Abstract
Phlomis bruguieri (P. bruguieri) is a large genus in the Lamiaceae family, with a wide variety distributed in Euro-Asia, Central Asia, Iran, and China. Phlomis flowers have been used as herbal tea for gastrointestinal disturbances, protection of liver and cardiovascular systems. The aim of this study was to analyse phytochemical of flavonoid constituents in semi polar fraction of P. bruguieri. Methanol extract of plant material (4 kg) yielded 361 g dark green concentrated extract gum. After preliminary fractionation by normal column chromatography on silica gel, Fr. 2 eluted with chloroform: methanol (90:10) selected as semi polar fraction and was more purified using different chromatography columns on silica gel, polyamide SC6 and Sephadex LH-20 adsorbents. Finally one new and three known flavonoids (1-4) were characterized in semi polar fraction. Isolated structures were identified using 1H-NMR, 13C-NMR, 31P-NMR, HSQC, HMBC, negative ESI mass, and UV spectra using different shift reagents. Using standard MTT assay, cytotoxicity of isolated new compound was done against michigan cancer foundation-7 (MCF-7) breast cancer cells. Phytochemical analysis of P. bruguieri resulted in identification of one new 4’-methoxy-luteolin-7-phosphate and three known flavones including luteolin, apigenin, and tricin for the first time in this plant. In MTT cytotoxicity test, 4’-methoxy-luteolin-7-phosphate showed cytotoxicity with IC50 value of 43.65 ± 8.56 μM agasint MCF-7 breast cancer cells.
Keywords: Breast cancer, Cytotoxicity, Flavone, MCF-7, Phlomis bruguieri
INTRODUCTION
The genus Phlomis from the Lamiaceae family are herbaceous, sub-shrub and rarely shrub with perennial growth. They are represented by 100 species from which 17-19 species are grown wild in Iran and specially in Zagros region(1,2). The genus is distributed mostly in world in Eur-Asia, Northwest of Africa, Turkey, Iran, central Asia, and China(3). In the case of secondary metabolites, the genus is rich in iridoids, diterpenes, flavonoids, phenylpropanoid glycosides, and phenylethanoid glycosides(4,5,6,7). Phlomis species are used in traditional medicine as analgesic, diuretic, emetic, and emmenagogue(8). Plant flowers are used in folk medicine as herbal tea for gastrointestinal problems and protection of liver and cardiovascular systems(9).
Phlomis bruguieri (P. bruguieri) is one of the species distributed in flora of Iran, Turkey and Iraq(1). Previous studies on this plant have reported its antioxidant, antimicrobial against Streptococcus sanguis and Staphylococcus aureus and α-amylase inhibitory activities(10,11,12). In the recent study, cytotoxicity of six Phlomis species against different human cancer cell lines was studied of which P. bruguieri showed interesting cytotoxic activity against MCF7 breast cancer cell line(13).
The main constituents of essential oils included germacrene D, apiole, and myristicin(9).
In another study by high performance liquid chromatography electrospray ionization tandem mass spectrometry (HPLC-ESI/MS) analysis screening on aerial parts of P. bruguieri, phenylethanoid glycosides including verbascoside, isoverbascoside, leucosceptosides A and martynoside were reported and quantified(14). In a study conducted by Kaaji et al, it was shown that Phlomis species have a wide variation in flavonoid contents including flavones, isoflavonones, and chalcones. Therefore, the paper in hand aimed to isolate and characterize flavonoid constituents in P. bruguieri in addition to cytotoxic evaluation of isolated new compound against breast cancer cells.
MATERIALS AND METHODS
General procedures
Nuclear magnetic resonance analysis (NMR) was done on a Bruker AV400 spectrometer (Billerica, MA, USA) using deuterated dimethyl sulfoxide (DMSO-d6) as the solvent at 400 MHz for proton nuclear magnetic resonance (1H-NMR), 100 MHz for carbon-13 (13C)-NMR, and 31 MHZ for phosphorus-31 (31P)-NMR. Mass spectra (electrospray ionization (ESI)) were performed on Shimadzu 2010EV liquid chromatography-mass spectrometry (LC-MS) system (Shimadzu, Japan), and are reported in m/z. Thin layer chromatography (TLC) was done on Merck TLC silica gel allufoils (Germany) and visualized with 1% natural product reagent (2-aminoethyl diphenylborinate) and 1% ceric sulfate solution in 10% sulfuric acid followed by heating by hair dryer for about 2 min. Column chromatographies were done on silica gel (60-200 tm, Merck, Germany), polyamide SC6 (Roth, Germany), Sephadex-LH (Pharmacia fine chemicals, Uppsala, Sweden). Sephadex LH-20 was swelled in hexane: methanol: acetone (30:60:10) in a glass column 45 cm in length and 2 cm i.d., fitted with teflon stopcock and rinsed with the same solvent system with a flow of 3 cm/h.
