Abstract
Cyclophosphamide (CP), as a chemotherapy drug, induces hepatotoxicity through causing oxidative stress. Atorvastatin (ATV) at a low dose has antioxidant and anti-inflammatory properties. The present study was designed to investigate the protective effects of ATV against CP-induced hepatotoxicity in rat. In this experimental study, 32 rats were treated with ATV orally at a dose of 10 mg/kg for 10 consecutive days, 5 days before and 5 days after the administration of a single intraperitoneal injection of CP (150 mg/kg). The hepatoprotective effect of ATV was evaluated by measuring liver function markers, oxidative markers, histological and immunohistochemical assays. The biochemical results showed that administration of CP increased hepatic biomarkers enzymes as aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) levels. CP increased malondialdehyde (MDA), protein carbonyl (PC) and decreased glutathione (GSH) content in rats. Moreover, administration of CP was associated with periportal leucocyte infiltration, dilation sinusoids, hepatocyte vacuolation, congestion and hemorrhage in livers of rats. CP significantly increased immunoreactivity of caspase-3 as a marker of apoptosis in liver tissue. ATV markedly mitigated liver injury through reduction in oxidative stress biomarkers, histopathological findings and apoptosis. The antioxidant and anti-apoptotic activities of ATV are main proposed mechanisms involved in its hepatoprotective effects against CP-induced hepatic injury.
Keywords: Atorvastatin, Caspase-3, Cyclophosphamide, Hepatotoxicity, Oxidative stress
INTRODUCTION
Cyclophosphamide (CP), as one of the most common anticancer drug, causes side effects on normal tissues such as heart, kidney and liver(1,2). Phosphoramide mustard and acrolein are main active metabolites of CP that induce oxidative stress in tissues(3). CP is enable to produce free radicals and inhibit the activities of endogenous antioxidant enzymes such as superoxide dismutase (SOD), glutathione (GSH) and catalase (CAT)(4,5). The exogenous antioxidants are beneficial in reducing the oxidative stress induced by toxic substances(6,7).
Atorvastatin (ATV), the most common synthetic statins, reduces the level of plasma lipoproteins and cholesterol by inhibiting enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase(8). In addition, ATV has the beneficial biological effects such as antioxidant and anti-inflammatory properties that are independent of lowering cholesterol levels. These effects are observed at low doses of ATV(9). The protective effects of ATV were seen in ovarian(10), gastrointestinal tract(11) and kidney damages followed by torsion/detorsion(12), testicular toxicity(13), and cardiotoxicity(14). ATV protects cell structure against oxidative stress and inhibits the reduction of endogenous antioxidant enzymes.
However, the acute liver injury was seen in high dose of ATV(15), the protective effect of ATV on the hepatotoxicity induced by ischemia-reperfusion and ethanol was established at low dose(16,17). ATV protected liver damage by inhibiting inflammation and oxidative stress parameters(16). Considering the above issues, it is hypothesized that ATV could reduce liver damage induced by CP. Therefore, the aim of this study was to investigate the protective effect of ATV against CP-induced hepatotoxicity in the rat model with the histological, biochemical, histochemical, and immunohistochemical evaluations.
MATERIALS AND METHODS
Chemicals
Atorvastatin was purchased from Sobhan Pharmaceutical Company (Rasht, I.R. Iran) and cyclophosphamide was manufactured by the Baxter Company of Germany.
Animals
Although gender affects pharmacokinetics, pharmacodynamics, side effects of drugs, and its toxicity(18), but Massafra has reported in his study that gender has no effect on antioxidant enzyme system(19). Thus, 32 female Wistar rats (weighing 150-180 g) were obtained from Animal Research Center of Mazandaran University of Medical Sciences, Sari, I.R. Iran. For adaptation to the experiment environment, the animals were maintained on a 12:12 h dark/light cycle, 55% ± 5% humidity and 22-24 °C for one week. They had free access to food and water during the study period. All the experimental procedures were designed in accordance with the Institutional Animal Ethics Committee of the Mazandaran University of Medical Sciences, Mazandaran, I.R. Iran (ethical ID: IR.MAZUMS.REC.1396.S217).
Study design
In this study, the animals were randomly divided into four groups of 8 animals each. Group I, rats were received normal saline (same volume with other groups); Group II, rats were received 10 mg/kg ATV daily by gavage for 10 consecutive days(18) ; Group III, on the fifth day, rats were injected a single dose of CP (150 mg/kg) intraperitoneally(19) ; Group IV, rats were received ATV and CP with the same dose as administer to groups II and III. ATV was administrated daily for five consecutive days before and after CP injection.
The doses of ATV and CP were selected according to previous studies(20,21). CP was dissolved in distilled water and ATV was suspended in normal saline. In ATV + CP group, ATV was administered 1 h prior to CP treatment. The most appropriate time for the assessment of histochemistry is 24 to 72 h after receiving the latest treatment(22). Thus, in this study, two days after receiving the latest drug treatment, biochemical, histological, and immunohistochemical assays were performed.
