Abstract
Context:
Docosahexaenoic acid (DHA) is an omega-3 fatty acid essential for cardiovascular health, brain development, and reproductive function. Due to hydrophobicity and low DHA bioavailability, new microencapsulated DHA formulations are under development.
Aim:
This study aims to evaluate DHA pharmacokinetics (PKs) and biological oxidation parameters in volunteers ingesting a newly developed lutein-containing lycosomal formulation of DHA (LF-DHA).
Materials and Methods:
A total of 32 healthy volunteers (40–65 years old) with signs of oxidative stress (OS) and subclinical hypoxia were orally supplemented for a month with 250 mg of regular DHA (1st group) or a combination of lutein (7.0 mg) and zeaxanthin (1.4 mg) (2nd group). The third group received regular DHA (250 mg) co-ingested with lutein/zeaxanthin (7.0/1.4 mg), whereas the 4th group was given LF-DHA containing lutein/zeaxanthin (7.0/1.4 mg). PK, OS, and oxygenation parameters were analyzed.
Results:
LF-DHA improved the PKs of DHA enhancing its serum concentrations time dependently by 34.6% and 94.1% after 2nd and 4th weeks, respectively. DHA and lutein ingested either alone or simultaneously as two separate formulations reduced the levels of OS markers. However, LF-DHA inhibited the malonicdialdehyde (MDA) and oxidized low-density lipoprotein values were better than other formulations. LF-DHA also enhanced the plasma oxygen and tissue oxygen saturation. This effect was significantly higher than in other groups.
Conclusion:
LF-DHA eliminates the need in high-dose DHA supplementation protocols and confers a higher DHA bioavailability, thereby improving the parameters of biological oxidation and tissue respiration in affected individuals.
Keywords: Docosahexaenoic acid, lutein, oxidative stress, oxidized low-density lipoprotein, oxygenation
Introduction
Health benefits of the long-chain n-3 polyunsaturated fatty acids (LC n-3-PUFA) docosahexaenoic (DHA) and eicosapentaenoic (EPA) acid for the cardiovascular system, brain development and its support, sensory, and reproductive functions have been confirmed in multiple studies.[1,2,3] Mammals cannot synthesize LC n-3-PUFA and acquire them from foods (seafood, plants, eggs, and meat). Higher LC n-3-PUFA consumption is linked with lower incidence of cardiovascular and inflammatory diseases.[4,5] There is no consensus on optimum daily consumption of LC-3-PUFA. However, some authorities, in particular, the Academy of Nutrition and Dietetics, recommend daily intake of 500 mg n-3-PUFA.[6,7] At the same time, multiple studies suggest that actual daily intake of LC n-3-PUFA remains much lower even in developed countries.[7] Even well-designed nutritional strategies are often not sufficient enough to achieve a significant intake of LC n-3-PUFA from dietary sources. It can be related to the low bioavailability rate of LC fatty acids. All LC n-3-PUFAs are water-insoluble amphiphilic substances which require extensive emulsification with co-ingested fat by pancreatic lipase and can be transported through intestinal epitheliocytes only via mechanisms of energy-dependent transport.[8,9] Thus, the overwhelming popularity and widespread adherence to low-fat diets in the developed countries may have also limit LC n-3-PUFA intestinal absorption rates since dietary fat is essential for postprandial assimilation of omega-3 fatty acids.[10] Moreover, a significant part of LC n-3-PUFA undergoes extensive oxidation in the intestinal lumen and serves as a substrate for mitochondrial beta oxidation in the intestine and liver.[8] Bioavailability of LC n-3-PUFA becomes even more compromised in the aging individuals due to age-related decline in pancreatic lipase production.[11] To overcome the bioavailability problem, various nutraceutical formulations of LC n-3-PUFA with an enhanced bioavailability have been recently proposed.[12]
As we have shown below, microencapsulated lycosomal formulation of DHA has not only enhanced its bioavailability rate in middle-aged individuals, but also normalizes peripheral tissue oxygen saturation (StO2) and reduces blood markers of oxidative damage after a month intake.
To create these lycosomes, we used a combination of carotenoids (lutein and zeaxanthin), which could provide some level of DHA protection from stomach acidity as well as enzymatic and other gastrointestinal (GI) factors upon microencapsulation.
