Abstract
Background
India has the highest number of HIV-infected adolescents in Asia, however, little is known about their treatment outcomes. We assessed rates and factors associated with loss to follow-up (LTFU) and mortality among Indian adolescents.
Methods
Analysis included adolescents (10–19 years) starting ART, between 2005 and 2014, at BJ Government Medical College, Pune, India. LTFU was defined as missing >3 monthly visits. Competing-risks method was used to calculate sub-distribution hazard ratios (SHR) of predictors for LTFU, with death as the competing risk. Cox proportional hazard models were used to identify predictors of mortality.
Results
Of 717 adolescents starting ART, 402 with complete data were included in the analysis. Of these, 61% were male, 80% were perinatally infected, median baseline CD4 was 174 cells/µl. LTFU and mortality rates were 4.4 and 4.9/100-person years, respectively. Cumulative LTFU incidence increased from 6% to 15% over six years. Age ≥15 years (adjusted SHR (aSHR) 2.44; 95% CI:1.18 – 5.02), was a risk factor for LTFU. Cumulative mortality increased from 9.5% to 17.9% over six years. WHO Stage III and IV (aHR: 2.26; 95% CI:1.14 – 4.48) and CD4 count/100 (aHR: 0.59; 95% CI: 0.43 – 0.83) were associated with mortality.
Conclusions
A third of adolescents were LTFU or died by follow-up year 6. Older age, was a risk factor for LTFU and advanced clinical disease for death. Strategies to strengthen retention counselling for older adolescents and closer clinical monitoring of all adolescents, must be considered.
Keywords: HIV/AIDS, adolescents, LTFU, mortality, India
Introduction
Globally, there were approximately 36.7 (34.0–39.8) million people living with HIV at the end of 2015.1 Of these, over 2.1 million were adolescents between the ages of 10–19 years. Adolescents account for about 6 per cent of all people living with HIV (PLHIV) and 15 per cent of new HIV infections. Since 2004, access to paediatric antiretroviral treatment (ART) has expanded worldwide, resulting in a substantial decline in mortality rates in HIV-infected children.2 Consequently, this has led to more HIV-infected children surviving to adolescence.3, 4
Outside sub-Saharan Africa, India has the highest number of adolescents living with HIV.5 According to UNAIDS figures, 126,000 adolescents in India are HIV- infected,6 translating to 60% of the adolescent HIV-burden in South Asia. Adolescence is a time of transformation and experimentation.7 This period is also marked by heightened self-consciousness and a need to conform to peer behaviour that contributes to increased vulnerability. Studies from Africa have shown that HIV-infected adolescents often disengage from HIV care, undermining conventional preventive and treatment strategies that rely on regular interactions with the health care system.7, 8 Furthermore, studies from Uganda showed that mortality rates among adolescents were similar to that of adults at 6.7%.9 However little data are available on the risk and factors associated with loss to follow-up (LTFU) and mortality in adolescents living with HIV from Asia. Understanding the reasons associated with LTFU and mortality in HIV-infected adolescents may provide opportunities to design specific interventions to improve retention in care and avert mortality. Using a large programmatic dataset of HIV-infected adolescents, this paper sought to determine rates and factors affecting LTFU and mortality in western India.
Methods
Study design and population
This study was a retrospective review of programmatic data routinely collected at the antiretroviral treatment (ART) centre of Byramjee Jeejeebhoy Government Medical College (BJGMC) and Sassoon General Hospitals (SGH), Pune, India. The BJGMC-SGH ART centre was established in 2004, and has over 36,000 registered HIV-infected patients (including HIV-infected children), with approximately 350 patients accessing care every day. For the purpose of this analysis, data of all HIV-infected adolescents (10–19 years) starting treatment at the ART centre between May 2005 and August 2014 were used. BJGMC and Johns Hopkins University ethics committees approved the project.
Data variables and definitions
Standard procedure for data collection under the Indian HIV program involves the use of a structured paper form, which is then transcribed into an electronic database. Data collected include demographic and clinical characteristics for each participant at registration and select clinical characteristics at subsequent visits. We abstracted key available variables obtained at registration, for all adolescents. These included age, gender, mode of HIV acquisition, date of ART start, CD4 counts, WHO category, place of residence, the latest ART regimen, treatment outcomes (LTFU, death) and information on whether they were transferred to another ART centre.