Plant material
Aerial parts of P. bruguieri Desf. (Lamiaceae) was collected from Zagros region in Kermanshah, west of Iran. Plant material was identified by taxonomist according to the voucher specimen (2182) deposited in the herbarium of biology department, Faculty of Science, University of Isfahan, Isfahan, I.R. Iran.
Extraction and isolation
The air-dried powder of the plant material (4 Kg) was extracted trice with methanol (20 L × 3) at room temperature for one week. Combined extracts were concentrated to a dark brownish gum (361 g) by rotary evaporator attached to vacuum pump (60 mbar) at 40 °C. The extract was suspended in water and defatted in a separating funnel by hexane. Defatted aqueous phase was concentrated (80 g) and adsorbed on pre-adsorbent (Celite) in equal weight and then applied on silica-gel column chromatography (400 g) for preliminary fractionation. Liquid chromate-graphy using solvent systems of hexane:chloroform (70:30), chloroform: methanol (90:10), and methanol (100%) yielded three fractions (Frs.1-3). Fr. 1 eluted with hexane:chloroform (70:30) containing fatty acids and Fr.3, eluted with methanol (100%) containing polar glycoside secondary metabolites were put aside to evaluate later. Semi-polar fraction, Fr. 2 eluted with chloroform:methanol (90:10) was selected and subjected on silica gel column chromatography (hexane:acetone, 90:10, 85:15, 80:20, 70:30, and 50:50) and yielded 5 sub-fractions (Frs. 2A-2E). After taking preliminary 1H-NMR spectra of different fractions, Fr. 2C, Fr. 2D, and Fr. 2E showing flavonoid profiles were selected and subjected to more purification on polyamide SC6 column chromatography using stepwise gradient of chloroform:methanol (98:2, 96:4, 94:6, 92:8, 90:10, and 80:20). Based on TLC profiles visualized by natural product reagent, sub-fractions with yellowish spots in TLC was indicative of flavonoid content and were further purified by size exclusion chromatography on a Sephadex LH-20 column (2 × 45) using hexane:methanol:acetone (30:60:10) as the mobile phase. Finally, Fr. 2C6a (12.1 mg), Fr. 2D3 (8.3 mg), Fr. 2D5b (6.8 mg), and Fr. 2E7a (11.2 mg) were obtained as pure compounds called 1-4 and submitted for identification to NMR, mass spectra(15).
Cytotoxicity assay
Because of anti-estrogenic properties of flavones, isolated new flavonoid was subjected to MTT assay on estrogen sensitive MCF-7 breast cancer cells. Pasteur Institute of Iran (Tehran, I.R. Iran) provided MCF-7 cell line which was grown adherently in RPMI-1640 with 10% fatal calf serum (FCS), 100 units/mL penicillin and 100 μg/mL streptomycin at 37 °C in 5% CO2. Cells were seeded at 5000 cells per well in 5% CO2 at 37 °C in RPMI with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin, in 96-well plates. Compound 2 (2 mg) was dissolved in DMSO (1 mL) for preparation of stock sample (5.27 mM).
Different concentrations of tested compound were prepared by serial dilution method using RPMI as diluent. After 24 h incubation, cells exposed with concentrations of 0.1, 1, 10, 50, and 100 μM of tested compound for 48 h. Doxorubicin as positive control was used at concentrations 0.001, 0.01, 0.1, 1, 10, and 20 μM. MTT was added to the wells and incubated for another 4 h. Experiment was done in triplicate and the absorbance was read by the microplate reader (Bio-Rad, Hercules, CA, USA) at 570 nm. Cell viability percentages were calculated by the following equation and were expressed as percent of control cells which were not treated(16,17).