Specimen collections
The animals were anesthetized with ketamine (50 mg/kg) and xylazine (5 mg/kg) two days after receiving the latest treatment. The blood samples were collected from the heart and their serum were separated by centrifugation at 3000 × g (13). The serum samples were stored at -20 °C for measurement of serum liver enzymes as alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH). Then animals were sacrificed and liver was immediately removed. It was divided into two pieces, washed with phosphate buffer saline (PBS), and weighed out. One of these pieces was freshly used for biochemical evaluation. The other piece was fixed in 10% buffer formalin for histological and immunohistochemical analyses.
Biochemical analysis
The level of liver lipid peroxidation was measured by determination of malondialdehyde (MDA) level using thiobarbituric acid. The liver sample (0.2 mL) was mixed with phosphoric acid (0.25 mL, 0.05 M) and 0.2% thiobarbituric acid (TBA, 0.3 mL). Samples were boiled in water bath for 30 min. The sample tubes were placed to an ice-bath and then 0.4 mL of n-butanol was added to each sample. The mixtures were centrifuged at 3500 rpm for 10 min and then MDA level were measured based on reacting with thiobarbituric acid (an MDA-TBA complex). MDA in each sample was calculated in the supernatant at 532 nm with ELISA reader (Tecan, Rainbow Thermo, Austria). MDA content was expressed as nmol/mg protein. Tetramethoxypropane (TEP) was used as standard(23).
Protein carbonyls were measured by the method reported by Fathi et al.(23). The protein carbonyl was measured using 2,4-dinitrophenyl-hydrazine (DNPH) reagent. After determination of tissue protein, 500 μL of trichloroacetic acid (20% w/v) was added to the samples and stored at 4 °C for 15 min. Then precipitated protein was centrifuged at 6500 × g for 10 min and the supernatant was discarded. Soluble protein (0.5 mL) was reacted with DNPH 10 mM (0.5 mL) in HCl (2 M) for 1 h at room temperature. Precipitate was washed with 1 mL of a mixture of ethanol and ethyl acetate 1:1 (v/v) and then was centrifuged at 6500 × g for 10 min and the supernatant was removed. The final protein deposition solubilized in 200 μL guanine hydrochloride solution and was centrifuged at 16000 × g for 5 min to remove any trace of insoluble material.
The protein carbonyl was assessed spectrophotometrically at 365 nm with an absorption coefficient of 22,000 M-1 cm-1 was expressed as a nmol of DNPH per milligram of protein.
Content of the glutathione in the samples was determined by spectrophotometer (UV-1601 PC, Shimadzu, Japan) with 5,5’-dithiobis-2-nitrobenzoic acid (DTNB) as an indicator at 412 nm and expressed as μM(23).
Mitochondrial function, as mitochondria viability, was assessed using 3-45- dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay based on reduction of MTT to its formazan product by mitochondrial dehydrogenase activity of liver tissue. In this way, after preparation of the samples, MTT was dissolved in PBS (pH 7.2, at 2.5 mg/mL) and this solution was added to the samples(24). After incubation at 37 °C for 30 min, mitochondrial toxicity was measured by assessing the reduction of MTT by mitochondria at 580 nm.
Serum biochemical assay
The enzymes activities as ALT, AST, and LDH were determined using the quantitative detection kit and according to the instructions of the manufacturer, Pars Azmoon, AST (Cat. No. 1 400 018), ALT (Cat. No. 1 400 019) and LDH (Targa BT 3000, Cat. No. 12201), I.R. IRAN. Content of these enzymes was measured by a spectrophotometer with L-alanine, 2-oxoglutarate, pyruvate, and NADH as an indicator at 340 nm and expressed as U/L.
Histopathological assay
The liver samples were fixed in 10% (w/v) buffer formalin for 24 h. After processing and embedding in paraffin using standard protocol, sections with 5 μm thickness stained with hematoxylene and eosin (H & E) for evaluation of liver damage. Sample sections were evaluated using 40 × magnification for assessment of the degree of liver injury by a histologist who was blinded to the treatment groups. For the quantitative analysis, histological photomicrographs were evaluated by scoring system. According to extent of sinusoidal dilatation, inflammatory cell infiltration, congestion, degeneration and cytoplasmic vacuolization scored as 0 (normal), 1 (mild), 2 (moderate), or 3 (severe)(25).
Immunohistochemical assay
Immunohistochemical technique was performed according to the manufacturer's instructions (Abcam Company, USA). The serial sections of tissues were deparaffinized with xylene and then rehydrated in alcohol series. After that, they were incubated with H2O2 (0.3%) in methanol to block endogenous peroxidase activity for 15 min. After this steps, the tissue sections were incubated with protein blocker for 10 min. After incubation at 4 °C overnight with primary antibodies (anti-caspase-3 rabbit polyclonal antibody, 1:100 in PBS, v/v, Abcam, Lat: GR224831-2), serial sections of tissues were incubated with secondary antibody conjugated with horseradish peroxidase (mouse and rabbit specific HRP/DAB, Abcam, Lat: GR2623314-4) for 20 min. The sections were incubated with diaminobenzidine tetrahydrochloride for 5 min(26). Then the slides were dehydrated in alcohol series and mounted. Primary antibodies were omitted for negative controls. Finally, all the slides were assessed under light microscope with a magnification of × 40. For the quantitative analysis, immunohistochemical photomicro-graphs were assessed using MacBiophotonics ImageJ 1.41a software by densitometry method. The positive staining severity was assessed as the ratio of the stained area to the entire field of assessment.