Materials and Methods
The study was conducted at the Institute of Cardiology, the Ministry of Health of the Russian Federation (Saratov, RF). The trial was a part of broader nutraceutical trials registered (NCT01199549 and ISRCTN89815519) and approved by the local ethical committee. All patients were fully informed about the purpose of the study and had given written consent to their participation in the clinical trial.
The present study was a part of a larger multi-arm trials which included 89 clinically healthy middle-aged persons with reduced level of peripheral tissue StO2 and positive for presence of oxidative damage blood markers, who were not taking any medications or supplements, containing omega-3 fatty acids, lutein, or other antioxidants. Oxidative stress (OS) was defined as a status characterized by detection in the serum of the presence of oxidized low-density lipoprotein (LDL) and markers of inflammatory oxidative damage (IOD). Subclinical hypoxia, or reduced tissue oxygenation, was defined as a state with age-associated reduction of peripheral tissue StO2 and reduced level of plasma oxygen transport. Individuals positive for both groups of markers (subclinical hypoxia and OS) were recruited for the study and underwent thorough clinical and anamnestic investigation. After 2 weeks, all patients were rescreened and randomized into four study groups of eight patients per group) and given 2 weeks’ supply of the study products. All tests were performed at the baseline and intermediate time point, 14 days of the intake of investigational products, and at the completion of the study, 28th day of the trial. Patients were given another 2 weeks’ supply of the study products at the intermediate visit. Clinical tests and examination were carried out in the beginning, at the trial, and at its end. Twelve patients were unable to complete the study for various reasons not related to the intake of the test products and were replaced with qualifying individuals from the prescreened pool. Patient illegibility was determined by the following inclusion/exclusion criteria.
Inclusion criteria
Caucasian male or female individuals aged 40–65 years with the following criteria: (a) gave signed consent form; not on anti-hypertensive, lipid-lowering, or anti-diabetic drugs; serum positivity for markers of inflammation and oxidation such as oxidized LDL-Px ELISA ≥200 U, IOD ≥40 μM of MDA, as well as willingness and ability to comply with the protocol for the duration of the study; (b) Abnormal values of oxygen turnover which include tissue StO2 <80% (area under the curve [AUC]/min) and plasma oxygen transport <700 (×5 μM O2/L).
Exclusion criteria
Exclusion criteria included unwillingness to sign the informed consent, inability to comply with the protocol of the study, significant medical condition that would impact safety considerations (severe cardiovascular disease, hepatitis, severe dermatitis, uncontrolled diabetes, cancer, severe GI disease, fibromyalgia, renal failure, recent cerebrovascular accident, pancreatitis, respiratory diseases, epilepsy, HIV/AIDS, and compulsive alcohol abuse [>10 drinks weekly], or regular exposure to other substances of abuse, concurrent participation in other dietary studies or clinical studies, allergy to omega-3 supplements or fish oil). Individual dietary patterns were questioned and recorded during the enrollment process and used for the randomization procedure. All participants were asked to adhere to habitual dietary pattern during the study. No adverse effects were reported during the study.
Study products
(1) Conventional formulation of DHA: Individuals from the first group were given once daily a capsule containing 250 mg of DHA (DSM, Switzerland), (2) Lutein: volunteers from the second group were advised to take once daily 1 capsule containing a combination of 7 mg of lutein (Lycored, Switzerland) and 1.4 mg of zeaxanthin (PIVEG, USA), (3) Co-ingestion of DHA and lutein-zeaxanthin: two separate capsules, one containing 250 mg of DHA and the other one containing 7 mg of lutein with 1.4 mg of zeaxanthin, were given to volunteers in the 3rd group at the same time, (4) Lycosome formulation of DHA: individuals from the 4th group were instructed to ingest once daily one capsule containing 250 mg of DHA microencapsulated in the lutein-zeaxanthin lycosomes with their dose per capsule at 7 and 1.4 mg, respectively.[13] Use of lycosome microencapsulation technology has been shown in our previous work to promote overall bioavailability of nutraceuticals by increasing their resistance to oxidation in the stomach and GI tract, enhancing their intestinal absorption rate, and optimizing their delivery to the internal organs and tissues.[13,14,15,16,17,18] All the study products were ingested once daily in the morning hours with breakfast meals for 4 weeks (28 subsequent days).