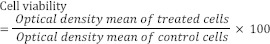
Statistical analysis
Biological data are reported as mean ± SD and the IC50 values were calculated using Excel worksheet processor. T-test with two samples assuming equal variances as well as Dunnet test one way ANOVA were used for analysis of MTT cytotoxicity results and P < 0.05 was considered to indicate statistically significant differences.
RESULTS
Methanol extract of plant material (4 Kg) yielded 361 g dark green gum. After preliminary fractionation by silica gel column, Fr. 2 eluted with chloroform:methanol (90:10) was selected and more purified using different chromatography adsorbents including silica gel, polyamide SC6 and Sephadex LH-20. Finally one new and three known flavonoids (1-4) were characterized in semi polar fraction (Fig. 1). Isolated structures were identified using 1H-NMR, 13C-NMR, 31P-NMR, heteronuclear single quantum correlation (HSQC), heteronuclear multiple bond correlation (HMBC), negative ESI-mass, and UV shift reagents as well as comparing with literature data (Fig. 2).
Fig. 1.
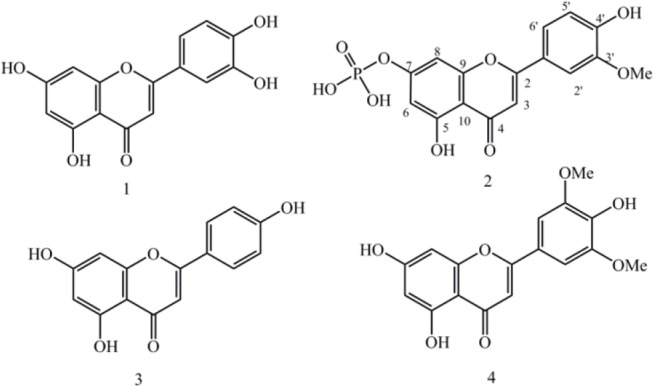
Flavones isolated from Phlomis bruguieri.
Fig. 2.
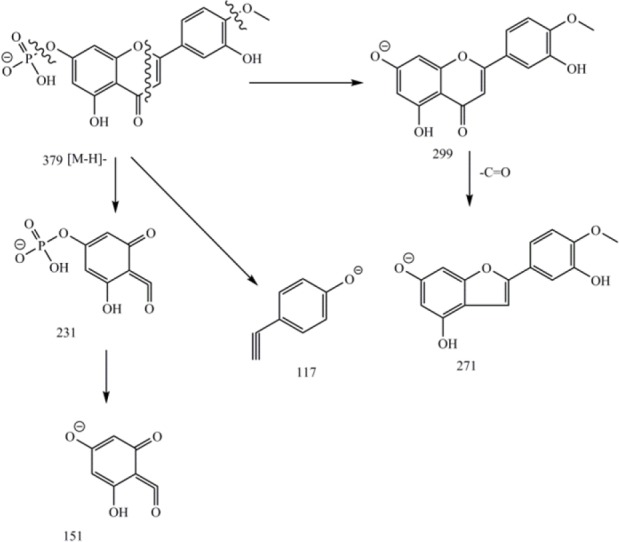
Electron ionization mass fragmentation pattern of 4′-methoxy-luteolin-7-phosphate. Cleavage of ring C by retro-Diels-Alder (RDA) mechanism led to A (m/z 231) and B (m/z 117) ions(16).
Spectral data of isolated compounds
Compound 1: Pale yellow powder, 1H-NMR in DMSO-d6 (400 MHz) ppm δ 5.39 (1H, d, J = 2.0 Hz, H-8), 5.62 (1H, d, J = 2.0 Hz, H-6), 5.72 (1H, s, H-3), 6.10 (1H, d, J = 8. 8 Hz, H-5’), 6.56 (1H, overlapped, H-2’), 6.58 (1H, dd, J = 2.0, 8.8, H-6’); 13C-NMR (100 MHz, DMSO-d6) δC: 181.6 (C4), 164.1 (C2), 163.9 (C7), 161.4 (C5), 157.2 (C9), 149.7 (C4’), 145.7 (C3’), 121.4 (C1’), 118.9 (C6), 116.0 (C5’), 113.3 (C2’), 103.6 (C10), 102.8 (C3), 98.8 (C6), 93.8 (C8), 55.9 (4’-OMe). Negative ESI mass (m/z) 285 [M-H]-.
