Abstract
Human breath, along with urine and blood, has long been one of the three major biological media for assessing human health and environmental exposure. In fact, the detection of odor on human breath, as described by Hippocrates in 400 BC, is considered the first analytical health assessment tool. Although less common in comparison to contemporary bio-fluids analyses, breath has become an attractive diagnostic medium as sampling is non-invasive, unlimited in timing and volume, and does not require clinical personnel. Exhaled breath, exhaled breath condensate (EBC), and exhaled breath aerosol (EBA) are different types of breath matrices used to assess human health and disease state. Over the past 20 years, breath research has made many advances in assessing health state, overcoming many of its initial challenges related to sampling and analysis. The wide variety of sampling techniques and collection devices that have been developed for these media are discussed herein. The different types of sensors and mass spectrometry instruments currently available for breath analysis are evaluated as well as emerging breath research topics, such as cytokines, security and airport surveillance, cellular respiration, and canine olfaction.
Keywords: breath research, exhaled breath condensate (EBC), exhaled breath aerosol (EBA), volatile organic compounds (VOCs), analytical techniques, biomarkers
Graphical abstract

Abbreviations1
1.Introduction
Before the advent of even the simplest laboratory instrumentation, the human nose served as the original chemical detector for disease diagnosis. It all started with Hippocrates (born 460 BC) who taught his students to use breath odor to identify patients with liver disease, uncontrolled diabetes, and failing kidneys.1There are a few additional pivotal moments in breath history that bring us to the present. In 1971, Linus Pauling published a seminal article demonstrating analytical methodology used to identify approximately 250 compounds in breath in addition to nitrogen, oxygen, water and carbon dioxide.2 The final step in ushering in the modern era of breath research came from the work of Lance Wallace of the U.S. Environmental Protection Agency, who recognized that breath analysis could unambiguously document recent exposures to environmental contaminants.3 Michael Phillips (St. Vincent’s Medical Center, New York and Mensanna Research Inc., New Jersey) also contributed by developing case-control tudies starting in 1987 using breath constituents and patterns to discern a variety of adverse health states including lung and breast cancer as well as cardio-pulmonary disease.4–5
Over the years, the wide use of different methods for breath media analyses has revealed the need for consistency and standardization in breath research. Researchers have proposed that breath analyses should undergo a series of checks, including standard parameters, specifications, and test methods, as well as risk analysis. These are measurable criteria by which research studies can be characterized and evaluated.6 A wide variety of compounds, such as volatile organic compounds (VOCs), NO, CO2, NH3, cytokines, and hydrogen peroxide (H2O2) are commonly analyzed in exhaled breath, exhaled breath condensate (EBC), and exhaled breath aerosol (EBA). These compounds can be analyzed using different techniques, including GC-MS, ion mobility mass spectrometry (IMS), chemical ionization-mass spectrometry (CI-MS), spectroscopy, and sensors.7 The use of mass spectrometers that measure the integer mass of non- target compounds instead of the exact mass (to the fourth or fifth decimal place) can make it challenging to identify non-target compounds in breath samples since many compounds share the same integer mass despite having different chemical formulas. The use of high-resolution mass spectrometry, including GC × GC-MS and GC-time-of-flight (TOF)-MS, can alleviate this problem for non-targeted analysis.8 However, the wide range of available analytical techniques and target compounds make it difficult to apply universal standards for sampling and analysis that are suitable across all methods.7
Despite these challenges, breath research has made many advances in both analytical techniques and health-related applications throughout its history. Traditional gas-phase breath spectrometry, including GC × GC-MS and GC-time-of-flight (TOF)-MS, can alleviate this analysis as well as EBC and EBA have become valuable breath matrices for disease detection and environmental exposure.9,10,11 The variety of breath sample collection devices and methods that have been developed, ranging from large canisters and polymer bags to easily portable thermal desorption (TD) tubes for VOC analysis, have evolved to suit the needs of the changingfield.12 The analytical instrumentation for detection of compounds in exhaled breath, EBC, and EBA has also improved to include GC-MS, LC-MS, Direct MS, and a variety of sensors, including electronic noses.13,14,15,16 These detection methods have been utilized to advance research in human health and disease, including alcohol breath testing,17,18 lung cancer,19,20 asthma,21,22 and chronic obstructive pulmonary disorder (COPD),22,23,24 as well as to identify biomarkers of health state in breath matrices. These topics are discussed in this review within the context of the history of breath research from 1995–2016. Papers were curated using PubMed searches. The data from these searches were utilized to construct bar graphs for many of the classic breath topics to discern trends in the number of publications over time. All of the topics show an increase in publications, especially in recent years. These data are utilized as a supplement to explain the significance of breath research over time, but investigation of all publications in this time range is outside the scope of this review. This review briefly discusses many of the main topics in breath research, providing an overview of some of the history and interesting applications in recent years. Novel applications of breath research for security, cellular respiration, organ-on-chip, canine olfaction, and crowd breath show the evolution of the field from standard VOC analysis of breath toward cellular and animal models with promising future directions.
2. Publication Curation Methods
Publications reporting breath research data in the various topic areas were gathered using PubMed advanced searches. All keyword searches were limited to the Title/Abstract of the paper to ensure that breath research was the focus of the article. Papers were date restricted from 01/01/1995 to 12/31/2016 for all categories except alcohol, which was searched from 01/01/1960 to 12/31/2016. The year range from 1995–2016 was selected because breath research became more prominent in 1995 in a wider range of applications. For alcohol research, a longer time span was chosen because alcohol research began earlier than many of the other types of breath research. Initial keyword searches were inspected to determine the relevance of the returned articles versus the topic area, and “NOT” statements were added to the search strings to eliminate topics unrelated to the analysis of exhaled breath. These “NOT” statements included “shortness of breath,” “short of breath,” “breath sounds,” and “gastric emptying.” These phrases tended to be present in articles describing medical procedures that did not involve the analysis of exhaled breath. The PubMed database was accessed in July 2017. Aerosol, condensate, and alcohol searches only included the search string for exhaled breath, as these topic areas involved sampling from just humans. Searches for GC-MS, LC-MS, Direct-MS, and sensors also included volatile organic compounds and headspace from biological media, including blood, urine, feces, faeces, milk, cell line, cell culture, bacteria, or saliva. Detailed search strings for all topics that were used to compose the figures can be found in the accompanying Data in Brief article. The number of manuscripts published per year in each category as well as the appendix of articles curated using each search can also be found elsewhere (Wallace et al. submitted).
3. Types of Breath Matrices
3.1 Gas-Phase Exhaled Breath
The traditional venue of breath testing has always revolved around the gas-phase. After inhalation and exposure, VOCs are retained within different parts of the body, depending on their breath-blood-fat partition coefficients. VOCs located in fatty tissues are released to the blood and are then exchanged into the breath through the alveoli and airways in the lungs. A portion of VOCs are also retained within the respiratory tract after exposure.25,26 Thus, breath concentrations of VOCs are representative of blood concentrations, but samples can be obtained non-invasively with little discomfort to the individual.26 VOCs may originate from exogenous environmental exposures through inhalation, ingestion, or skin absorption (e.g., gasoline, food, lotions) or endogenous sources from within the body (e.g., microbe metabolism, byproducts, cancerous tumors).27 Breath contains both dead space air and alveolar air. Dead space air originates from the mouth, trachea, and bronchi, while alveolar air is from the lungs.28,26 Alveolar breath only consists of a 350 mL volume at the tail end of breath. Many breath sampling techniques have been designed to capture only the alveolar air, as this contains the VOCs that have been exchanged across the blood-breath barrier.26 Since VOCs are gases, they are easier to access in the breath compared to a condensed medium.29
3.2 Exhaled Breath Condensate (EBC)
The breath, however, is more complex than gas exchange. The lungs humidify exhaled breath, and because the human body is usually warmer than the environment, the exhaled water vapor tends to condense on the way out. Furthermore, the surfaces in all parts of the lung down to the alveoli are coated with an aqueous mucous layer that can be aerosolized and carry along a variety of non-volatile constituents.9,30 This was originally exploited for assessing lung status via dissolved ions (pH).31,32 The pH provides an indication of the acidobasic balance of the compounds that compose EBC.9 Access to this fraction of the breath was developed using a technique wherein multiple breaths were passed through a chilled tube and condensate was recovered for analysis. Termed exhaled breath condensate (EBC) in the literature, it was quickly recognized as a matrix providing a completely new set of analytes that were previously unknown.11–33 EBC is now used regularly in breath research as a complement to gas-phase analyses.
EBC consists mostly of condensed water vapor with dispersed nonvolatile molecules from the respiratory tract within it.11–34 EBC represents a relatively non-invasive sample that provides important information about the constituents in the fluid lining the airway.35 Hydrogen peroxide, ammonia, adenosine, leukotrienes, isoprostanes, nitrogen oxides, peptides and cytokines are all examples of compounds found in EBC.10, 34,9 Recently, simple measurements of EBC acidity (pH) have been developed for “at home” collection and monitoring of children’s asthma status.36,37 Another promising application for EBC analysis is the collection of condensate from ventilated patients in hospitals. This is of particular interest for preterm infants, as they cannot be regularly sampled for blood or urine analyses in sufficient volumes.38,39,40,41 Publications of EBC analyzed from patients on respiratory support have continued to grow ever since.42,20 This is equally important for assessment of EBC from critically ill mechanically ventilated patients with acute respiratory distress syndrome (ARDS).43,44
3.3 Exhaled Breath Aerosols (EBA)
EBA represents a fraction of total EBC, and is targeted to larger molecules, such as fatty acids and cytokines, as well as cellular fractions, proteins, viruses, and bacteria instead of the gas-phase.45,46,47,48,49 This technique has the advantage of simplicity in the field as there are no requirements for freezing out the sample; subjects or patients can simply wear standard hospital/medical masks for a given time (e.g., 10 min) and these can be shipped back to thelaboratory and processed for a variety of targeted compounds analyses.49,48 EBA has been measured in breath over the past 20 years, with a steady number of articles published per year from 2008–2015 and a significant spike in publications in 2012. Figure 1 compares the publication trend in the number of peer-reviewed articles published featuring EBA versus EBC media. While both EBC and EBA publications have increased over time, EBC has been reported in the literature more frequently than EBA, showing a faster upward trend in the number of publications from 2003–2009 (see Figure 1). The usefulness of EBA for breath research appears to still be evolving, while EBC is a more established technique.
Figure 1:
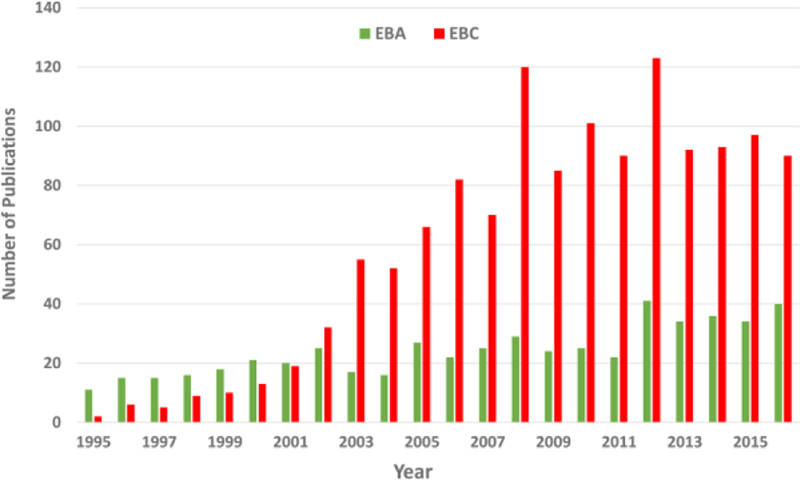
Comparison of EBC and EBA publication trends
Publications featuring EBA and EBC media have increased over the past 20 years, although EBC publication rates have accelerated past those of EBA, with more than double the number of EBC publications than EBA from 2003–2016.
In addition to biological media, particulate matter (PM) and nanoparticles have also been detected using EBA. Exposure to PM from air pollution causes inflammation in the lungs.50 Exhaled breath particles (EBPs) are formed when closed airways open upon inhalation.51 EBPs have also been evaluated in individuals with asthma and allergies, children with asthmatic symptoms, patients on mechanical ventilators, smokers, and patients with chronic obstructive pulmonary disease (COPD).51,50,52,53,54,55 Occupational exposure to titanium dioxide and iron oxide nanoparticles has been investigated using breath analysis. Although particle concentrations in air did not exceed the national airborne exposure limit, employees consistently exposed to nanoparticles showed significantly more titanium and oxidative stress markers in EBC samples compared with controls.56;57,58
Breath aerosol sampling has also been applied to develop drug tests. Unlike testing blood or urine, breath sampling represents a noninvasive technique that can be used for routine sampling in work places.59 For drug testing, bio-aerosols from breath are collected on a filter when the subject breathes through the sampling device. The drug compounds are then released into solution when the filter is immersed in methanol.60,61 An LC-MS method was developed to detect drugs from breath samples, and the method was employed to monitor amphetamine, methamphetamine, tetrahydrocannabinol (THC), and cocaine.61 Another study used an ExaBreath sampler to measure methadone in drug addicts to evaluate exposure. The ExaBreath sampler collects aerosols (particles) onto a filter as an individual exhales through the sampling device. However, breath detection was limited to methadone, whereas urine samples collected from the same subjects tested positive for a variety of additional substances, including morphine, neuroleptics, and benzoylecgonine.60 Breath samples were found to only reflect the most recent exposure, whereas urine samples were needed to determine drugs that had been in the system for a longer duration.60,61
4. Breath Sample Collection
4.1 Polymer Bags
Popular methods for volatile breath sampling include polymer bags, canisters, and thermal desorption tubes. Polymer bags are widely used due to their low cost, easy maneuverability, and potential for reuse. Examples of polymer bags include Tedlar, Teflon, FlexFoil, and Nalophan, although Tedlar is most common.28 Polymer bags are composed of inert materials to reduce reactions.28 Some polymer bags are covered with outer layers of black Tedlar to block UV rays that may damage samples.62 Tedlar bags have been used to analyze acetone as a marker for diabetes,63 isoprene as a marker of cholesterol synthesis,64 as well as volatiles from healthy individuals and lung cancer patients to identify biomarkers.65 While Tedlar bags have been shown to pollute the contents of breath samples, introducing some minor contaminants to the sample that are worse when it is subjected to heat,28,62 these contaminants have been shown to be less than 10% of the total values from human breath.62 Common contaminants from Tedlar bags include phenol, N,N-dimethylacetamide, carbon disulfide, and carbonyl sulfide.28
4.2 Canisters
Stainless steel inert canisters are used to collect and store breath samples without corrosion, exposure to light, or the potential for interactions with the vessel. Canisters are advantageous for breath sample collection because they are durable and easy to use.66 However, a disadvantage of canisters is their high cost, the need for prior evacuation before sampling, and requirements for specialized cleaning equipment.28 Electropolished SUMMA canisters have been used to collect alveolar breath samples in 1 L volumes with parts-per-billion by volume (ppbv) sensitivity.66 After releasing the dead-space air at the end of a breath, the individual opens the canister valve and expires the reserve air from the lungs into the canister before closing it. Most VOCs are stable in canisters for at least 30 days after sample collection.67 Canisters have been used to analyze compounds in breath to determine exposure to VOCs in groundwater, gasoline, and swimming pools.68,66,69,70,71
4.3 Sorbent Tubes
TD tube sampling is another method commonly used for breath sampling. Individuals exhale end-tidal breath into a device such as a polymer bag or a Markes Bio-VOC concentrator, and the sample is then plunged onto a thermal desorption tube to collect the VOCs.72,12 Other breath samplers, such as the ReCIVA unit (Owlstone Ltd.) are available, which capture end-tidal breath directly onto sorbent tubes.73 Modifications of the Haldane-Priestley tube have also been used for such applications, in which insulated aluminum tubes are connected to polymer bags.28 After sample collection, the tubes are capped and analyzed by automated thermal desorption (ATD)-GC/MS. Many different types of thermal desorption tubes with different sorbents are available, depending upon the volatile compounds of interest. Tenax and carbograph sorbents are commonly utilized in breath research.74,75,28 TD has been used to analyze VOCs and other compounds originating from diverse groups such as firefighters, chronic kidney disease patients, and cellular headspace.68,76,77
4.4 EBC Collection
Exhaled breath, which consists largely of water vapor and aerosol particles, can be collected through condensation using a breathing tube, and the fractions of EBC and EBA can be used experimentally. EBC is typically collected by 10 min of tidal breathing through a long tube that is cooled with ice (0 °C) or dry ice (−80 °C) in order to condense the aqueous fraction of the exhaled air, depending on the analysis method and the stability of the substances to be measured.10,9 A volume of 1–2 mL is usually collected at a time.34,11 Three commercial devices that are often used for collecting EBC are the RTube, Turbodecss, and EcoScreen. The RTube is advantageous because it is a disposable kit with a one-way non-rebreathing valve connected to a collection tube.37 The Turbodecss is also portable with a disposable collection cell and a non-rebreathing valve, allowing this sampler to also be used for in-home collection.9 The EcoScreen system has a two-way valve that prevents mixing of inhaled and exhaled air as the exhaled air is cooled in the condenser.37 However, as opposed to the RTube and Turbodecss, the EcoScreen is not portable, limiting its ability to be used at home.20 The EcoScreen has therefore been used primarily in research laboratories.9 A Loccioni instrument can also be used during collection of EBC and EBA samples. This device gives the individual feedback about their breathing pattern during sample collection, reducing variability in sample volumes and biomarker levels that can be caused by hyperventilation.78,79
4.5 EBA Collection
EBA can be collected using sampling devices or different forms of filter materials at room temperature. Teflon filters have been used for collection, and EBA particles can be extracted using solvent.45 Particles can then be analyzed using LC-MS.46 The Gesundheit II (G- II) sampling device has been utilized to collect breath aerosol while subjects sit in a booth andbreath through the cone of the sampler.80 Subjects can wear masks or respirators, and breathe, talk, or cough as they normally would. The breath enters a slit impactor, which collects particles larger than 5.0 µm aerodynamic diameter and captures then on a Teflon substrate from which they are extracted and analyzed by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). This device is commercially available from SKC BioSampler and has been utilized to assess influenza and levels of other viruses in breath.80 Other commercially available devices, such as the ExaBreath sampler mentioned above, can also be used for convenient collection of EBA.60 Silicon wafers with cascade impactors have also been used to collect phospholipids from exhaled breath based on their inertia. This technique was used to compare phospholipid compositions of patients with asthma, cystic fibrosis, and healthy controls.81 For a more simplistic collection approach, individuals can wear plastic masks/respirators or paper hospital masks for a specified length of time, and the surface of the mask (or the entire paper mask) can be sampled using a moistened piece of filter paper, which can then be extracted with solvent for LC-MS analysis.82
5. Detection Methods: Sensors
5.1 Electronic Nose
