Abstract
Objective
Mild forms of HIV-associated neurocognitive disorder (HAND) remain prevalent in the combination anti-retroviral therapy (cART) era. This study’s objective was to identify neuropsychological subgroups within the Multicenter AIDS Cohort Study (MACS) based on the participant-based latent structure of cognitive function and to identify factors associated with subgroups.
Design
The MACS is a four-site longitudinal study of the natural and treated history of HIV disease among gay and bisexual men.
Methods
Using neuropsychological domain scores we used a cluster variable selection algorithm to identify the optimal subset of domains with cluster information. Latent profile analysis was applied using scores from identified domains. Exploratory and post-hoc analyses were conducted to identify factors associated with cluster membership and the drivers of the observed associations.
Results
Cluster variable selection identified all domains as containing cluster information except for Working Memory. A three-profile solution produced the best fit for the data. Profile 1 performed below average on all domains, Profile 2 performed average on executive functioning, motor, and speed and below average on learning and memory, Profile 3 performed at or above average across all domains. Several demographic, cognitive, and social factors were associated with profile membership; these associations were driven by differences between Profile 1 and the other profiles.
Conclusions
There is an identifiable pattern of neuropsychological performance among MACS members determined by all domains except Working Memory. Neither HIV nor HIV-related biomarkers were related with cluster membership, consistent with other findings that cognitive performance patterns do not map directly onto HIV serostatus.
Keywords: HIV, model-based clustering, latent profile analysis, epidemiology, cognition
Introduction
Since early in the HIV epidemic, it has been clear that HIV infection affects the nervous system[1]. Immediately following infection, an aseptic meningitis can develop which can be symptomatic, and which usually clears without treatment in 4 to 6 weeks[2, 3]. With persistent infection, particularly in the absence of effective suppression of viral replication, neurological and cognitive symptomatology can manifest. The cluster of signs and symptoms fall under the rubric of HIV-associated neurocognitive disorder (HAND)[4, 5], and in the absence of effective treatment the condition may progress to an HIV-associated dementia which can result in death within six months[6]. While the use of combination antiretroviral therapies (cART) has reduced the prevalence of HAD, milder forms of HAND persist[6–9]. Indeed, some form of HAND is still present in approximately 40% of HIV-infected individuals[10]. Risk factors for HAND, both host- and virus-based, have been identified and include age, low educational attainment, presence of depression, hepatitis C virus (HCV) co-infection, AIDS, intravenous drug use, alcohol abuse, and the presence of other severe medical comorbidities, among others[1, 4, 11].
In the early days of the epidemic, the neurological and neuropsychological abnormalities resulted in a relatively coherent cluster of signs and symptoms such that the milder form of dysfunction was referred to as Minor Cognitive/Motor Disorder[12, 13]. Patients became slow, weak, and the cognitive deficits appeared similar to those found in “subcortical” dementia syndromes [13, 14]. More recently, however, even with access to large pools of data, it has been more difficult to identify an HIV-related phenotype of cognitive dysfunction[15–18]. This is important because patterns of impairment should, at least to some extent, reflect the central nervous system dysfunction that is the underlying cause of the cognitive impairment. Further, understanding the cognitive/behavioral phenotype and factors that influence risk is critical for developing rational pharmacological and nonpharmacological prevention and treatment/management strategies. This investigation analyzed patterns of cognitive function among a large group of gay/bisexual men in order to determine how these men cluster in patterns of cognitive functions and the factors associated with cluster membership.
The Multicenter AIDS Cohort Study (MACS) is a four-center study of the natural and treated history of HIV infection among gay/bisexual men. The MACS has tracked cognitive test performance for over 30 years in a subcohort, including participants infected with HIV and seronegative participants at risk of infection[19]. Participants in this Neuropsychological (NP) substudy are regularly assessed with a battery tests measuring performance in each of six cognitive domains.
We chose to use model-based clustering because it is an increasingly popular approach in diverse areas, including epidemiology[20] and clinical psychology[21]. Unlike more traditional clustering techniques, model-based clustering approaches the choice of the best clustering solution as a model choice problem that can be solved with goodness-of-fit techniques. While previous analyses have used cluster analyses to identify patterns in neuropsychological data among HIV/AIDS populations[15–17], to the best of our knowledge this is the first time it has been done with a model-based approach. The aim of this study is to identify neuropsychological phenotypes via model-based clustering of MACS participants based on cognitive domain scores at the first neuropsychological classification and to identify factors associated with identified phenotypes.
Methods
Participants
The MACS enrolled a total of 6972 men, irrespective of serostatus, from sites in Baltimore, Washington D.C., Chicago, Los Angeles, and Pittsburgh at three separate time points: 4954 men enrolled in 1984-85, 668 men enrolled in 1987-91, and 1350 men enrolled in 2001-03[22, 23]. MACS participants return every 6 months for an interview, physical examination, and collection of blood for laboratory testing. The interview covers physical health, medical treatments, and sexual and substance use disorders. A more detailed description of MACS study enrollment has been previously reported[19, 24]. This study uses data from the NP substudy of the MACS that were collected on or before September 30, 2014.