Statistical analysis
Statistical data analysis was done using SPSS 19 version (Chicago, USA). All of the data are expressed as mean ± standard deviation (M ± SD). Different groups were compared with each other using One-Way ANOVA and Tukey tests. P < 0.05 was considered statistically significant.
RESULTS
Effects of atorvastatin on oxidative stress in cyclophosphamide-treated rats
Malondialdehyde, GSH, PC levels, and cell viability rate in liver tissues are presented in Fig. 1. The MDA level, as the final product of lipid peroxidation, was significantly increased while GSH content was decreased in CP treated group as compared with control group. In addition, cell viability rate were significantly decreased and PC increased in CP-treated rats when compared with control group. In contrast, ATV pretreatment in CP-treated rats significantly decreased the MDA and PC levels when compared with CP group, whereas the GSH contents were significantly increased. Besides, the cell viability rate was also significantly improved in ATV + CP group as compared with CP alone group.
Fig. 1.
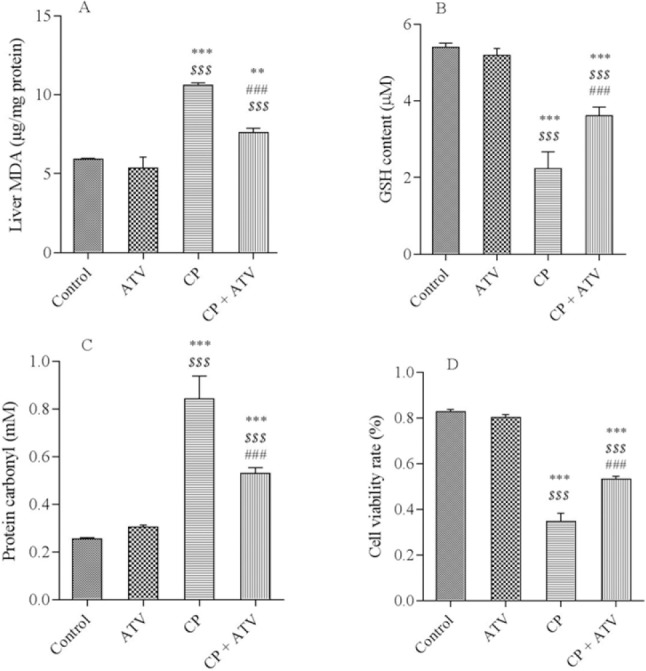
A, Malondialdehyde (MDA); B, glutathione (GSH); C, protein carbonyl (PC) levels; and D, represent cell viability in all groups. Rats treated with cyclophosphamide (CP) showed an increase in the MDA, PC, and a decrease in GSH content and MTT compared with control group. Pretreatment with atorvastatin (ATV) in CP- treated rats significantly decreased concentration of MDA, PC and increased GSH content and MTT in the liver tissue compared with CP group. All values are expressed as mean ± SD. **, ***significantly different from control group (P < 0.01, P < 0.001, respectively); $$$ significantly different from ATV (P < 0.001); and ### significantly different from CP groups (P < 0.001).
Effects of atorvastatin on serum enzymes in cyclophosphamide-treated rats
The levels of the serum enzymes as AST, ALT, and LDH were significantly increased in the CP-treated rats as compared to the control group. ATV administration in CP-treated rats significantly decreased the level of these markers as compared to the CP alone group (Table 1).
Table 1.
Aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) levels in control and cyclophosphamide (CP), atorvastatin (ATV), and ATV + CP groups.

Effect of atorvastatin on histopathology of liver in cyclophosphamide-treated rats
The photomicrographs of liver are presented in Fig. 2. Normal histoarchitecture of liver (hepatocytes, sinusoids, and Kupffer cells) and normal hepatic lobules were observed in control group (Fig. 2A).
Fig. 2.