Biochemistry and inflammatory markers
Blood was collected in the morning hours between 8 am and 9 am after night fast and before breakfast, from arm veins of the patients. The serum was separated by centrifugation, and then aliquots were stored at −80°C prior to analysis. Glucose, total cholesterol, triglycerides, high-density cholesterol, LDL, and C-reactive protein were measured using commercially available analytical kits according to the manufacturers’ recommendations (Applied BioSystems, Foster City, CA, USA, R&D Systems, Minneapolis, MN, USA).
Pharmacokinetic (PK) blood sampling was performed after ingestion of a single oral dose of lycosome-formulated DHA or regular DHA. Both formulations were given at a 50 mg dosage. Each formulation was tested in six volunteers. The blood specimen sampling included the following time points: 30 min, 1, 2, 4, 6, 9, and 12 h after oral dosing. For each individual, there was a baseline adjustment conducted by subtracting the predose value from each time point variable of DHA and EPA during the postingestion period. All adjustments were patient specific. Negative values were converted to “0.” The obtained parameters were used for the calculation of overall AUC0-t and Cmax values which represent the maximum serum concentration during the whole sampling period. Moreover, average serum concentration (Cavg) during the post-dosing period was calculated along with time (tmax) at which the highest DHA/EPA levels were observed. AUC0-t, Cmax, and Cavg were presented as geometric mean (geometric CV%), whereas tmax was calculated as median value with confidence intervals.
48 h after completion of acute ingestion protocol, the volunteers were given the study products and the 4-week ingestion study was initiated according to the protocol described above.
Lutein and zeaxanthin concentrations in all serum samples were measured in duplicate by high-performance liquid chromatography (HPLC),[19] with modifications. Briefly, 400 μl of serum was mixed with 400 μl of ethanol and was extracted twice with 2 ml of hexane. The combined hexane layers were evaporated to dryness under vacuum (Scan Speed 32 centrifuge) and the residue reconstituted to the volume of 100 μl with sample solution (absolute ethanol–methylene chloride, 5:1, v/v). The specimens were centrifuged again (15 min at 10,000 g) and the clear supernatant was transferred into HPLC vials. The separation module was an Aquity UPLC H-Class (Waters, USA). Five microliters of the extract was injected into a YMC Carotenoid S-3 2.0 × 150 mm × 3 μm column, preceded by a YMC Carotenoid S-3 2.0 mm × 20 mm guard cartridge (YMC, Japan) and eluted at 30° with a mobile phase consisting of acetonitrile (A), 0.08% phosphoric acid solution (B), methanol (C), and tert-Butyl methyl ether (D) at a flow rate of 0.4 ml/min. The following gradient elution was used: 10% A, 1% B, 79% C, and 10% D, 0–4.0 min; 0% A, 0% B, 30% C, and 70% D, 5.0–7.0 min; and returned to 10% A, 1% B, 79% C, and 10% D after 7.1 min. The lutein peak was detected by a photodiode array detector (Waters, USA) at 444 nm. The peak area was measured using the Empower 3 software (Waters, Milford. MA, USA). The lutein concentrations in serum samples were calculated on the basis of the standard concentrations. Analytical standard (Lutein, Fluka 07168) was used in the assay.
EPA and DHA concentration in all serum samples was measured in duplicate by gas–liquid chromatography,[20] with slight modifications. Briefly, 2 ml of stock solution required for each sample included 1.9 ml of methanol and 100 μl of acetyl chloride. Briefly, 100 μl of serum and 2 ml of the stock solution were combined in screw-capped glass tubes. The tubes were capped and heated at 100°C for 60 min. The tubes were allowed to cool to room temperature; after that, the extraction with 1 ml hexane was repeated twice. The combined hexane solution was evaporated under vacuum (Scan Speed 32 centrifuge) and the residue reconstituted to a volume of 50 μl of hexane, transferred to gas-chromatography vials, and capped under nitrogen.
Fatty acid analysis was performed with a fused silica capillary column (HP-5) of 30 mm × 0.32 mm inner diameter and 0.25-μm film thickness (Hewlett Packard, USA), a Shimadzu GC 2010 Gas chromatograph with Flame Ionization Detector, and manual injection system (Shimadzu, Japan). Temperature program was as follows: initial: 130°C with a 4-min hold and ramp: 4°C/min to 280°C with a 2-min hold. Carrier gas was He, with a linear velocity of 30 cm/s. Fatty acid analysis was performed by injection of 1 μl of each sample at a split ratio of 50:1. The flame ionization detector and the injection port temperature was 300°C. The sampling frequency was 40 Hz.