Compound 2: Pale yellow powder, 1H-NMR in DMSO-d6 (400 MHz) ppm δ 3.95 (1H, s, 4’-OMe), 6.26 (1H, d, J = 2.0 Hz, H-8), 6.58 (1H, d, J = 2.0 Hz, H-6), 6.96 (1H, s, H-3), 6.99 (1H, d, J = 8. 8 Hz, H-5’), 7.61 (1H, overlapped, H-2’), 7.63 (1H, overlapped, H-6’); 13C-NMR (100 MHz, DMSO-d6) δC: 181.8 (C4), 164.1 (C2), 163.6 (C7), 161.4 (C5), 157.3 (C9), 150.7 (C4’), 148.0 (C3’), 121.5 (C1’), 120.3 (C6’), 115.7 (C5’), 110.1 (C2’), 103.7 (C10), 103.2 (C3), 98.8 (C6), 94.0 (C8). 31P-NMR in DMSO-d6 (162 MHz) ppm δ 0.18 (C7-H2PO4). Negative ESI mass (m/z): 379 [M-H]-, 343 (379-2H2O)-, 299 (379-H2PO3)-, 271(299-CO), 257, 231, 151, 117.
Compound 3: Pale yellow powder, 1H-NMR in pyridine-d6 (400 MHz) ppm: 6.10 (1H, d, J = 2.0 Hz, H-8), 6.36 (1H, d, J = 2.0 Hz, H-6), 6.49 (1H, s, H-3), 6.83 (2H, d, J = 8. 8 Hz, H-6’,2’) and 7.75 (2H, d, J = 8.8 Hz, H-5’,3’); 13C-NMR (100 MHz, pyridine-d6) δC: 183.2 (C4), 166.4 (C2), 163.8 (C7), 163.3 (C5), 163.2 (C4’), 159.0 (C9), 129.4 (C5’, C3’), 117.3 (C6’, C2’), 122.8 (C1’), 106.5 (C10), 104.4 (C3), 100.5 (C6), 95.3 (C8). Negative ESI mass (m/z): 269 [M-H]-.
Compound 4: 1H-NMR (400 MHz, DMSO-d6) ppm 4.04 (s, 6H, 3’-OMe, 5’-OMe), 6.30 (1H, d, J = 2.0 Hz, H-6), 6.55 (1H, d, J = 2.0 Hz, H-8), 6.69 (1H, s, H-3), 7.32 (2H, s, H-6’,2’); 13C-NMR (100 MHz, pyridine-d6) δC: 183.7 (C4), 166.1 (C2), 165.6 (C7), 163.3 (C4’), 163.3 (C5’), 159.2 (C9), 150.1 (C5’, C3’), 141.7 (C1’), 123.4 (C10), 106.2 (C6’, C2’), 105.5 (C3), 100.7 (C6), 96.1 (C8), 58.2 (3’-OMe, 5’-OMe). Negative ESI mass (m/z): 329 [M-H]-.
Result of cytotoxicity assay
The MTT assay was done on compound 2 as a new compound to check its cytotoxicity against MCF-7 breast cancer cells (Fig. 3). MTT results showed moderate cytotoxicity with IC50 value of 43.65 ± 8.56 μM while doxorubicin as standard drug showed IC50 value of 0.32 ± 0.08 μM.
Fig. 3.