Neuropsychological Evaluation
The MACS NP evaluation includes tests from multiple cognitive domains related to the classification of HAND (Supplementary Table 1)[5, 24–27]: Executive Functioning (Trails B, Stroop Interference), Speed of Information Processing (Symbol Digit Substitution Task, Stroop Color Naming), Attention and Working Memory (2 indices of Choice Reaction Time), Learning (Rey Auditory Verbal Learning Test Sum of Trials 1-5, Rey Complex Figure Immediate Recall), Memory (Rey Auditory Verbal Learning Test Delayed Recall, Rey Complex Figure Delayed Recall), and Motor Speed/Coordination (Grooved Pegboard)[19]. Data from HIV seronegative participants were used to create statistical models to derive T-scores for each participant that are adjusted for age, years of education, ethnicity (white or non-white), and number of times the test has been administered. For each cognitive domain, a summary T-score was calculated by averaging all-available T-scores for that domain or, in the case of the Motor domain, using the lowest Grooved-Pegboard score[28]. Individuals who completed tests in at least four of the six domains were classified as cognitively normal, mildly impaired, or severely impaired according to the Antinori criteria (Supplementary Table 2) [5]. Briefly, an individual was classified as Normal if one or fewer domains had T-scores 1 SD or more below the mean; Mildly Impaired if two or more domains had T-scores 1 SD or more below the mean and criteria for Severely Impaired were not fulfilled; and Severely Impaired if two or more domains had T-scores 2 SDs or more below the mean or one domain had a T-score of 2.5 SDs or more below the mean.
To be included in this study, participants needed to have a domain-specific T-score in each of the six domains at the time of their first cognitive classification. Further, men with extremely high or low scores (T-score less than 10 or greater than 90) on the motor and speed domains were truncated at 10 or 90. Of the 6972 men in the MACS, 4318 have participated in the NP study and completed enough testing to be cognitively classified. Of these, 2904 met the inclusion criteria. The differences between men who did and did not meet these criteria are summarized in Table 1. Briefly, the men included in these analyses are, on average, older, less likely to identify as Caucasian, have fewer years of education and lower estimated IQ, less likely to belong to the first enrollment cohort, more likely to use drugs, and less likely to be classified as cognitively normal. Among those excluded from the analysis due to missing domain scores, the most commonly missing domain score was Working Memory followed by Executive Functioning, Memory, Learning, Motor, and Speed respectively. Men from the first enrollment cohort were more likely to be excluded in part because of the Reaction Time measures that contributed to the Working Memory domain and the Stroop Interference Test that contributed to the Executive Functioning and Speed of Information Processing domains that were not part of the original test battery.
Table 1.
Comparison of characteristics of excluded v. included participants
| Status | Statistic | ||
|---|---|---|---|
| Excluded | Included | χ2 or F (p value) | |
| N= | 1414 | 2904 | |
| Agea | 38.23 (8.8) | 39.70 (9.8) | 22.81(< 0.001) |
| Educationa | 15.94 (6.1) | 15.19 (4.2) | 20.85 (<0.001) |
| Raceb | 79 (1120) | 69 (2009) | 47.42 (<0.001) |
| HIV Infectedb | 58 (823) | 53 (1531) | 11.31 (<0.001) |
| Cohort 1b | 76 (1069) | 61 (1770) | 89.99 (<0.001) |
| Smokinga | 12.05 (17.4) | 12.40 (17.8) | 0.34 (0.56) |
| Drug/Alcohol Use | |||
| Marijuanab | 43 (593) | 42 (1199) | 0.28 (0.59) |
| Cocaineb | 16 (222) | 19 (534) | 4.24 (0.04) |
| Uppersb | 6 (82) | 9 (241) | 9.35 (0.002) |
| Opiatesb | 1 (13) | 3 (83) | 14.15 (0.0002) |
| Intravenous Drug Useb | 2.2 (29) | 4.7 (136) | 15.56 (<0.001) |
| Estimated IQa | 106.04 (11.7) | 104.44 (13.2) | 11.59 (<0.001) |
| CES-Da | 11.11 (10.5) | 11.40 (10.6) | 0.70 (0.41) |
| CD4+ Counta | 494.07 (320) | 506.33 (272) | 3.31 (0.069) |
| Log (Viral Load)a | 3.43 (1.55) | 3.16 (1.5) | 12.89 (<0.001) |
| NP T-Scores | |||
| Motora | 46.04 (10.6) | 46.88 (10.5) | 6.17 (0.013) |
| Learninga | 49.01 (8.9) | 49.69 (9.3) | 4.99 (0.026) |
| Memorya | 49.50 (9.5) | 49.88 (9.5) | 1.46 (0.27) |
| Speeda | 50.74 (9.6) | 49.71 (8.8) | 12.35 (<0.001) |
| Executivea | 49.10 (9.9) | 49.95 (9.4) | 5.68 (0.017) |
| Normal Cognitionb | 80 (1134) | 76 (2202) | 14.92 (<0.001) |
Continuous variables: data provided as mean (SD), and the test statistic is provided F test statistic (p-value)
Categorical variables: data provided as percent (N), and the test statistic is provided as X2 test statistic (p-value)
Procedure
Prior to implementing the latent profile analysis, we ran a variable selection procedure on the six domain scores in order to identify those variables that were most likely to make a significant contribution to the model. Including unnecessary variables can reduce model fit and/or make it difficult to identify a reasonable clustering solution. We utilized a “greedy search” algorithm[29] that screens the variables to identify those that most improve the clustering solution as measured by the Bayesian Information Criterion (BIC)[20, 30] . In a stepwise manner, it determines which of the clustering variables can be dropped from the model, and stops when there is no change in the clustering solution.