The electronic nose, or e-nose, detects odors, flavors, and VOCs using non-specific gas sensor arrays and pattern recognition tools.83 The first e-nose was developed in 1964 by Wilkens and Hatman using redox reactions of odorants on an electrode; in 1965 this design was quickly followed by other designs that utilized conductivity of odorants and variation of odorant contact potential.84,85 ,86 In 1982, Persaud and Dodd developed an electronic nose using semiconductor transducers that was capable of discriminating between different odorants,87 and in 1985 Ikegami and Kaneyasu devised an e-nose composed of semiconductor oxide that produced different patterns in response to odors.88 These publications in the 1980s showcased the e-nose as a sensitive and useful chemical array sensor system, and from this point on the use of e-noses increased.14
Sensors in e-noses function by detecting physical or chemical changes within the array in response to the components in exhaled breath. Metal-oxide sensors were first introduced in the 1960s and have low parts-per-million by volume (ppmv) detection levels.89 Metal oxide sensors have long-term performance and stability, making them advantageous for e-nose designs.90 Metal oxide sensors are heated with a ceramic support tube coated in metal, typically SnO2. Gas molecules that interact with the metal cause changes in conductivity due to oxygen exchange.89 Metal-oxide-semiconductor field-effect transistor (MOSFET) sensor arrays are commonly used to devise e-noses and utilize transistors to amplify and alter electronic signals. Gases, such as hydrogen, enter the sensor array and interact with the metal, changing the electric field inside the device to produce a signal that is electronically recorded.91 The SpiroNose sensor, which was designed for medical testing, contains five metal-oxide semiconductor sensor arrays, two of which are used as reference arrays to analyze ambient VOCs and three to detect VOCs in breath samples.92
Other sensors can be used to develop e-nose devices, such as conducting polymers, polymer composites, acoustic waves, optical fiber arrays, electrochemical gas (EC), and colorimetric sensor arrays.93 The Cyranose 320 is a widely-used, portable, hand-held e-nose device that utilizes a carbon black conducting polymer sensor array to achieve low ppmv detection limits.94 Upon exposure to gases or vapors, the polymer undergoes changes in electrical resistance that reduce the number of conducting pathways. Surface acoustic wave sensors are usually developed using piezoelectric substrate materials such as ZnO, lithium niobate or quartz with transmitting and receiving transducers on either side.91 The surface acoustic wave sensor has been utilized in an e-nose to compare volatile profiles of lung cancer patients with controls.83 Optical fibers coated in fluorescent dye have been utilized as sensors, in which wavelength changes, intensity alterations, and spectrum changes are tracked as indicators of interaction with a gas or vapor.91 Metalloporphyrin dye dots have been printed on paper sensor cartridges to mcreate colorimetric sensor arrays, and pattern changes in the dye colors as a result of interactions with the chemicals in the breath samples have been captured by photographs.93 In EC sensors, volatiles are detected after electrochemical oxidation or reduction at the electrode surface, making these sensors selective for a small number of reactive gases.89
E-noses have been applied to detect pattern differences in a variety of clinical and research samples, including military, food industry, and environmental applications.83 The e-nose has been utilized to differentiate Alzheimer’s disease, Parkinson’s disease, and healthy control samples, and organic compounds with significant differences between the disease states were identified by IMS and used to create a predictive model for sample classification.95 A study of obstructive sleep apnea (OSA) revealed that the e-nose could distinguish OSA breath samples from controls in the morning but not in the evening, indicating that the sampling time may affect the ability of the e-nose to detect the disorder.96 Breathprints are the chemical signatures of compounds and molecules that are found within an individual’s exhaled breath. The SpiroNose was able to discriminate between breathprints of healthy controls and patients with asthma, COPD, and lung cancer with cross-validation values from 78–88%.92 Recently, aspergillus fumigatus colonization was found to produce a distinct breathprint in e-nose samples from cystic fibrosis patients, providing a non-invasive test for infection.97
While e-nose publications remain steady in the field, there is still a need for standardization. Some studies have shown that e-noses lack the accuracy and repeatability needed to make them useful for diagnosis. A recent study investigated the effect of different exhalation flow rates and breath holding times on the breathprints of healthy individuals and individuals diagnosed with lung cancer during sample collection using the Cyranose 320 and found that the patterns were significantly altered for healthy adults but not for lung cancer patients.94 This study shows how inconsistencies in sample collection between individuals and research groups can significantly alter the ability to discriminate healthy versus diseased individuals. In another study, e-noses were found to have greater within-day than between-day repeatability (P < 0.0001), but sample transportation and storage on sorbent tubes prior to e-nose analysis has not been found to significantly affect quantitation of target compounds.98,99
5.2 Mid-infrared Sensors
Mid-infrared quantum cascade lasers are advantageous for exhaled breath analysis due to their reliability, compactness, tunability, and low power requirements.100 Mid-infrared lasers and sensors have been utilized over the past fifteen years to detect exhaled compounds in breath. Mid-infrared lasers have a wavelength range from 3–20 μm with a tuning range of greater than 2 μm, giving them high spectral brightness. These properties make them ideal for accurately determining the isotope ratio of (12)CO2/(13)CO2, which is an important biomarker of glucose metabolism during sepsis.101,102 (12)CO2/(13)CO2 isotope ratios have been monitored in mouse breath using hollow waveguide gas cells and external cavity quantum cascade lasers (EC- QCLs).102,101 A (13)CO2 isotope ratio-meter has also been recently developed to monitor breath levels during moderate exercise for individuals attempting to lose weight, as altered (13)CO2 isotope ratios indicate changes in macronutrient metabolism.103
Mid-infrared sensors are often utilized to monitor exhaled levels of volatiles, such as nitric oxide (NO), hydrogen sulfide, isoprene, and acetone. NO is a biomarker for asthma and respiratory disorders and is therefore frequently monitored in clinical settings. Sensitive sensors capable of real-time measurements of NO in breath have been using the mid-infrared spectral region.104,105 A miniaturized mid-infrared one-dimensional (1D) photonic crystal cavity sensor with a sensitivity of 10 ppbv with comparable performance to commercial sensors was recently developed.106 Real time gas sensing of hydroge sulfide was accomplished using substrate- integrated hollow waveguides coupled to a Fourier transform infrared spectrometer with applications for development into a hand-held instrument for industrial monitoring of hydrogen sulfide exposure based on breath levels of this toxic gas.107 Hollow waveguide mid-infrared sensors have also been utilized to measure isoprene breath levels, and acetone has been detected at sub-ppmv levels using QCL infrared spectrometers.108,109 Breath profiles of dialysis patients have also been assessed using mid-infrared absorption spectroscopy to quantify levels of volatiles, such as carbon dioxide and ammonia to establish reference values for use in case-control studies.110
5.3 Novel Sensors and Sampling Devices
In addition to electronic noses and mid-infrared sensors, a series of novel sensors and sampling devices that use similar technology have also been developed within the last ten years for breath monitoring applications. A gas phase biosensor was developed that can measure breath acetone to determine lipid metabolism after exercise.111 Another portable breath analyzer capable of measuring acetone levels was developed that utilizes the cavity ringdown spectroscopy technique for online breath analysis.112 Gold nanoparticle-based flexible sensors that can detect ppbv levels of VOCs have been utilized to distinguish exhaled breath collected from ovarian cancer patients versus healthy controls.113.
6. Detection Methods: Mass Spectrometry
Mass spectrometry is a useful analytical tool for breath research that provides essential information about the compounds in different types of breath samples. Breath articles were searched under three main types of mass spectrometry applications: GC-MS, LC-MS, and Direct MS. These breath searches also included applications of gas-phase analysis of biological headspace from blood, urine, milk, saliva, feces, cell lines, and bacteria. A category for sensors, which included electronic noses, laser spectroscopy, and mid-infrared sensors was also included for comparison (discussed in detail in a later section).
As seen in Figure 2, all mass spectrometry and sensor publications show a general upward trend over the past 20 years. Unsurprisingly, the number of publications reporting GC- MS applications has been greater than the number of articles utilizing LC-MS or Direct-MS techniques. This result was expected as breath is a gas-phase medium that easily lends itself to GC-MS analysis. Interestingly, in the past four years, the number of publications utilizing sensors for breath research has caught up to the GC-MS publication output. This indicates that sensors are becoming more popular for breath, which may be due to the wide range and availability of simple and easy to use sensors such as electronic noses and laser spectroscopy.92,94,101 Direct-MS publications also appear to have steadily increased in the past few years in contrast to LC-MS publications, which remained constant. LC-MS techniques are more commonly utilized for exhaled breath condensate analysis, while Direct MS provides fast, real-time measurements of breath samples in the field, but is not useful when high resolution data are required.35,114,13. Several of the interesting breath research publications curated through these PubMed searches of mass spectrometry applications and their impact on the field are highlighted below.
Figure 2:
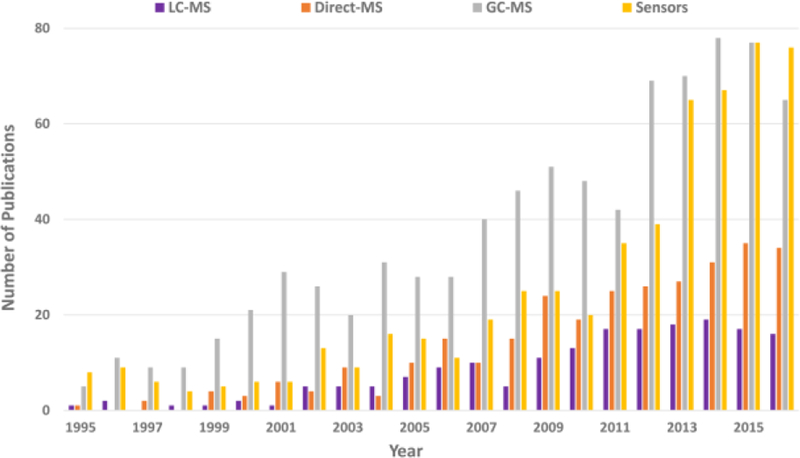
Mass spectrometry publication comparisons
This chart compares the number of publications for LC-MS, Direct-MS, GC-MS and sensors over the past 20 years. All publications in breath research show an upward trend, with most publications reporting breath research results utilizing GC-MS and sensors in recent years.
6.1 GC-MS
6.1.1 GC-TOF-MS
Gas chromatography time-of-flight mass spectrometry (GC-TOF-MS) is faster and provides higher mass resolution than conventional GC-MS instruments and has been used in breath research applications to achieve sub-ppbv limits of detection with high precision.13,115,116 Ion separation in the TOF analyzer is based on ion mass and the length of the flight tube as well as the electric charge. In addition to the advantage of high mass resolution, the TOF also acquires multiple spectra within a short timeframe.117 An example of a GC-TOF-MS with an automated thermal desorption (ATD) front end is shown in Figure 3(B), which includes a variety of GC/MS and LC/MS instruments that can be used for breath research analyses of exhaled breath, EBC, and EBA. The GC/MS instruments shown in Figure 3(A) and (B) can be used to analyze sorbent tubes for exhaled breath analysis.
Figure 3:

High-throughput GC-MS and LC-MS instrumentation for analysis of breath matrices
A) ATD-GC/MS; B) ATD-GC-TOF/MS; C) QqQ/MS; D) Q-TOF/MS. Instruments A and B have been utilized to analyze sorbent tubes to assess VOC levels in exhaled breath samples, while C and D have been used to analyze liquid filter extractions to detect EBA collected on different types of breathing masks. Photo credits: Chuck Gaul (U.S. EPA).
GC-TOF-MS has been utilized in breath research to obtain data with high resolution and accuracy. A method for EBC sample preparation for metabolomic analysis utilizing liquid-liquid extraction and analysis by GC-TOF-MS has been recently developed by Peralbo-Molina et al.,118 reporting a high percentage of fatty acids, methyl esters, amides, and volatile prenol lipids in analyzed samples. GC-TOF-MS has been applied to detect differences between disease states in patient samples in order to identify biomarkers. Non-targeted metabolomics analysis of EBC samples from lung cancer patients, smokers, and healthy controls was conducted to identify signatures that discirimate the groups.119 Breath samples from patients with suspected ventilator- associated pneumonia were compared to identify 12 VOCs that differentiated between the diseased and non-diseased groups.120 In a study of breath biomarkers of Crohn’s disease, 10 VOCs were found to discriminate between active CD and remission using GC-TOF-MS analysis.121 GC-TOF-MS analysis has also been utilized to analyze the cellular headspace of eosinophils and neutrophils to identify VOCs associated with inflammation and oxidative stress.122.
6.1.2 GC×GC-TOF-MS
Two-dimensional gas chromatography coupled with time-of-flight mass spectrometry (GC×GC-TOF-MS) is a useful high resolution multidimensional technology that has increased the number of detectable VOCs in breath samples. Multidimensional GC is advantageous compared to one-dimensional GC because it separates co-eluting VOCs using two capillary GC columns and has increased the coverage of VOCs in the metabolome by an order of magnitude.16 The two columns utilized typically have different polarities and lengths, with the first longer column containing a nonpolar stationary phase and the second shorter column a more polar stationary phase to increase resolution.123 GC×GC-TOF-MS has been applied to identify and characterize VOCs from healthy volunteers and individuals with medical conditions. In a recent study, 2000 VOCs were identified in alveolar breath samples from healthy volunteers, some of which had not been previously detected in breath.124 GC×GC-TOF-MS was utilized to analyze the breath of patients undergoing cardiac surgery to identify biomarkers, drugs, and contaminants.125 VOCs from children with allergic asthma and healthy controls were also characterized using GC×GC-TOF-MS to identify six alkanes that were unique to the asthmatic group.126 Gender-specific VOCs have also been identified in breath samples from healthy volunteers using GC×GC-TOF-MS. In this study, eleven VOCs capable of distinguishing between male and female participants were identified.127 Additionally, biomarkers of radiation exposure have also been identified using GC×GC-TOF-MS technology,128 demonstrating the potential of this technique for exposure assessment.
6.2 LC-MS
LC-MS has been commonly used to analyze breath and EBC samples in addition to GC-MS, and several LC-MS instruments that can be used for different breath research analyses are shown in Figure 3(C) and (D). These LC-MS instruments, a triple quadrupole-MS (QqQ-MS) and a Q-ToF-MS/MS, can be used to analyze EBC and EBA samples. While gas-phase breath can be analyzed relatively easily using GC-MS instrumentation, EBC and EBA samples often require extensive preparation procedures due to the low concentration of the components within the matrices, making these more difficult samples to manipulate than exhaled breath.15 As mentioned previously, EBC and EBA samples can both be obtained in liquid form, and therefore are amenable to LC-MS analyses. The samples can be injected directly into the instrument, or extracted for further analyses.129 More analytes have the potential to be measured using LC/MS than GC/MS; however, LC/MS data can be more variable due to large retention time drifts.73 High resolution MS instrumentation, such as Q-ToF-MS/MS or orbitrap-MS/MS, provide the opportunity for non-targeted analyses of breath samples, while QqQ-MS can be utilized to quantitate target compounds of interest.130
Orbitrap mass analyzers can achieve high resolution and accurate mass, providing excellent platforms for proteomics and metabolomics studies. In 2005, the linear trap quadrupole (LTQ)-Orbitrap mass spectrometer was first introduced, combining linear ion trap with an Orbitrap mass analyzer for sensitive ion detection, stable mass accuracy, and fragmentation capabilities.130,131 The Exactive mass spectrometer was released in 2008 as a more compact and affordable benchtop instrument.131 In this Orbitrap instrument, the ion source is directly linked to the C-trap, which is an external ion storage device.130 A modification of the Exactive instrument, the Q Exactive, is a quadrupole/Orbitrap hybrid with increased accurate mass MS/MS speed compared to the LTQ Orbitrap. The Q Exactive has been utilized for proteomics and high- throughput screening.130 Introduced in 2011, the Orbitrap Elite instrument has improved MS/MS acquisition rates and resolving power, which was increased almost 4-fold to 240,000 at m/z 400 compared to versions of the LTQ Orbitrap instrument.130 The Orbitrap Fusion, a more recent combination instrument, contains a quadrupole, orbitrap and linear ion trap mass analyzer, allowing for simultaneous isolation and detection of ions in different mass analyzers.131 Orbitrap mass analyzers have been successfully utilized for proteomics analysis of breath samples.
Several studies have been conducted to analyze the protein profiles of EBC samples using high resolution LC-MS. Muccilli et al.132 pooled EBC samples from nine healthy subjects and separated the proteins by 1D-SDS-PAGE prior to LC-MS/MS analysis of digested proteins using an Orbitrap-Elite mass spectrometer. A total of 163 gene products were identified, of which cytokeratins were found to be the most abundant.132 Similarly, Fumagalli et al.114 assessed pooled EBC samples from non-smokers, healthy smokers, and individuals with COPD and pulmonary emphysema using a linear trap quadrupole (LTQ)-Orbitrap mass spectrometer. Proteomic analysis was utilized to develop a fingerprint of proteins present in each pooled sample, representing a different disease state.114 EBC proteins have also been investigated as biomarkers for asthma by comparing protein profiles from healthy and diseased children as well as by monitoring levels of asymmetric dimethylarginine, a contributor to asthma pathogenesis by LC-MS.133,134
LC-MS and LC-MS/MS have also been utilized to evaluate drugs of abuse in EBA. A recently developed method was capable of analyzing 28 drugs in EBA with limits of quantification between 1 and 66 pg/filter for most substances of abuse.135 Cocaine and its metabolites have also been quantified in exhaled breath samples using Q Exactive high resolution LC-MS.136 SensAbues (Huddinge, Sweden) breath samplers were used to collect EBAm from volunteers and individuals using drugs or participating in elimination studies for sports drug testing. Twelve drugs of interest were characterized by LC-MS/MS with detection limits between 5 and 100 pg/filter.137 Exhaled drugs including amphetamine, metamphetamine, methadone, and THC have all been detected in exhaled breath samples using LC-MS.129
6.3 SESI-MS
Secondary electrospray ionization coupled to high resolution mass spectrometry (SESI-MS) is a fast analysis method whereby proton transfer reactions between the electrospray solution and the volatile components in the sample form ions in the gas phase. This technique is advantageous because it does not require sample pretreatment.138 SESI-MS was employed to study human exposure to benzothiazoles, a common industrial chemical, in exhaled breath, achieving part-per- trillion by volume (pptv) detection limits for this method.139 SESI-MS has also been applied to detect volatiles indicative of breast cancer by comparing them to breath samples from healthy 520 controls.140 Fatty acids have also been identified in human breath samples using SESI-MS.141 SESI-MS applications have also extended to mouse breath for the determination of bacterial lung infections using volatile fingerprints.142,143,144
6.4 Direct MS
6.4.1 PTR-MS
Proton transfer reaction mass spectrometry (PTR-MS) is a fast direct MS method capable of real-time analysis with breath-to-breath resolution.145 An example of a PTR-MS is shown in Figure 4(A), which includes several other Direct MS instruments and some immunoassay instruments. In PTR-MS, H3O+ions undergo non-dissociative proton transfer with compounds with a higher protein affinity than water (VOCs) without reacting with components in air, such as nitrogen, oxygen, and carbon dioxide.145,146 In PTR-MS, pptv detection limits have been achieved.13, 146 Some PTR-MS instruments contain quadrupole mass analyzers (PTR-QMS). PTR-QMS has been utilized to assess breath levels of VOCs such as ethanol and acetone,145 as well as VOCs from carbonic alcohols and short chain fatty acids that are indicative of glucose metabolism.147 Levels of isoflurane fragment ions have been detected in the breath of anaesthetized patients several days and weeks following surgery using PTR-QMS, showing that the drug is not eliminated as quickly as expected.148
Figure 4:
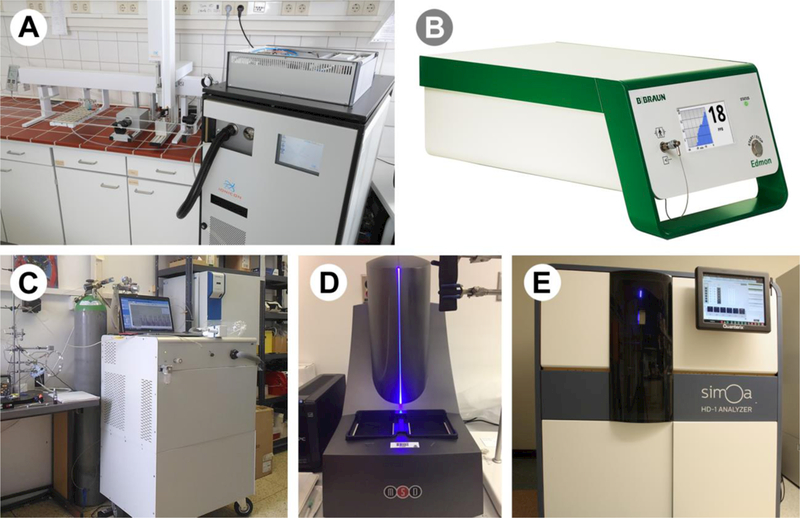
Direct-MS and immunoassay instrumentation utilized in breath research
A) PTR-MS; B) IMS; C) SIFT-MS; D) MSD; E) SIMOA HD-1 Analyzer. These instruments can be used for fast analysis of breath samples to analyze VOCs (A, B, and C) and cytokines (D and E). Photo credits: A) Dr. Jonathan Beauchamp; B) Dr. Jorg Ingo Baumbach; C) Dr. Patrik Spanel; D) Brett Winters; E) Chuck Gaul (U.S. EPA).