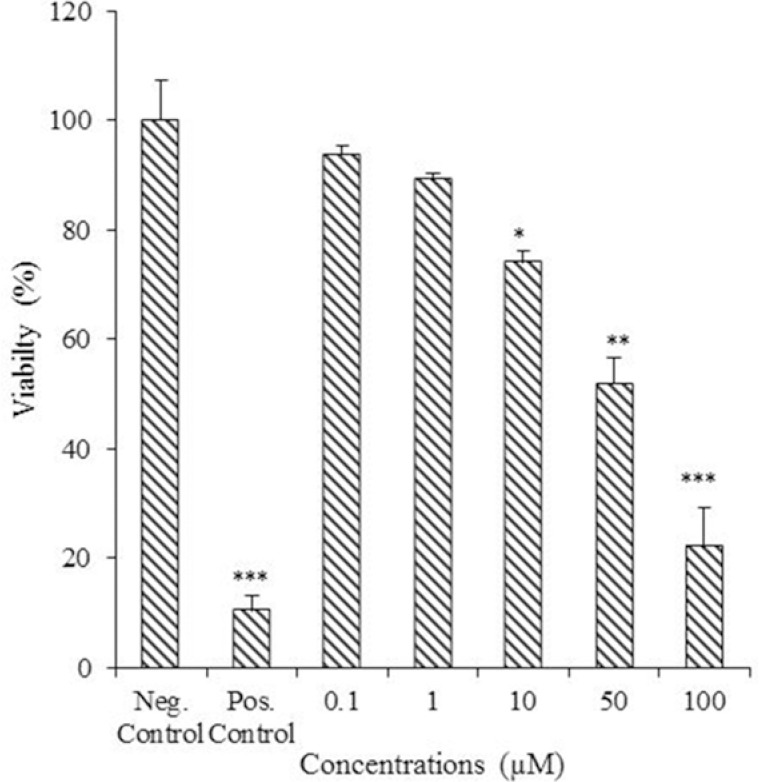
Cytotoxicity effect of 4′-methoxy-luteolin-7-phosphate against MCF-7 breast cancer cells. Cells were treated with different concentrations of tested compound (0.1, 1, 10, 50, and 100 μM ) in three replicates. Doxorubicin (10 μM), and vehicle were used as positive and negative controls, respectively. (* P < 0.5; ** P < 0.01; *** P < 0.001 vs control).
DISCUSSION
Compound 1 was isolated as a pale yellowish solid with positive reaction to FeCl3 and flavonoid natural product reagent. Molecular formula was determined by 13C-NMR data (broad band and distortionless enhancement by polarization transfer (DEPT)) and negative ESI/MS m/z 285 [M-H]- as C15H10O6. The UV spectrum showed absorption maxima at 264 and 350 nm characteristic of flavones(16). The 1H-NMR spectrum displayed two meta coupled doublets at δH 6.26 (1H, d, J = 1.6 Hz) and 6.45 (1H, d, J = 1.6 Hz) attributed to H-6 and H-8, a singlet proton at δH 6.51 (1H, s, H-3) two ortho coupled proton signals at δH 6.97 (1H, d, J = 8. 4 Hz) and 7.33 (1H, dd, J = 8.4, 2.0 Hz), corresponding to H-5′ and H-6′ as well as δ 7.41 (1H, d, J = 2.0 Hz) corresponding to H-2′ proton, indicated that compound 1 is 3′,4′,57 tetrahydroxy flavone known as luteolin in agreement with literature data and co-TLC with authenticated sample(18,19).
Compound 2 showed positive reaction to flavonoid natural product reagent. 1H-NMR spectra resonated at δH 6.26 (1H, d, J = 2.0, H-6), 6.58 (1H, d, J = 2.0, H-8), 6.96 (1H, s, H-3), in addition to an ABX spin system at δH 6.99 (1H, d, J = 8. 8 Hz), 7.63 (1H, dd, overlapped), and 7.62 (1H, bd, overlapped) corresponding to H-5′, H-6′, and H-2′ showed closed similarities to those of compound 1 but an additional methoxy signal at δH 3.95 (3H, s) and an additional phosphate group at δP 0.18 ppm which made slight downfield shifts in aromatic protons resonances in ring A. 2,3JC-H HMBC correlations of methoxy singnal with δH 3.95 (3H, s) and δC 150.7 ppm confirmed the location of methoxy group at C-4′ in ring B. Structural assignment of compound 2 was initially assumed to be the corresponding sulfate analog on the basis of the facts that sulphate esters of flavonoids were well reported in literature, but it was eventually identified by 31P-NMR to be the corresponding phosphate ester. Presence of a phosphate group was confirmed through 31P-NMR with proton decoupling at δP 0.18 ppm (Fig. 2). Negative ESI/MS showed m/z 379 as molecular ion [M-H]- together with fragment ions of m/z 343 (379 - 2H2O)-, 299 (379 - H2PO3)-, and retro-Diels-Alder (RDA) fragments of 231, and 117 ions were indicative of a flavone with one phosphate on ring A and one methoxy and one hydroxyl group on ring B (Fig. 2). 13C-NMR showed 16 decoupled signals including one methoxy at δC 55.9 (4′-OMe), 6 olefin methines and 8 quaternary sp2 carbons from which 6 were oxygenated (experimental section), and a carbonyl carbon (δC 181.8 ppm). The UV spectrum showed absorption maxima at 267 and 350 nm characteristic of flavones(16). Bathochromic shifts of band III at 386 nm in AlCl3/HCl was about 36 nm indicative of a free hydroxyl group at C-5(16). Addition of NaOAc following with BH3 did not change band I and III and was indicative of lack of free hydroxyl group at C-7 in ring A. Therefore, based on these data, the structure of compound 2 is proposed as 4’-methoxy-luteolin-7-phosphate but needs more analytical analysis for excat structure.