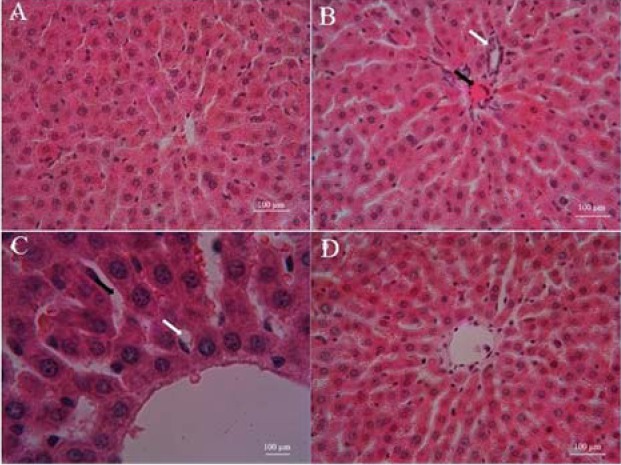
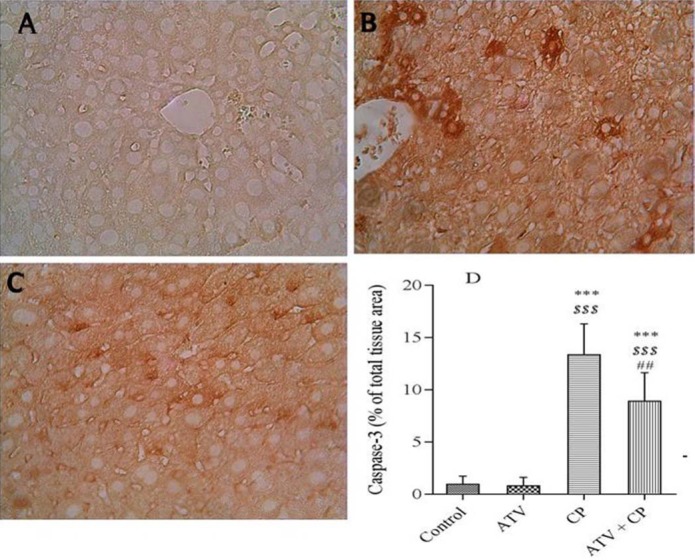
Photomicrographs showed the effect of atorvastatin (ATV) pre-treatment and cyclophosphamide (CP) on the histological architecture of liver in various groups. A, Control; B and C, CP; and D, ATV + CP groups. Normal structure in control group, disorganization, hepatic tissue periportal inflammation (B, white arrow), dilation sinusoids (C, black arrow), hemorrhage, congestion (B, black arrow) and vacuolization (C, white arrow), in CP group. Treatment with ATV improved these changes. Hematoxylene and eosin (H & E), liver sections with magnification; A, B, and D ×40, C ×100. Scale bar = 100 μm.
The structure of the liver in ATV-treated rats was similar to control group. The sections of liver in CP-treated group showed periportal leucocyte infiltration (Fig. 2B, white arrow), congestion and hemorrhage (Fig. 2B, black arrow), fatty degeneration, dilation sinusoids (Fig. 2C, black arrow), focal necrosis with pyknotic and enlarged nuclei, and hepatocyte vacuolation (Fig. 2C, white arrow). However, ATV administration in CP-treated rats mitigated the pathological changes as compared with CP-treated group (Fig. 2D). Liver injury's mean scores of all groups are shown in Fig. 3. Liver injury score increased in CP-treated rats. Score of liver injury was lower in ATV + CP group as compared to CP group, but this decline was not statistically significant.
Fig. 3.
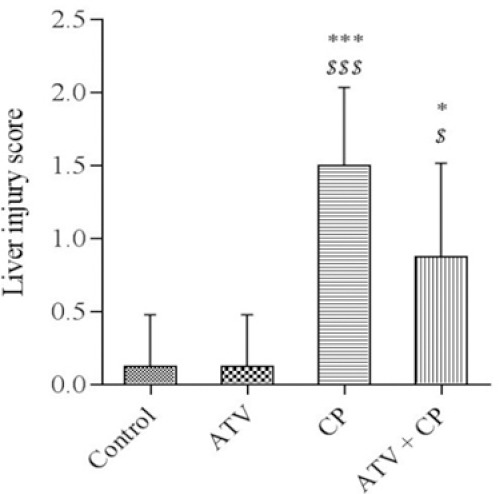
Liver injury scores in liver tissue. Data are presented as mean ± SD. The highest score belongs to cyclophosphamide (CP) group. Atorvastatin (ATV) was able to reduce liver injury score in the ATV + CP group, but was not significant. * and *** significantly different from the control and $ and $$$ significantly different from ATV groups. * and $P < 0.05, *** and $$$P < 0.001.
Effect of atorvastatin on immunoreactivity of caspase-3 in cyclophosphamide-treated rats
Immunohistochemical photomicrographs of the livers are shown in Fig. 4. Section of liver in the control group showed no caspase-3 immunoreactivity.
Fig. 4.
A, Immunohistochemical staining demonstrated no caspase-3 immunoreactivity in the control group. B, Cyclophosphamide (CP) increased caspase-3 immunoreactivity that were remarkable in hepatocyts. C, Atorvastatin (ATV) treatment diminished caspase-3 immunoreactivity in CP treated mice. D, Densitometry analysis of immunohistochemical staining for caspase-3. Data are presented as a percentage of total tissue area. Immunoreactivity level of caspase-3 in the control and ATV alone groups was similar. Data are presented as mean ± SD. *** significantly different from the control, $$$ significantly different from ATV, and ## significantly different from CP groups. ##P < 0.01 and *** and $$$P < 0.001.