LTFU information was directly available from the database and no additional modifications were made to the definition. The National AIDS Control Organization (NACO), India’s HIV program, defines LTFU as missing more than three consecutive monthly visits.10 As most baseline characteristics had been measured only at registration, we included only those adolescents whose duration between registration at the ART clinic and ART start was not more than 6 months, to account for time varying covariates (Age, CD4 counts, WHO staging, Residence). Since CD4 counts under the Indian HIV program are done once in six months, we believe this to be an appropriate duration to be used, while also maintaining an effective compromise between sample size and potential unmeasured confounders. Furthermore, we included only those adolescents who had not received ART prior to registry at the ART centre and had at least one subsequent follow-up visit following treatment initiation. Adolescents who started treatment after May 31, 2014, were excluded to allow sufficient time for the participant to be LTFU, according to the definition. Tracing of all participants deemed LTFU or transferred to another centre, was attempted by making phone calls to numbers that participants had provided at registration.
Statistical Analyses
We used descriptive statistics to describe baseline characteristics by participant status (Alive on treatment, LTFU, Died, Transferred to another centre). For person-time calculation, we considered the date of administrative censoring (August 31, 2014) as the last date for all those who were alive and on treatment. The last available visit date was used as the last date for participants transferred to another centre or LTFU. When available, we used the recorded death dates ascertained by family members of the deceased, else the last visit date was used as a surrogate for the death date. We included the time period only till when the participant was an adolescent, that is, we did not include the person time beyond age 19. If a participant was LTFU, transferred to another centre or had died after age 19, we considered such a participant to be alive at 19, and changed the status accordingly. Competing risks method as defined by Fine and Gray11 were used for the calculation of subdistribution hazard ratios (SHR), with death taken as the competing risk, for the LTFU analysis. Age and CD4 counts were categorized on the basis of published literature, sex on biological sex at birth, WHO categories on clinical presentation at registration and ART regimen on NNRTI used. Approximations of distance of participant residence from the ART centre, were done down to the neighbourhood, using Google Maps. We used the same covariates to assess for association with mortality. However, since death precludes LTFU from occurring, a Cox-proportional hazard model was fitted for mortality. Covariates associated with LTFU and/or death in literature and those with univariate associations p-value<0.1 were included in multivariable models. Sensitivity analyses to test for the robustness of our six-month assumption for time-varying covariates, was performed by checking for the value and direction of estimates when the difference between registration and ART start date was varied between one and twelve months. Statistical significance was defined as a two-sided p-value<0.05. All analyses were performed using Stata version 14 (StataCorp, College Station, Texas).
Results
Baselines Characteristics
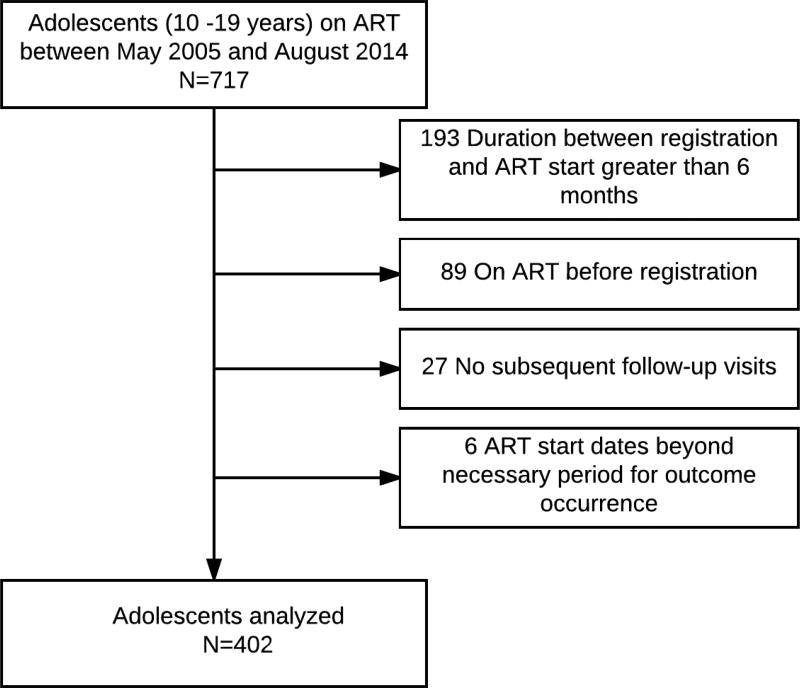
Of 717 HIV-infected adolescents started on ART between 2005 and August 2014, 315 were excluded for reasons shown in Figure 1. Of 402 adolescents who met the study criteria, 246 (61%) were males and the baseline median age was 13 years (IQR, 11–15 years). Most adolescents (n=321, 80%) acquired infection perinatally, 14% reported behavioural acquisition and 6% were unaware of the route of acquisition. Median CD4 count was 171 (IQR, [97–245 cells/µl]). Overall 243 (60%) started ART during 2005–09 and 159 (40%) during 2010 –14. Table 1 shows the overall characteristics of adolescents who were retained in care, LTFU, died and transferred to another centre.