Phosphate‐transferring phosphatases made covalent phospho-intermediates including phosphate and pyrophosphate esters of secondary metabolite as biosynthetic intermediates like erythrose-4-phospate, inositol-phosphate, glyceraldehyde phosphate, and etc., which are hydrolyzed at the end of biosynthesis pathway. Therefore, phospho-intermediates found in small amounts and a limited number of them are identified till now. In comparison with phosphate estrs, inorganic sulphates conjugated forms of flavonoids like apigenine-7-sulphate, and quercetin-3-sulphate are more reported in literature. In a study conducted by Harborn et al. they reviewed and reported distribution and structural variations of inorganic sulphate esters of flavonoids(20).
Compound 3 was isolated with a pale yellowish color and positive reaction to natural product reagent. The UV spectrum showed two intense absorption maxima at 266 and 339 nm characteristics of flavones. Based on NMR data and negative mode of ESI/MS m/z 269 [M-H]- molecular formula was proposed as C15H10O5. The 1H-NMR spectrum displayed two meta doublets at δ 6.10 (1H, d, J = 2.0 Hz), and 6.36 (1H, d, J = 2.0 Hz) coupled to each other similar to those of H-8 and H-6 in flavones in addition to a singlet proton at 6.49 (H-3), and two ortho-coupled signals at δ 6.83 (2H, d, J = 8. 8 Hz) and 7.75 (2H, d, J = 8.8 Hz), describing to H-6′,2′ and H-5′, 3′ related to AA′BB′ spin system indicated that compound 3 is 4′,57-trihydroxyflavone or apigenin(19).
Compound 4 with pale color showed UV spectrum pattern of flavones. The 1H-NMR spectrum displayed two coupled meta doublets at δ 6.30 (1H, d, J = 2.0 Hz), and 6.55 (1H, d, J = 2.0 Hz), and a singlet signal at 6.69 (1H, s), related to H-6, H-8, and H-3 similar to apigenin but different in ring B in AA′ spin system at δ 7.32 (2H, s) instead of AA′BB′ system ascribing to H-6′,2′ protons, as well as a singlet signal at 4.04 (6H, s) related to two symmetric methoxy groups located at C-5′, and C-3′. Therefore, based on 1H- and 13C-NMR data (see experimental), ESI-negative mass m/z 329 [M-H], and literature data it was identified as 4′,57-trihydroxy-3′, 5′-dimethoxyflavone or tricin(21).
These results were in agreement with previous report on presence of different classes of flavonoids in Phlomis species. In a study conducted by Aghakhani and coworkers using liquid chromatography tandem mass spectrometry as a fast but not very certain screening method, they proposed presence of different flavonoids including kaempferol, luteolin, chrysoeriol, naringenin, and 4-hydroxy-5,7-dimethoxy flavanone in P. bruguieri leaves(22).
In biological assay, compound 2 showed cytotoxic activity with IC50 value of 43.65 ± 8.56 μM against estrogen sensitive MCF-7 breast cancer cells. MTT result was in agreement with antiestrogenic and antiproliferative activities of flavonoids against MCF-7 breast cancer cells(23,24,25). In a study done by Bail et al. they evaluated flavonoids for their cytotoxicity against estrogen-dependent (MCF-7) breast cancer cells(26). In their study, 7-methoxyflavanone and 7,8-dihydroxyflavones showed anti-estrogenic and cytotoxic properties. In another study on luteolin by Wang, luteolin inhibited MCF-7 cells proliferation which was induced by IGF-1 pathway dependent estrogen receptor-α(23). It regulated also estrogen signaling and cell cycle pathway genes(26). In another study on chrysin (7,5-dihydroxyflavone) and its phosphate esters on HeLa cancer cells, the results showed both compounds suppressed HeLa cells proliferation by apoptotic induction. In comparison between parent and its phosphate ester, chrysyl-7-phophate showed more cytotoxic properties which is probably due to its better solubility(27). Low aqueous solubility is one of the main problems of flavonoid applications leads to their poor oral bioavailability which could be improved by flavonoid phosphate esters and making their potassium or sodium salts(28).