PTR-MS has also been combined with TOF technology to increase mass resolving power.149 While PTR-QMS has a mass resolution of 1, PTR-TOF-MS has a mass resolution between 4000–5000.13 PTR-TOF-MS has been applied to assess the effect of body position and breath holding during sampling on the concentrations of VOCs in the samples.150,151 PTR-TOF- MS is also valuable for biomarker detection. A biomarker of kidney malfunction was identified in kidney transplant patients that showed good correlation with serum creatinine.152 Exhaled breath from mechanically ventilated patients has also been continuously monitored using PTR- TOF-MS, and over 300 mass traces were evaluated. Ammonia, acetone, isoprene, benzene, and sevoflurane are some of the VOCs that were monitored.153 Exhaled breath samples from Celiac disease patients on a gluten-free diet were analyzed by PTR-TOF-MS in a discovery mode to look for differences from healthy controls.154 PTR-MS has been applied to analyze urine headspace to identify differences in metabolism caused by strenuous walking.155 Exhaled breath samples from mice on different diets and animal cell culture headspace have also been monitored by PTR-MS, demonstrating that PTR-MS applications extend beyond human breath analyses.156,157
6.4.2 SIFT-MS
Selected ion flow tube mass spectrometry (SIFT)-MS (Figure 4(C)) provides real-time measurements of volatile compounds in breath using chemical ionization of positive (H3O+, NO+, and O2+) or negative reagent ions (O-, OH-, O2-, NO2-, and NO3-).158 These precursor ions react with the molecules present in breath, such as VOCs and inorganic gases, but are unreactive with N2, O2, and Ar (common components in air).159 The product ions are then detected by the mass spectrometer. SIFT-MS can detect pptv levels of volatile compounds in breath, making this a sensitive technique for fast analysis.159 A variation of SIFT-MS, selection ion flow-drift tube-MS (SIFDT-MS), incorporated an electric field in the axis of the flow tube where the ions interact with one another. A significant advantage of this modified technique is that a lower pump speed is needed for SIFDT-MS compared with SIFT-MS, reducing the analysis cost and the size of the instrument.160
SIFT-MS has been used to improve medical diagnostics and provide timely test results. SIFT-MS has been operated in full scan mode to create linear models to estimate chronic kidney disease parameters using principal component analysis with intensity screening.161 Identification of hydrogen cyanide on the breath of cystic fibrosis patients has been deemed a signature of Pseudomonas aeruginosa infection in the respiratory tract.162 Isoprene has been found to be a specific biomarker of advanced fibrosis in chronic liver disease patient samples analyzed by SIFT-MS.126 SIFT-MS has also been applied to assess the toxicity of aspartame by monitoring one of its breakdown products, methanol, in the exhaled breath of volunteers and to identify VOCs indicative of obesity in children.163,164 Exhaled breath VOC profiles have also been found to be capable of distinguishing Crohn’s disease, ulcerative colitis, and inflammatory bowel disease.165 SIFDT-MS has been utilized to measure acetone and isoprene in exhaled breath.160 In addition to exhaled breath applications, SIFT-MS has also been utilized to monitor bacteria VOCs and feces headspace for colorectal cancer-monitoring applications.166,167,168.
6.4.3 Ion Mobility Spectrometry
Ion mobility spectrometry (IMS) utilizes a multicapillary column (MCC) to generate two- dimensional peak patterns. IMS instruments are compact, transportable, and advantageous for field research and detection of aldehydes and ketones at sub-ppbv concentrations.13,169 IMS has also been implemented for use in medical devices. The Exhaled Drug Monitor Edmon®, shown in Figure 4B, can be used to monitor propofol concentrations in the exhaled air of patients under anaesthesia or sedated with propofol during medical procedures.170,171,172 With quick and reliable detection of compounds in breath, IMS has been utilized to distinguish patients with respiratory disease from healthy controls.173 An IMS method was recently developed that can detect acetone, acetaldehyde, and acetonitrile in human breath at low ppbv to low ppmv levels.174 MCC-IMS has been applied to make kinetic measurements, and the results have been shown to be complementary to those obtained by PTR-MS, demonstrating the reliability of the technique, especially considering the small size and portability of the instrument.175 IMS has also been utilized to identify biomarkers in breath samples by comparing the VOCs of diseased patients versus healthy controls for COPD, lung cancer, Alzheimer’s disease and Parkinson’s disease by determining differences in breath patterns.176,177,95 Similarly, IMS has been applied to detect differences in the urinary headspace of samples from patients with Celiac disease and irritable bowel syndrome.178
7. Detection Methods: Immunoassay Instrumentation
7.1 Traditional ELISA
While traditionally breath research has focused on characterizing volatile compounds, recent applications have been extended to include the analysis of non-volatile compounds, such as cytokine proteins. Previously only available from blood analyses, this new technology is becoming extremely valuable for assessing inflammatory response directly in the lung.179,180 EBC and EBA are both utilized as breath media for cytokine analysis. The traditional ELISA, which utilizes a solid-phase immunoassay to detect the presence of an antigen in a liquid sample, was first developed by Eva Engvall and Peter Perlmann in 1971.181 ELISA has been traditionally applied to cytokine analyses in EBC applications. ELISA has been utilized to measure cytokines in EBC samples of asthma patients to assess correlations between interleukin levels and inflammation.182,183,184 Cytokine concentrations in EBC have been reported in the ng range, while concentrations in bronchoalveolar lavage (BAL) and sputum have been found in the pg range.184 ELISA has also been utilized to detect levels of the pro-inflammatory cytokines interleukin (IL)- 6, tumor necrosis factor α (TNF-α), IL-1β, and IL-8 in EBC of children with inflammatory bowel disease.185 Cytokine levels have also been analyzed in EBC samples of patients with sarcoidosis, allergic rhinitis, hereditary 1,25-dihydroxyvitamin D-resistant rickets, and individuals with tetraplegia to assess inflammatory responses.186,187,188,182
7.2 Mesoscale Discovery (MSD)
MSD SECTOR instruments are sensitive electrochemiluminescence plate readers that can be utilized for immunoassay applications (shown in Figure 4(D)). The MSD SECTOR PR 400 and 100 operate using photodiode array technology, while the SECTOR S 600 and QuickPlex SQ 120 utilize charge coupled device (CCD) cameras, enhancing the capacity for multiplexing. Most MSD instruments can read plates in 1.5–3 min, allowing for high throughput analyses. Additionally, the simplicity of the design lends itself to minimal requirements for routine maintenance, and the instrument does not require calibration. MSD offers a variety of human cytokine and chemokine multiplex assays, including several 4-plex, 7-plex, and 10-plex kits. An advantage of MSD is the small amount of sample volume needed for analysis; the 384-well plates only require 10 μL, while the 96-well plates need 25 μL of sample for analysis (www.mesoscale.com). The sensitivity level for cytokines in EBC samples has been reported in the 0.05–0.10 pg mL−1 range, and between-plate variance has been attributed to random error.179 A comparison of Th1/Th2 cytokine levels in blood plasma, EBC, and urine samples using MSD showed that the cytokine levels among the three different biological media did not share the same pattern, which may be due to biological differences in the location of the three media.179 Cytokine assays have also been employed in environmental exposure applications to assess ultrafine particulate matter exposure in school children, and cytokine levels in EBC samples in subjects exposed to diesel exhaust, ozone, and a mixture of diesel and ozone in order to elucidate the effects of multi-pollutant exposures.189,190,180
7.3 Luminex
Another alternative to traditional ELISA, Luminex offers bead-based multiplexing xMAP technology with color-coded beads that are read in compact laser or LED-based analyzers to determine individual microsphere interactions. Luminex has a throughput of 1000 samples a day and can multiplex 1 to 500 tests per sample (www.luminexcorp.com). Luminex multiplex assays have been utilized to measure cytokines in EBC of preschool children with asthma and patients with non-small cell lung cancer.191,192 A Luminex 21-plex assay plate was also used to quantify cytokines in BAL and sputum samples from children with severe therapy-resistant asthma (STRA) to determine if airway inflammation is caused by T helper 2 (TH2) cytokines. However, the cytokine levels in patients with STRA and controls were comparable, indicating that cytokines were not playing a significant role in inflammation.193
7.4 Cytometric Bead Array (CBA)
CBA is an emerging immunoassay technique for analyzing cytokines, chemokines, growth factors, and phosphorylated cell signaling proteins. CBA is a flow cytometry technique that utilizes antibody-coated beads to capture analytes and has a reduced analysis time and sample volume requirements compared with traditional ELISA. CBA can be used to analyze up to 30 proteins at the same time using only 25 to 30 μL of sample with detection limits as low as 0.27 pg mL−1 in multiplex assays (https://www.bdbiosciences.com/sg/research/cytometricbeadarray/). While CBA can multiplex more proteins than MSD, the detection limit of MSD is lower (0.05–0.10 pg mL−1). CBA has been used for several different applications, including measuring cytokine levels in EBC and BAL fluid samples from mechanically ventilated patients.194,195
7.5 Quanterix Single Molecule Array (Simoa) HD-1 Analyzer
The Quanterix Simoa HD-1 Analyzer is an automated robotic immunoassay platform that operates by capturing single protein molecules on magnetic beads and labeling them with standard ELISA reagents (see Figure 4(E)). The beads are combined with the enzyme substrate, loaded into wells, and sealed with oil. The fluorophores are digitally counted, and the percentage of beads with immunocomplexes is determined using a Poisson distribution (www.quanterix.com). This technique has achieved single-molecule resolution at sub- femtomolar concentrations with as much as 200- to 1000-times as much sensitivity as current multiplex immunoassays.196,197 While the Simoa HD-1 Analyzer has been previously demonstrated for cytokine analysis in blood plasma and serum samples, the technique was recently utilized to analyze cytokines in EBC samples, which is challenging due to the dilute nature of the medium.196,197,198,199 An advantage of the Simoa HD-1 Analyzer is that the system is fully automated, eliminating inconsistencies and errors caused by pipetting and sample handling. However, because the instrument requires a minimum sample volume of 100 μL and a sample dead volume of 50 μL, automating the Simoa HD-1 Analyzer requires more sample volume than for manual sample preparation. In a previous study, sample dilution was required prior to analysis, and researchers found that EBC samples should be diluted with buffer, as diluting with water caused signal suppression issues due to clumping of the beads.199 At the time these experiments were performed, no multiplexed cytokine kits were available from Quanterix, increasing the need for EBC sample dilution in order to analyze more cytokines using the individual assay kits.
8. Applications to Human Health and Disease
8.1 Alcohol Dependence
One traditional application of breath research with which the general public is familiar with is breath testing for blood alcohol levels. Alcohol dependence and abuse remains a major disease that affects 208 million people worldwide.200 Many individuals who suffer from alcohol dependence struggle to control the amount of alcohol that they consume and often end up driving and performing other tasks while impaired. The use of alcohol breath tests allows law enforcement to identify individuals who have consumed levels of alcohol that are unsafe for driving and operating heavy machinery.
Dr. R. N. Harger developed the first breath alcohol test in 1938, named “The Drunkometer”.17 The portable breath testing device that most people are more familiar with, the “Breathalyzer”, was developed in 1954 by Robert Borkenstein.18 While breath applications involving alcohol date back all the way to the 1930s, only a handful of publications were in existence at this time. More breath alcohol applications started appearing in the 1960s-1970s and gained popularity in the 1980s (Figure 5). Publications in the 1960s included basic assessments of breath alcohol levels, with a focus on the development of novel measurement devices for assessing drunk drivers.201-202 Alcohol breath tests also extended to the clinic in the 1970s to determine the effects of alcoholism on hepatic function and to evaluate the absorption of a tuberculosis drug.203-204 In 1982, an important publication monitored exhaled breath alcohol concentrations and temperatures to show that ethanol dissolves in mouth membranes and equilibrates with breath.205 New applications have been steadily developed over the years, with a spike in publications from 2013–2015 (see Figure 5). A significant focus of alcohol-related breath research still revolves around reducing instances of drunk driving, although the focus has shifted away from the development of novel breath tests toward large-scale surveys of the effects of alcohol consumption using previously established breathalyzers.206,207
Figure 5:
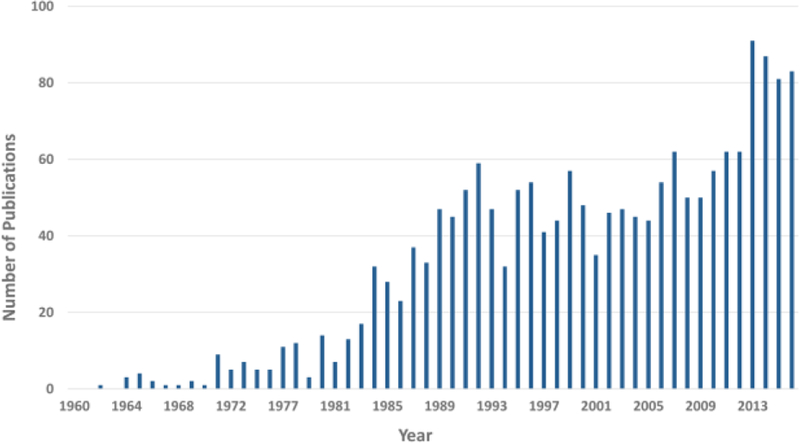
Trends in breath alcohol research publications
This figure shows the number of papers published from 1960–2016 that discuss breath analysis of alcohol or ethanol. In general, publications have increased over time, with a significant spike in alcohol-related breath publications from 2012–2016.
8.2 Lung Cancer
Another familiar application of breath testing is lung cancer research, which has steadily gained momentum over the past 20 years. Lung cancer research has made headway in both the clinic and the research laboratory, as both patient samples have been investigated and new assays have been developed to detect lung cancer using breath biomarkers. Lung cancer has the highest death rate among cancers,208 with non-small cell lung cancer (NSCLC) being particularly deadly due to the late stage at which it is typically detected.209 This makes the search for a simple and accurate diagnostic test for early stage lung cancer a worthy cause.209 Breath testing for lung cancer is attractive because it is noninvasive, and the breath provides a direct link to the current state of the lungs.
While a majority of the lung cancer research has revolved around exhaled breath analysis, EBC has become a more popular breath medium for early lung cancer testing to measure proteins linked to pathogenesis.210 In 2002, EBC from NSCLC patients was analyzed to find that interleukin-6 (IL-6) levels were increased compared to healthy controls, indicating that IL-6 could serve as a potential biomarker for early cancer detection.211 In 2004, increased endothelin- 1 peptide concentrations and mutations in the p53 gene were detected in DNA extracted from the EBC of patients with NSCLC, which provided additional indicators of disease state.212-213 Canine olfaction has also been utilized to detect biomarkers of lung cancer in EBC and distinguish cancerous and healthy samples.214,215 These studies have indicated that there are likely only low concentrations of biomarkers in EBC, presenting a challenge for developing a reproducible detection method.215
After 2004, the number of lung cancer publications in breath research began to increase rapidly. In addition to EBC analyses, studies were conducted using exhaled breath to compare the VOC profiles of healthy individuals and patients with NSCLC to look for potential biomarkers of disease state.216,217,218 Wang et al. compared the VOC profiles of breath from non-small cell carcinoma patients before and after tumor resection to healthy controls, finding caprolactam and propanoic acid as potential biomarkers.216 Poli et al. compared VOCs from NSCLC patients, smokers, non-smokers and patients with COPD. While no individual VOC discriminated the groups, the patterns of the 13 VOCs studies allowed for correct classification of disease state.217 Fu et al. reported that 2-butanone levels were much higher in the breath of NSCLC patients with stages II-IV compared to stage I.218 2-Butanone along with 1-propanol have been identified as a the best discriminators of lung cancer amongst all identified biomarkers.19 Oral glucose tolerance tests (OGTT) have been performed on LC patients with adenocarcinoma, squamous, small cell carcinoma, and controls, with exhaled breath samples taken before and after testing to obtain the volatile signatures of each disease state. OGTT was found to have little effect on LC VOC signatures, but a significant effect on the VOC patterns of the control group because the cancerous group already exists in a hyperglycolytic state.219.Extensive lists of VOC breath biomarkers of lung cancer can be found in Saalberg et al.19 and Dent et al.214 Additional information about lung cancer biomarkers is available in the Literature 20,19.