Figure 1.
Flowchart showing inclusion criteria
Table 1.
Summary of the adolescents characteristics
| Overall, n(%) | In-care, n(%) | LTFU, n(%) | Deaths, n(%) | Transfers, n(%) | |
|---|---|---|---|---|---|
|
| |||||
| 402 | 174 (43.3) | 37 (9.2) | 42 (10.4) | 147 (36.6) | |
|
| |||||
| Median age at ART start (IQR) [years] | 13 (11–15) | 14 (11–17) | 13 (12–17) | 11 (10 –14) | 12 (11–13) |
|
| |||||
| Sex | |||||
| Male | 246 (61.2) | 94 (54.0) | 27 (73.0) | 30 (71.4) | 93 (63.3) |
| Female | 156 (38.8) | 80 (46.0) | 10 (27.0) | 12 (28.6) | 54 (36.7) |
|
| |||||
| Mode of HIV exposure | |||||
| Perinatal | 321 (80.2) | 126 (72.8) | 26 (70.3) | 34 (80.9) | 133 (91.1) |
| Behavioral | 56 (14.0) | 38 (21.9) | 5 (13.5) | 6 (14.3) | 7 (4.8) |
| Unknown | 23 (5.8) | 9 (5.2) | 6 (16.2) | 2 (4.8) | 6 (4.1) |
|
| |||||
| Median CD4_count at ART start (IQR) [cells/µL] | 171 (97 –245) | 182 (109 – 274) | 158 (90 – 281) | 126 (41 – 223) | 161 (96 –233) |
|
| |||||
| WHO status at ART start | |||||
| I & II | 210 (54.4) | 97 (58.8) | 14 (38.9) | 12 (30.8) | 85 (59.0) |
| III & IV | 176 (45.6) | 68 (41.2) | 22 (61.1) | 27 (69.2) | 59 (41.0) |
|
| |||||
| Residence from ART centre | |||||
| <5 km | 82 (20.8) | 40 (23.7) | 11 (29.7) | 11 (26.2) | 19 (13.2) |
| 5 to <10 km | 102 (25.9) | 52 (30.8) | 8 (21.6) | 22 (26.2) | 31 (21.5) |
| ≥10 to < 20 km | 65 (16.5) | 30 (17.8) | 6 (16.2) | 5 (11.9) | 24 (16.7) |
| ≥20 km | 145 (36.8) | 47 (27.8) | 12 (32.4) | 15 (35.7) | 70 (48.6) |
|
| |||||
| NNRTI regimen | |||||
| Nevirapine based | 272 (71.8) | 99 (64.7) | 21 (58.3) | 26 (61.9) | 124 (84.9) |
| Efavirenz based | 107 (28.2) | 54 (35.3) | 15 (41.7) | 16 (38.1) | 22 (15.1) |
|
| |||||
| Year of ART start | |||||
| 2005 – 2009 | 243 (60.4) | 67 (38.5) | 21 (56.8) | 32 (76.2) | 122 (83.0) |
| 2010 – 2014 | 159 (39.6) | 107 (61.5) | 16 (43.2) | 10 (23.8) | 25 (17.0) |
|
| |||||
| Median duration of follow-up (IQR) [months] | 16.2 (6.3 – 44) | 25.8 (9.8 – 68.5) | 6.8 (2.9 – 24.1) | 3.4 (1.3 – 15.8) | 16.8 (6.9 – 34.6) |
|
| |||||
| Median age at administrative censoring# (IQR) [years] | 15.4 (13.1 – 18.1) | 17.8 (14.9 – 19.7) | 16.2 (14.1 – 17.4) | 12.3 (11.1 – 16.1) | 14.1 (12.6 – 16.2) |
All statuses are reflective of the time that the participants were adolescents, i.e if a participant was lost at 20 years of age, that participant was still considered alive when he/she was 19 years and the status changed accordingly. Two participants stopped treatment completely and these have not been shown.
Observations administratively censored at August 31, 2014.