Expression of caspase-3 was similar in the ATV and control groups (Fig. 4A). Increased immunoreactivity level of caspase-3 was observed in CP-treated rats. Immunoreactivity staining was shown in the hepatocytes (Fig. 4B). Mild immunoreactivity staining of caspase-3 displayed in ATV + CP group (Fig. 4C) as compared to CP alone group. The histograms of the semi-quantitative analysis of caspase-3 staining in all groups is shown in Fig. 4D.
The most intense immunoreactivity of caspase-3 was determined by semi-quantitative analysis in CP-treated rats (13.35 ± 2.94) compared with the other groups (P < 0.05). ATV administration mitigated the severity of immunoreactivity of caspase-3 (8.9 ± 2.72). Immunoreactivity level of caspase-3 in the control group was similar to ATV group.
DISCUSSION
Cyclophosphamide, as an alkylating drug, has severe toxic effects on normal organs. Phosphoramide mustard and acrolein are active metabolites of CP, that are metabolized in the liver(27). Previous studies have shown that antioxidants can protect normal tissues against CP-induced toxicity(28). In the present study, CP increased the levels of the hepatic associated enzymes in the serum, which confirms liver damage. ATV administration clearly preserved the level of these enzymes against CP-induced hepatotoxicity. Administration of ATV in CP-treated animals resulted in a decrease in MDA, protein carbonyl levels and an increase in the cell viability rate and GSH as compared to CP alone group. Moreover, ATV caused the decreased immunoreactivity level of caspase-3 (apoptosis) and preserved hepatic histoarchitecture.
The oxidative stress and high reactive oxygen species (ROS) production are the main mechanisms involved in hepatotoxicity induced by CP. Reactive oxygen species causes lipid peroxidation of the cell membrane and loss of integrity of cell membrane(29). Our findings showed an increased MDA level in the liver of CP-treated rat which is associated with hepatic damage. Treatment with ATV decreased the lipid peroxidation in CP-treated rats which could be attributed to the free radical scavenging activity of ATV as well as suppressing oxidative stress. We have already shown that ATV mitigated oxidative stress induced by ionizing radiation in the testis of mice(13). In addition, in this study, CP treatment led to a significant reduction in GSH content and an increase in PC contents when compared to the control group liver. The increase in lipid peroxidation and PC attributed to the elevation of free radicals. GSH, as a strong antioxidant, plays a crucial role against the tissue damage caused by oxidative stress. GSH moderates cellular damage that is related to the increase of ROS and modulates the apoptosis(30). Our findings showed that hepatoprotective effect of ATV against CP-induced hepatic damage is related to the suppression of oxidative stress through inhibiting GSH degradation.
In CP-induced hepatotoxicity, cellular damage led to the increased cytosolic enzymes levels (AST, ALT, and LDH) in the blood(31). In this study, a rise in the hepatic index such as ALT, AST, and LDH in serum were observed in CP-induced heptatotoxicy, that were consistent with previous studies(32). Antioxidants are effective in the protection of hepatotoxicity undergoing chemotherapy(4). ATV at low dose is well known for its antioxidant and anti-inflammatory properties(14,13,26). In previous study it was demonstrated that ATV administration at dose of 10 mg/kg for 1 h before ischemia/reperfusion could have protective effects on liver tissue damage(16). ATV mediates its antioxidant property through inhibition of the generation of ROS(33). Protective effect of ATV has been proven in the intestines(11), brain(34), kidney(12), ovary(10), injury induced by ischemia/reperfusion and genotoxicity induced by ionizing radiation(35).
Chemoprotective and therapeutic effects of ATV has been seen against doxorubicin-induced hepato-renal toxicity(18) and CP-induced testicular toxicity(13). Our findings showed protective effect of ATV against CP-induced hepatotoxicity. Although, in the human study, ATV at high doses exhibited an increased risk of significant hepatotoxicity(36).
The protective effect of ATV at low doses against hepatotoxicity induced ischaemia-reperfusion injury has been shown in previous studies(16,37). The key mechanisms of statins are suppression of inflammation (NF-κB, TNF-a, and IL-6) and microvascular protection(16). Furthermore, statins increase antioxidant enzyme activities(38). Atorvastatin with inhibition of vasoconstrictors and upregulation of nitric oxide, improved microcirculation and oxygen delivery to tissue(39). Wiggers showed ATV pretreatment protected hepatotoxicity induced by I/R injury but was not able to protect hepatocellular damage in cholestatic livers(39). Mohamed expressed ATV protected doxorubicin-induced hepato-renal damage via antioxidant, anti- nitrosative, anti-inflammatory, and anti-apoptotic mechanisms(18).
