time to surgery.
Time to surgery (months) for patients prior to implemented changes (baseline group), those exposed to some changes (intermediate subgroup of the postchanges group), and those exposed to all process changes, including addition of a nurse navigator (navigator subgroup) of the postchanges group. A nurse navigator was associated with a shorter time to surgery compared to the baseline group (p = 0.0211).
DISCUSSION
While guidelines exist that advise on the components of a comprehensive ES program, it is difficult to find literature that has quantified efforts to efficiently structure such a program. Our data provide evidence that addition of specific staff, clinics, and conferences can streamline a necessarily complex process and may have implications for other multidisciplinary subspecialty programs—such as the multidisciplinary evaluations required for consideration of deep brain stimulation in the movement disorders population.
Guidelines created by national organizations, including the National Association of Epilepsy Centers (NAEC) in the United States (Guidelines for Essential Services, Personnel, and Facilities in Specialized Epilepsy Centers, 2010),6,7 or the National Institute for Clinical Excellence in the United Kingdom (The Diagnosis and Care of Children and Adults With Epilepsy, 2012),8 summarize elements of necessary diagnostic testing for patients with epilepsy, and essential staffing and competencies of epilepsy centers.7,8 For example, the NAEC guideline published in 2010 specified that a Level 4 facility is expected to offer complex neurodiagnostic monitoring and comprehensive evaluations for ES, including specialized neuroimaging (e.g., PET) and intracranial monitoring, with a team of experienced nurses, technologists, epileptologists, neurosurgeons, neuropsychologists, and psychologists, as well as various surgical procedures for epilepsy.7 However, the structure and processes necessary to run a comprehensive ES program effectively are not delineated.
Review of the workup for ES at our institution revealed a number of steps associated with delays. More frequent conferences, better coordination and communication with other departments, as well as dedicated staff and clinics resulted in shorter times to critical preoperative evaluation milestones and surgery. Of course, there are departments that have a well-organized process in place, but there are others in which the described tools could be employed and improve patient flow, or those that are considering starting an ES program. While the process improvement interventions were basic and their effectiveness not really surprising, this report includes measuring the effect of procedural changes providing a clearer idea of how to organize an ES program or another similar subspecialty program more efficiently. One should be prepared, though, that each introduced component may require an adjustment period in staff behavior, necessary to overcome habitual practice established over years prior to any changes.
An NN informs about the tests, schedules appointments, creates a liaison with other departments and their schedulers to facilitate timely and bundled testing, and communicates with insurance companies. Since the presurgical process consists of multiple steps and can be lengthy and confusing for patients, a coordinator is a reassuring presence and by tracking tests unnecessary delays to diagnostic procedures and presentation at PCC can be avoided. At our institution, the NN took on the essential duties of ascertaining that patients have orders placed, have appointments made, and actually go through testing and conference presentation. Physicians within our epilepsy division indicated that prior to an NN they would lose track of surgery candidates among the hundreds of clinic visits, which left some of the patients stranded, until another clinic appointment reminded the respective provider to resume the presurgical work-up.
Navigators are key additions to an ES program or other subspecialty programs, as they can act, e.g., in Great Britain, as independent care providers, facilitate access to specialized clinics, and play an important role in patient education.9,10 As a result, they increase the volume of evaluated and treated patients and improve patient satisfaction.11 Furthermore, they decrease the cost for the institution and health care system by optimizing care and its availability.10 The creation and funding of this position can be challenging in times of increasing resource stewardship. However, coordinator positions are expected to easily pay for themselves, as the results presented here underscore the utility of an NN in building a more efficient program, resulting in greater numbers of surgeries and increasing the contribution margin of a subspecialty program. Review of salary range and reimbursement for surgical procedures at our institution revealed that salary and benefits for an NN position ranges from $65,000 to $90,000 annually, and average net revenue for 1 ES is $59,000. Of course, these numbers can only be seen as examples, as they may vary depending on multiple factors and therefore cannot be universally applied. Yet, with the addition of an NN, the number of annual surgeries more than doubled from around 15 to more than 30 per year, creating enough reimbursement to help justify the position in negotiations with local hospital administrators.
Increasing patient care conferences (PCC) from monthly to weekly helped channel more patients towards surgery, when considering that each PCC affords enough time to thoroughly discuss 2 to 4 patients. Times to IAT decreased as these investigations were scheduled more quickly by using appointment slots that were blocked for epilepsy patients considered for surgery.
The ES clinic helped focus staff efforts and provided an opportunity to educate patients with respect to epilepsy, testing, and surgical and nonsurgical treatment options. Patients were scheduled for this clinic by the NN after tests were completed or as decision points were reached, and they were appreciative of considerable time spent explaining in detail the purpose and meaning of procedures, results, and outcomes.
Limitations of this project include its retrospective nature and the lack of a control group, obligating comparison to a historical baseline. The data represent the experience during a practice improvement project at a single institution and as such are not generalizable. It was difficult to determine the changes related to each individual component, as interventions were staggered but close enough in time to cause overlapping effects of several factors. This was because elements were introduced as quickly as each became available because the goal of the process changes was to improve patient care without delay. While time to surgery was reduced from 10 months to 5 months, efforts are continuing to identify other ways to improve and shorten the process further. Even though added staff has taken over portions of the process, we have not yet done a formal assessment on how much administrative physician time was saved or how tasks were redistributed to have them assigned more appropriately to the professional level, e.g., of a nurse or physician. Finally, the increase in surgery numbers is expected to be due in part to a faster process addressing a backlog of patients waiting to undergo presurgical testing and surgery, producing a steeper increase in surgical volume than without such a conceivable accumulation. Aspects that were not addressed in our analyses were psychosocial features of our patients, which may have affected their willingness and drive to undergo surgery.