Fatty acid identification was performed with SUPELCO 37 COMPONENT FAME MIX (Supelco, USA). DHA and EPA concentrations in serum samples were calculated on the basis of the standard concentrations. Analytical standards (EPA methyl ester and DHA; Sigma, USA) were used in the assay.
As a tissue target for the assessment of StO2, or combined level of oxygenated hemoglobin and myoglobin, we used the thenar eminence and forearm muscles of the patients.[21] StO2 was analyzed by continuous wavelength near-infrared spectroscopy, with wide-gap second derivative (In Spectra, Hutchinson Technology, MN, USA). The measurements were made at different time points. The recording began following 15 min rest in a supine position before occlusion of the brachial artery. It then continued during stagnant ischemia induced by rapidly inflating the cuff to 50 mmHg above systolic blood pressure. The ischemia lasted for 3 min, and the recording period lasted for another 5 min after that until StO2 was stabilized.
Then, the area under curve of the recorded signal for the settling time in the postocclusion period was calculated in % O2/min.
Plasma oxygen transport parameters were measured as described elsewhere.[15,22]
For the IOD assessment, plasma samples were incubated overnight in 0.05 M phosphate-buffered saline (PBS) acetate buffer (pH 5.6) which would imitate the type of oxidative damage which occurs during the release of lysosomes following neutrophil degranulation. The following morning, the reaction was terminated using trichloroacetic acid. The concentration of the end products such as MDA, and other possible thiobarbituric acid-reactive substances, was then measured by colorimetric methods using reagents and kits from Cayman Chemical (MC, USA). Oxidized LDL (LDL-Px) and activity of serum LDL peroxidase proteins, which include IgG with superoxide dismutase activity, were measured as described earlier.[15]
For the statistical assessment of normally distributed parameters, the Shapiro–Wilk method was used. Student's t-test was then applied both for paired and unpaired samples. In cases where parameters were not normally distributed, Mann–Whitney U-test and Kruskal–Wallis test were used. Analysis of variance and analysis of covariance were used with post hoc analysis (Statistica 9 suit and StatSoft; Inc, StatSoft, Palo Alto, CA, USA). Statistical significance between two-tailed parameters was considered to be P < 0.05.
Results
Table 1 shows that all baseline parameters of the volunteers enrolled in the trial were comparable and there were no statistically significant deviations in gender, age, and body mass index among the four major groups of the study. Additionally, plasma glucose and major lipid classes (results not shown) were closely matched among the groups.
Table 1.
Baseline characteristic of the enrolled patients (average±standard deviations)
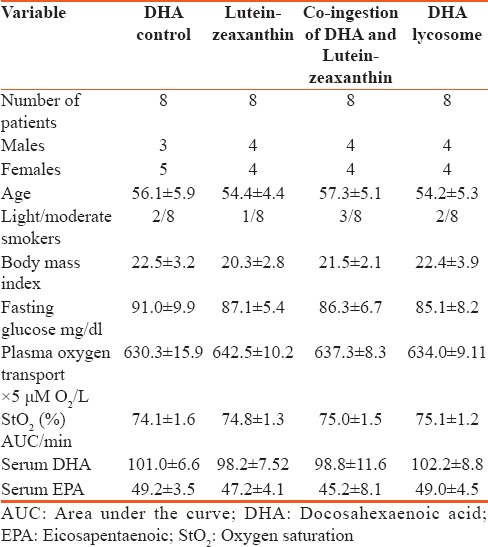
It is important to mention that all DHA/EPA concentration levels were similar in all the four groups of the study.
Table 2 shows the baseline-adjusted PK parameters after single-dose acute ingestion of lycosome-formulated and control-unformulated DHA.
Table 2.