Histopathological examination revealed that CP caused disorganization of hepatic structure, degeneration of hepatocytes and vacuolization, necrosis in hepatocyte cells, peripotal leucocyte infiltration, sinusoid dilatation, congestion, and hemorrhage. These pathological changes were in agreement with previous studies(21). In addition, the mean hepatic injury scoring in CP group was higher as compared to the control and ATV groups. Damage in hepatic tissue structure may be attributed to CP-induced lipid peroxidation, oxidative stress, and subsequently disruption in the structure and function of liver. These findings are consistent with the results of other researchers(40). In the present study, administrations of ATV to CP-treated rat revealed marked preservation of the hepatic tissue structure compared with the CP group. In ATV + CP group, hepatic tissue showed mild inflammation that further reflecting the anti-inflammatory activity of ATV at low doses.
Phosphoramide mustard causes apoptosis through DNA cross-links and acrolein, an extremely reactive molecule, induces toxicity and disruption of normal cell function(41). Increased free radicals with binding to DNA activate caspase-3 signaling and subsequently promote cell death(42). CP induced apoptosis in liver with elevation of pro-inflammatory cytokines and reduction of anti-inflammatory cytokines. The use of antioxidants exhibited protective effect against CP-induced liver damage(43). In the current study, mild immunoreactivity level of caspase-3 was found in ATV + CP group as compared to severe immunoreactivity of caspase-3 in CP group. Pre- and post-treatment with ATV, as an antioxidant, 5 days before and 5 days after administration of CP decreased apoptosis in hepatocytes. This finding was supported by another study stating that ATV has anti-apoptotic property(13,44).
CONCLUSION
Our study showed that CP induced oxidative stress in the liver and ATV administration effectively improved hepatotoxicity induced by CP through free radicals scavenging, decrease of lipid peroxidation and decrease of oxidative stress. In addition, hepatoprotective effect may be attributed to its anti-apoptotic activity that is mediated through alleviating immunoreactivity of caspase 3 in liver tissue.
ACKNOWLEDGMENTS
This work was financially supported by a research grant (IR.MAZUMS.REC. 1396.S217) provided by the Vice Chancellery of Research and Student Research Committee of Mazandaran University of Medical Sciences, Sari, I.R. Iran.
REFERENCES
- 1.Perini P, Calabrese M, Rinaldi L, Gallo P. The safety profile of cyclophosphamide in multiple sclerosis therapy. Expert opin drug saf. 2007;6(2):183–190. doi: 10.1517/14740338.6.2.183. [DOI] [PubMed] [Google Scholar]
- 2.Senthilkumar S, Devaki T, Manohar BM, Babu MS. Effect of squalene on cyclophosphamide-induced toxicity. Clin Chim Acta. 2006;364(1-2):335–342. doi: 10.1016/j.cca.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 3.Tripathi DN, Jena GB. Intervention of astaxanthin against cyclophosphamide-induced oxidative stress and DNA damage: a study in mice. Chem biol interact. 2009;180(3):398–406. doi: 10.1016/j.cbi.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Sheweita SA, El-Hosseiny LS, Nashashibi MA. Protective effects of essential oils as natural antioxidants against hepatotoxicity induced by cyclophosphamide in mice. PloS One. 2016;11(11):e0165667. doi: 10.1371/journal.pone.0165667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kocahan S, Dogan Z, Erdemli E, Taskin E. Protective effect of quercetin against oxidative stress-induced toxicity associated with doxorubicin and cyclophosphamide in rat kidney and liver tissue. Iran J Kidney Dis. 2017;11(2):124–131. [PubMed] [Google Scholar]
- 6.Tripathi D, Jena G. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: role of Nrf2, p53, p38 and phase-II enzymes. Mutat Res. 2010;696(1):69–80. doi: 10.1016/j.mrgentox.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Wei X, Su F, Su X, Hu T, Hu S. Stereospecific antioxidant effects of ginsenoside Rg3 on oxidative stress induced by cyclophosphamide in mice. Fitoterapia. 2012;83(4):636–642. doi: 10.1016/j.fitote.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Schrott HG, Knapp H, Davila M, Shurzinske L, Black D. Effect of atorvastatin on blood lipid levels in the first 2 weeks of treatment: a randomized, placebo-controlled study. Am Heart J. 2000;140(2):249–252. doi: 10.1067/mhj.2000.108245. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Q, Liao JK. Pleiotropic effects of statins. Basic research and clinical perspectives. Circ J. 2010;74(5):818–826. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parlakgumus HA, Bolat FA, Kilicdag EB, Simsek E, Parlakgumus A. Atorvastatin for ovarian torsion: effects on follicle counts, AMH, and VEGF expression. Eur J Obstet Gynecol Reprod Biol. 2014;175:186–190. doi: 10.1016/j.ejogrb.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Ozacmak VH, Sayan H, Igdem AA, Cetin A, Ozacmak ID. Attenuation of contractile dysfunction by atorvastatin after intestinal ischemia reperfusion injury in rats. Eur J pharmacol. 2007;562(1-2):138–147. doi: 10.1016/j.ejphar.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 12.Gottmann U, Brinkkoetter PT, Hoeger S, Gutermann K, Coutinho ZM, Ruf T, et al. Atorvastatin donor pretreatment prevents ischemia/reperfusion injury in renal transplantation in rats: possible role for aldose-reductase inhibition. Transplantation. 2007;84(6):755–762. doi: 10.1097/01.tp.0000281410.85659.48. [DOI] [PubMed] [Google Scholar]
- 13.Naeimi RA, Talebpour Amiri F, Khalatbary AR, Ghasemi A, Zargari M, Ghesemi M, et al. Atorvastatin mitigates testicular injuries induced by ionizing radiation in mice. Reprod Toxicol. 2017;72:115–121. doi: 10.1016/j.reprotox.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 14.Hamzeh M, Talebpour Amiri F, Karimpour Malekshah A, Yaghubi Beklar S, Hosseinimehr SJ. Protective effect of atorvastatin against cardiotoxicity and hematotoxicity induced by cyclophosphamide in rat. J Mazandaran Univ Med Sci. 2017;27(151):1–11. [Google Scholar]
- 15.Carrascosa MF, Salcines-Caviedes JR, Lucena MI, Andrade RJ. Acute liver failure following atorvastatin dose escalation: Is there a threshold dose for idiosyncratic hepatotoxicity? J Hepatol. 2015;62(3):751–752. doi: 10.1016/j.jhep.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Ajamieh H, Farrell GC, McCuskey RS, Yu J, Chu E, Wong HJ, et al. Acute atorvastatin is hepatoprotective against ischaemia‐reperfusion injury in mice by modulating eNOS and microparticle formation. Liver Int. 2015;35(9):2174–2186. doi: 10.1111/liv.12827. [DOI] [PubMed] [Google Scholar]
- 17.Zamani E, Mohammadbagheri M, Fallah M, Shaki F. Atorvastatin attenuates ethanol-induced hepatotoxicity via antioxidant and anti-inflammatory mechanisms. Res pharm sci. 2017;12(4):315–321. doi: 10.4103/1735-5362.212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- 19.Massafra C, Gioia D, De Felice C, Picciolini E, De Leo V, Bonifazi M, et al. Effects of estrogens and androgens on erythrocyte antioxidant superoxide dismutase, catalase and glutathione peroxidase activities during the menstrual cycle. Journal of Endocrinology. 2000;167(3):447–452. doi: 10.1677/joe.0.1670447. [DOI] [PubMed] [Google Scholar]
- 20.El-Moselhy MA, El-Sheikh AA. Protective mechanisms of atorvastatin against doxorubicin-induced hepato-renal toxicity. Biomed Pharmacother. 2014;68(1):101–110. doi: 10.1016/j.biopha.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Fouad AA, Albuali WH, Jresat I. Protective effect of hesperidin against cyclophosphamide hepatotoxicity in rats. Int J Biol Food Vet Agric Eng. 2014;8(7):722–5. [Google Scholar]
- 22.Radu M, Munteanu MC, Petrache S, Serban AI, Dinu D, Hermenean A, et al. Depletion of intracellular glutathione and increased lipid peroxidation mediate cytotoxicity of hematite nanoparticles in MRC-5 cells. Acta Biochim Pol. 2010;57(3):355–360. [PubMed] [Google Scholar]
- 23.Fathi H, Ebrahimzadeh MA, Ziar A, Mohammadi H. Oxidative damage induced by retching; antiemetic and neuroprotective role of Sambucus ebulus L. Cell Biol Toxico. 2015;31(4-5):231–239. doi: 10.1007/s10565-015-9307-8. [DOI] [PubMed] [Google Scholar]
- 24.Ghazi‐Khansari M, Mohammadi‐Bardbori A, Hosseini MJ. Using janus green B to study paraquat toxicity in rat liver mitochondria. Ann N Y Acad Sci. 2006;1090(1):98–107. doi: 10.1196/annals.1378.010. [DOI] [PubMed] [Google Scholar]
- 25.Akbulut S, Elbe H, Eris C, Dogan Z, Toprak G, Otan E, et al. Cytoprotective effects of amifostine, ascorbic acid and N-acetylcysteine against methotrexate-induced hepatotoxicity in rats. World J Gastroenterol. 2014;20(29):10158–10165. doi: 10.3748/wjg.v20.i29.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talebpour Amiri F, Hamzeh M, Naeim RA, Ghasemi A, Hosseinimehr SJ. Radioprotective effect of atorvastatin against ionizing radiation-induced nephrotoxicity in mice. Int J Radiat Biol. 2018;94(2):106–113. doi: 10.1080/09553002.2018.1420926. [DOI] [PubMed] [Google Scholar]