Cumulative Incidence, rates, and factors for Loss to follow-up
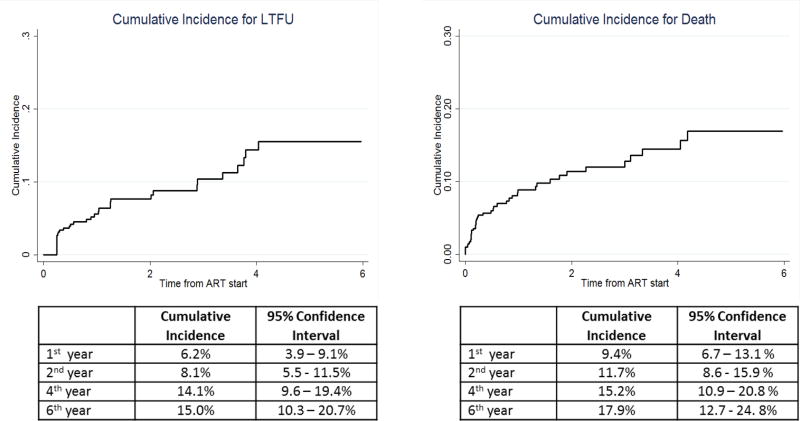
The overall median duration of follow-up was 16.2 months (IQR, 6.3–44 months). Thirty-seven (9.2%) were LTFU, contributing to a crude LTFU rate of 4.4 (95% CI, 3.2–6.0) per 100 person-years (PY). Median CD4 count was 158 cells/µl (IQR: 90–281) among those LTFU compared to 182 cells/µl (IQR:109–274,) for those retained in care (p= 0.51). A higher proportion of male adolescents were LTFU as compared to females (p<0.05) and a higher proportion of those who started ART between 2005 and 2009 were lost as compared to those who started ART later (p<0.05). The cumulative LTFU incidence at 1, 2, 4, and 6 years of follow up from ART start was 6.2%(95% CI, 3.9% – 9.1%), 8.1% (95% CI, 5.5% – 11.5%), 14.1% (95% CI, 9.6%–19.4%), and 15% (95% CI, 10.3% – 20.7%), respectively (Figure 2).
Figure 2.
Cumulative Incidence for LTFU and mortality
In univariate analysis, older adolescents (15–19 years) (SHR, 2.98; 95% CI, 1.61–5.53), unknown mode of HIV acquisition (SHR, 4.21; 95% CI, 1.84–9.59), usage of Efavirenz- based regimens (SHR, 2.37; 95% CI, 1.23 – 4.58) and ART initiation between 2010 and 2014 (SHR, 2.10; 95% CI, 1.13–3.93) were associated with increased risk of LTFU. In multivariable analysis adjusted for age, sex, mode of HIV acquisition, CD4 count, NNRTI regimen and year of ART start, only older age (aSHR: 2.44; 95% CI, 1.18 – 5.02) was associated with LTFU. (Table 2).
Table 2.
Characteristics associated with loss to follow-up (with death as the competing event)
| Characteristics (n=402) |
LFU, n | Person Years (PY) |
Crude loss to follow-up rate per 100 PY (95% CI) |
Univariate analysis | Multivariable analysis* | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| SHR (95% CI) | p-value | aSHR (95% CI) | p-value | ||||
|
| |||||||
| Overall | 37 | 844 | 4.4 (3.2 – 6.0) | - | - | - | - |
|
| |||||||
| Age at ART initiation | |||||||
| 10–14 years | 20 | 700 | 2.8 (1.8 – 4.4) | 1 | 1 | ||
| 15–19 years | 17 | 144 | 11.8 (7.4 – 19.1) | 2.98 (1.61 – 5.53) | 0.001 | 2.44 (1.18 – 5.02) | 0.016 |
|
| |||||||
| Sex | |||||||
| Male | 27 | 517 | 5.2 (3.6 – 7.6) | 1.59 (0.77 – 3.28) | 0.212 | 1.82 (0.81 – 4.09) | 0.146 |
| Female | 10 | 327 | 3.1 (1.6 – 5.7) | 1 | 1 | ||
|
| |||||||
| Mode of HIV acquisition$ | |||||||
| Perinatal | 26 | 752 | 3.4 (2.3 – 5.1) | 1 | 1 | ||
| Behavioral | 5 | 56 | 8.9 (3.7 – 21.3) | 1.59 (0.61 –4.12) | 0.342 | 0.57 (0.16 –2.08) | 0.399 |
| Unknown | 6 | 33 | 18.0 (8.1 – 40.1) | 4.21 (1.84 – 9.59) | 0.001 | 2.15 (0.81 –5.74) | 0.125 |
|
| |||||||
| Residence from ART centre | |||||||
| <5 km | 11 | 197 | 5.6 (3.1 – 10.1) | 1.40 (0.62 –3.16) | 0.411 | ||
| 5 to <10 km | 8 | 227 | 3.5 (1.8 – 7.0) | 0.90 (0.37 – 2.17) | 0.819 | - | - |