After the variable selection step we implemented a latent profile analysis (LPA) using the domain-specific T-scores from the time of participants’ first cognitive classification. The use of model-based clustering approaches is driven by the idea that the observed data from a population actually arose from two or more subpopulations. Finite mixture models estimate the overall population as a weighted combination of these subpopulations. In a Gaussian finite mixture model, we assume that each subpopulation is normally distributed and the overall population is a combination of these distributions. Bayes’ rule can be used to identify the subpopulations within the overall population by assigning each observation to the cluster for which it has a maximum posterior probability. This is done using the Expectation-Maximization algorithm to maximize the likelihood function. The BIC is used to select the appropriate number of clusters such that the model that corresponds to the largest BIC value has the best fit[20, 30].
Following the latent profile analysis, chi-square tests for independence and analyses of variance (ANOVA) were conducted to examine differences in demographic, HIV/AIDS, behavioral, educational, and emotional factors of interest across cluster. For those factors that were found to be significantly associated with profile membership, post-hoc analyses were conducted to determine which pairwise difference (s) were driving the effects.
Results
The variable selection procedure[29] identified five (out of six) domain T scores that we used in subsequent analyses. Only the Working Memory domain score was excluded because it did not contribute significantly to the clustering solution.
The Mclust package[31, 32] in R[33] was used to conduct the latent profile analysis. The five domain-specific T-scores from the first NP cognitive classification visit were entered. The best fitting model was a three-cluster solution with variable distribution, variable volume, equal shape and orientation, a VVE solution, and the solution had a BIC value of −100650.2. The next best-solution was a four-cluster VVE solution with a BIC value of −100700.1. The difference between these two models in BIC was 49.93, which is positive support for the better model[21] . All other solutions corresponded to smaller BIC solutions than the four-cluster VVE solution.
The best fitting model produced clusters with 578 (19.9%), 1012 (34.8%), and 1314 (45.2%) participants, respectively. The distribution of domain-specific T-scores is shown in Table 2 and Figure 1.
Table 2.
Characteristics of Individual Profiles
| Profile | Statistics | ||||
|---|---|---|---|---|---|
| One | Two | Three | χ2 or F (p value) | Effect sizes 1 vs. 3 Cohen’s d or h | |
| N= | 578 | 1012 | 1314 | ||
| Age (years)a | 41.4 (11) | 40.1 (9.4) | 38.7 (9.7) | 16.8(< 0.001) | 0.27 |
| Educationa | 14.8 (5.9) | 15.2 (3.8) | 15.37 (3.6) | 3.4 (0.03) | 0.13 |
| Race (white)b | 56 (326) | 72 (724) | 73 (959) | 55.8 (< 0.001) | 0.22 |
| HIV Infectedb | 53 (307) | 52 (530) | 53 (694) | 0.09 (0.96) | 0 |
| Cohort 1b | 49 (283) | 64 (648) | 64 (839) | 43.6 (< 0.001) | 0.18 |
| Smokinga | 13.58 (19.28) | 13.59 (18.17) | 10.96 (16.69) | 15.6 (< 0.001) | 0.15 |
| Alcohol Use (4+ Drinks/Week)b | 33 (185) | 40 (394) | 42 (539) | 21.0 (0.002) | 0.10 |
| Marijuanab | 37 (211) | 44 (439) | 42 (549) | 7.09 (0.029) | −.057 |
| Cocaineb | 18 (105) | 20 (199) | 18 (230) | 1.82 (0.40) | 0.007 |
| Uppersb | 7.8 (43) | 8.7 (83) | 9.3 (115) | 1.08 (0.58) | −.015 |
| Opiatesb | 3.5 (19) | 3.6 (33) | 2.7 (31) | 1.82 (0.40) | 0.008 |
| Intravenous Drug Useb | 6 (36) | 5 (47) | 4 (53) | 4.4 (0.11) | 0.02 |
| Estimated IQa | 98 (16) | 104 (13) | 108 (11) | 160.4 (< 0.001) | 0.43 |
| CES-Da | 13 (12) | 11 (10) | 11 (10) | 11 (< 0.001) | 0.23 |
| CD4+ Count*a | 523.69 (286.86) | 520.42 (279.12) | 487.82 (260.57) | 5.77 (0.06) | 0.13 |
| Nadir CD4+ Count*a | 299.86 (232.8) | 247.89 (206.3) | 240.31 (208.8) | 8.72 (0.00017) | 0.28 |
| Log (Viral Load)*a | 3.05 (1.49) | 3.19 (1.52) | 3.19 (1.50) | 0.66 (0.52) | −.09 |
| NP T-Scores | |||||
| Motora | 36.1 (12.1) | 49.4 | 49.7 (7.5) | 597 (<0.0001) | 1.5 |
| Learninga | 44.6 (9.3) | 43.8 (7.2) | 56.5(5.6) | 1116 (<0.0001) | 1.7 |
| Memorya | 44.7 (9.1) | 43.6 (7.3) | 57 (5.4) | 1283 (<0.0001) | 1.8 |
| Speeda | 45.9 (12) | 49.5 (7) | 51.5 (7.7) | 90 (<0.0001) | 0.61 |
| Executivea | 42.9 (12) | 50.2 (7.5) | 52.8 (7.8) | 263 (<0.0001) | 1.1 |
| Normal Cognitionb | 37 (213) | 74 (744) | 95(1245) | 864.7 (< 0.001) | 0.87 |
Continuous variables: data provided as mean (SD, test statistic provided as F (p), effect size reported as Cohen’s d.