- 27.McDonald GB, Slattery JT, Bouvier ME, Ren S, Batchelder AL, Kalhorn TF, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101(5):2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 28.Ghobadi E, Moloudizargari M, Asghari MH, Abdollahi M. The mechanisms of cyclophosphamide-induced testicular toxicity and the protective agents. Expert Opin Drug Metab Toxicol. 2017;13(5):525–536. doi: 10.1080/17425255.2017.1277205. [DOI] [PubMed] [Google Scholar]
- 29.Mahmoud AM. Hesperidin protects against cyclophosphamide-induced hepatotoxicity by upregulation of PPARγ and abrogation of oxidative stress and inflammation. Can J Physiol Pharmacol. 2014;92(9):717–724. doi: 10.1139/cjpp-2014-0204. [DOI] [PubMed] [Google Scholar]
- 30.Aksu E, Kandemir FM, Özkaraca M, Ömür AD, Küçükler S, Çomaklı S. Rutin ameliorates cisplatin‐induced reproductive damage via suppression of oxidative stress and apoptosis in adult male rats. Andrologia. 2017;49(1) doi: 10.1111/and.12593. DOI: 10.1111/and.12593. [DOI] [PubMed] [Google Scholar]
- 31.Cuce G, Çetinkaya S, Koc T, Esen HH, Limandal C, Balcı T, et al. Chemoprotective effect of vitamin E in cyclophosphamide-induced hepatotoxicity in rats. Chem Biol Interact. 2015;232:7–11. doi: 10.1016/j.cbi.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 32.El-Kholy AA, Elkablawy MA, El-Agamy DS. Lutein mitigates cyclophosphamide induced lung and liver injury via NF-κB/MAPK dependent mechanism. Biomed Pharmacother. 2017;92:519–527. doi: 10.1016/j.biopha.2017.05.103. [DOI] [PubMed] [Google Scholar]
- 33.Wassmann S, Laufs U, Müller K, Konkol C, Ahlbory K, Bäumer AT, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002;22(2):300–305. doi: 10.1161/hq0202.104081. [DOI] [PubMed] [Google Scholar]
- 34.Elewa HF, Kozak A, El-Remessy AB, Frye RF, Johnson MH, Ergul A, et al. Early atorvastatin reduces hemorrhage after acute cerebral ischemia in diabetic rats. J Pharmacol Exp Ther. 2009;330(2):532–540. doi: 10.1124/jpet.108.146951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosseinimehr SJ, Izakmehri M, Ghasemi A. In vitro protective effect of atorvastatin against ionizing radiation induced genotoxicity in human lymphocytes. Cell Mol Biol (Noisy-le-grand) 2015;61(1):68–71. [PubMed] [Google Scholar]
- 36.Clarke AT, Johnson PC, Hall GC, Ford I, Mills PR. High dose atorvastatin associated with increased risk of significant hepatotoxicity in comparison to simvastatin in UK GPRD cohort. PloS One. 2016;11(3):e0151587. doi: 10.1371/journal.pone.0151587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ajamieh H, Farrell G, Wong HJ, Yu J, Chu E, Chen J, et al. Atorvastatin protects obese mice against hepatic ischemia-reperfusion injury by Toll‐like receptor‐4 suppression and endothelial nitric oxide synthase activation. J Gastroenterol Hepatol. 2012;27(8):1353–1361. doi: 10.1111/j.1440-1746.2012.07123.x. [DOI] [PubMed] [Google Scholar]
- 38.Kocak FE, Kucuk A, Ozyigit F, Tosun M, Kocak C, Kocak A, et al. Protective effects of simvastatin administered in the experimental hepatic ischemia-reperfusion injury rat model. J Surg Res. 2015;199(2):393–401. doi: 10.1016/j.jss.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 39.Wiggers JK, van Golen RF, Verheij J, Dekker AM, van Gulik TM, Heger M. Atorvastatin does not protect against ischemia-reperfusion damage in cholestatic rat livers. BMC Surg. 2017;17(1):35. doi: 10.1186/s12893-017-0235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selvakumar E, Prahalathan C, Mythili Y, Varalakshmi P. Mitigation of oxidative stress in cyclophosphamide-challenged hepatic tissue by DL-alpha-lipoic acid. Mol Cell Biochem. 2005;272(1-2):179–185. doi: 10.1007/s11010-005-7322-4. [DOI] [PubMed] [Google Scholar]
- 41.Frew JW, Davatchi CC, Murrell DF. Cyclophosphamide in autoimmune blistering diseases: safety, efficacy and evidence base. Blistering Diseases: Springer; 2015. pp. 507–13. [Google Scholar]
- 42.Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update. 2005;11(2):162–177. doi: 10.1093/humupd/dmi001. [DOI] [PubMed] [Google Scholar]
- 43.Shi L, Liu Y, Tan D, Yan T, Song D, Hou M, et al. Blueberry anthocyanins ameliorate cyclophosphamide-induced liver damage in rats by reducing inflammation and apoptosis. J Funct Foods. 2014;11:71–81. [Google Scholar]
- 44.Bao XM, Wu CF, Lu GP. Atorvastatin inhibits homocysteine-induced oxidative stress and apoptosis in endothelial progenitor cells involving Nox4 and p38MAPK. Atherosclerosis. 2010;210(1):114–121. doi: 10.1016/j.atherosclerosis.2009.11.032. [DOI] [PubMed] [Google Scholar]