Advances in diagnostic technology have also improved lung cancer detection. GC/MS and sensor technologies, including electronic noses and colorimetric sensors, have all been widely used approaches.208 Gas sensors based on gold nanoparticles have been developed for lung cancer detection,220,221,214 which are capable of distinguishing the breath of lung cancer patients from negative controls with 100% accuracy.221 The Cyranose 320, another sensor, has been used to distinguish the VOCs from lung cancer and controls with 90% accuracy.214,222 More recently, lung cancer research has extended to the evaluation of “cell breath” in addition to human exhaled air, in which volatiles from tumors and cell lines have been studied in an attemptto better understand their contribution to disease state.223,224,225,226
8.3 Asthma
Asthma is one of the most prevalent human pulmonary diseases manifested as periodic inflammatory events resulting in swelling of the bronchioles that cause severe restrictions in breathing. This disease has a range of severity and is characterized by wheezing, coughing, and shortness of breath.9 As a disease of the lung, exhaled breath is considered the most proximate bio-fluid for diagnosing and monitoring asthma. Many biomarkers of asthma have been identified. Exhaled nitric oxide (NO) can be used to identify patients with asthma, as asthmatics end to have elevated NO levels due to reduced airways.227 Hydrogen peroxide (H2O2) is also a classic breath biomarker of asthma that has been discovered in increased levels in the EBC of patients with asthma compared to healthy subjects.20 More specific biomarkers have also been discovered, including urates as biomarkers of airway inflammation in children.228 Additionally, 8-iso-prostaglandin E2 has been detected as a biomarker of aspirin hypersensitivity in sensitive asthmatics,229 and leukotrienes have been used to monitor airway inflammation and determine pharmacological treatment.230,231 E-noses have also been utilized to detect VOCs from patients with asthma. Classification accuracies of 87–87.5% have been achieved for asthmatics versus healthy controls using this approach.27,232,92
Analyses of particulate matter (PM) from EBA and EBC have also been significant for asthma research, as PM often exacerbates asthma symptoms. For example, EBA from individuals with asthma who were exposed to birch pollen showed a reduced number of exhaled particles due to inflammation.51 EBC analysis in children exposed to ultrafine particulate matter (UFP) showed an increase in particle content with worsening respiratory symptoms and decreased lung function during exercise due to lower nitrate levels in EBC.50,233 Exposure to diesel exhaust particles has also been shown to increase airway inflammation in individuals with asthma.234,235 For extensive reviews of breath analysis for asthma research see Zhou et al.21 and Snell et al.22
8.4 COPD
COPD is a progressive respiratory disease that includes both bronchitis and emphysema,9 and is currently the fifth highest cause of death worldwide.23 COPD is characterized by coughing, wheezing, excess mucous production, shortness of breath and airway inflammation.9 The highest contributing factor for developing COPD is smoking, although environmental exposure to chemicals and air pollutants, childhood respiratory infections, and genetic factors, such as alpha-1 antitrypsin deficiency have also been found to play a role in developing the disease.236 Case-control studies that look for differences in chemicals, proteins, pH, and other properties in EBC and EBA samples from healthy individuals, patients with COPD, and sometimes smokers without COPD have been performed in order to identify biomarkers of COPD.237,238,176,239 In one study, breath samples were collected from non-smokers, COPD ex-smokers, smokers without COPD, and smokers with COPD and analyzed by TD-GC-MS to identify 14 COPD-related VOCs, including butanone, toluene, phenol, and m,p-cresol. 89.4% of the COPD patients were correctly classified in the non/ex-smoking group, and 82.6% were classified correctly in the actively smoking group.239 In a GC-atmospheric pressure chemical ionization (APCI)-MS study, 2-pentanone was identified as a VOC biomarker that showed differences between COPD patients and healthy controls 240, although this VOC was not deemed a COPD-related biomarker in the previous study.239
In contrast to GC-MS, e-noses have also been applied to COPD research. For COPD patients, detection of viral versus bacterial infections is critical for proper diagnosis and medical treatment. Van Geffen et al. demonstrated that an Aeonose e-nose could discriminate between COPD patients with viral infections versus controls (sensitivity 83%) and COPD patients with bacterial infections versus controls (sensitivity 73%).241 A Cyranose 320 e-nose was recently used to distinguish the breathrpints of COPD from those of obstructive sleep apnea (OSA) alone and overlap syndrome, which is a combined syndrome in which OSA is associated with COPD. While the OSA patient breathprints were distinct from those of OVS and COPD, the breathprints from OVS patients were similar to those of COPD patients, indicating that the technique could be utilized to distinguish OSA from COPD.242 Similarly, another study sought to differentiate the VOC patterns of COPD from those of NSCLC patients and healthy controls using the Cyranose 320. With a cross-validated correct classification rate of 85%, COPD breathprints were differentiated from those of NSCLC.27,222
Non-volatile compounds have also been utilized as biomarkers of COPD. C-reactive protein (CRP) levels have been identified as a biomarker capable of predicting risk of death and hospitalization for COPD patients.22 COPD breath research has included the analysis of cytokines in EBC samples as a measure of airway inflammation, a common symptom of the disease.243,244,245 Using an EcoScreen device, Ko et al.244 collected EBC from patients with acute exacerbations of COPD (AECOPD) and monitored levels of TNF-α while patients received steroids and antibiotic treatment, showing that cytokines could be used to non-invasively monitor the severity of the disease. Eosinophils, which are markers of airway inflammation, have been detected in higher levels in COPD patients,243 as have leukotriene B4 and prostaglandin E2.245 Airway obstruction in COPD has also been evaluated by analyzing the number and distribution of exhaled particles.53 Reviews of COPD research can be found in Snell et al.,22 Cazzola et al.24 and Christiansen et al.23
8.5 Biomarkers of Environmental Exposure
Volatile organic compounds (VOCs) represent a major environmental exposure issue for the human population. VOCs in air have myriad sources including consumer products, combustion of fossil fuels, water disinfection, and a variety of industrial and manufacturing processes.246,247,248,249. Airborne chemicals in the environment such as benzene, toluene, ethylbenzene, xylene,246 ethyl acetate, propylene glycol monomethyl ether acetate, and inorganic acids 249 impact human health primarily through the inhalation pathway, and as such, are also present during the exhalation cycle. In fact, exhaled breath provides a direct window into blood-borne VOCs through the gas exchange mechanisms of the alveoli.31–29 Advantages of using breath to assess VOC exposures (in contrast to blood or urine) are that breath can be monitored in any time frame (minutes or hours) or even continuously without reaching a sampling limit. This has provided insight into the classical pharmacokinetics of VOCs exposure with respect to absorption, distribution, metabolism, and excretion (ADME), which are of critical importance in determining acquired dose and ultimate health risk.
8.6 Disease Detection using EBA Patterns
Exhaled aerosol patterns can be utilized to identify fingerprints and signatures of conditions and diseases. Computational fluid dynamics approaches with fractal analyses have been applied to distinguish between aerosol fingerprints of normal, tracheal tumor, bronchial tumor, and different levels of asthma severity in lung models.250,251 In these physiological-based models, exhaled particles form unique patterns on filters. The fractal, or repetitive, properties of these images have been used to identify disease location and severity.250 Asthma models created using this technique have been shown to have 100% diagnosis accuracy under perfect conditions, and 99.1% accuracy under more realistic sampling conditions.251 Fingerprints of bacterial, viral, and healthy individuals can also be distinguished from one another using exhaled breath and volatile profiles. Differences in infected and uninfected fingerprints of bacteria strains have been demonstrated in a mouse model, and methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive S. aureus (MSSA) breathprints were investigated to distinguish features using principal component analysis (PCA).252 Intensity differences in the shared dominant peaks and the presence of several low abundance peaks were found to be important for distinguishing MRSA and MSSA.253
9.0 Emerging Topics in Breath Research
9.1 Security and Airport Surveillance
Breath research has several unique applications toward security and airport surveillance implementations. During disaster scenarios, such as earthquakes, tornadoes, fires, or explosions, individuals may become entrapped inside collapsed buildings. Portable sensors and devices can be utilized to detect VOCs emitted from human breath, sweat, skin, and biological fluids in order to locate lost individuals who may be injured or nonresponsive.254 Acetone, isoprene, dimethylsulfide, dimethyldisulfide, and trimethylamine have been proposed as target VOCs that could be used to detect individuals within collapsed buildings. These VOCs would be advantageous for detection from exhaled breath, blood, urine, or sweat, all of which would be emitted from entrapped individuals.255
Portable sensors also have applications for border security and airport surveillance. Aportable quadrupole mass spectrometer with a heated membrane probe has been utilized to detect chemical signatures of volunteers in a container simulator. VOCs, ammonia, carbon dioxide, and water were measured from exhaled breath, sweat, and skin excretions. These compounds were all found to be useful probes for discovering humans within confined spaces. Therefore, this portable mass spectrometer could be used to detect humans hiding in boxes or suitcases to increase border control and decrease instances of illegal immigration.256,257 A method for multiplex surface enhanced Raman scattering (SERS) has recently been developed that has the potential to be utilized in a portable sensor device to detect VOCs, such as acetone and ethanol, emitted from human breath.258 SERS operates using metallic nanostructures that create an electromagnetic field at surface plasmon hot-spots for single molecule detection.258 These portable devices could be used in airport security and border control to detect humans hiding in boxes or suitcases to improve safety regulations and decrease instances of illegal immigration.255,258 ,256,257
Detection and identification of drugs and other harmful substances could also be accomplished using portable sensors for security applications. The portable membrane inlet mass spectrometer was tested for the ability to detect narcotics, explosives, and chemical warfare agents. Methyl benzoate, a signature of cocaine, and piperidine were successfully detected using the portable spectrometer, indicating that the device can be utilized to determine if individuals have handled or consumed drugs. A breakdown product of trinitrotoluene (TNT), 2-nitrotoluene, and a chemical used in plastic explosives, cyclohexanone were also identified in addition to chemicals used to produce sarin and soman nerve agents and sulfur mustard gas. Low ppbv LODs were determined for each of these compounds.257 This spectrometer could be utilized as a fast, non-invasive technique to screen for illegal and harmful substances in airports as well as in law enforcement applications. Hand-held screening devices can also be used in law enforcement to monitor substance levels. A new breath alcohol analyzer has been developed that does not require contact with the subject. The device uses carbon dioxide as a biomarker and has been shown to meet industrial standards.259 This non-invasive breath sampler can be used for law enforcement applications to measure breath alcohol levels and determine levels of consumption to enforce alcohol safety laws.
9.2 Cellular Respiration and Microbiome
Breath VOCs have been frequently used to detect alterations in cellular respiration. While VOC profiles of breath have been extensively investigated, research has recently expanded to include biological and cellular headspace as a useful model of exhaled breath. VOC profiles of breath, saliva, milk, blood, urine, skin secretions, and feces have all been compiled, and clear differences between compound abundance in certain biological media were noted.260,261 VOCs emitted from breath and biological media can be important indicators of health state, as they may be produced by adverse outcome pathways (AOPs) within the body. An AOP is a model consisting of biological events that result in an adverse effect and can be used to assess exposure risk.262 Cellular models can be used for in vitro systems to identify VOCs that are altered by the presence of external stressors.263 Headspace samples of four bacteria were analyzed, demonstrating that the microorganisms could be identified based on their unique VOC profiles. Applications such as this show potential for non-invasive breath tests for diagnosis of bacterial infections.264
Breath VOCs have also been found to change based on diet and disease onset. Adhering to a gluten-free versus a normal diet for four weeks altered the volatile profiles excreted in breath, suggesting that the gluten-free diet reversibly altered metabolism.265 The effect of diet on breath VOC profiles was also investigated by feeding mice low and high fat diets, which showed differences in exhaled VOC levels that may be linked to metabolic functions.266 Exhaled VOCs have also been linked to cancer, and VOCs have been studied for indications of viral infections and pneumonia in mechanically ventilated patients.267,268,269 Identifying VOCs that are produced by bacteria or fungi, such as hydrogen cyanide, which is produced by Pseudomonas aeruginosa, in breath samples can indicate the presence of an infection.270
Liver function can also be assessed using breath biomarkers by monitoring data for probe molecules. Probe molecules are exogenous compounds with known kinetic data that can be introduced to an in vitro or in vivo system to produce an expected metabolite. An example probe molecule is methyl tertiary butyl ether (MTBE), which is metabolized to tertiary butyl alcohol (TBA) by the CYP 2A6 pathway in the liver.262 Sevoflurane (SEV), an anesthetic used during surgery, has also been demonstrated as a probe molecule for liver function. SEV is metabolized to hexafluoroisopropanol (HFIP), which is eliminated in exhaled breath.271 Potentially liver toxic compounds of interest can be added to cellular in vitro systems in the presence of MTBE or SEV to see if production of TBA or HFIP, respectively, is disrupted by the presence of the test compound.262,271
9.3 Canine Olfaction
An interesting application of breath research that has often baffled researchers is the use of canine olfaction for pre-clinical cancer and disease detection. Some dogs can tell the difference between breath samples from healthy and diseased patients, aiding in sample discrimination and diagnosis (Figure 6). The reason for this ability is attributed to a dog’s enhanced sense of smell.272 Dogs have been recently used to differentiate lung cancer patient samples from healthy controls in several different studies.273,274,275 However, dogs are not perfect diagnosticians, and often generate random results, presumably due to boredom, distraction, hunger, fatigue, or a lack of sufficient training.274,272 While using dogs as breath samplers for cancer and disease detection is certainly not optimized at this point in time, canine olfaction has promise as a future tool for breath research diagnostics because it is affordable, relatively simple, and does not require complex instrumentation, in contrast to some of the other methods currently available. However, identifying the chemicals that dogs identify as cancerous or attributed to a disease-state would be advantageous for understanding the biological processes undergoing diseases as well as for developing instruments capable of detecting the same compounds. The current state of thought is that dogs must be detecting compounds in EBA, as the VOCs in exhaled air would quickly dissipate within the environment.276 For an extensive review of canine olfaction for breath research, see Pirrone et al.277
Figure 6:

Canine olfaction in breath sample classification
Canine sniffing of biological samples, including breath, has become a viable method for cancer and disease detection. Dogs are trained to differentiate samples from diseased versus healthy individuals based on scent, providing a potential method for early stage disease diagnosis. Photo credit: Glenn Ferguson from Cancerdogs.
9.4 Recent Applications and Future Directions
Several other interesting and unique breath applications have emerged in the last several years. These applications hold promise for future development to extend breath research beyond its current scope. Additional research areas for future development include:
• Nose breath
• Organ-on-chip
• Crowd breath
Pure nose breath has been utilized for breath sampling instead of mouth breath to avoid the contribution of mouth bacteria to the analysis. This is not to be confused with e-nose measurements, which use mouth breath to mimic the sensory functions of the nose.278 Nasal breath has been typically utilized to measure NO in clinical settings and is a sign of airway inflammation leading to asthma.279,280 Nose breath can be used in situations where sample purity is critical and contamination is a concern due to illness or other factors.
The development of lung-on-chip models have also revolutionized drug discovery, toxicity testing, and evaluation of disease states. Prototypes of human lungs using the lung’s air sac linings and blood vessels on flexible membranes have been designed to mimic breathing at the cellular level.281,282 These lung-on-chips have been used to investigate pulmonary edema, inflammatory disorders, asthma, and lung cancer drugs, among others.283,284,285 These devices also have the potential to replace animals as more predictable models of human breath.281
The analysis of crowd breath as opposed to individual sampling is a future direction of the field of breath research. In crowd breath sampling, the air surrounding large groups of people is measured using a direct-MS approach (e.g., PTR-MS, IMS, or SIFT-MS) as opposed to taking separate breath samples from each individual within the room. Common breath biomarkers, such as carbon dioxide or acetone can be measured in group settings to evaluate trends over time. Changes in the steady state levels of these volatiles can indicate that a chemical exposure has occurred or that a threat has been introduced. Crowd breath technology would be particularly advantageous in national security settings, airports, or for drug and toxicity screening. While it would be unnecessary and time consuming to test each individual separately in such scenarios, a baseline for the crowd breath room air could be established, and individual sampling could be conducted only if a change in the VOC profile was detected that could be indicative of a threat.286
10. Conclusion
In the last 20 years, breath research has seen significant improvements in sampling techniques, available instrumentation for analysis of different sample media, and the wide range of applications of breath applications, including investigations of disease state, exposure assessment, environmental research, impairment, and security. While breath research may have started off with a narrow range of relevant applications, such as alcohol breath testing, lung cancer research, and the detection of basic compounds in breath, the field has grown to include EBC and EBA in addition to exhaled breath, widening the range of relevant applications. The future for breath research is promising, with an increase in health applications, including organ-on-chip and canine olfaction on the horizon. EBA analysis is also likely to be pursued in the future, with an increase in instruments and techniques capable of analyzing these types of samples currently underway. In the midst of all of these advances, one challenge for breath research going forward is the implementation of consistent protocols and standards across research laboratories.
Acknowledgments
The authors would like to acknowledge Chuck Gaul, Dr. Jonathan Beauchamp, Dr. Patrik Spanel, Dr. Jorg Ingo Baumbach, and Brett Winters for supplying photographs for the publication figures. The authors acknowledge Glenn Ferguson from Cancerdogs for the photograph of Indie the dog (http://www.cancerdogs.ca/contact/). The United States Environmental Protection Agency through its Office of Research and Development has subjected this article to Agency administrative review and approved it for publication. Mention of trade names or commercial products does not constitute endorsement for use.
Footnotes
Abbreviations:
1D one-dimensional
ADME absorption, distribution, metabolism, and excretion
AOP adverse outcome pathway
ARDS acute respiratory distress syndrome
ATD automated thermal desorption
BAL bronchoalveolar lavage
CBA cytometric bead array
CI-MS chemical ionization-mass spectrometry
charge coupled device (CCD)
CO2 carbon dioxide
COPD chronic obstructive pulmonary disease
EBA exhaled breath aerosol
EBC exhaled breath condensate
EBP exhaled breath particle
EC-QCL external cavity quantum cascade lasers
e-nose electronic nose
GCxGC two-dimensional gas chromatography
HFIP hexafluoroisopropanol
HRMS high resolution mass spectrometry
IL interleukin
IMS ion mobility mass spectrometry
H2O2 hydrogen peroxide
LTQ linear trap quadrupole
MCC multicapillary column
MOSFET metal-oxide-semiconductor field-effect transistor
MRSA methicillin=resistant Staphylococcus aureus
MSD mesoscale discovery
MSSA methicillin sensitive S. aureus
MTBE methyl tertiary butyl ether
NH3 ammonia
NO nitric oxide
OSA obstructive sleep apnea
PCA principal component analysis
pptv part per trillion by volume
PTR proton transfer reaction
SIFDT selected ion flow drift tube
SIFT selected ion flow tube
SIMOA single molecule array
SELDI surface-enhanced laser desorption/ionization
SERS surface enhanced Raman scattering
SESI secondary electrospray ionization
SEV sevoflurane
STRA severe therapy-resistant asthma
TBA tertiary butyl alcohol
TD thermal desorption
TH T helper
THC tetrahydrocannabinol
TOF time-of-flight
TNF-α tumor necrosis factor α
TNT trinitrotoluene
UFP ultrafine particulate matter
volatile organic compound (VOC)
Conflict of Interest Disclosure
The authors declare no competing financial interest.