CONCLUSION
Phytochemical analysis of P. bruguieri resulted in identification of one new 4’-methoxy-luteolin-7-phosphate and three known flavones including luteolin, apigenin, and tricin for the first time in this plant. In MTT cytotoxicity test, 4’-methoxy-luteolin-7-phosphate showed moderae toxicity agasint MCF-7 breast cancer cells.
ACKNOWLEDGMENTS
The content of this paper is extracted from the Pharm. D thesis (No. 394687) submitted by Zeinab Delazar which was financially supported by Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Rechinger KH, editor. Flora Iranica. Graz: Akademische Drucku Verlagsanstalt; 1982. pp. 292–317. [Google Scholar]
- 2.Aghakhani Kaaji, Kharazian N. Flavonoid diversity and morphological variations among seven Phlomis species in Zagros, Iran. Iran J Sci Technol Trans Sci. 2017:1–7. [Google Scholar]
- 3.Azizian D, Moore DM. Morphological and palynological studies in Phlomis L., Eremostachys Bunge and Paraphlomis Prain (Labiatae) Bot J Linn Soc. 1982;85(4):225–248. [Google Scholar]
- 4.Modaressi M, Delazar A, Nazemiyeh H, Fathi‐Azad F, Smith E, Rahman MM, et al. Antibacterial iridoid glucosides from Eremostachys laciniata. Phytother Res. 2009;23(1):99–103. doi: 10.1002/ptr.2568. [DOI] [PubMed] [Google Scholar]
- 5.Delazar A, Byres M, Gibbons S, Kumarasamy Y, Modarresi M, Nahar L, et al. Iridoid glycosides from Eremostachys glabra. J Nat Prod. 2004;67(9):1584–1587. doi: 10.1021/np040044b. [DOI] [PubMed] [Google Scholar]
- 6.Sarkhail P, Nikan M, Sarkheil P, Gohari AR, Ajani Y, Hosseini R, et al. Quantification of verbascoside in medicinal species of Phlomis and their genetic relationships. Daru. 2014;22(1):32–36. doi: 10.1186/2008-2231-22-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SJ, Jin SW, Lee GH, Kim YA, Jeong HG. Evaluation of estrogenic activity of extract from the herbal mixture Cynanchum wilfordii Hemsley, Phlomis umbrosa Turczaninow, and Angelica gigas Nakai. Toxicol Res. 2017;33(1):71–77. doi: 10.5487/TR.2017.33.1.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duke JA. Handbook of Medicinal Herbs. 2nd ed. CRC press; 2002. pp. 777–778. [Google Scholar]
- 9.Amor IL, Boubaker J, Sgaier MB, Skandrani I, Bhouri W, Neffati A, et al. Phytochemistry and biological activities of Phlomis species. J Ethnopharmacol. 2009;125(2):183–202. doi: 10.1016/j.jep.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Delnavazi MR, Safaei M, Biuki S, Tavakoli S, Aghaahmadi M, Hadjiakhoondi A, et al. Coumaroyl flavone glycosides and cinammic acid derivatives from the aerial parts of Phlomis bruguieri Desf. RJP. 2017;4(4):17–22. [Google Scholar]
- 11.Morteza-Semnani K, Saeedi M, Mahdavi MR, Rahimi F. Antimicrobial studies on extracts of three species of Phlomis. Pharm Biol. 2006;44(6):426–429. doi: 10.4103/0250-474X.43021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safamansouri H, Nikan M, Amin G, Sarkhail P, Gohari AR, Kurepaz-Mahmoodabadi M, et al. α-Amylase inhibitory activity of some traditionally used medicinal species of Labiatae. J Diabetes Metab Disord. 2014;13(1):114–117. doi: 10.1186/s40200-014-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkhail P, Sahranavard S, Nikan M, Gafari S, Eslami-Tehrani B. Evaluation of the cytotoxic activity of extracts from six species of Phlomis genus. J Appl Pharm Sci. 2017;7(2):180–184. [Google Scholar]
- 14.Kırmızıbekmez H, Montoro P, Piacente S, Pizza C, Dönmez A, Çalış İ. Identification by HPLC‐PAD‐MS and quantification by HPLC‐PAD of phenylethanoid glycosides of five Phlomis species. Phytochem Anal. 2005;16(1):1–6. doi: 10.1002/pca.802. [DOI] [PubMed] [Google Scholar]