Summary of baseline-adjusted serum total eicosapentaenoic + docosahexaenoic acid pharmacokinetic parameters
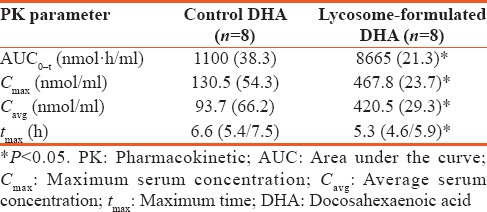
As can be seen, lycosome-formulated DHA caused an 8-fold increase in AUC0-t parameters in the post-dosing period. It was accompanied by nearly 4-fold increase of both the serum maximum concentration and average EPA + DHA concentration in the blood of volunteers ingesting with the lycosome-formulated DHA. It was interesting that the highest level of DHA + EPA in volunteers ingesting with lycosome-formulated DHA was seen at earlier time point as compared to individuals who were given regular formulation of DHA.
These results are in good agreement with data summarized in Table 3 which presents the serum concentration of DHA and EPA in the four major groups of the study [Table 3]. As shown, ingestion of the regular formulation of DHA led to a very modest but still statistically significant increase in serum DHA level after 14 days of supplementation (median increase by 17.0 μg/ml, P < 0.05) while there was no significant change in DHA level after 4 weeks of treatment (P > 0.05). As one would expect, ingestion of the lutein-zeaxanthin combination as a singular formulation did not affect serum DHA levels. Co-ingestion of these carotenoids and DHA as two separate capsules did not affect the magnitude of DHA buildup seen in the “DHA-alone” group. However, ingestion of lycosome formulation of DHA increased the serum level of DHA, especially after 4 weeks of ingestion by two fold. Interestingly, the increase in DHA level at the mid-point of the study in volunteers ingesting with lycosome formulation of DHA was also significantly higher than that in the “DHA-alone” group.
Table 3.
Median of serum docosahexaenoic and eicosapentaenoic acid levels with 95.0% confidence intervals)
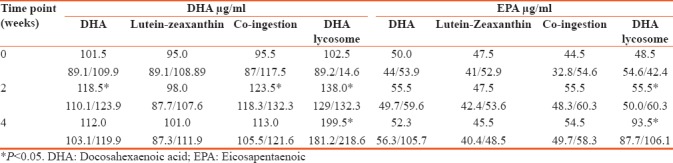
EPA levels changed less significantly. There was no increase in EPA concentration at all time points in volunteers ingesting DHA alone or when DHA was taken with the blend of lutein-zeaxanthin in two separate capsules. A borderline increase in EPA level was seen after 2 weeks of ingestion of lycosome-formulated DHA although 4-week consumption was accompanied by a 2-fold increase in serum EPA level [Table 2].
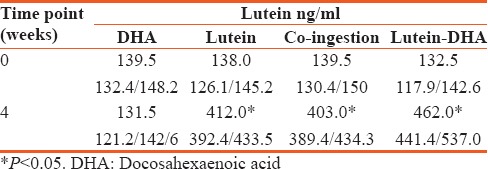
Table 4 shows the changes in serum lutein level in the course of the study. Ingestion of DHA alone did not affect serum lutein concentration. Ingestion of carotenoids alone resulted in increase of its serum concentration by approximately three folds. Similar increase took place in volunteers ingesting DHA and lutein in two capsules. Interestingly, intake of the lutein as a part of the DHA lycosomes resulted in more significant buildup of this carotenoid in the serum (P < 0.05) than after taking it alone or with DHA but in separate capsules.
Table 4.
Serum lutein levels (medians with 95% confidence intervals)
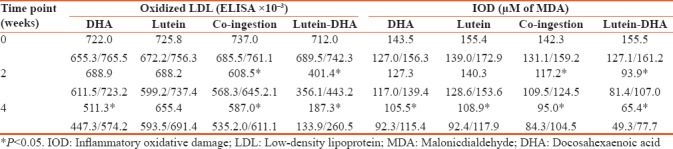
Some important changes were observed in the serum oxidative parameters. As shown in Table 5, ingestion of DHA alone led to reduction of oxidized LDL and IOD level by 29.1% and 26.4% respectively, at the end of the study. In contrast, 1-month ingestion of lutein alone did not affect oxidized LDL values, but reduced IOD levels by 29.9% which had more profound effect on serum oxidation parameters. In particular, there was a reduction of medians reflecting serum oxidized LDL by 128.5 and 150.0 points after 2 and 4 weeks of supplementation, respectively. Similar reduction took place in the IOD values at the same time points (reduction by 17.3 and 33.2%% from the control). However, supplementation with the lutein-zeaxanthin DHA lycosomes resulted in the most profound changes in serum oxidation parameters. In particular, this led to the reduction of oxidized LDL by 43.6 and 73.6%%; and IOD level by 39.6 and 57.9%% after 2 and 4 weeks of the trial, respectively.