References
- 1.Phillips M, Breath tests in medicine. Sci Am 1992, 267 (1), 74–79. [DOI] [PubMed] [Google Scholar]
- 2.Pauling L; Robinson AB; Teranishi R; Cary P, Quantitative Analysis of Urine Vapor and Breath by Gas-Liquid Partition Chromatography. Proceedings of the National Academy of Sciences 1971, 68 (10), 2374–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace LA; Pellizzari ED; Hartwell TD; Sparacino CM; Sheldon LS; Zelon H, Personal exposures, indoor-outdoor relationships, and breath levels of toxic air pollutants measured for 355 persons in New Jersey. Atmospheric Environment (1967) 1985, 19 (10), 1651–1661. [Google Scholar]
- 4.Phillips M; Gleeson K; Hughes JMB; Greenberg J; Cataneo RN; Baker L; McVay WP, Volatile organic compounds in breath as markers of lung cancer: a cross-sectional study. The Lancet 1999, 353 (9168), 1930–1933. [DOI] [PubMed] [Google Scholar]
- 5.Risby TH; Pleil JD, Breath analysis—past, present and future: a special issue in honour of Michael Phillips’ 70th birthday. J. Breath Res 2013, 7 (1), 010201. [DOI] [PubMed] [Google Scholar]
- 6.Herbig J; Beauchamp J, Towards standardization in the analysis of breath gas volatiles. Journal of breath research 2014, 8 (3), 037101. [DOI] [PubMed] [Google Scholar]
- 7.Beauchamp J, Current sampling and analysis techniques in breath research—results of a task force poll. Journal of Breath Research 2015, 9 (4), 047107. [DOI] [PubMed] [Google Scholar]
- 8.Pleil JD; Isaacs KK, High-resolution mass spectrometry: basic principles for using exact mass and mass defect for discovery analysis of organic molecules in blood, breath, urine and environmental media. J Breath Res 2016, 10 (1), 012001. [DOI] [PubMed] [Google Scholar]
- 9.Kubáň P; Foret F, Exhaled breath condensate: Determination of non-volatile compounds and their potential for clinical diagnosis and monitoring. A review. Analytica Chimica Acta 2013, 805, 1–18. [DOI] [PubMed] [Google Scholar]
- 10.MUTLU GM; GAREY KW; ROBBINS RA; DANZIGER LH; RUBINSTEIN I, Collection and Analysis of Exhaled Breath Condensate in Humans. American Journal of Respiratory and Critical Care Medicine 2001, 164 (5), 731–737. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadzai H; Huang S; Hettiarachchi R; Lin J-L; Thomas PS; Zhang Q, Exhaled breath condensate: a comprehensive update. Clinical chemistry and laboratory medicine 2013, 51 (7), 1343– 1361. [DOI] [PubMed] [Google Scholar]
- 12.Sun X; Shao K; Wang T, Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Analytical and Bioanalytical Chemistry 2016, 408 (11), 2759–2780. [DOI] [PubMed] [Google Scholar]
- 13.Calenic B; Amann A, Detection of volatile malodorous compounds in breath: current analytical techniques and implications in human disease. Bioanalysis 2014, 6 (3), 357–376. [DOI] [PubMed] [Google Scholar]
- 14.Gardner JW; Bartlett PN, A brief history of electronic noses. Sensors and Actuators B: Chemical 1994, 18 (1–3), 210–211. [Google Scholar]
- 15.Fernández-Peralbo MA; Calderón Santiago M; Priego-Capote F; Luque de Castro MD, Study of exhaled breath condensate sample preparation for metabolomics analysis by LC–MS/MS in high resolution mode. Talanta 2015, 144, 1360–1369. [DOI] [PubMed] [Google Scholar]
- 16.Eiserbeck C; Nelson RK; Reddy CM; Grice K, Advances in comprehensive two-dimensional gas chromatography (GC× GC). Royal Society of Chemistry: London: 2014; Vol. 4. [Google Scholar]
- 17.Harger E; Forney RB; Barnes HB, Estimation of the level of blood alcohol from analysis of breath. The Journal of laboratory and clinical medicine 1950, 36 (2), 306–318. [PubMed] [Google Scholar]
- 18.Jones A, Measuring alcohol in blood and breath for forensic purposes-a historical review. Forensic science review 1996, 8, 13–44. [PubMed] [Google Scholar]
- 19.Saalberg Y; Wolff M, VOC breath biomarkers in lung cancer. Clinica Chimica Acta 2016, 459, 5– 9. [DOI] [PubMed] [Google Scholar]
- 20.Konstantinidi EM; Lappas AS; Tzortzi AS; Behrakis PK, Exhaled Breath Condensate: Technical and Diagnostic Aspects. The Scientific World Journal 2015, 2015, 435160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou M; Liu Y; Duan Y, Breath biomarkers in diagnosis of pulmonary diseases. Clinica Chimica Acta 2012, 413 (21), 1770–1780. [DOI] [PubMed] [Google Scholar]
- 22.Snell N; Newbold P, The clinical utility of biomarkers in asthma and COPD. Current Opinion in Pharmacology 2008, 8 (3), 222–235. [DOI] [PubMed] [Google Scholar]
- 23.Anders C; Jesper Rømhild D; Ingrid T; Jørgen V; Jan B, A systematic review of breath analysis and detection of volatile organic compounds in COPD. Journal of Breath Research 2016, 10 (3), 034002. [DOI] [PubMed] [Google Scholar]
- 24.Cazzola M; Novelli G, Biomarkers in COPD. Pulmonary pharmacology & therapeutics 2010, 23 (6), 493–500. [DOI] [PubMed] [Google Scholar]
- 25.Haick H; Broza YY; Mochalski P; Ruzsanyi V; Amann A, Assessment, origin, and implementation of breath volatile cancer markers. Chemical Society reviews 2014, 43 (5), 1423–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Z; Liu Y; Duan Y, Breath analysis: technical developments and challenges in the monitoring of human exposure to volatile organic compounds. Journal of Chromatography B 2015, 1002 (Supplement C), 285–299. [DOI] [PubMed] [Google Scholar]
- 27.Bos LD; Sterk PJ; Fowler SJ, Breathomics in the setting of asthma and chronic obstructive pulmonary disease. Journal of Allergy and Clinical Immunology 2016, 138 (4), 970–976. [DOI] [PubMed] [Google Scholar]
- 28.Alonso M; Sanchez JM, Analytical challenges in breath analysis and its application to exposure monitoring. TrAC Trends in Analytical Chemistry 2013, 44 (Supplement C), 78–89. [Google Scholar]
- 29.Cao W; Duan Y, Breath analysis: potential for clinical diagnosis and exposure assessment. Clinical chemistry 2006, 52 (5), 800–811. [DOI] [PubMed] [Google Scholar]
- 30.Davis MD; Montpetit A; Hunt J, Exhaled Breath Condensate—an overview. Immunology and allergy clinics of North America 2012, 32 (3), 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shier D; Butler J; Lewis R, Hole’s essentials of human anatomy and physiology McGraw-Hill; New York: 2006. [Google Scholar]
- 32.Vaughan J; Ngamtrakulpanit L; Pajewski T; Turner R; Nguyen T; Smith A; Urban P; Hom S; Gaston B; Hunt J, Exhaled breath condensate pH is a robust and reproducible assay of airway acidity. European Respiratory Journal 2003, 22 (6), 889–894. [DOI] [PubMed] [Google Scholar]
- 33.Dwyer TM, Sampling airway surface liquid: non-volatilesin the exhaled breath condensate. Lung 2004, 182 (4), 241–250. [DOI] [PubMed] [Google Scholar]
- 34.Horváth I; Hunt J; Barnes PJ, Exhaled breath condensate: methodological recommendations and unresolved questions. European Respiratory Journal 2005, 26 (3), 523–548. [DOI] [PubMed] [Google Scholar]
- 35.Fumagalli M; Dolcini L; Sala A; Stolk J; Fregonese L; Ferrari F; Viglio S; Luisetti M; Iadarola P, Proteomic analysis of exhaled breath condensate from single patients with pulmonary emphysema associated to α 1-antitrypsin deficiency. Journal of proteomics 2008, 71 (2), 211–221. [DOI] [PubMed] [Google Scholar]
- 36.Montuschi P, Review: Analysis of exhaled breath condensate in respiratory medicine: methodological aspects and potential clinical applications. Therapeutic Advances in Respiratory Disease 2007, 1 (1), 5–23. [DOI] [PubMed] [Google Scholar]
- 37.Soyer O; Dizdar E; Keskin O; Lilly C; Kalayci O, Comparison of two methods for exhaled breath condensate collection. Allergy 2006, 61 (8), 1016–1018. [DOI] [PubMed] [Google Scholar]
- 38.Hitka P; Cerny M; Vizek M; Wilhelm J; Zoban P, Assessment of exhaled gases in ventilated preterm infants. Physiological research 2004, 53 (5), 561–564. [PubMed] [Google Scholar]
- 39.Cheah FC; Darlow BA; Winterbourn CC, Problems Associated with Collecting Breath Condensate for the Measurement of Exhaled Hydrogen Peroxide from Neonates on Respiratory Support. Neonatology 2003, 84 (4), 338–341. [DOI] [PubMed] [Google Scholar]
- 40.Kononikhin AS; Starodubtseva NL; Chagovets VV; Ryndin AY; Burov AA; Popov IA; Bugrova AE; Dautov RA; Tokareva AO; Podurovskaya YL; Ionov OV; Frankevich VE; Nikolaev EN; Sukhikh GT, Exhaled breath condensate analysis from intubated newborns by nano HPLC coupled to high resolution MS. Journal of Chromatography B 2017, 1047 (Supplement C), 97–105. [DOI] [PubMed] [Google Scholar]
- 41.Urs R; Simpson S; Pillow J; Hall G; Clarke M, Exhaled breath condensate: Measuring inflammation and oxidative stress in preterm infants. Eur Respiratory Soc: 2016. [Google Scholar]
- 42.Gessner C; Hammerschmidt S; Kuhn H; Seyfarth H-J; Sack U; Engelmann L; Schauer J; Wirtz H, Exhaled breath condensate acidification in acute lung injury. Respiratory Medicine 2003, 97 (11), 1188–1194. [DOI] [PubMed] [Google Scholar]
- 43.Vaschetto R; Corradi M; Goldoni M; Cancelliere L; Pulvirenti S; Fazzini U; Capuzzi F; Longhini F; Mutti A; Della Corte F, Sampling and analyzing alveolar exhaled breath condensate in mechanically ventilated patients: a feasibility study. Journal of breath research 2015, 9 (4), 047106. [DOI] [PubMed] [Google Scholar]
- 44.Fermier B; Blasco H; Godat E; Bocca C; Moënne-Loccoz J; Emond P; Andres CR; Laffon M; Ferrandière M, Specific Metabolome Profile of Exhaled Breath Condensate in Patients with Shock and Respiratory Failure: A Pilot Study. Metabolites 2016, 6 (3), 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beck O; Olin A-C; Mirgorodskaya E, Potential of mass spectrometry in developing clinical laboratory biomarkers of nonvolatiles in exhaled breath. Clinical chemistry 2016, 62 (1), 84–91. [DOI] [PubMed] [Google Scholar]
- 46.Tinglev ÅD; Ullah S; Ljungkvist G; Viklund E; Olin A-C; Beck O, Characterization of exhaled breath particles collected by an electret filter technique. Journal of breath research 2016, 10 (2), 026001. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell AB; Mourad B; Tovey E; Buddle L; Peters M; Morgan L; Oliver BG, Spirometry filters can be used to detect exhaled respiratory viruses. Journal of breath research 2016, 10 (4), 046002. [DOI] [PubMed] [Google Scholar]
- 48.Li W; Pi X; Qiao P; Liu H, Collecting Protein Biomarkers in Breath Using Electret Filters: A Preliminary Method on New Technical Model and Human Study. PloS one 2016, 11 (3), e0150481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pleil JD, Breath biomarkers in toxicology. Archives of toxicology 2016, 90 (11), 2669–2682. [DOI] [PubMed] [Google Scholar]
- 50.Benor S; Alcalay Y; Domany KA; Gut G; Soferman R; Kivity S; Fireman E, Ultrafine particle content in exhaled breath condensate in airways of asthmatic children. Journal of breath research 2015, 9 (2), 026001. [DOI] [PubMed] [Google Scholar]
- 51.Larsson P; Lärstad M; Bake B; Hammar O; Bredberg A; Almstrand AC; Mirgorodskaya E; Olin AC, Exhaled particles as markers of small airway inflammation in subjects with asthma. Clinical physiology and functional imaging 2015. [DOI] [PubMed] [Google Scholar]
- 52.Wan G-H; Wu C-L; Chen Y-F; Huang S-H; Wang Y-L; Chen C-W, Particle size concentration distribution and influences on exhaled breath particles in mechanically ventilated patients. PloS one 2014, 9 (1), e87088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwarz K; Biller H; Windt H; Koch W; Hohlfeld JM, Characterization of exhaled particles from the human lungs in airway obstruction. Journal of aerosol medicine and pulmonary drug delivery 2015, 28 (1), 52–58. [DOI] [PubMed] [Google Scholar]
- 54.Sauvain J-J; Hohl MSS; Wild P; Pralong JA; Riediker M, Exhaled breath condensate as a matrix for combustion-based nanoparticle exposure and health effect evaluation. Journal of aerosol medicine and pulmonary drug delivery 2014, 27 (6), 449–458. [DOI] [PubMed] [Google Scholar]
- 55.Ari A; Fink JB; Pilbeam SP, Secondhand aerosol exposure during mechanical ventilation with and without expiratory filters: An in-vitro study 2016. [Google Scholar]
- 56.Pelclova D; Zdimal V; Fenclova Z; Vlckova S; Turci F; Corazzari I; Kacer P; Schwarz J; Zikova N; Makes O, Markers of oxidative damage of nucleic acids and proteins among workers exposed to TiO2 (nano) particles. Occupational and environmental medicine 2016, 73 (2), 110–118. [DOI] [PubMed] [Google Scholar]
- 57.Pelclova D; Barosova H; Kukutschova J; Zdimal V; Navratil T; Fenclova Z; Vlckova S; Schwarz J; Zikova N; Kacer P, Raman microspectroscopy of exhaled breath condensate and urine in workers exposed to fine and nano TiO2 particles: a cross-sectional study. Journal of breath research 2015, 9 (3), 036008. [DOI] [PubMed] [Google Scholar]
- 58.Pelclova D; Zdimal V; Kacer P; Fenclova Z; Vlckova S; Syslova K; Navratil T; Schwarz J; Zikova N; Barosova H, Oxidative stress markers are elevated in exhaled breath condensate of workers exposed to nanoparticles during iron oxide pigment production. Journal of breath research 2016, 10 (1), 016004. [DOI] [PubMed] [Google Scholar]
- 59.Wallace MAG; Kormos TM; Pleil JD, Blood-borne biomarkers and bioindicators for linking exposure to health effects in environmental health science. Journal of Toxicology and Environmental Health, Part B 2016, 19 (8), 380–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kintz P; Mathiaux F; Villéger P; Gaulier J. m., Testing for Drugs in Exhaled Breath Collected With ExaBreath in a Drug Dependence Population: Comparison With Data Obtained in Urine After Liquid Chromatographic-Tandem Mass Spectrometric Analyses. Therapeutic drug monitoring 2016, 38 (1), 135– 139. [DOI] [PubMed] [Google Scholar]
- 61.Stephanson N; Sandqvist S; Lambert MS; Beck O, Method validation and application of a liquid chromatography–tandem mass spectrometry method for drugs of abuse testing in exhaled breath. Journal of Chromatography B 2015, 985, 189–196. [DOI] [PubMed] [Google Scholar]
- 62.Steeghs MM; Cristescu SM; Harren FJ, The suitability of Tedlar bags for breath sampling in medical diagnostic research. Physiological measurement 2006, 28 (1), 73. [DOI] [PubMed] [Google Scholar]
- 63.Deng C; Zhang J; Yu X; Zhang W; Zhang X, Determination of acetone in human breath by gas chromatography–mass spectrometry and solid-phase microextraction with on-fiber derivatization. Journal of Chromatography B 2004, 810 (2), 269–275. [DOI] [PubMed] [Google Scholar]
- 64.Hyšpler R. r.; Crhová Š; Gasparič J; Zadák Z; Čĺž ková M; Balasová V, Determination of isoprene in human expired breath using solid-phase microextraction and gas chromatography–mass spectrometry. Journal of Chromatography B: Biomedical Sciences and Applications 2000, 739 (1), 183– 190. [DOI] [PubMed] [Google Scholar]
- 65.Bajtarevic A; Ager C; Pienz M; Klieber M; Schwarz K; Ligor M; Ligor T; Filipiak W; Denz H; Fiegl M; Hilbe W; Weiss W; Lukas P; Jamnig H; Hackl M; Haidenberger A; Buszewski B; Miekisch W; Schubert J; Amann A, Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009, 9 (1), 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pleil JD; Lindstrom AB, Measurement of volatile organic compounds in exhaled breath as collected in evacuated electropolished canisters. Journal of Chromatography B: Biomedical Sciences and Applications 1995, 665 (2), 271–279. [DOI] [PubMed] [Google Scholar]
- 67.Lindstrom AB; Pleil JD, A review of the USEPA’s single breath canister (SBC) method for exhaled volatile organic biomarkers. Biomarkers 2002, 7 (3), 189–208. [DOI] [PubMed] [Google Scholar]
- 68.Fent KW; Evans DE, Assessing the risk to firefighters from chemical vapors and gases during vehicle fire suppression. Journal of environmental monitoring 2011, 13 (3), 536–543. [DOI] [PubMed] [Google Scholar]
- 69.Lindstrom A; Highsmith V; Buckley T; Pate W; Michael L, Gasoline-contaminated ground water as a source of residential benzene exposure: a case study. Journal of exposure analysis and environmental epidemiology 1993, 4 (2), 183–195. [PubMed] [Google Scholar]
- 70.Lindstrom AB; Pleil JD; Berkoff DC, Alveolar breath sampling and analysis to assess trihalomethane exposures during competitive swimming training. Environmental Health Perspectives 1997, 105 (6), 636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pleil JD; Lindstrom AB, Collection of a single alveolar exhaled breath for volatile organic compounds analysis. American Journal of Industrial Medicine 1995, 28 (1), 109–121. [DOI] [PubMed] [Google Scholar]
- 72.Kwak J; Fan M; Harshman SW; Garrison CE; Dershem VL; Phillips JB; Grigsby CC; Ott DK, Evaluation of Bio-VOC sampler for analysis of volatile organic compounds in exhaled breath. Metabolites 2014, 4 (4), 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beale DJ; Jones OA; Karpe AV; Dayalan S; Oh DY; Kouremenos KA; Ahmed W; Palombo EA, A review of analytical techniques and their application in disease diagnosis in breathomics and salivaomics research. International journal of molecular sciences 2016, 18 (1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woolfenden E, Monitoring VOCs in air using sorbent tubes followed by thermal desorption capillary GC analysis: summary of data and practical guidelines. Journal of the Air & Waste Management Association 1997, 47 (1), 20–36. [Google Scholar]
- 75.Dettmer K; Engewald W, Adsorbent materials commonly used in air analysis for adsorptive enrichment and thermal desorption of volatile organic compounds. Analytical and bioanalytical chemistry 2002, 373 (6), 490–500. [DOI] [PubMed] [Google Scholar]
- 76.Pleil JD; Stiegel MA; Fent KW, Exploratory breath analyses for assessing toxic dermal exposures of firefighters during suppression of structural burns. Journal of breath research 2014, 8 (3), 037107. [DOI] [PubMed] [Google Scholar]
- 77.Grabowska-Polanowska B; Faber J; Skowron M; Miarka P; Pietrzycka A; Śliwka I; Amann A, Detection of potential chronic kidney disease markers in breath using gas chromatography with mass-spectral detection coupled with thermal desorption method. Journal of chromatography A 2013, 1301, 179–189. [DOI] [PubMed] [Google Scholar]