Categorical variables: data provided as percent (N), test statistic provided as X2 (p), effect size reported as Cohen’s h
Restricted to HIV+ participants only
Figure 1.
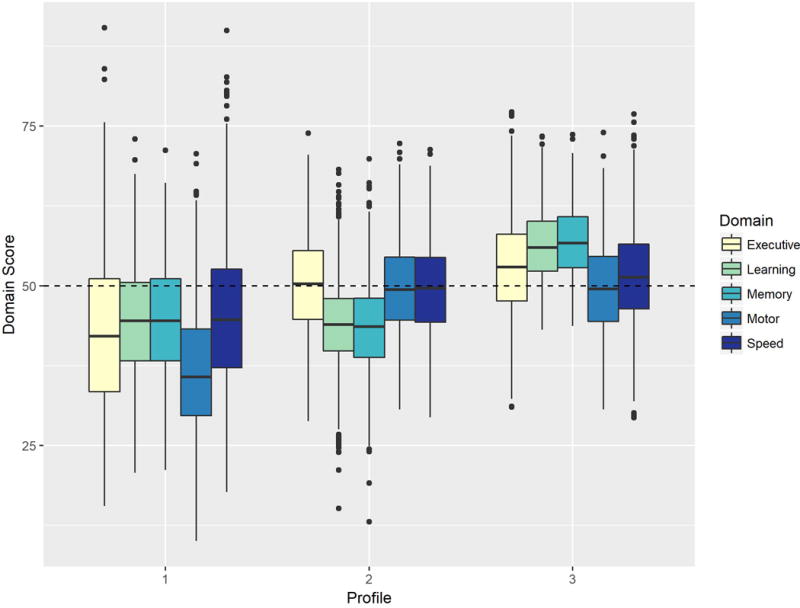
The pattern of domain score distributions for each of the three latent profiles. The horizontal line within each box indicates the median score for the domain, while the hinges of each box respectively indicate the scores corresponding to the 25th and 75th percentiles. The range of values within the whiskers represent scores that fall within 1.5*IQR of the nearest hinge.
In a normative sample, the cognitive domain T-score distribution has a mean of 50 with a standard deviation of 10. Scores of 45-55 are considered normal; 40-44 are borderline; 35-39 are Mildly Impaired; 30-34 are Mild/Moderately Impaired; 25-29 are Moderately Impaired; 20-24 are Moderately/Severely Impaired; a score less than 20 is Severely Impaired.
Participants in Profile 1 performed below average on all domains except for Motor skills where the average score was in the impaired range. For Profile 2, performance was within the normal range (at or slightly below a T score of 50) for all of the individual domain scores except for Memory and Learning, which were below the normal range with average scores of 43.6 and 43.8 respectively. By contrast, Profile 3 showed at or above average performance in all domains.
The participants classified in Profile 1 performed more poorly on measures of Motor speed than their other four domain scores, and had significantly lower motor speed than participants classified in Profiles 2 or 3. This group was the oldest, reported more symptoms of depression, had the lowest estimated IQ (based on the Shipley Institute of Living Scale Vocabulary Subtest) and had completed the fewest number of years of education (note, however, that the average was more than two years of college). Fifty-one percent of the participants classified in Profile 1 were drawn from the 2001/2003 enrollment cohort which is the highest of the three profiles. In addition, only 56% of the individuals in this profile self-identified as Caucasian, which is significantly lower than the proportions in the other two profiles. Approximately half of the participants in Profile 1 were HIV-infected. Only 37% of the individuals are classified as cognitively normal, and 20% were classified as severely impaired (both proportions differ significantly from those seen in the other two profiles (for details see Table 2)).
For the individuals classified in Profile 2, their performance on measures of Learning and of Memory were lower than those for the other three domains, and were significantly lower than the Learning and Memory domain scores seen in Profile 3 (but not Profile 1). On average, their age was midway between that of Profiles 1 and 3, as well as the number of items endorsed on the CES-D. Their estimated IQ and years of education were also midway between the other two profiles. 74% of the individuals in this group were classified as cognitively normal, and only 2% were classified as severely impaired. Like Profile 1 approximately 50% of individuals were HIV-infected. Only 36% of these participants were drawn from the 2001/2003 cohort, and 72% of the participants classified themselves as Caucasian.