Additional elements that may affect ET are as follows: (1) insurance type and associated length of time required to gain access to the dedicated ES clinic and preoperative testing; (2) neurosurgeon scheduling practices and availability; (3) socioeconomic factors12; and (4) patient and physician attitudes towards surgery.13–16 For example, a study by Erba et al.16 showed that patient acceptance of surgery is dependent on educational level, leading to rejection of a surgical option especially in patients with low socioeconomic status and less than college education. Surveys focusing on physicians indicated that some knowledge and experience with indication and outcomes of ES was lacking in 32%–60% of neurologists and 53% of family practitioners.13–15
The process changes introduced in our institution led to an increased number of ES and faster process times. Similar strategies could be implemented in other centers to increase volume and reduce wait time to surgery, thereby improving patient safety—due to lower epilepsy-related morbidity and mortality—as well as patient satisfaction. Correspondingly, other multidisciplinary processes could consider modeling a program using comparable components.
Additional efforts are underway to improve the process further to allow even faster access to all intra-institutional providers and departments, to educate patients and outside providers about the indications and benefits of early surgery in selected cases, and to assess how a more efficient process influences outcomes—seizures and overall well-being—as well as cost.
AUTHOR CONTRIBUTIONS
C. Drees conceptualized the project and drafted the original manuscript. S. Sillau provided statistical analysis and interpretation of the data. M.-G. Brown participated in analysis or interpretation of the data and revised the manuscript for intellectual content. A. Abosch participated in analysis or interpretation of the data and revised the manuscript for intellectual content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
C. Drees, S. Sillau, and M.-G. Brown report no disclosures. A. Abosch serves on a scientific advisory board for the Disease Modification in Early Parkinson's Disease Working Group (sponsored by APDA and PDF; Vanderbilt MC); has received speaker honoraria from RuiJen Hospital, Shanghai, China; serves as an Associate Editor for Stereotactic & Functional Neurosurgery, Journal of Neurosurgery, Neurology, and Psychiatry, and Neurosurgery; is author on a provisional patent re: Imaging Table-to-Head Frame Adaptor; and serves as a consultant for Medtronic. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319. [DOI] [PubMed] [Google Scholar]
- 2.Wiebe S, Blume WT, Girvin JP, Eliasziw M; Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311–318. [DOI] [PubMed] [Google Scholar]
- 3.Engel J Jr, McDermott MP, Wiebe S, et al. ; Early Randomized Surgical Epilepsy Trial (ERSET) Study Group. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 2012;307:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burneo JG, Shariff SZ, Liu K, Leonard S, Saposnik G, Garg AX. Disparities in surgery among patients with intractable epilepsy in a universal health system. Neurology 2016;86:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.England MJ, Liverman CT, Schultz AM, Strawbridge LM, eds. Epilepsy Across the Spectrum: Promoting Health and Understanding. Washington, DC: National Academy of Sciences; 2012. [PubMed] [Google Scholar]
- 6.Labiner DM, Bagic AI, Herman ST, Fountain NB, Walczak TS, Gumnit RJ; National Association of Epilepsy Centers. Essential services, personnel, and facilities in specialized epilepsy centers: revised 2010 guidelines. Epilepsia 2010;51:2322–2333. [DOI] [PubMed] [Google Scholar]
- 7.NAEC. Guidelines for Essential Services, Personnel, and Facilities in Specialized Epilepsy Centers. 2012. Available at: naec-epilepsy.org/wp-content/uploads/GuidelinesEpilepsia.pdf. Accessed March 23, 2017. [Google Scholar]
- 8.NICE GNIfHaCE. Epilepsies: Diagnosis and Management. NICE Guideline [CG 137]. 2012. Available at: nice.org.uk/guidance/cg137. Accessed March 23, 2017; Updated February 2014; Cited March 27, 2016. [Google Scholar]
- 9.Goodwin M, Higgins S, Lanfear JH, Lewis S, Winterbottom J. The role of the clinical nurse specialist in epilepsy: a national survey. Seizure 2004;13:87–94. [DOI] [PubMed] [Google Scholar]
- 10.Camicia M, Chamberlain B, Finnie RR, et al. The value of nursing care coordination: a white paper of the American Nurses Association. Nurs Outlook 2013;61:490–501. [DOI] [PubMed] [Google Scholar]
- 11.Pfafflin M, Schmitz B, May TW. Efficacy of the epilepsy nurse: results of a randomized controlled study. Epilepsia 2016;57:1190–1198. [DOI] [PubMed] [Google Scholar]
- 12.Begley C, Basu R, Lairson D, et al. Socioeconomic status, health care use, and outcomes: persistence of disparities over time. Epilepsia 2011;52:957–964. [DOI] [PubMed] [Google Scholar]
- 13.Hakimi AS, Spanaki MV, Schuh LA, Smith BJ, Schultz L. A survey of neurologists' views on epilepsy surgery and medically refractory epilepsy. Epilepsy Behav 2008;13:96–101. [DOI] [PubMed] [Google Scholar]
- 14.Kumlien E, Mattsson P. Attitudes towards epilepsy surgery: a nationwide survey among Swedish neurologists. Seizure 2010;19:253–255. [DOI] [PubMed] [Google Scholar]
- 15.Cothros N, Burneo JG, Steven DA. Knowledge and attitudes about epilepsy surgery among family doctors in Ontario. Can J Neurol Sci 2016;43:672–677. [DOI] [PubMed] [Google Scholar]
- 16.Erba G, Messina P, Pupillo E, Beghi E; OPTEFF Group. Acceptance of epilepsy surgery among adults with epilepsy: what do patients think? Epilepsy Behav 2012;24:352–358. [DOI] [PubMed] [Google Scholar]