| ≥10 to < 20 km | 6 | 130 | 4.6 (2.1 – 10.2) | 1.20 (0.45 – 3.23) | 0.711 | ||
| ≥20 km | 12 | 274 | 4.4 (2.5 – 7.7) | 1 | |||
|
| |||||||
| CD4 category at ART start¥ | |||||||
| <200 | 20 | 478 | 4.2 (2.7 – 6.5) | 0.57 (0.23 – 1.43) | 0.232 | 0.79 (0.30 – 2.07) | 0.627 |
| 200–350 | 11 | 300 | 3.7 (2.0 – 6.6) | 0.55 (0.20 – 1.52) | 0.250 | 0.68 (0.22 – 2.07) | 0.498 |
| >350 | 5 | 61 | 8.2 (3.4 – 19.6) | 1 | 1 | ||
|
| |||||||
| WHO category at ART start¥ | |||||||
| I & II | 14 | 410 | 3.4 (2.0 – 5.8) | 1 | - | - | |
| III & IV | 22 | 412 | 5.3 (3.5 – 8.1) | 1.60 (0.82 – 3.14) | 0.170 | ||
|
| |||||||
| NNRTI regimen | |||||||
| Nevirapine based | 21 | 644 | 3.3 (2.1 – 5.0) | 1 | 1 | ||
| Efavirenz based | 15 | 155 | 9.6 (5.8 – 16.0) | 2.37 (1.23 – 4.58) | 0.010 | 1.89 (0.91 – 3.93) | 0.087 |
|
| |||||||
| Year of ART start | |||||||
| 2005 –2009 | 21 | 677 | 3.1 (2.0 – 4.8) | 1 | 1 | ||
| 2010 – 2014 | 16 | 167 | 9.6 (5.9 – 15.6) | 2.10 (1.13 – 3.93) | 0.020 | 1.54 (0.69 – 3.42) | 0.287 |
The CD4 variable has 6 missing values, NNRTI regimen 5 missing values WHO category 16 missing values. One person categorized as LTFU has one values missing for the aforementioned variables, hence the total number of LTFU in these three variables is 36. Using continuous CD4 counts per 100 cell increase, does not appear to be associated with LTFU and the interpretation remains the same as when using categorical CD4 counts. Hence this has not been shown separately.
Behavioral modes of HIV acquisition include heterosexual, IV drug use and blood transfusion
Adjusted for age, sex, mode of HIV acquisition, CD4 categories and year of ART start
Cumulative Incidence, Rates and Factors for Mortality
Overall, 42 (10.4%) adolescents died contributing to a crude mortality rate of 4.9 per 100 person-years [95% CI, 3.7–6.7]. Thirty (71.4%) were males and median age at death was 12.3 years (IQR, 11.1–16.1). Adolescents who died were younger (p<0.05) and had lower median CD4 counts compared to those retained in care (126 cells/µl [IQR:41–223] vs.82 cells/µl [IQR:109–274], p<0.05). The cumulative mortality incidence at 1, 2, 4, 6 years of follow-up after ART start was 9.4% (95% CI, 6.7% – 13.1%), 11.7% (95% CI, 8.6 –15.9%), 15.2% (95% CI, 10.9% –20.8%), 17.9 (95% CI, 12.7% –24.8%) respectively (Figure 2).
In univariate analysis, an increase in CD4 count by 100 cells (SHR, 0.68; 95% CI, 0.50 – 0.92), usage of Efavirenz-based NNRTI regimens (SHR, 2.09; 95% CI, 1.12 – 3.93) and WHO categories III and IV (SHR, 2.54; 95% CI, 1.28 – 5.02) were associated with mortality. In multivariable analysis adjusted for age, sex, WHO stages, CD4 count per 100 cell increase and NNRTI regimens, WHO categories III and IV (aSHR, 2.26; 95% CI, 1.14 – 4.48) and CD4 count per 100 cell increase (aSHR, 0.59; 95% CI, 0.43 – 0.83) were associated with mortality. (Table 3).
Table 3.