Finally, Profile 3 represents what might be the “healthiest” of the three profiles. Mean performance on all five domains was at or above normal, with Memory and Learning being one standard deviation unit higher than that seen in Profiles 1 and 2. This group of individuals was younger, reported fewer symptoms of depression, had the highest estimated IQ, and the greatest number of years of education. Ninety-five percent of the individuals in this profile were classified as cognitively normal, and none were classified as severely impaired. Fifty-three percent of the sample were HIV-infected like Profiles 1 and 2, 36% of the sample were drawn from Cohort Three, and 73% self-identified as Caucasian.
Among the HIV-infected individuals only, there was a significant difference in nadir CD4 + cell counts, such that the individuals in Profile 3, the highest performing profile, had the lowest levels. In multinomial logistic regression models of profile membership, however, nadir CD4 no longer significantly contributed to the model after adjustment for recruitment cohort in the main analysis (Likelihood Ratio Test: X2=1.824, p=0.402) or analyses restricted to HIV-infected invdividuals (Likelihood Ratio Test: X2=4.891, p=0.087). The mean log10 viral load ranged from 3.05 to 3.19, with no significant difference among the three profiles. At first cognitive classification, 61% of the individuals in Profile 1 had used cART, whereas 70% of the individuals in Profiles 2 and 3 had used these medication regimens. The proportion of individuals who met criteria for AIDS ranged from 4 to 7%, and was not different across profiles.
Post-hoc pairwise comparisons adjusted for multiple comparisons between the three profiles reveal that the significance of the associations between covariates and profile membership described above are generally driven by the differences between Profile 1 and the other two profiles (Table 3). Profile 1 was significantly different from Profiles 2 and 3 with respect to age, CES-D, IQ, HAART use, enrollment cohort membership, race, and cognitive classification. Profile 1 was different from Profile 3 but not Profile 2 in terms of years of education, pack-years of smoking, and alcohol use. Profile 2 was significantly different from Profile 3 with respect to age, IQ, smoking, and cognitive classification. None of the pairwise comparisons between profiles were significant with respect to HIV status, CD4 count, or viral load.
Table 3.
Post-hoc Pairwise Analyses
| Variable | Profile 1 v. 2 | Profile 1 v. 3 | Profile 2 v. 3 |
|---|---|---|---|
| Agea | 2.5726 (0.0307) | 5.2887 (<0.0001) | 3.4562 (0.0017) |
| CES-Da | 3.4477 (0.0017) | 4.3205 (<0.0001) | 0.9725 (0.9923) |
| Estimated IQa | −6.4697 (<0.0001) | −11.475 (<0.0001) | −6.7716 (<0.0001) |
| Education Yearsa | −1.3166 (0.5651) | −2.0587 (0.1197) | −1.2147 (0.6739) |
| Smoking Pack Yearsa | −0.0121 (>0.999) | 2.7972 (0.0158) | 3.5497 (0.0012) |
| CD4 Counta | 0.1588 (>0.999) | 1.8567 (0.1918) | 2.0644 (0.1176) |
| Nadir CD4 Counta | 3.2354 (0.0039) | 3.8417 (0.0004) | 0.6311 (>0.999) |
| Log Viral Loada | −1.0622 (0.8660) | −1.1421 (>0.7620) | −0.0185 (>0.999) |
| HAARTb | 7.344 (0.020) | 9.2661 (0.0070) | 0.0238 (1.000) |
| Cohortb | 33.807 (<0.0001) | 36.257 (<0.0001) | 0.0022 (>0.999) |
| HIV+b | 0.054 (>0.999) | 0.0049 (>0.999) | 0.0292 (>0.999) |
| Race (White)b | 36.927 (<0.0001) | 49.897 (<0.0001) | 0.5423 (>0.999) |
| IDUb | 1.5931 (0.6207) | 3.9063 (0.1443) | 0.3850 (>0.999) |
| Alcoholb | 7.8802 (0.1457) | 19.983 (0.0005) | 5.0719 (0.4998) |
| Potb | 6.6395 (0.0299) | 4.1464 (0.1252) | 0.5613 (>0.999) |
| Cocaineb | 0.3946 (>0.999) | 0.1070 (>0.999) | 1.6587 (0.5933) |
| Uppersb | 0.2658 (>0.999) | 0.8882 (>0.999) | 0.1643 (>0.999) |
| Neuro NBAb | 264.8 (<0.0001) | 779.4 (<0.0001) | 211.24 (<0.0001) |
Continuous variables: data provided as pairwise t-test statistic (p-value) corrected for multiple testing using Bonferroni adjustment
Categorical variables: data provided as pairwise X2 test statistic (p-value) corrected for multiple testing using Bonferroni adjustment
Discussion
There are three main findings from these analyses. First, there are three major groups of participants which cluster together based on performance on standard neuropsychological measures of Executive Functioning, Speed of Information Processing, Learning, Memory, and Motor speed/coordination. Second, a variety of factors differed among the three profiles, including age, education, race, enrollment cohort, and mood-related symptoms. Third, there were no differences among the clusters in terms of rate of HIV-infection, rate of AIDS diagnosis, or markers of immunocompetence or viral replication.