Table 5.
Oxidized low-density lipoprotein and inflammatory oxidative damage (medians with 95% confidence intervals)
Furthermore, there were noticeable changes in the plasma and tissue oxygen balance of the volunteers [Table 6]. By the end of the observational period, there were increases in medians reflecting plasma oxygen transport in patients supplemented with lutein-zeaxanthin (increase by 151 points, P < 0.05), whereas DHA alone had no effect on the plasma oxygen. However, simultaneous intake of DHA and these carotenoids in two separate capsules resulted in additive effect of these compounds on this parameter. In this group, plasma oxygen transport median was upregulated by 312 points (P < 0.05). However, the most significant increase in plasma oxygen transport took place in patients supplemented with lycosome formulation of DHA when medians of plasma oxygen transport spiked by 454 points.
Table 6.
Plasma oxygen transport and tissue oxygen saturation (medians with 95% confidence intervals)
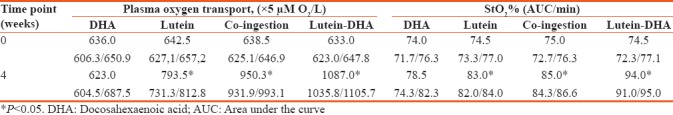
Similar pattern of changes were observed in the tissue oxygen saturation. Lutein alone modestly increased tissue oxygen saturation (by 8.5 points) in the volunteers. Interestingly, simultaneous but in separate capsules’ intake of DHA and carotenoids increased tissue oxygen saturation by 10 points (P < 0.05). Once again, lycosome formulation of DHA caused the most significant increase of tissue oxygen saturation after 4 weeks of ingestion, improving the control value by 19.5 points (P < 0.05).
Discussion
Although there is a significant body of scientific evidence arising from epidemiological and clinical studies that n-3 PUFA has a favorable impact on heart, brain, and vision functions, the majority of world populations lack to achieve adequate dietary intake of n-3 PUFA to maintain optimal health status.[23,24] It is widely accepted that 500 mg/day of DHA/EPA dose is adequate for maintaining cardiovascular health, although much higher amounts of n-3-PUFA (from 1000 to 5000 mg/day) are recommended for individuals with compromised health status.[6] High doses of DHA/EPA in the form of fish oil or as synthetic formulations are beneficial but known to be associated with a number of side effects ranging from heartburn, nausea, and diarrhea to pancreatitis, arrhythmias, and kidney failure.[25,26,27] Increased level of plasma LDL represents another side effect in excessive DHA use.[28]
The major conclusion from our work is that new nutraceutical formulations with an increased bioavailability of DHA/EPA, in particular in their lycosome formulation, may allow reducing the effective dose of DHA/EPA without compromising the health benefits of DHA/EPA supplementation. At the stage of planning of our work, we intentionally used a suboptimal dose of DHA/EPA (250 mg/day) for a month-long treatment of the volunteers. As described above, DHA intake in a conventional form at the dose of 250 mg/day does not translate into sustainable increase in serum DHA level. In contrast, lycosome formulation of DHA remarkably improves PK of DHA and enhances its serum concentrations in the time-dependent fashion in volunteers by 34.6% and 94.1% after 2nd and 4th weeks of supplementation, respectively. Serum EPA level followed the similar trend. Comparison of median distributions in the “DHA-alone” group versus lycosome-formulated DHA group allows to conclude that use of the lycosomes increased the DHA bioavailability rate by 2.1 and 9.2 folds, respectively, on the 2nd and 4th weeks of the study.
Deeper insight into the bioavailability of lycosome-formulated DHA was obtained in the acute ingestion protocol when it was compared with the convectional form of DHA. Although both products were ingested at an equal dose of 250 mg of DHA, the AUC values reflecting serum DHA + EPA level were 8-fold higher in individuals who were given the lycosome formulation. These results were in a good agreement with the higher maximum and average serum concentrations of DHA + EPA in the postingestion period when individuals were administered with the lycosome DHA. Finally, it is interesting to mention that the absorption peak of DHA in volunteers, who ingested the lycosome product, took place at earlier time point than that was seen with the conventional DHA.