- 78.Winters BR; Pleil JD; Angrish MM; Stiegel MA; Risby TH; Madden MC, Standardization of the collection of exhaled breath condensate and exhaled breath aerosol using a feedback regulated sampling device. Journal of Breath Research 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Solga SF; Mudalel M; Spacek LA; Lewicki R; Tittel F; Loccioni C; Russo A; Risby TH, Factors influencing breath ammonia determination. Journal of breath research 2013, 7 (3), 037101. [DOI] [PubMed] [Google Scholar]
- 80.McDevitt JJ; Koutrakis P; Ferguson ST; Wolfson JM; Fabian MP; Martins M; Pantelic J; Milton DK, Development and performance evaluation of an exhaled-breath bioaerosol collector for influenza virus. Aerosol Science and Technology 2013, 47 (4), 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Almstrand A-C; Ljungström E; Lausmaa J; Bake B; Sjövall P; Olin A-C, Airway monitoring by collection and mass spectrometric analysis of exhaled particles. Analytical chemistry 2008, 81 (2), 662–668. [DOI] [PubMed] [Google Scholar]
- 82.Pleil JD; Wallace A; Madden MC, Exhaled breath aerosol (EBA): the simplest non-invasive medium for public health and occupational exposure biomonitoring. Journal of Breath Research 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dragonieri S; Pennazza G; Carratu P; Resta O, Electronic Nose Technology in Respiratory Diseases. Lung 2017, 195 (2), 157–165. [DOI] [PubMed] [Google Scholar]
- 84.Wilkens WF; Hartman JD, An Electronic Analog for the Olfactory Processesa. Journal of Food Science 1964, 29 (3), 372–378. [DOI] [PubMed] [Google Scholar]
- 85.Buck TM; Allen FG; Dalton JV, Detection of Chemical Species by Surface Effects on Metals and Semiconductors. Bell Telephone Laboratories: 1965. [Google Scholar]
- 86.Dravnieks A; Trotter PJ, Polar vapour detector based on thermal modulation of contact potential. Journal of Scientific Instruments 1965, 42 (8), 624. [Google Scholar]
- 87.Persaud K; Dodd G, Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299 (5881), 352–355. [DOI] [PubMed] [Google Scholar]
- 88.Ikegami A; Kaneyasu M, Olfactory detection using integrated sensors. Digest of Technical Papers, Transducers 1985, 85, 136–139. [Google Scholar]
- 89.Wilson AD; Baietto M, Applications and advances in electronic-nose technologies. Sensors 2009, 9 (7), 5099–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Romain A-C; Nicolas J, Long term stability of metal oxide-based gas sensors for e-nose environmental applications: An overview. Sensors and Actuators B: Chemical 2010, 146 (2), 502–506. [Google Scholar]
- 91.Arshak K; Moore E; Lyons G; Harris J; Clifford S, A review of gas sensors employed in electronic nose applications. Sensor review 2004, 24 (2), 181–198. [Google Scholar]
- 92.De Vries R; Brinkman P; van der Schee M; Fens N; Dijkers E; Bootsma S; de Jongh F; Sterk P, Integration of electronic nose technology with spirometry: validation of a new approach for exhaled breath analysis. Journal of breath research 2015, 9 (4), 046001. [DOI] [PubMed] [Google Scholar]
- 93.Thaler ER; Lee DD; Hanson CW, Diagnosis of rhinosinusitis with a colorimetric sensor array. Journal of breath research 2008, 2 (3), 037016. [DOI] [PubMed] [Google Scholar]
- 94.Bikov A; Hernadi M; Korosi BZ; Kunos L; Zsamboki G; Sutto Z; Tarnoki AD; Tarnoki DL; Losonczy G; Horvath I, Expiratory flow rate, breath hold and anatomic dead space influence electronic nose ability to detect lung cancer. BMC pulmonary medicine 2014, 14 (1), 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bach J-P; Gold M; Mengel D; Hattesohl A; Lubbe D; Schmid S; Tackenberg B; Rieke J; Maddula S; Baumbach JI, Measuring Compounds in Exhaled Air to Detect Alzheimer’s Disease and Parkinson’s Disease. PloS one 2015, 10 (7), e0132227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kunos L; Bikov A; Lazar Z; Korosi BZ; Benedek P; Losonczy G; Horvath I, Evening and morning exhaled volatile compound patterns are different in obstructive sleep apnoea assessed with electronic nose. Sleep and Breathing 2015, 19 (1), 247–253. [DOI] [PubMed] [Google Scholar]
- 97.de Heer K; Kok M; Fens N; Weersink E; Zwinderman A; van der Schee M; Visser C; van Oers M; Sterk P, Detection of Airway Colonization by Aspergillus fumigatus by Use of Electronic Nose Technology in Patients with Cystic Fibrosis. Journal of clinical microbiology 2016, 54 (3), 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bofan M; Mores N; Baron M; Dabrowska M; Valente S; Schmid M; Trové A; Conforto S; Zini G; Cattani P, Within-day and between-day repeatability of measurements with an electronic nose in patients with COPD. Journal of breath research 2013, 7 (1), 017103. [DOI] [PubMed] [Google Scholar]
- 99.Van der Schee M; Fens N; Brinkman P; Bos L; Angelo M; Nijsen T; Raabe R; Knobel H; Vink T; Sterk P, Effect of transportation and storage using sorbent tubes of exhaled breath samples on diagnostic accuracy of electronic nose analysis. Journal of breath research 2012, 7 (1), 016002. [DOI] [PubMed] [Google Scholar]
- 100.Zhang L; Tian G; Li J; Yu B, Applications of absorption spectroscopy using quantum cascade lasers. Applied spectroscopy 2014, 68 (10), 1095–1107. [DOI] [PubMed] [Google Scholar]
- 101.Wörle K; Seichter F; Wilk A; Armacost C; Day T; Godejohann M; Wachter U; Vogt J; Radermacher P; Mizaikoff B, Breath analysis with broadly tunable quantum cascade lasers. Analytical chemistry 2013, 85 (5), 2697–2702. [DOI] [PubMed] [Google Scholar]
- 102.Seichter F; Wilk A; Wörle K; Kim S-S; Vogt JA; Wachter U; Radermacher P; Mizaikoff B, Multivariate determination of 13CO2/12CO2 ratios in exhaled mouse breath with mid-infrared hollow waveguide gas sensors. Analytical and bioanalytical chemistry 2013, 405 (14), 4945–4951. [DOI] [PubMed] [Google Scholar]
- 103.Butz DE; Weidmann D; Brownsword R; Cook ME; Schoeller DA; Whigham LD In Immediate biofeedback for energy balance via expired breath δ 13 CO 2, Engineering in Medicine and Biology Society (EMBC), 2015 37th Annual International Conference of the IEEE, IEEE: 2015; pp 8205– 8208. [DOI] [PubMed] [Google Scholar]
- 104.Bakhirkin YA; Kosterev AA; Roller C; Curl RF; Tittel FK, Mid-infrared quantum cascade laser based off-axis integrated cavity output spectroscopy for biogenic nitric oxide detection. Applied optics 2004, 43 (11), 2257–2266. [DOI] [PubMed] [Google Scholar]
- 105.McCurdy MR; Bakhirkin Y; Wysocki G; Tittel FK, Performance of an exhaled nitric oxide and carbon dioxide sensor using quantum cascade laser-based integrated cavity output spectroscopy. Journal of Biomedical Optics 2007, 12 (3), 034034–034034-9. [DOI] [PubMed] [Google Scholar]
- 106.Conteduca D; Dell’Olio F; Ciminelli C; Armenise M, New miniaturized exhaled nitric oxide sensor based on a high Q/V mid-infrared 1D photonic crystal cavity. Applied optics 2015, 54 (9), 2208– 2217. [DOI] [PubMed] [Google Scholar]
- 107.da Silveira Petruci JF; Fortes PR; Kokoric V; Wilk A; Raimundo IM; Cardoso AA; Mizaikoff B, Monitoring of hydrogen sulfide via substrate-integrated hollow waveguide mid-infrared sensors in real-time. Analyst 2014, 139 (1), 198–203. [DOI] [PubMed] [Google Scholar]
- 108.Perez-Guaita D; Kokoric V; Wilk A; Garrigues S; Mizaikoff B, Towards the determination of isoprene in human breath using substrate-integrated hollow waveguide mid-infrared sensors. Journal of breath research 2014, 8 (2), 026003. [DOI] [PubMed] [Google Scholar]
- 109.Ciaffoni L; Hancock G; Harrison JJ; van Helden J-PH; Langley CE; Peverall R; Ritchie GA; Wood S, Demonstration of a mid-infrared cavity enhanced absorption spectrometer for breath acetone detection. Analytical chemistry 2012, 85 (2), 846–850. [DOI] [PubMed] [Google Scholar]
- 110.Hannemann M; Antufjew A; Borgmann K; Hempel F; Ittermann T; Welzel S; Weltmann K; Völzke H; Röpcke J, Influence of age and sex in exhaled breath samples investigated by means of infrared laser absorption spectroscopy. Journal of breath research 2011, 5 (2), 027101. [DOI] [PubMed] [Google Scholar]
- 111.Ye M; Chien P-J; Toma K; Arakawa T; Mitsubayashi K, An acetone bio-sniffer (gas phase biosensor) enabling assessment of lipid metabolism from exhaled breath. Biosensors and Bioelectronics 2015, 73, 208–213. [DOI] [PubMed] [Google Scholar]
- 112.Sun M; Jiang C; Gong Z; Zhao X; Chen Z; Wang Z; Kang M; Li Y; Wang C, A fully integrated standalone portable cavity ringdown breath acetone analyzer. Review of Scientific Instruments 2015, 86 (9), 095003. [DOI] [PubMed] [Google Scholar]
- 113.Kahn N; Lavie O; Paz M; Segev Y; Haick H, Dynamic nanoparticle-based flexible sensors: Diagnosis of ovarian carcinoma from exhaled breath. Nano letters 2015, 15 (10), 7023–7028. [DOI] [PubMed] [Google Scholar]
- 114.Fumagalli M; Ferrari F; Luisetti M; Stolk J; Hiemstra PS; Capuano D; Viglio S; Fregonese L; Cerveri I; Corana F, Profiling the proteome of exhaled breath condensate in healthy smokers and COPD patients by LC-MS/MS. International journal of molecular sciences 2012, 13 (11), 13894–13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rudnicka J; Kowalkowski T; Ligor T; Buszewski B, Determination of volatile organic compounds as biomarkers of lung cancer by SPME–GC–TOF/MS and chemometrics. Journal of Chromatography B 2011, 879 (30), 3360–3366. [DOI] [PubMed] [Google Scholar]
- 116.Gaspar EM; Lucena AF; da Costa JD; das Neves HC, Organic metabolites in exhaled human breath—a multivariate approach for identification of biomarkers in lung disorders. Journal of Chromatography A 2009, 1216 (14), 2749–2756. [DOI] [PubMed] [Google Scholar]
- 117.Buszewski B; Grzywinski D; Ligor T; Stacewicz T; Bielecki Z; Wojtas J, Detection of volatile organic compounds as biomarkers in breath analysis by different analytical techniques. Bioanalysis 2013, 5 (18), 2287–2306. [DOI] [PubMed] [Google Scholar]
- 118.Peralbo-Molina A; Calderón-Santiago M; Priego-Capote F; Jurado-Gámez B; de Castro ML, Development of a method for metabolomic analysis of human exhaled breath condensate by gas chromatography–mass spectrometry in high resolution mode. Analytica chimica acta 2015, 887, 118– 126. [DOI] [PubMed] [Google Scholar]
- 119.Peralbo-Molina A; Calderón-Santiago M; Priego-Capote F; Jurado-Gámez B; Castro M. D. L. d., Metabolomics analysis of exhaled breath condensate for discrimination between lung cancer patients and risk factor individuals. Journal of Breath Research 2016, 10 (1), 016011. [DOI] [PubMed] [Google Scholar]
- 120.Schnabel R; Fijten R; Smolinska A; Dallinga J; Boumans M-L; Stobberingh E; Boots A; Roekaerts P; Bergmans D; van Schooten FJ, Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Scientific Reports 2015, 5, 17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bodelier AGL; Smolinska A; Baranska A; Dallinga JW; Mujagic Z; Vanhees K; van den Heuvel T; Masclee AAM; Jonkers D; Pierik MJ; van Schooten FJ, Volatile Organic Compounds in Exhaled Air as Novel Marker for Disease Activity in Crohn’s Disease: A Metabolomic Approach. Inflammatory Bowel Diseases 2015, 21 (8), 1776–1785. [DOI] [PubMed] [Google Scholar]
- 122.Florence NS; Jan WD; Monique H; Emiel FMW; Renaud L; Frederik JVS, Volatile organic compounds discriminate between eosinophilic and neutrophilic inflammation in vitro. Journal of Breath Research 2016, 10 (1), 016006. [DOI] [PubMed] [Google Scholar]
- 123.Xu M; Tang Z; Duan Y; Liu Y, GC-based techniques for breath analysis: current status, challenges, and prospects. Critical Reviews in Analytical Chemistry 2016, 46 (4), 291–304. [DOI] [PubMed] [Google Scholar]
- 124.Phillips M; Cataneo RN; Chaturvedi A; Kaplan PD; Libardoni M; Mundada M; Patel U; Zhang X, Detection of an extended human volatome with comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. PloS one 2013, 8 (9), e75274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mieth M; Schubert JK; Gröger T; Sabel B; Kischkel S; Fuchs P; Hein D; Zimmermann R; Miekisch W, Automated needle trap heart-cut GC/MS and needle trap comprehensive two-dimensional GC/TOF-MS for breath gas analysis in the clinical environment. Analytical chemistry 2010, 82 (6), 2541– 2551. [DOI] [PubMed] [Google Scholar]
- 126.Caldeira M; Perestrelo R; Barros AS; Bilelo MJ; Morête A; Câmara JS; Rocha SM, Allergic asthma exhaled breath metabolome: A challenge for comprehensive two-dimensional gas chromatography. Journal of Chromatography A 2012, 1254, 87–97. [DOI] [PubMed] [Google Scholar]
- 127.Das MK; Bishwal SC; Das A; Dabral D; Varshney A; Badireddy VK; Nanda R, Investigation of Gender-Specific Exhaled Breath Volatome in Humans by GCxGC-TOF-MS. Analytical Chemistry 2014, 86 (2), 1229–1237. [DOI] [PubMed] [Google Scholar]
- 128.Phillips M; Byrnes R; Cataneo RN; Chaturvedi A; Kaplan PD; Libardoni M; Mehta V; Mundada M; Patel U; Ramakrishna N, Detection of volatile biomarkers of therapeutic radiation in breath. Journal of breath research 2013, 7 (3), 036002. [DOI] [PubMed] [Google Scholar]
- 129.Berchtold C; Bosilkovska M; Daali Y; Walder B; Zenobi R, Real‐time monitoring of exhaled drugs by mass spectrometry. Mass spectrometry reviews 2014, 33 (5), 394–413. [DOI] [PubMed] [Google Scholar]
- 130.Zubarev RA; Makarov A, Orbitrap mass spectrometry. ACS Publications: 2013. [DOI] [PubMed] [Google Scholar]
- 131.Eliuk S; Makarov A, Evolution of orbitrap mass spectrometry instrumentation 2015. [DOI] [PubMed] [Google Scholar]
- 132.Muccilli V; Saletti R; Cunsolo V; Ho J; Gili E; Conte E; Sichili S; Vancheri C; Foti S, Protein profile of exhaled breath condensate determined by high resolution mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis 2015, 105, 134–149. [DOI] [PubMed] [Google Scholar]
- 133.Bloemen K; Van Den Heuvel R; Govarts E; Hooyberghs J; Nelen V; Witters E; Desager K; Schoeters G, A new approach to study exhaled proteins as potential biomarkers for asthma. Clinical & Experimental Allergy 2011, 41 (3), 346–356. [DOI] [PubMed] [Google Scholar]
- 134.Carraro S; Giordano G; Piacentini G; Kantar A; Moser S; Cesca L; Berardi M; Di Gangi IM; Baraldi E, Asymmetric dimethylarginine in exhaled breath condensate and serum of children with asthma. CHEST Journal 2013, 144 (2), 405–410. [DOI] [PubMed] [Google Scholar]
- 135.Ullah S; Sandqvist S; Beck O, A liquid chromatography and tandem mass spectrometry method to determine 28 non-volatile drugs of abuse in exhaled breath. Journal of Pharmaceutical and Biomedical Analysis 2017. [DOI] [PubMed] [Google Scholar]
- 136.Ellefsen KN; Concheiro M; Beck O; Gorelick DA; Pirard S; Huestis MA, Quantification of cocaine and metabolites in exhaled breath by liquid chromatography-high-resolution mass spectrometry following controlled administration of intravenous cocaine. Analytical and bioanalytical chemistry 2014, 406 (25), 6213–6223. [DOI] [PubMed] [Google Scholar]
- 137.Thevis M; Krug O; Geyer H; Schänzer W, Expanding Analytical Options in Sports Drug Testing: Mass Spectrometric Detection of Prohibited Substances in Exhaled Breath. Rapid Communications in Mass Spectrometry 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bean HD; Zhu J; Hill JE, Characterizing bacterial volatiles using secondary electrospray ionization mass spectrometry (SESI-MS). JoVE (Journal of Visualized Experiments) 2011, (52), e2664- e2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.García-Gómez D; Bregy L; Nussbaumer-Ochsner Y; Gaisl T; Kohler M; Zenobi R, Detection and quantification of benzothiazoles in exhaled breath and exhaled breath condensate by real-time secondary electrospray ionization–high-resolution mass spectrometry and ultra-high performance liquid chromatography. Environmental science & technology 2015, 49 (20), 12519–12524. [DOI] [PubMed] [Google Scholar]
- 140.Sinues PM-L; Landoni E; Miceli R; Dibari VF; Dugo M; Agresti R; Tagliabue E; Cristoni S; Orlandi R, Secondary electrospray ionization-mass spectrometry and a novel statistical bioinformatic approach identifies a cancer-related profile in exhaled breath of breast cancer patients: a pilot study. Journal of breath research 2015, 9 (3), 031001. [DOI] [PubMed] [Google Scholar]
- 141.Martinez-Lozano P; Zingaro L; Finiguerra A; Cristoni S, Secondary electrospray ionization- mass spectrometry: breath study on a control group. Journal of breath research 2011, 5 (1), 016002. [DOI] [PubMed] [Google Scholar]
- 142.Bean HD; Jiménez-Díaz J; Zhu J; Hill JE, Breathprints of model murine bacterial lung infections are linked with immune response. European Respiratory Journal 2015, 45 (1), 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhu J; Jiménez-Díaz J; Bean HD; Daphtary NA; Aliyeva MI; Lundblad LK; Hill JE, Robust detection of P. aeruginosa and S. aureus acute lung infections by secondary electrospray ionization-mass spectrometry (SESI-MS) breathprinting: from initial infection to clearance. Journal of breath research 2013, 7 (3), 037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhu J; Bean HD; Jiménez-Díaz J; Hill JE, Secondary electrospray ionization-mass spectrometry (SESI-MS) breathprinting of multiple bacterial lung pathogens, a mouse model study. Journal of applied physiology 2013, 114 (11), 1544–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bikov A; Paschalaki K; Logan-Sinclair R; Horváth I; Kharitonov SA; Barnes PJ; Usmani OS; Paredi P, Standardised exhaled breath collection for the measurement of exhaled volatile organic compounds by proton transfer reaction mass spectrometry. BMC pulmonary medicine 2013, 13 (1), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lindinger W; Hansel A; Jordan A, On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int J Mass Spectrom Ion Processes 1998, 173. [Google Scholar]
- 147.Beate G; Stefan K; Thomas G; Georg M; Wilfried S; Ralf Z, Breath gas monitoring during a glucose challenge by a combined PTR-QMS/GC×GC-TOFMS approach for the verification of potential volatile biomarkers. Journal of Breath Research 2016, 10 (3), 036003. [DOI] [PubMed] [Google Scholar]