The analyses described here support three distinct latent profiles of neuropsychological test performance, which were determined based on cognitive domain scores in Learning, Memory, Speed, Motor Skills, and Executive Functioning at the time of first cognitive classification. The results indicate that approximately 20% of participants identify most closely with Profile 1, 35% identify most closely with Profile 2, and 45% identify most closely with Profile 3. Profile 1 is characterized by below average or impaired functioning across domains, Profile 2 is characterized by average to slightly below average functioning across domains, and Profile 3 is characterized by average or above average functioning across domains.
One of the key strengths of this study is that it was conducted using model-based clustering rather than more traditional hierarchical or k-means based clustering. Model-based clustering is advantageous in that it allows the data to determine the optimal number of clusters, and therefore is less subject to a priori assumptions about the latent structure. Additionally, model-based clustering assigns membership weights for each profile rather than strictly assigning each subject to a specific profile, which is more flexible and allows participants to be ideal members of a single profile or mixed members of multiple profiles.
Another strength of this study is the utilization of data from the MACS. The MACS is a large, longitudinal cohort study with rich data that has enrolled multiple sub-cohorts over time. Because of these characteristics, we are able to differentiate effects of HIV-related factors from effects of demographic and other factors that have shifted among the HIV-infected population over the course of the HIV epidemic.
A key limitation of this study is that those who met inclusion criteria were significantly different than those who did not with respect to many factors, including age, race, years of education, illicit drug use, enrollment cohort, and neurocognitive test performance. The differences between the included versus excluded individuals are likely a reflection of the differences between the enrollment cohorts. Due to strong competing risks during the initial epidemic and the timing of the initiation of the NP substudy, fewer participants in the first enrollment cohort were able to participate in the NP substudy. As previously described[19], the first enrollment cohort differs greatly from the second enrollment cohort with respect to many of the same factors that differ between individuals included versus excluded from this study.
Another potential limitation of this study is that the participants are exclusively men who have sex with men and, as such, their primary risk factor for becoming HIV-infected is through unprotected sexual intercourse with other men. If either sex or the route of infection modifies the effect of HIV on neurocognitive performance, this would limit the generalizability of these results. As our cohorts consist solely of men who have sex with men, we are unable to explore the extent to which these results would differ in other at-risk populations.
In spite of attempts, we were unable to develop a model that accurately predicted profile membership. We believe this was largely due to a limitation of the data, as different variables began to be collected at different time points over the course of the MACS such that many variables are missing at the time of first neurocognitive classification for participants enrolled in the earlier sub-cohorts. For instance, although variables known to be related to cognitive performance, such as markers of cardiometabolic health, may have improved predictive performance of our model, we had insufficient data at the time of the relevant neurocognitive classification to consider these factors. This limitation reinforces the need to consistently collect information beyond the variables traditionally considered in NeuroHIV work, as these alone appear to be inadequate for predicting cognitive performance.
The analyses described here are not dissimilar to the results of the mixed membership trajectory model (MMTM) previously described by our group[34], which identified three canonical trajectories describing the probabilities of normal, mildly impaired, and severely impaired cognition over time in the MACS. Figure 2 displays the proportion of participants identifying with each MMTM trajectory in each LPA profile among the nearly 2700 participants included in both analyses. Approximately half of participants who most closely identify with the ‘unhealthy’ MMTM trajectory also most closely identify with LPA Profile 1, the profile with the lowest average cognitive domain scores. Similarly, participants who most closely identify with the ‘healthy’ or ‘premature aging’ MMTM trajectories are most likely to identify with LPA Profile 3 followed by LPA Profile 2, the highest and second highest performing LPA profiles respectively. The similarities between the two different methodologies suggests some degree of convergent validity.
Figure 2.
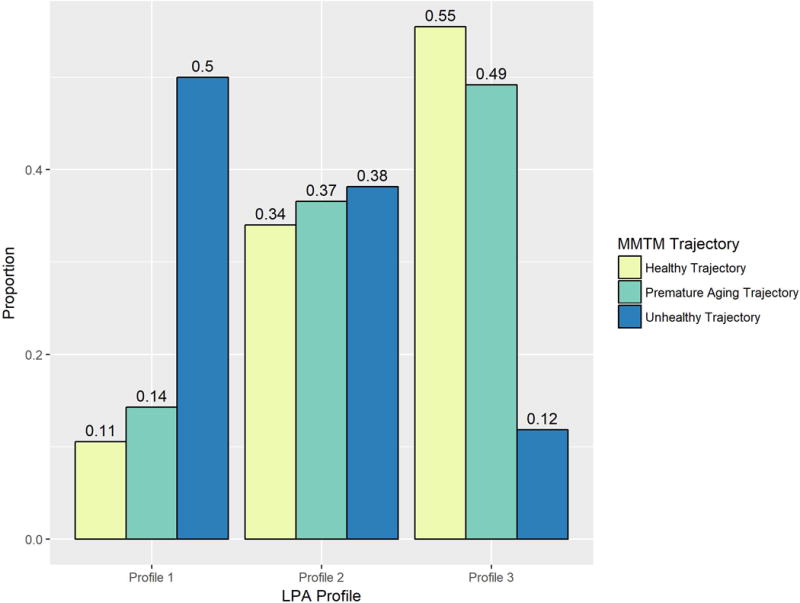
The proportion of individuals identifying most closely with each MMTM canonical trajectory among those who identify most closely with a given latent profile. The bars for each respective MMTM trajectory sum to 1.