- 15.Baniadam S, Rahiminejad MR, Ghannadian M, Saeidi H, Ayatollahi AM, Aghaei M. Cycloartane triterpenoids from Euphorbia macrostegia with their cytotoxicity against MDA-MB48 and MCF-7 cancer cell lines. Iran J Pharm Res. 2014;13(1):135–139. [PMC free article] [PubMed] [Google Scholar]
- 16.Zarei SM, Ayatollahi AM, Ghanadian M, Kobarfard F, Aghaei M, Choudhary MI, et al. Unusual ingenoids from Euphorbia erythradenia Bioss. with pro-apoptotic effects. Fitoterapia. 2013;91:87–94. doi: 10.1016/j.fitote.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Markham KR, Chari VM. Carbon-13 NMR Spectroscopy of Flavonoids. In: Harborne JB, Mabry TJ, editors. The flavonoids: Advances in Research. London, New Yourk: Chapman and Hall; 1975. pp. 45–127. [Google Scholar]
- 18.Agrawal PK, editor. Elsevier Science. 2013. Carbon-13 NMR of Flavonoids. Vol 39; pp. 134–140. [Google Scholar]
- 19.Sajjadi SE, Ghanadian M, Haghighi M. Isolation and identification of two phenolic compounds from a moderately cytotoxic fraction of Cousinia verbascifolia Bunge. Adv Biomed Res. 2017;6:66–74. doi: 10.4103/2277-9175.190980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harborne JB. Flavonoid sulphates: a new class of sulphur compounds in higher plants. Phytochemistry. 1975;14(5-6):1147–1155. [Google Scholar]
- 21.Bhattacharyya J, Stagg D, Mody NV, Miles DH. Constituents of Spartina cynosuroides: isolation and 13C‐NMR analysis of tricin. J Pharm Sci. 1978;67(9):1325–1326. doi: 10.1002/jps.2600670938. [DOI] [PubMed] [Google Scholar]
- 22.Aghakhani F, Kharazian N, Gooini ZL. flavonoid constituents of Phlomis (lamiaceae) species using liquid chromatography mass spectrometry. Phytochem Anal. 2018;29(2):180–95. doi: 10.1002/pca.2733. [DOI] [PubMed] [Google Scholar]
- 23.Wang LM, Xie KP, Huo HN, Shang F, Zou W, Xie MJ. Luteolin inhibits proliferation induced by IGF-1 pathway dependent ERα in human breast cancer MCF-7 cells. Asian Pac J Cancer Prev. 2012;13(4):1431–1437. doi: 10.7314/apjcp.2012.13.4.1431. [DOI] [PubMed] [Google Scholar]
- 24.Markaverich BM, Shoulars K, Rodriguez MA. Luteolin regulation of estrogen signaling and cell cycle pathway genes in MCF-7 human breast cancer cells. Int J Biomed Sci. 2011;7(2):101–105. [PMC free article] [PubMed] [Google Scholar]
- 25.Mojaddami A, Sakhteman A, Fereidoonnezhad M, Faghih Z, Najdian A, Khabnadideh S, et al. Binding mode of triazole derivatives as aromatase inhibitors based on docking, protein ligand interaction fingerprinting, and molecular dynamics simulation studies. Res Pharm Sci. 2017;12(1):21–30. doi: 10.4103/1735-5362.199043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Bail JC, Varnat F, Nicolas JC, Habrioux G. Estrogenic and antiproliferative activities on MCF-7 human breast cancer cells by flavonoids. Cancer Lett. 1998;130(1-2):209–216. doi: 10.1016/s0304-3835(98)00141-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhang T, Chen X, Qu L, Wu J, Cui R, Zhao Y. Chrysin and its phosphate ester inhibit cell proliferation and induce apoptosis in Hela cells. Bioorg Med Chem. 2004;12(23):6097–6105. doi: 10.1016/j.bmc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Hsu C, Wu BY, Chang YC, Chang CF, Chiou TY, Su NW. Phosphorylation of isoflavone by Bacillus subtilis bcrc 80517 may represent xenobiotic metabolism. J Agric Food Chem. 2018;66:127–113. doi: 10.1021/acs.jafc.7b04647. [DOI] [PubMed] [Google Scholar]