Characteristics associated with mortality
| Characteristics (n=402) |
Deaths, n | Person Years (PY) |
Crude mortality rate per 100 PY (95% CI) |
Univariate analysis | Multivariable analysis* | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| HR (95% CI) | p-value | aHR (95% CI) | p-value | ||||
|
| |||||||
| Overall | 42 | 844 | 4.9 (3.7 – 6.7) | - | - | - | - |
|
| |||||||
| Age at ART start | |||||||
| 10–14 years | 32 | 700 | 4.5 (3.2 – 6.5) | 1.05 (0.51 – 2.16) | 0.891 | 1.01 (0.48 – 2.15) | 0.974 |
| 15–19 years | 10 | 144 | 6.8 (3.7 – 12.9) | 1 | 1 | ||
|
| |||||||
| Sex | |||||||
| Male | 30 | 517 | 5.8 (4.0 – 8.3) | 1.54 (0.79 – 3.02) | 0.201 | 1.66 (0.83 – 3.53) | 0.155 |
| Female | 12 | 327 | 3.7 (2.1 – 6.5) | 1 | 1 | ||
|
| |||||||
| Mode of HIV acquisition | |||||||
| Perinatal | 34 | 752 | 4.5 (3.2 – 6.3) | 1 | |||
| Behavioral$ | 6 | 56 | 10. 6 (4.8 – 23.7) | 1.43 (0.6 – 3.4) | 0.422 | - | - |
| Unknown | 2 | 33 | 5.9 (1.5 – 23.9) | 1.05 (0.25 – 4.38) | 0.945 | ||
|
| |||||||
| Residence from ART centre | |||||||
| <5 km | 11 | 196 | 5.6 (3.1 – 10.1) | 1. 24 (0.57 – 2.69) | 0.594 | ||
| 5 to <10 km | 11 | 227 | 4.8 (2.7 – 8.7) | 1.01 (0.46 – 2.19) | 0.990 | - | - |
| ≥10 to < 20 km | 5 | 130 | 3.8 (1.6 – 9.2) | 0.76 (0.28 – 2.11) | 0.605 | ||
| ≥20 km | 15 | 273 | 5.4 (3.3 – 9.1) | 1 | |||
|
| |||||||
| WHO category at ART start | |||||||
| I & II | 12 | 410 | 2.9 (1.7 – 5.2) | 1 | 1 | ||
| III & IV | 27 | 412 | 6.5 (4.5 – 9.5) | 2.54 (1.28 – 5.02) | 0.007 | 2.26 (1.14 – 4.48) | 0.020 |
|
| |||||||
| Year of ART start | |||||||
| 2005 –2009 | 32 | 677 | 4.7 (3.3 – 6.7) | 1.40 (0.68 – 2.89) | 0.361 | - | - |
| 2010 – 2014 | 10 | 167 | 5.9 (3.2 – 11.1) | 1 | |||
|
| |||||||
| NNRTI regimen | |||||||
| Nevirapine based | 26 | 644 | 4.0 (2.7 – 5.9) | 1 | 1 | ||
| Efavirenz based | 16 | 155 | 10.3 (6.1 – 16.8) | 2.09 (1.12 – 3.93) | 0.021 | 1.89 (0.97 – 3.71) | 0.062 |
|
| |||||||
| CD4 category at ART start | |||||||
| <200 | 29 | 478 | 6.1 (4.2 – 8.7) | 4.40 (0.60 – 32.37) | 0.145 | ||
| 200–350 | 12 | 300 | 3.9 (2.3 – 7.0) | 3.21 (0.42 –24.78) | 0.263 | - | - |
| >350 | 1 | 61 | 1.6 (0.2 – 11.6) | 1 | |||
| CD4/100 cells# | - | - | - | 0.68 (0.50 – 0.92) | 0.012 | 0.59 (0.43 – 0.83) | 0.012 |
Behavioral modes of HIV acquisition include heterosexual, IV drug use and blood transfusion
Continuous CD4 counts per 100 cell increment. Using CD4 categorically does not seem to be associated with mortality, but using them as a continuous variable, appears to have an association with mortality.
Adjusted for age, sex, WHO categories, NNRTI regimen and CD4 increment by 100 cells.
Discussion
Our study is among the first to describe a largely perinatally infected adolescent cohort receiving standard of care in a large public sector ART centre in India, the country with the third largest burden of HIV.12 Notably, we observed adolescents had a low CD4 count and by 6 years in care the LTFU rate was 15% and mortality rate was 18%. Furthermore, we identified older adolescents (15–19 years) to be at highest risk of LTFU and not surprisingly those with most advanced clinical stage to be at highest risk of mortality. Clearly with one-third of the cohort being LTFU or dying by 6 years despite ART initiation, more attention and resources are needed to reduce LTFU and death in this vulnerable population of youth.