Several cluster analyses using neuropsychological test performance have been conducted among other cohorts that included HIV-infected individuals [15–17]. These analyses all use different neuropsychological batteries and different cluster analysis techniques. As such, the different studies are not directly comparable, but overall, this body of literature generally supports the idea of a small number of latent neuropsychological performance profiles among people living with HIV. The analyses reported here are the first to indicate that such profile membership may be driven by demographic, mood, and lifestyle characteristics rather than factors related to HIV infection.
These data reinforce the importance for clinicians to consider all possible causes of cognitive impairment in their HIV-infected patients. While these results do not show that HIV has no impact on cognitive function, they add to the growing body of literature that shows that other factors, some related to HIV disease (or treatment) and others not, may be more important than the infection in terms of driving cognitive impairment. Self-reported mood state, use of cART, and predispositional risk factors should be considered in designing treatment plans.
Supplementary Material
Acknowledgments
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Jay Bream, Todd Brown, Adrian Dobs, Michelle Estrella, W. David Hardy, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), Sheila Badri, Dana Gabuzda, Frank J. Palella, Jr., Sudhir Penugonda, John P. Phair, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (PI), Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Lawrence A. Kingsley, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D’Souza (PI), Alison Abraham, Keri Althoff, Michael Collaco, Priya Duggal, Sabina Haberlen, Eithne Keelaghan, Heather McKay, Alvaro Muñoz, Derek Ng, Anne Rostich, Eric C. Seaberg, Sol Su, Pamela Surkan, Nicholas Wada. Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://aidscohortstudy.org/.
Source of funding: Additional support for the analysis of these data and preparation ofthe manuscript was provided by funds from the NIH to J.T.B. (AG034852 and MH098745).
Disclosure: E.N.M. is the author of the reaction time software used in this study (CalCAP) and has a financial interest in the software.
The members of the Neuropsychology Working Group include J.T.B., Pim Brouwers, Christopher Cox, Jenna Fahey, Rebecca Godfrey, K.G., Robin Huebner, A.J.L., E.M., Donna M. Martineck, Eric N. Miller, Ann Ragin, S.R., JoanaDarc Roe, Ned Sacktor, Janet Schollenberger, Eric Seaberg, and Matthew Wright.
NIH Funding: Funding for the MACS: U01-AI35042, U01-AI35039, U01-AI35040, U01-AI35041, UM1-AI35043, UL1-TR001079; Funding for these analyses: NIH to J.T.B. (AG034852 and MH098745).
Footnotes
Contributions of authors: S.A.M., conceptualization or design of the study, analysis and interpretation of data, drafting the manuscript; Y.C. conceptualization or design of the study, analysis and interpretation of data, revising the manuscript; L.K. acquisition of study data, analysis and interpretation of data, revising the manuscript; L.J. revising the manuscript; A.J.L. acquisition of study data, revising the manuscript; E.M. acquisition of study data, revising the manuscript; E.N.M. acquisition of study data, revising the manuscript; C.M. acquisition of study data, revising the manuscript; A.R. acquisition of study data, revising the manuscript; N.S. acquisition of study data, revising the manuscript; J.T.B. acquisition of study data, conceptualization or design of the study, analysis and interpretation of data, revising the manuscript.
Conflicts of interest: None declared.