- 148.del Río RF; O’Hara M; Pemberton P; Whitehouse T; Mayhew C, Elimination characteristics of post-operative isoflurane levels in alveolar exhaled breath via PTR-MS analysis. Journal of breath research 2016, 10 (4), 046006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Herbig J; Müller M; Schallhart S; Titzmann T; Graus M; Hansel A, On-line breath analysis with PTR-TOF. Journal of breath research 2009, 3 (2), 027004. [DOI] [PubMed] [Google Scholar]
- 150.Sukul P; Trefz P; Kamysek S; Schubert JK; Miekisch W, Instant effects of changing body positions on compositions of exhaled breath. Journal of Breath Research 2015, 9 (4), 047105. [DOI] [PubMed] [Google Scholar]
- 151.Sukul P; Trefz P; Schubert JK; Miekisch W, Immediate effects of breath holding maneuvers onto composition of exhaled breath. Journal of breath research 2014, 8 (3), 037102. [DOI] [PubMed] [Google Scholar]
- 152.Kohl I; Beauchamp J; Cakar-Beck F; Herbig J; Dunkl J; Tietje O; Tiefenthaler M; Boesmueller C; Wisthaler A; Breitenlechner M, First observation of a potential non-invasive breath gas biomarker for kidney function. Journal of breath research 2013, 7 (1), 017110. [DOI] [PubMed] [Google Scholar]
- 153.Beate B; Svend K; Josephine S; Phillip T; Jochen KS; Wolfram M, Monitoring of breath VOCs and electrical impedance tomography under pulmonary recruitment in mechanically ventilated patients. Journal of Breath Research 2017, 11 (1), 016005. [DOI] [PubMed] [Google Scholar]
- 154.Aprea E; Cappellin L; Gasperi F; Morisco F; Lembo V; Rispo A; Tortora R; Vitaglione P; Caporaso N; Biasioli F, Application of PTR-TOF-MS to investigate metabolites in exhaled breath of patients affected by coeliac disease under gluten free diet. Journal of Chromatography B 2014, 966, 208– 213. [DOI] [PubMed] [Google Scholar]
- 155.Samudrala D; Geurts B; Brown PA; Szymańska E; Mandon J; Jansen J; Buydens L; Harren FJ; Cristescu SM, Changes in urine headspace composition as an effect of strenuous walking. Metabolomics 2015, 11 (6), 1656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schmidberger T; Gutmann R; Bayer K; Kronthaler J; Huber R, Advanced online monitoring of cell culture off‐gas using proton transfer reaction mass spectrometry. Biotechnology progress 2014, 30 (2), 496–504. [DOI] [PubMed] [Google Scholar]
- 157.Szymczak W; Rozman J; Höllriegl V; Kistler M; Keller S; Peters D; Kneipp M; Schulz H; Hoeschen C; Klingenspor M, Online breath gas analysis in unrestrained mice by hs-PTR-MS. Mammalian genome 2014, 25 (3–4), 129–140. [DOI] [PubMed] [Google Scholar]
- 158.Hera D; Langford VS; McEwan MJ; McKellar TI; Milligan DB, Negative Reagent Ions for Real Time Detection Using SIFT-MS. Environments 2017, 4 (1), 16. [Google Scholar]
- 159.Perkins MJ; Langford VS; McEwan MJ, High-Throughput VOC and Inorganic Gas Analysis: Automated SIFT-MS. AMERICAN LABORATORY 2017, 49 (1), 17–19. [Google Scholar]
- 160.Spesyvyi A; Smith D; Španěl P, Selected ion flow-drift tube mass spectrometry: quantification of volatile compounds in air and breath. Analytical Chemistry 2015, 87 (24), 12151–12160. [DOI] [PubMed] [Google Scholar]
- 161.Wang MH; Lau SY-F; Chong KC; Kwok C; Lai M; Chung AH; Ho CS; Szeto C-C; Zee BC-Y, Estimation of clinical parameters of chronic kidney disease by exhaled breath full-scan mass spectrometry data and iterative PCA with intensity screening algorithm. Journal of Breath Research 2017, 11 (3), 036007. [DOI] [PubMed] [Google Scholar]
- 162.Smith D, From molecules in space to molecules in breath. Paediatric respiratory reviews 2016, 17, 50–52. [DOI] [PubMed] [Google Scholar]
- 163.Španěl P; Dryahina K; Vicherková P; Smith D, Increase of methanol in exhaled breath quantified by SIFT-MS following aspartame ingestion. Journal of Breath Research 2015, 9 (4), 047104. [DOI] [PubMed] [Google Scholar]
- 164.Alkhouri N; Eng K; Cikach F; Patel N; Yan C; Brindle A; Rome E; Hanouneh I; Grove D; Lopez R, Breathprints of childhood obesity: changes in volatile organic compounds in obese children compared with lean controls. Pediatric obesity 2015, 10 (1), 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hicks LC; Huang J; Kumar S; Powles ST; Orchard TR; Hanna GB; Williams HR, Analysis of exhaled breath volatile organic compounds in inflammatory bowel disease: a pilot study. Journal of Crohn’s and Colitis 2015, jjv102. [DOI] [PubMed] [Google Scholar]
- 166.Batty CA; Cauchi M; Lourenco C; Hunter JO; Turner C, Use of the analysis of the volatile faecal metabolome in screening for colorectal cancer. PloS one 2015, 10 (6), e0130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smith D; Chippendale TW; Španěl P, Reactions of the selected ion flow tube mass spectrometry reagent ions H3O+ and NO+ with a series of volatile aldehydes of biogenic significance. Rapid Communications in Mass Spectrometry 2014, 28 (17), 1917–1928. [DOI] [PubMed] [Google Scholar]
- 168.Shestivska V; Dryahina K; Nunvář J; Sovová K; Elhottová D; Nemec A; Smith D; Španěl P, Quantitative analysis of volatile metabolites released in vitro by bacteria of the genus Stenotrophomonas for identification of breath biomarkers of respiratory infection in cystic fibrosis. Journal of breath research 2015, 9 (2), 027104. [DOI] [PubMed] [Google Scholar]
- 169.Fink T; Baumbach JI; Kreuer S, Ion mobility spectrometry in breath research. Journal of breath research 2014, 8 (2), 027104. [DOI] [PubMed] [Google Scholar]
- 170.Kreuder A-E; Buchinger H; Kreuer S; Volk T; Maddula S; Baumbach J, Characterization of propofol in human breath of patients undergoing anesthesia. International Journal for Ion Mobility Spectrometry 2011, 14 (4), 167–175. [Google Scholar]
- 171.Maurer F; Lorenz D; Pielsticker G; Volk T; Sessler D; Baumbach JI; Kreuer S, Adherence of volatile propofol to various types of plastic tubing. Journal of breath research 2017, 11 (1), 016009. [DOI] [PubMed] [Google Scholar]
- 172.Maurer F; Walter L; Geiger M; Baumbach JI; Sessler DI; Volk T; Kreuer S, Calibration and validation of a MCC/IMS prototype for exhaled propofol online measurement. Journal of pharmaceutical and biomedical analysis 2017, 145, 293–297. [DOI] [PubMed] [Google Scholar]
- 173.Brodrick E; Davies A; Neill P; Hanna L; Williams EM, Breath analysis: translation into clinical practice. Journal of breath research 2015, 9 (2), 027109. [DOI] [PubMed] [Google Scholar]
- 174.Allafchian AR; Majidian Z; Ielbeigi V; Tabrizchi M, A novel method for the determination of three volatile organic compounds in exhaled breath by solid-phase microextraction–ion mobility spectrometry. Analytical and bioanalytical chemistry 2016, 408 (3), 839–847. [DOI] [PubMed] [Google Scholar]
- 175.Ruzsanyi V, Ion mobility spectrometry for pharmacokinetic studies–exemplary application. Journal of breath research 2013, 7 (4), 046008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Besa V; Teschler H; Kurth I; Khan AM; Zarogoulidis P; Baumbach JI; Sommerwerck U; Freitag L; Darwiche K, Exhaled volatile organic compounds discriminate patients with chronic obstructive pulmonary disease from healthy subjects. International journal of chronic obstructive pulmonary disease 2015, 10, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Handa H; Usuba A; Maddula S; Baumbach JI; Mineshita M; Miyazawa T, Exhaled breath analysis for lung cancer detection using ion mobility spectrometry. PloS one 2014, 9 (12), e114555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Arasaradnam RP; Westenbrink E; McFarlane MJ; Harbord R; Chambers S; O’Connell N; Bailey C; Nwokolo CU; Bardhan KD; Savage R, Differentiating Coeliac disease from irritable bowel syndrome by urinary volatile organic compound analysis–a pilot study. PloS one 2014, 9 (10), e107312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Stiegel MA; Pleil JD; Sobus JR; Morgan MK; Madden MC, Analysis of inflammatory cytokines in human blood, breath condensate, and urine using a multiplex immunoassay platform. Biomarkers 2015, 20 (1), 35–46. [DOI] [PubMed] [Google Scholar]
- 180.Stiegel MA; Pleil JD; Sobus JR; Madden MC, Inflammatory cytokines and white blood cell counts response to environmental levels of diesel exhaust and ozone inhalation exposures. PloS one 2016, 11 (4), e0152458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Engvall E; Perlmann P, Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry 1971, 8 (9), 871–874. [DOI] [PubMed] [Google Scholar]
- 182.Radulovic M; Bauman WA; Wecht JM; LaFountaine M; Kahn N; Hobson J; Singh K; Renzi C; Yen C; Schilero GJ, Biomarkers of inflammation in persons with chronic tetraplegia. Journal of breath research 2015, 9 (3), 036001. [DOI] [PubMed] [Google Scholar]
- 183.Brzozowska A; Majak P; Jerzyńska J; Smejda K; Bobrowska-Korzeniowska M; Stelmach W; Koczkowska M; Stelmach I, Exhaled nitric oxide correlates with IL-2, MCP-1, PDGF-BB and TIMP-2 in exhaled breath condensate of children with refractory asthma. Postep Derm Alergol 2015, 32, 107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu H-C; Lu M-C; Lin Y-C; Wu T-C; Hsu J-Y; Jan M-S; Chen C-M, Differences in IL-8 in serum and exhaled breath condensate from patients with exacerbated COPD or asthma attacks. Journal of the Formosan Medical Association 2014, 113 (12), 908–914. [DOI] [PubMed] [Google Scholar]
- 185.Krenke K; Peradzyńska J; Lange J; Banaszkiewicz A; Łazowska‐Przeorek I; Grzela K; Radzikowski A; Kulus M, Inflammatory cytokines in exhaled breath condensate in children with inflammatory bowel diseases. Pediatric pulmonology 2014, 49 (12), 1190–1195. [DOI] [PubMed] [Google Scholar]
- 186.Ahmadzai H; Cameron B; Chui J; Lloyd A; Wakefield D; Thomas PS, Measurement of neopterin, TGF-β1 and ACE in the exhaled breath condensate of patients with sarcoidosis. Journal of breath research 2013, 7 (4), 046003. [DOI] [PubMed] [Google Scholar]
- 187.Zagórska W; Grzela K; Kulus M; Sobczyński M; Grzela T, Nitric oxide IL ‐6 and IL‐13 are increased in the exhaled breath condensates of children with allergic rhinitis. Acta Paediatrica 2014, 103 (4), e148–e153. [DOI] [PubMed] [Google Scholar]
- 188.Bar-Yoseph R; Bentur L; Goldbart A; Livnat G; Hakim F; Weisman Y; Tiosano D, A mutated vitamin D receptor in hereditary vitamin D-resistant rickets prevents induction of bronchial hyperreactivity and inflammation. The Journal of Clinical Endocrinology & Metabolism 2014, 99 (9), E1610–E1616. [DOI] [PubMed] [Google Scholar]
- 189.De Prins S; Dons E; Van Poppel M; Panis LI; Van de Mieroop E; Nelen V; Cox B; Nawrot TS; Teughels C; Schoeters G, Airway oxidative stress and inflammation markers in exhaled breath from children are linked with exposure to black carbon. Environment international 2014, 73, 440–446. [DOI] [PubMed] [Google Scholar]
- 190.Pieters N; Koppen G; Van Poppel M; De Prins S; Cox B; Dons E; Nelen V; Panis LI; Plusquin M; Schoeters G, Blood pressure and same-day exposure to air pollution at school: associations with nano-sized to coarse PM in children. Environmental health perspectives 2015, 123 (7), 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Klaassen EM; Kant KD; Jöbsis Q; Penders J; Schooten FJ; Quaak M; Hartog GJ; Koppelman GH; Schayck CP; Eys G, Integrative genomic analysis identifies a role for intercellular adhesion molecule 1 in childhood asthma. Pediatric Allergy and Immunology 2014, 25 (2), 166–172. [DOI] [PubMed] [Google Scholar]
- 192.Brussino L; Culla B; Bucca C; Giobbe R; Boita M; Isaia G; Heffler E; Oliaro A; Filosso P; Rolla G, Inflammatory cytokines and VEGF measured in exhaled breath condensate are correlated with tumor mass in non-small cell lung cancer. Journal of breath research 2014, 8 (2), 027110. [DOI] [PubMed] [Google Scholar]
- 193.Bossley CJ; Fleming L; Gupta A; Regamey N; Frith J; Oates T; Tsartsali L; Lloyd CM; Bush A; Saglani S, Pediatric severe asthma is characterized by eosinophilia and remodeling without T H 2 cytokines. Journal of Allergy and Clinical Immunology 2012, 129 (4), 974–982. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Korovesi I; Papadomichelakis E; Orfanos SE; Giamarellos-Bourboulis EJ; Livaditi O; Pelekanou A; Sotiropoulou C; Koutsoukou A; Dimopoulou I; Ekonomidou F, Exhaled breath condensate in mechanically ventilated brain-injured patients with no lung injury or sepsis. The Journal of the American Society of Anesthesiologists 2011, 114 (5), 1118–1129. [DOI] [PubMed] [Google Scholar]
- 195.Boshuizen M; Leopold JH; Zakharkina T; Knobel HH; Weda H; Nijsen TM; Vink TJ; Sterk PJ; Schultz MJ; Bos LD, Levels of cytokines in broncho-alveolar lavage fluid, but not in plasma, are associated with levels of markers of lipid peroxidation in breath of ventilated ICU patients. Journal of breath research 2015, 9 (3), 036010. [DOI] [PubMed] [Google Scholar]
- 196.Rivnak AJ; Rissin DM; Kan CW; Song L; Fishburn MW; Piech T; Campbell TG; DuPont DR; Gardel M; Sullivan S, A fully-automated, six-plex single molecule immunoassay for measuring cytokines in blood. Journal of immunological methods 2015, 424, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rissin DM; Kan CW; Song L; Rivnak AJ; Fishburn MW; Shao Q; Piech T; Ferrell EP; Meyer RE; Campbell TG, Multiplexed single molecule immunoassays. Lab on a Chip 2013, 13 (15), 2902–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wu D; Milutinovic MD; Walt DR, Single molecule array (Simoa) assay with optimal antibody pairs for cytokine detection in human serum samples. Analyst 2015, 140 (18), 6277–6282. [DOI] [PubMed] [Google Scholar]
- 199.Pleil JD; Angrish MM; Madden MC, Immunochemistry for high-throughput screening of human exhaled breath condensate (EBC) media: implementation of automated Quanterix SIMOA instrumentation. Journal of Breath Research 2015, 9 (4), 047108. [DOI] [PubMed] [Google Scholar]
- 200.Organization, W. H.; Unit, W. H. O. M. o. S. A., Global status report on alcohol and health, 2014. World Health Organization: 2014. [Google Scholar]
- 201.Kitagawa T; Wright BM, A Quantitative Detector-tube Method for Breath-alcohol Estimation. British Medical Journal 1962, 2 (5305), 652–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Begg TB; Hill ID; Nickolls LC, Breathalyzer And Kitagawa-Wright Methods Of Measuring Breath Alcohol. The British Medical Journal 1964, 1 (5374), 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Macdougall BRD; Dordoni B; Thompson RPH; Davis M; Williams R, [1–14C] Ethanol breath test in alcoholic liver disease. Clinica Chimica Acta 1978, 86 (1), 121–127. [DOI] [PubMed] [Google Scholar]
- 204.Mattila MJ; Linnoila M; Seppälä T; Koskinen R, Effect of aluminium hydroxide and glycopyrrhonium on the absorption of ethambutol and alcohol in man. British Journal of Clinical Pharmacology 1978, 5 (2), 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jones AW, Quantitative measurements of the alcohol concentration and the temperature of breath during a prolonged exhalation. Acta Physiologica Scandinavica 1982, 114 (3), 407–412. [DOI] [PubMed] [Google Scholar]
- 206.Voas RB; Lacey JH; Jones K; Scherer M; Compton R, Drinking drivers and drug use on weekend nights in the United States. Drug and alcohol dependence 2013, 130 (1), 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ashdown HF; Fleming S; Spencer EA; Thompson MJ; Stevens RJ, Diagnostic accuracy study of three alcohol breathalysers marketed for sale to the public. BMJ open 2014, 4 (12), e005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nardi-Agmon I; Peled N, Exhaled breath analysis for the early detection of lung cancer: recent developments and future prospects. Lung Cancer: Targets and Therapy 2017, 8, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mozzoni P; Banda I; Goldoni M; Corradi M; Tiseo M; Acampa O; Balestra V; Ampollini L; Casalini A; Carbognani P; Mutti A, Plasma and EBC microRNAs as early biomarkers of non-small- cell lung cancer. Biomarkers 2013, 18 (8), 679–686. [DOI] [PubMed] [Google Scholar]
- 210.Hayes SA; Haefliger S; Harris B; Pavlakis N; Clarke SJ; Molloy MP; Howell VM, Exhaled breath condensate for lung cancer protein analysis: a review of methods and biomarkers. Journal of breath research 2016, 10 (3), 034001. [DOI] [PubMed] [Google Scholar]
- 211.Carpagnano G; Resta O; Foschino-Barbaro M; Gramiccioni E; Carpagnano F, Interleukin-6 is increased in breath condensate of patients with non-small cell lung cancer. The International journal of biological markers 2002, 17 (2), 141–145. [DOI] [PubMed] [Google Scholar]
- 212.Carpagnano GE; Foschino-Barbaro MP; Resta O; Gramiccioni E; Carpagnano F, Endothelin-1 Is Increased in the Breath Condensate of Patients with Non-Small-Cell Lung Cancer. Oncology 2004, 66 (3), 180–184. [DOI] [PubMed] [Google Scholar]
- 213.Gessner C; Kuhn H; Toepfer K; Hammerschmidt S; Schauer J; Wirtz H, Detection of p53 gene mutations in exhaled breath condensate of non-small cell lung cancer patients. Lung Cancer 2004, 43 (2), 215–222. [DOI] [PubMed] [Google Scholar]
- 214.Dent AG; Sutedja TG; Zimmerman PV, Exhaled breath analysis for lung cancer. Journal of thoracic disease 2013, 5 (Suppl 5), S540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.de Castro ML; Fernandez-Peralbo M, Analytical methods based on exhaled breath for early detection of lung cancer. TrAC Trends in Analytical Chemistry 2012, 38, 13–20. [Google Scholar]