A wide range of LTFU rates (4.8 –14.7 per 100 PY) have been reported from sub-Saharan Africa and our rates fall below this range.13–16 However, compared to these cohorts, that were chiefly female (52% to 66% female), our cohort had a higher proportion of male adolescents. As HIV-infected men have been shown to be more likely to be LTFU,17 our finding of low LTFU rates in a predominantly male cohort, necessitates further investigation of cultural and behavioural differences between male adolescents in our cohort and their sub-Saharan counterparts, in future.
As has been seen in adults, our cumulative LTFU incidence increased during longer follow ups.18,19 This can be explained by our observation that older adolescents aged 15–19 years were more likely to be LTFU.13, 20 We hypothesize that a number of synergistic factors contribute to this observation. Firstly, the fear of social ostracisation, resulting from greater awareness about stigmatization around HIV in late adolescence, could negatively impact attendance at an ART center.21 Secondly, this period is associated with sexual debut for some adolescents;7 apprehension about inadvertent partner disclosure, if seen at the ART centre, along with reduced adult supervision, could further limit follow-up.22 Lastly, the transition into adulthood may be associated with greater familial responsibilities for some adolescents, relegating personal health to a secondary priority.23, 24 Greater access to social protection, incentivizing in person follow-ups with cash or kind benefits, could be considered to improve retention among older adolescents.25, 26
The mortality rate in our cohort is higher than the mortality rate reported in some studies from South Africa9, 13, 15 and was observed to be higher in the first few years after starting ART. Late presentation to care as characterized by over 45% of adolescents presenting in either WHO stage III or IV disease and non-availability of regular viral load testing to optimize treatment regimens, could be reasons for the high mortality rates observed. However, in the more recent years, there has been a promising trend in the proportion of adolescents presenting much earlier (Supplementary Fig.1). In this regard, NACO’s efforts in increasing HIV awareness among adolescents through its Adolescent Education Program27 should be commended and its wider expansion championed.
Our study relied on programmatic data, thus was limited by incompletely collected data on status of caregivers, adherence to ART and cause of death. Also, although only 1/5th of the adolescents self-reported to behavioural acquisition of HIV, it is possible that responses were biased by social-desirability, consequently providing an underestimate of the proportion of adolescents who were behaviourally infected. Furthermore, the data collected is from an urban centre catering to surrounding semi-urban and rural areas and may not be representative of all HIV-infected adolescents in India. Unavailability of routinely collected viral loads, under the Indian HIV program also precluded us from drawing associations between viral suppression and the treatment outcomes under consideration. Finally, even though telephonic tracing of all individuals LTFU and transferred to other centres was attempted, contact was established with only 23% of them or their relatives, limiting us from making reasonable estimates on actual mortality rates for those LTFU and transferred out (Supplementary Fig 2 & Fig 3). It is therefore conceivable that mortality rates are actually higher than what we have reported.
Over the past decade, India has rapidly rolled out ART to all children and adolescents and has established several ART centres, limiting LTFU due to distance and providing easy access to ART. Our report highlights this effort in that over 36 % of the adolescents were transferred out to different ART centres in the region. However, despite these advances, we demonstrate that a third of HIV-infected adolescents who start ART are either LTFU or die by the sixth year of follow-up, representing a huge loss of an economically and socially productive group. Our report also brings to light a small group of adolescents, who are unaware of their mode of HIV-acquisition. While the exact mechanisms for this unawareness is difficult to comment upon, a primary driver could be non-disclosure of HIV status, as reported by a previous study from our group.28 The existence of such a group brings to the forefront the necessity of discourse regarding disclosure guidelines in India.
With demographic transition and improvement in HIV care, occurring hand in hand in India, it is more crucial now than ever to sensitize healthcare workers to adolescent specific needs. Along with this, strengthening of counselling and support services for older adolescents incorporating destigmatization mitigation techniques and close monitoring of adolescents with advanced clinical disease, are some strategies that could benefit this vulnerable population. Future longitudinal studies should confirm our study findings.
Supplementary Material
Acknowledgments
We would like to thank NACO, the staff at the ART centre of BJGMC & SGH that were involved in data collection. We also extend our gratitude to Tata Consultancy Services for assisting us with data cleaning for this study.