Conclusion
In this PK analysis, the results obtained in the acute and 4-week studies reveal and confirm that the lycosome formulation of DHA has a significantly higher bioavailability rate when compared to the regular DHA. Therefore, introduction of lycosome-formulated DHA may eliminate the need in high-dose DHA treatment protocols which might be harmful for patients.
The increase in DHA bioavailability was translated in our study into important changes in the oxidative status of volunteers. According to our results, DHA and lutein ingested either alone or simultaneously but as two separate capsules reduced the level of markers of oxidative damage in volunteers in a comparable fashion. However, the lycosome formulation of DHA inhibited the value of IOD to a greater extent than in the other groups. That decline, according to our results, translated into reduction of serum oxidized LDL. It was noticed that either intake of the carotenoids and DHA alone, or simultaneously but in separate capsules, resulted only insignificant decrease in oxidized LDL concentration. Most pronounced reduction in the level of oxidized LDL, by 71%, took place when the lycosome formulation of DHA was ingested.
Study limitations
Our study has some limitations. To start with, a larger cohort of the patients required to confirm the clinical efficacy and pharmacodynamic parameters of the lycosome-formulated DHA. A longer treatment period would be beneficial to evaluate the effect of lycosome-formulated DHA not only on oxygen plasma and tissue parameters and OS markers but also on other physiological and clinical parameters. All these questions need to be answered in further research.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bowen KJ, Harris WS, Kris-Etherton PM. Omega-3 fatty acids and cardiovascular disease: Are there benefits? Curr Treat Options Cardiovasc Med. 2016;18:69. doi: 10.1007/s11936-016-0487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Königs A, Kiliaan AJ. Critical appraisal of omega-3 fatty acids in attention-deficit/hyperactivity disorder treatment. Neuropsychiatr Dis Treat. 2016;12:1869–82. doi: 10.2147/NDT.S68652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lalia AZ, Lanza IR. Insulin-sensitizing effects of omega-3 fatty acids: Lost in translation? Nutrients. 2016;8 doi: 10.3390/nu8060329. pii: E329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimmig LM, Karalis DG. Do omega-3 polyunsaturated fatty acids prevent cardiovascular disease? A review of the randomized clinical trials. Lipid Insights. 2013;6:13–20. doi: 10.4137/LPI.S10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson LE, Mazurak VC. N-3 polyunsaturated fatty acids: Relationship to inflammation in healthy adults and adults exhibiting features of metabolic syndrome. Lipids. 2013;48:319–32. doi: 10.1007/s11745-013-3774-6. [DOI] [PubMed] [Google Scholar]
- 6.Vannice G, Rasmussen H. Position of the academy of nutrition and dietetics: Dietary fatty acids for healthy adults. J Acad Nutr Diet. 2014;114:136–53. doi: 10.1016/j.jand.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Harika RK, Eilander A, Alssema M, Osendarp SJ, Zock PL. Intake of fatty acids in general populations worldwide does not meet dietary recommendations to prevent coronary heart disease: A systematic review of data from 40 countries. Ann Nutr Metab. 2013;63:229–38. doi: 10.1159/000355437. [DOI] [PubMed] [Google Scholar]
- 8.Backes J, Anzalone D, Hilleman D, Catini J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016;15:118. doi: 10.1186/s12944-016-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirillo A, Catapano AL. Update on the management of severe hypertriglyceridemia – Focus on free fatty acid forms of omega-3. Drug Des Devel Ther. 2015;9:2129–37. doi: 10.2147/DDDT.S67551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghasemifard S, Turchini GM, Sinclair AJ. Omega-3 long chain fatty acid “bioavailability”: A review of evidence and methodological considerations. Prog Lipid Res. 2014;56:92–108. doi: 10.1016/j.plipres.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Chantarojanasiri T, Hirooka Y, Ratanachu-Ek T, Kawashima H, Ohno E, Goto H, et al. Evolution of pancreas in aging: Degenerative variation or early changes of disease? J Med Ultrason (2001) 2015;42:177–83. doi: 10.1007/s10396-014-0576-2. [DOI] [PubMed] [Google Scholar]