- 216.Wang C; Dong R; Wang X; Lian A; Chi C; Ke C; Guo L; Liu S; Zhao W; Xu G; Li E, Exhaled volatile organic compounds as lung cancer biomarkers during one-lung ventilation. Scientific Reports 2014, 4, 7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Poli D; Carbognani P; Corradi M; Goldoni M; Acampa O; Balbi B; Bianchi L; Rusca M; Mutti A, Exhaled volatile organic compounds in patients with non-small cell lung cancer: cross sectional and nested short-term follow-up study. Respiratory Research 2005, 6 (1), 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fu X-A; Li M; Knipp RJ; Nantz MH; Bousamra M, Noninvasive detection of lung cancer using exhaled breath. Cancer Medicine 2014, 3 (1), 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Feinberg T; Alkoby-Meshulam L; Herbig J; Cancilla JC; Torrecilla JS; Gai Mor N; Bar J; Iluze M; Haick H; Peled N, Cancerous glucose metabolism in lung cancer-evidence from exhaled breath analysis. Breath Res 2016, 10, 026012. [DOI] [PubMed] [Google Scholar]
- 220.Peng G; Tisch U; Adams O; Hakim M; Shehada N; Broza YY; Billan S; Abdah-Bortnyak R; Kuten A; Haick H, Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat Nano 2009, 4 (10), 669–673. [DOI] [PubMed] [Google Scholar]
- 221.Barash O; Peled N; Hirsch FR; Haick H, Sniffing the Unique “Odor Print” of Non-Small-Cell Lung Cancer with Gold Nanoparticles. Small 2009, 5 (22), 2618–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dragonieri S; Annema JT; Schot R; van der Schee MP Spanevello A; Carratu P; Resta O; Rabe KF; Sterk PJ, An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer 2009, 64. [DOI] [PubMed] [Google Scholar]
- 223.Wojciech F; Anna F; Andreas S; Thomas S; Bettina Z; Clemens A; Ewa K; Hubert D; Alex P; Paolo L; Herbert J; Jakob T; Anton A, Comparative analyses of volatile organic compounds (VOCs) from patients, tumors and transformed cell lines for the validation of lung cancer-derived breath markers. Journal of Breath Research 2014, 8 (2), 027111. [DOI] [PubMed] [Google Scholar]
- 224.Schallschmidt K; Becker R; Jung C; Rolff J; Fichtner I; Nehls I, Investigation of cell culture volatilomes using solid phase micro extraction: Options and pitfalls exemplified with adenocarcinoma cell lines. Journal of Chromatography B 2015, 1006, 158–166. [DOI] [PubMed] [Google Scholar]
- 225.Filipiak W; Sponring A; Filipiak A; Ager C; Schubert J; Miekisch W; Amann A; Troppmair J, TD-GC-MS Analysis of Volatile Metabolites of Human Lung Cancer and Normal Cells In vitro. Cancer Epidemiology Biomarkers & Prevention 2010, 19 (1), 182–195. [DOI] [PubMed] [Google Scholar]
- 226.Wang Y; Hu Y; Wang D; Yu K; Wang L; Zou Y; Zhao C; Zhang X; Wang P; Ying K, The analysis of volatile organic compounds biomarkers for lung cancer in exhaled breath, tissues and cell lines. Cancer Biomarkers 2012, 11 (4), 129–137. [DOI] [PubMed] [Google Scholar]
- 227.Kim K-H; Jahan SA; Kabir E, A review of breath analysis for diagnosis of human health. TrAC Trends in Analytical Chemistry 2012, 33, 1–8. [Google Scholar]
- 228.Navratil M; Plavec D; Erceg D; Bulat Lokas S; Živković J; Turkalj M, Urates in exhaled breath condensate as a biomarker of control in childhood asthma. Journal of Asthma 2015, 52 (5), 437– 446. [DOI] [PubMed] [Google Scholar]
- 229.Mastalerz L; Januszek R; Kaszuba M; Wójcik K; Celejewska-Wójcik N; Gielicz A; Plutecka H; Oleś K; Stręk P; Sanak M, Aspirin provocation increases 8-iso-PGE 2 in exhaled breath condensate of aspirin-hypersensitive asthmatics. Prostaglandins & other lipid mediators 2015, 121, 163–169. [DOI] [PubMed] [Google Scholar]
- 230.Montuschi P; Santini G; Valente S; Mondino C; Macagno F; Cattani P; Zini G; Mores N, Liquid chromatography–mass spectrometry measurement of leukotrienes in asthma and other respiratory diseases. Journal of Chromatography B 2014, 964, 12–25. [DOI] [PubMed] [Google Scholar]
- 231.Syslová K; Böhmová A; Demirbağ E; Šimková K; Kuzma M; Pelclová D; Sedlák V; Čáp P; Martásek P; Kačer P, Immunomagnetic molecular probe with UHPLC–MS/MS: A promising way for reliable bronchial asthma diagnostics based on quantification of cysteinyl leukotrienes. Journal of pharmaceutical and biomedical analysis 2013, 81, 108–117. [DOI] [PubMed] [Google Scholar]
- 232.Montuschi P; Santonico M; Mondino C; Pennazza G; Mantini G; Martinelli E; Capuano R; Ciabattoni G; Paolesse R; Di Natale C; Barnes PJ; D’Amico A, Diagnostic Performance of an Electronic Nose, Fractional Exhaled Nitric Oxide, and Lung Function Testing in Asthma. Chest 2010, 137 (4), 790–796. [DOI] [PubMed] [Google Scholar]
- 233.Rundell KW; Slee JB; Caviston R; Hollenbach AM, Decreased lung function after inhalation of ultrafine and fine particulate matter during exercise is related to decreased total nitrate in exhaled breath condensate. Inhalation toxicology 2008, 20 (1), 1–9. [DOI] [PubMed] [Google Scholar]
- 234.Patel MM; Chillrud SN; Deepti K; Ross JM; Kinney PL, Traffic-related air pollutants and exhaled markers of airway inflammation and oxidative stress in New York City adolescents. Environmental research 2013, 121, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhang J; McCreanor JE; Cullinan P; Chung KF; Ohman-Strickland P; Han I-K; Järup L; Nieuwenhuijsen M, Health effects of real-world exposure to diesel exhaust in persons with asthma. Research report (Health Effects Institute) 2009, (138), 5–109; discussion 111–23. [PubMed] [Google Scholar]
- 236.Pauwels RA; Buist AS; Calverley PM; Jenkins CR; Hurd SS, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine 2012. [DOI] [PubMed] [Google Scholar]
- 237.MacNee W; Rennard SI; Hunt JF; Edwards LD; Miller BE; Locantore NW; Tal-Singer R, Evaluation of exhaled breath condensate pH as a biomarker for COPD. Respiratory medicine 2011, 105 (7), 1037–1045. [DOI] [PubMed] [Google Scholar]
- 238.Silva K. K. D. d.; Faria ACD; Lopes AJ; Melo P. L. d., Within-breath respiratory impedance and airway obstruction in patients with chronic obstructive pulmonary disease. Clinics 2015, 70 (7), 461– 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gaida A; Holz O; Nell C; Schuchardt S; Lavae-Mokhtari B; Kruse L; Boas U; Langejuergen J; Allers M; Zimmermann S; Vogelmeier C; Koczulla AR; Hohlfeld JM, A dual center study to compare breath volatile organic compounds from smokers and non-smokers with and without COPD. Journal of Breath Research 2016, 10 (2), 026006. [DOI] [PubMed] [Google Scholar]
- 240.Allers M; Langejuergen J; Gaida A; Holz O; Schuchardt S; Hohlfeld JM; Zimmermann S, Measurement of exhaled volatile organic compounds from patients with chronic obstructive pulmonary disease (COPD) using closed gas loop GC-IMS and GC-APCI-MS. Journal of Breath Research 2016, 10 (2), 026004. [DOI] [PubMed] [Google Scholar]
- 241.van Geffen WH; Bruins M; Kerstjens HA, Diagnosing viral and bacterial respiratory infections in acute COPD exacerbations by an electronic nose: a pilot study. Journal of breath research 2016, 10 (3), 036001. [DOI] [PubMed] [Google Scholar]
- 242.Dragonieri S; Quaranta VN; Carratu P; Ranieri T; Resta O, Exhaled breath profiling in patients with COPD and OSA overlap syndrome: a pilot study. Journal of breath research 2016, 10 (4), 041001. [DOI] [PubMed] [Google Scholar]
- 243.Larsson K, Inflammatory markers in COPD. The clinical respiratory journal 2008, 2 (s1), 84–87. [DOI] [PubMed] [Google Scholar]
- 244.Ko F; Leung T-F; Wong G; Ngai J; To KW; Ng S; Hui D, Measurement of tumor necrosis factor-alpha, leukotriene B4, and interleukin 8 in the exhaled breath condensate in patients with acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2009, 4 (1), 79– 86. [PMC free article] [PubMed] [Google Scholar]
- 245.Montuschi P; Kharitonov S; Ciabattoni G; Barnes P, Exhaled leukotrienes and prostaglandins in COPD. Thorax 2003, 58 (7), 585–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lim SK; Shin HS; Yoon KS; Kwack SJ; Um YM; Hyeon JH; Kwak HM; Kim JY; Kim TH; Kim YJ, Risk assessment of volatile organic compounds benzene, toluene, ethylbenzene, and xylene (BTEX) in consumer products. Journal of Toxicology and Environmental Health, Part A 2014, 77 (22–24), 1502–1521. [DOI] [PubMed] [Google Scholar]
- 247.Niri VH; Bragg L; Pawliszyn J, Fast analysis of volatile organic compounds and disinfection by-products in drinking water using solid-phase microextraction–gas chromatography/time-of-flight mass spectrometry. Journal of Chromatography A 2008, 1201 (2), 222–227. [DOI] [PubMed] [Google Scholar]
- 248.Chary NS; Fernandez-Alba AR, Determination of volatile organic compounds in drinking and environmental waters. TrAC Trends in Analytical Chemistry 2012, 32, 60–75. [Google Scholar]
- 249.Chein H; Chen TM, Emission characteristics of volatile organic compounds from semiconductor manufacturing. Journal of the Air & Waste Management Association 2003, 53 (8), 1029– 1036. [DOI] [PubMed] [Google Scholar]
- 250.Xi J; Si XA; Kim J; Mckee E; Lin E-B, Exhaled aerosol pattern discloses lung structural abnormality: a sensitivity study using computational modeling and fractal analysis. PloS one 2014, 9 (8), e104682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Xi J; Zhao W; Yuan JE; Kim J; Si X; Xu X, Detecting lung diseases from exhaled aerosols: Non-invasive lung diagnosis using fractal analysis and svm classification. PloS one 2015, 10 (9), e0139511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhu J; Bean HD; Wargo MJ; Leclair LW; Hill JE, Detecting bacterial lung infections: in vivo evaluation of in vitro volatile fingerprints. Journal of breath research 2013, 7 (1), 016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bean HD; Zhu J; Sengle JC; Hill JE, Identifying methicillin-resistant Staphylococcus aureus (MRSA) lung infections in mice via breath analysis using secondary electrospray ionization-mass spectrometry (SESI-MS). Journal of breath research 2014, 8 (4), 041001–041001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Giannoukos S; Agapiou A; Taylor S, Advances in chemical sensing technologies for VOCs in breath for security/threat assessment, illicit drug detection, and human trafficking activity. Journal of breath research 2017. [DOI] [PubMed] [Google Scholar]
- 255.Agapiou A; Mikedi K; Karma S; Giotaki Z; Kolostoumbis D; Papageorgiou C; Zorba E; Spiliopoulou C; Amann A; Statheropoulos M, Physiology and biochemistry of human subjects during entrapment. Journal of breath research 2013, 7 (1), 016004. [DOI] [PubMed] [Google Scholar]
- 256.Giannoukos S; Brkic B; Taylor S; France N, Monitoring of human chemical signatures using membrane inlet mass spectrometry. Analytical chemistry 2013, 86 (2), 1106–1114. [DOI] [PubMed] [Google Scholar]
- 257.Giannoukos S; Brkić B; Taylor S; France N, Membrane inlet mass spectrometry for homeland security and forensic applications. Journal of the American Society for Mass Spectrometry 2015, 26 (2), 231–239. [DOI] [PubMed] [Google Scholar]
- 258.Wong CL; Dinish U; Schmidt MS; Olivo M, Non-labeling multiplex surface enhanced Raman scattering (SERS) detection of volatile organic compounds (VOCs). Analytica chimica acta 2014, 844, 54–60. [DOI] [PubMed] [Google Scholar]
- 259.Ljungblad J; Hok B; Ekstrom M In Critical performance of a new breath alcohol analyzer for screening applications, Intelligent Sensors, Sensor Networks and Information Processing (ISSNIP), 2014 IEEE Ninth International Conference on, IEEE: 2014; pp 1–4. [Google Scholar]
- 260.de Lacy Costello B; Amann A; Al-Kateb H; Flynn C; Filipiak W; Khalid T; Osborne D; Ratcliffe NM, A review of the volatiles from the healthy human body. Journal of breath research 2014, 8 (1), 014001. [DOI] [PubMed] [Google Scholar]
- 261.Amann A; de Lacy Costello B; Miekisch W; Schubert J; Buszewski B; Pleil J; Ratcliffe N; Risby T, The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. Journal of breath research 2014, 8 (3), 034001. [DOI] [PubMed] [Google Scholar]
- 262.Angrish MM; Madden MC; Pleil JD, Probe Molecule (PrM) approach in Adverse Outcome Pathway (AOP) based High-Throughput Screening (HTS): In vivo discovery for developing in vitro target methods. Chemical research in toxicology 2015, 28 (4), 551–559. [DOI] [PubMed] [Google Scholar]
- 263.Baranska A; Smolinska A; Boots A; Dallinga J; Van Schooten F, Dynamic collection and analysis of volatile organic compounds from the headspace of cell cultures. Journal of breath research 2015, 9 (4), 047102. [DOI] [PubMed] [Google Scholar]
- 264.Boots A; Smolinska A; van Berkel J; Fijten R; Stobberingh E; Boumans M; Moonen E; Wouters E; Dallinga J; Van Schooten F, Identification of microorganisms based on headspace analysis of volatile organic compounds by gas chromatography–mass spectrometry. Journal of breath research 2014, 8 (2), 027106. [DOI] [PubMed] [Google Scholar]
- 265.Baranska A; Tigchelaar E; Smolinska A; Dallinga JW; Moonen EJ; Dekens JA; Wijmenga C; Zhernakova A; van Schooten FJ, Profile of volatile organic compounds in exhaled breath changes as a result of gluten-free diet. Journal of breath research 2013, 7 (3), 037104. [DOI] [PubMed] [Google Scholar]
- 266.Kistler M; Szymczak W; Fedrigo M; Fiamoncini J; Höllriegl V; Hoeschen C; Klingenspor M; de Angelis MH; Rozman J, Effects of diet-matrix on volatile organic compounds in breath in diet- induced obese mice. Journal of breath research 2014, 8 (1), 016004. [DOI] [PubMed] [Google Scholar]
- 267.Amann A; Mochalski P; Ruzsanyi V; Broza YY; Haick H, Assessment of the exhalation kinetics of volatile cancer biomarkers based on their physicochemical properties. Journal of breath research 2014, 8 (1), 016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Schivo M; Aksenov AA; Linderholm AL; McCartney MM; Simmons J; Harper RW; Davis CE, Volatile emanations from in vitro airway cells infected with human rhinovirus. Journal of breath research 2014, 8 (3), 037110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Filipiak W; Beer R; Sponring A; Filipiak A; Ager C; Schiefecker A; Lanthaler S; Helbok R; Nagl M; Troppmair J, Breath analysis for in vivo detection of pathogens related to ventilator- associated pneumonia in intensive care patients: a prospective pilot study. Journal of breath research 2015, 9 (1), 016004. [DOI] [PubMed] [Google Scholar]
- 270.Amann A; Miekisch W; Schubert J; Buszewski B; Ligor T; Jezierski T; Pleil J; Risby T, Analysis of exhaled breath for disease detection. Annual Review of Analytical Chemistry 2014, 7, 455– 482. [DOI] [PubMed] [Google Scholar]
- 271.Ghimenti S; Di Francesco F; Onor M; Stiegel M; Trivella M; Comite C; Catania N; Fuoco R; Pleil J, Post-operative elimination of sevoflurane anesthetic and hexafluoroisopropanol metabolite in exhaled breath: pharmacokinetic models for assessing liver function. Journal of breath research 2013, 7 (3), 036001. [DOI] [PubMed] [Google Scholar]
- 272.Hackner K; Pleil J, Canine olfaction as an alternative to analytical instruments for disease diagnosis: understanding ‘dog personality’to achieve reproducible results. Journal of Breath Research 2017, 11 (1), 012001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hackner K; Errhalt P; Mueller MR; Speiser M; Marzluf BA; Schulheim A; Schenk P; Bilek J; Doll T, Canine scent detection for the diagnosis of lung cancer in a screening-like situation. Journal of Breath Research 2016, 10 (4), 046003. [DOI] [PubMed] [Google Scholar]
- 274.Jezierski T; Walczak M; Ligor T; Rudnicka J; Buszewski B, Study of the art: canine olfaction used for cancer detection on the basis of breath odour. Perspectives and limitations. Journal of breath research 2015, 9 (2), 027001. [DOI] [PubMed] [Google Scholar]
- 275.Ehmann R; Boedeker E; Friedrich U; Sagert J; Dippon J; Friedel G; Walles T, Canine scent detection in the diagnosis of lung cancer: revisiting a puzzling phenomenon. European respiratory journal 2012, 39 (3), 669–676. [DOI] [PubMed] [Google Scholar]
- 276.Pleil J; Giese R, Integrating exhaled breath diagnostics by disease-sniffing dogs with instrumental laboratory analysis. Journal of Breath Research 2017, 11 (3), 032001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Pirrone F; Albertini M, Olfactory detection of cancer by trained sniffer dogs: A systematic review of the literature. Journal of Veterinary Behavior: Clinical Applications and Research 2017, 19 (Supplement C), 105–117. [Google Scholar]
- 278.Wang T; Pysanenko A; Dryahina K; Španěl P; Smith D, Analysis of breath, exhaled via the mouth and nose, and the air in the oral cavity. Journal of breath research 2008, 2 (3), 037013. [DOI] [PubMed] [Google Scholar]
- 279.Amann A; Smith D, Breath Analysis for Clinical Diagnosis and Therapeutic Monitoring:(With CD-ROM) World Scientific: 2005. [Google Scholar]
- 280.Krantz C; Janson C; Borres MP; Nordvall L; Alving K; Malinovschi A, Nasal nitric oxide is associated with exhaled NO, bronchial responsiveness and poor asthma control. Journal of breath research 2014, 8 (2), 026002. [DOI] [PubMed] [Google Scholar]
- 281.Huh D; Matthews BD; Mammoto A; Montoya-Zavala M; Hsin HY; Ingber DE, Reconstituting organ-level lung functions on a chip. Science 2010, 328 (5986), 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Esch EW; Bahinski A; Huh D, Organs-on-chips at the frontiers of drug discovery. Nature reviews Drug discovery 2015, 14 (4), 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Benam KH; Villenave R; Lucchesi C; Varone A; Hubeau C; Lee H-H; Alves SE; Salmon M; Ferrante TC; Weaver JC, Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nature methods 2016, 13 (2), 151–157. [DOI] [PubMed] [Google Scholar]
- 284.Chakir J; Pagé N; Hamid Q; Laviolette M; Boulet LP; Rouabhia M, Bronchial mucosa produced by tissue engineering: a new tool to study cellular interactions in asthma. Journal of allergy and clinical immunology 2001, 107 (1), 36–40. [DOI] [PubMed] [Google Scholar]
- 285.Xu Z; Gao Y; Hao Y; Li E; Wang Y; Zhang J; Wang W; Gao Z; Wang Q, Application of a microfluidic chip-based 3D co-culture to test drug sensitivity for individualized treatment of lung cancer. Biomaterials 2013, 34 (16), 4109–4117. [DOI] [PubMed] [Google Scholar]
- 286.Williams J; Pleil J, Crowd-based breath analysis: assessing behavior, activity, exposures, and emotional response of people in groups. Journal of breath research 2016, 10 (3), 032001. [DOI] [PubMed] [Google Scholar]