References
- 1.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13(11):976–986. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michaels J, Sharer LR, Epstein LG. Human immunodeficiency virus type 1 (HIV-1) infection of the nervous system: a review. Immunodefic Rev. 1988;1(1):71–104. [PubMed] [Google Scholar]
- 3.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125(4):257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Letendre S. Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top Antivir Med. 2011;19(4):137–142. [PMC free article] [PubMed] [Google Scholar]
- 5.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liner KJ, 2nd, Ro MJ, Robertson KR. HIV, antiretroviral therapies, and the brain. Curr HIV/AIDS Rep. 2010;7(2):85–91. doi: 10.1007/s11904-010-0042-8. [DOI] [PubMed] [Google Scholar]
- 7.Al-Khindi T, Zakzanis KK, van Gorp WG. Does antiretroviral therapy improve HIV-associated cognitive impairment? A quantitative review of the literature. J Int Neuropsychol Soc. 2011;17(6):956–969. doi: 10.1017/S1355617711000968. [DOI] [PubMed] [Google Scholar]
- 8.Joska JA, Gouse H, Paul RH, Stein DJ, Flisher AJ. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol. 2010;16(2):101–114. doi: 10.3109/13550281003682513. [DOI] [PubMed] [Google Scholar]
- 9.Spudich S. HIV and neurocognitive dysfunction. Curr HIV/AIDS Rep. 2013;10(3):235–243. doi: 10.1007/s11904-013-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Ronchi D, Faranca I, Berardi D, Scudellari P, Borderi M, Manfredi R, et al. Risk factors for cognitive impairment in HIV-1-infected persons with different risk behaviors. Arch Neurol. 2002;59(5):812–818. doi: 10.1001/archneur.59.5.812. [DOI] [PubMed] [Google Scholar]
- 12.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19(2):152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4(9):543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 14.Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27(1):86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- 15.Dawes S, Suarez P, Casey CY, Cherner M, Marcotte TD, Letendre S, et al. Variable patterns of neuropsychological performance in HIV-1 infection. J Clin Exp Neuropsychol. 2008;30(6):613–626. doi: 10.1080/13803390701565225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fazeli PL, Crowe M, Ross LA, Wadley V, Ball K, Vance DE. Cognitive Functioning in Adults Aging with HIV: A Cross-Sectional Analysis of Cognitive Subtypes and Influential Factors. J Clin Res HIV AIDS Prev. 2014;1(4):155–169. doi: 10.14302/issn.2324-7339.jcrhap-13-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lojek E, Bornstein RA. The stability of neurocognitive patterns in HIV infected men: classification considerations. J Clin Exp Neuropsychol. 2005;27(6):665–682. doi: 10.1081/13803390490918426. [DOI] [PubMed] [Google Scholar]
- 18.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-associated neurocognitive disorder–pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12(4):234–248. doi: 10.1038/nrneurol.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker JT, Kingsley LA, Molsberry S, Reynolds S, Aronow A, Levine AJ, et al. Cohort Profile: Recruitment cohorts in the neuropsychological substudy of the Multicenter AIDS Cohort Study. Int J Epidemiol. 2015;44(5):1506–1516. doi: 10.1093/ije/dyu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynt A, Daepp MI. Diet-related chronic disease in the northeastern United States: a model-based clustering approach. Int J Health Geogr. 2015;14:25. doi: 10.1186/s12942-015-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suveg C, Jacob ML, Whitehead M, Jones A, Kingery JN. A model-based cluster analysis of social experiences in clinically anxious youth: links to emotional functioning. Anxiety Stress Coping. 2014;27(5):494–508. doi: 10.1080/10615806.2014.890712. [DOI] [PubMed] [Google Scholar]
- 22.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 23.Kingsley LA, Detels R, Kaslow R, Polk BF, Rinaldo CR, Jr, Chmiel J, et al. Risk factors for seroconversion to human immunodeficiency virus among male homosexuals. Results from the Multicenter AIDS Cohort Study Lancet. 1987;1(8529):345–349. doi: 10.1016/s0140-6736(87)91725-9. [DOI] [PubMed] [Google Scholar]
- 24.Miller EN, Selnes OA, McArthur JC, Satz P, Becker JT, Cohen BA, et al. Neuropsychological performance in HIV-1-infected homosexual men: The Multicenter AIDS Cohort Study (MACS) Neurology. 1990;40(2):197–203. doi: 10.1212/wnl.40.2.197. [DOI] [PubMed] [Google Scholar]
- 25.Miller EN, Satz P, Visscher B. Computerized and conventional neuropsychological assessment of HIV-1-infected homosexual men. Neurology. 1991;41(10):1608–1616. doi: 10.1212/wnl.41.10.1608. [DOI] [PubMed] [Google Scholar]
- 26.Selnes OA, Miller EN. Development of a screening battery for HIV-related cognitive impairment. In: Grant I, Martin A, editors. Neuropsychology of HIV infection. New York: Oxford University Press; 1994. pp. 176–190. [Google Scholar]
- 27.Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26(6):759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 28.Becker JT, Martinson JJ, Penugonda S, Kingsley L, Molsberry S, Reynolds S, et al. No association between Apoepsilon4 alleles, HIV infection, age, neuropsychological outcome, or death. J Neurovirol. 2015;21(1):24–31. doi: 10.1007/s13365-014-0290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scrucca L, Raftery A. clustvarsel: A Package Implementing Variable Selection for Model-based CLustering in R. Journal of Statistical Software. 2015 doi: 10.18637/jss.v084.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scrucca L, Fop M, Murphy TB, Raftery AE. mclust 5: Clustering, Classification and Density Estimation Using Gaussian Finite Mixture Models. R J. 2016;8(1):289–317. [PMC free article] [PubMed] [Google Scholar]
- 31.Fraley C, Raftery A, Murphy T, Scrucca L. mclust Version 4 for R: Normal Mixture Modeling for Model-Based Clustering, Classification, and Density Estimation Technical Report No. 597. Department of Statistics, University of Washington; 2012. [Google Scholar]
- 32.Fraley C, Raftery A. Model-based Clustering, Discriminant Analysis and Density Estimation. Journal of the American Statistical association. 2002;97(458):611–631. [Google Scholar]
- 33.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 34.Molsberry SA, Lecci F, Kingsley L, Junker B, Reynolds S, Goodkin K, et al. Mixed membership trajectory models of cognitive impairment in the multicenter AIDS cohort study. AIDS. 2015;29(6):713–721. doi: 10.1097/QAD.0000000000000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