- 12.Pereira DM, Valentão P, Andrade PB. Nano- and microdelivery systems for marine bioactive lipids. Mar Drugs. 2014;12:6014–27. doi: 10.3390/md12126014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petyaev IM. Carotenoid particles and uses thereof. Patent WO 2012104576 A2. 2012 Aug 09; [Google Scholar]
- 14.Petyaev IM. Lycosome technology: Advances and perspectives. Am J Food Sci Nutr. 2016;3:18–23. [Google Scholar]
- 15.Petyaev IM, Dovgalevsky PY, Klochkov VA, Chalyk NE, Kyle N. Whey protein lycosome formulation improves vascular functions and plasma lipids with reduction of markers of inflammation and oxidative stress in prehypertension. ScientificWorldJournal 2012. 2012:269476. doi: 10.1100/2012/269476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bashmakov YK, Assaad-Khalil SH, Abou Seif M, Udumyan R, Megallaa M, Rohoma KH, et al. Resveratrol promotes foot ulcer size reduction in type 2 diabetes patients. ISRN Endocrinol 2014. 2014:816307. doi: 10.1155/2014/816307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petyaev IM, Dovgalevsky PY, Chalyk NE, Klochkov V, Kyle NH. Reduction in blood pressure and serum lipids by lycosome formulation of dark chocolate and lycopene in prehypertension. Food Sci Nutr. 2014;2:744–50. doi: 10.1002/fsn3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petyaev IM. Improvement of hepatic bioavailability as a new step for the future of statin. Arch Med Sci. 2015;11:406–10. doi: 10.5114/aoms.2015.50972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diwadkar-Navsariwala V, Novotny JA, Gustin DM, Sosman JA, Rodvold KA, Crowell JA, et al. A physiological pharmacokinetic model describing the disposition of lycopene in healthy men. J Lipid Res. 2003;44:1927–39. doi: 10.1194/jlr.M300130-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Bowen CL, Kehler J, Evans CA. Development and validation of a sensitive and selective UHPLC-MS/MS method for simultaneous determination of both free and total eicosapentaenoic acid and docosahexaenoic acid in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:3125–33. doi: 10.1016/j.jchromb.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Comerota AJ, Throm RC, Kelly P, Jaff M. Tissue (muscle) oxygen saturation (StO2): A new measure of symptomatic lower-extremity arterial disease. J Vasc Surg. 2003;38:724–9. doi: 10.1016/s0741-5214(03)01032-2. [DOI] [PubMed] [Google Scholar]
- 22.Lin LN, Wang LR, Wang WT, Jin LL, Zhao XY, Zheng LP, et al. Ischemic preconditioning attenuates pulmonary dysfunction after unilateral thigh tourniquet-induced ischemia-reperfusion. Anesth Analg. 2010;111:539–43. doi: 10.1213/ANE.0b013e3181e368d2. [DOI] [PubMed] [Google Scholar]
- 23.Salem N, Jr, Eggersdorfer M. Is the world supply of omega-3 fatty acids adequate for optimal human nutrition? Curr Opin Clin Nutr Metab Care. 2015;18:147–54. doi: 10.1097/MCO.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 24.Harris WS, Gonzales M, Laney N, Sastre A, Borkon AM. Effects of omega-3 fatty acids on heart rate in cardiac transplant recipients. Am J Cardiol. 2006;98:1393–5. doi: 10.1016/j.amjcard.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Serini S, Fasano E, Piccioni E, Cittadini AR, Calviello G. Dietary n-3 polyunsaturated fatty acids and the paradox of their health benefits and potential harmful effects. Chem Res Toxicol. 2011;24:2093–105. doi: 10.1021/tx200314p. [DOI] [PubMed] [Google Scholar]
- 26.Lien EL. Toxicology and safety of DHA. Prostaglandins Leukot Essent Fatty Acids. 2009;81:125–32. doi: 10.1016/j.plefa.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol. 2007;99:35C–43C. doi: 10.1016/j.amjcard.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Asztalos IB, Gleason JA, Sever S, Gedik R, Asztalos BF, Horvath KV, et al. Effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular disease risk factors: A randomized clinical trial. Metabolism. 2016;65:1636–45. doi: 10.1016/j.metabol.2016.07.010. [DOI] [PubMed] [Google Scholar]