Funding Acknowledgements: This work is supported by NIH/ Eunice Kennedy Shriver National Institute of Child Health & Human Development Foundation for AIDS Research (amfAR), supported by funds from the US National Institutes of Health (NIH) for contributions to the International Epidemiologic Databases to Evaluate AIDS (IeDEA) a project of the NIH National Institutes of Allergy and Infectious Diseases (NIAID) (U01AI069907), Fogarty International Center BJGMC JHU HIV TB Program, Fogarty International Center, National Institutes of Health (NIH) (D43TW009574), Johns Hopkins Baltimore-Washington-India Clinical Trials Unit for NIAID Networks BWI CTU (U01 AI069465), Gilead Foundation, and Ujala Foundation, Newtown Square, PA, USA
Footnotes
Authors’ Contribution: SN, IPM, NG, VM conceived the study idea. AK, SN, NG, AS, NS, framed the research question and provided scientific input on methodology. NG, BR, DK, AK helped in data acquisition, IPM, NG, AG, JG analysed and interpreted the data. BR, DK, AK, CV, provided technical expertise on the functioning of the national AIDS program, AG, AS, RB provided clinical scientific input to contextualise the study findings. SN, IPM, VM drafted preliminary manuscript. RB, AG, AS, AD, revised the manuscript critically for important intellectual content. All authors approve of the final revised version submitted for publication.
Competing Interest Statement: None to declare
References
- 1.UNAIDS. [Retrieved July 20, 2017];Fact sheet - latest statistics on the status of the aids epidemic. 2017 Jul; from http://www.unaids.org/en/resources/fact-sheet.
- 2.WHO, UNAIDS, & UNICEF. [Retrieved September 10, 2016];Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. 2010 from http://www.who.int/hiv/pub/2010progressreport/en/
- 3.Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: Children, adolescents, and young adults with perinatally acquired HIV infection. Annual Review of Medicine. 2010;61:169–185. doi: 10.1146/annurev.med.050108.151127. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee T, Pensi T, Banerjee D, Grover G. Impact of HAART on survival, weight gain and resting energy expenditure in HIV-1-infected children in india. Annals of Tropical Paediatrics. 2010;30(1):27–37. doi: 10.1179/146532810X12637745451915. [DOI] [PubMed] [Google Scholar]
- 5.UNICEF. [Retrieved July, 2017];Turning the tide against AIDS will require more concentrated focus on adolescents and young people. 2017 Jul; from https://data.unicef.org/topic/hivaids/adolescents-young-people/#, retrieved on 26 July 201.
- 6.UNAIDS. [Retrieved July 10, 2017];All In to #End Adolescent AIDS. 2015 from http://www.unaids.org/sites/default/files/media_asset/20150217_ALL_IN_brochure.pdf.
- 7.Kurapati S, Vajpayee M, Raina M, Vishnubhatla S. Adolescents living with HIV: An indian profile. AIDS Research and Treatment. 2012;2012:576149. doi: 10.1155/2012/576149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auld AF, Agolory SG, Shiraishi RW, Wabwire-Mangen F, Kwesigabo G, Mulenga M, Ellerbrock TV. Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults--seven african countries, 2004–2013. MMWR. Morbidity and Mortality Weekly Report. 2014;63(47):1097–1103. doi:mm6347a2 [pii] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakanda C, Birungi J, Mwesigwa R, Nachega JB, Chan K, Palmer A, Mills EJ. Survival of HIV-infected adolescents on antiretroviral therapy in uganda: Findings from a nationally representative cohort in uganda. PloS One. 2011;6(4):e19261. doi: 10.1371/journal.pone.0019261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National AIDS Control Organisation Department of AIDS Control Ministry of Health and Family Welfare Government of India New Delhi. [Retrieved April 27, 2016];Operational Guidelines For Link ART Centres And LAC PLUS. 2012 Jan; from http://www.naco.gov.in/sites/default/files/Operational%20Guidelines%20for%20LAC%20%20LAC%20plus%20Jan%202012.pdf.
- 11.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 12.AVERT. [Retrieved May 28, 2017];HIV AND AIDS IN INDIA. 2016 Dec 01; from https://www.avert.org/professionals/hiv-around-world/asia-pacific/india.
- 13.Evans D, Menezes C, Mahomed K, Macdonald P, Untiedt S, Levin L, Maskew M. Treatment Outcomes of HIV-Infected Adolescents Attending Public-Sector HIV Clinics Across Gauteng and Mpumalanga, South Africa. AIDS Research and Human Retroviruses. 2013;29(6):892–900. doi: 10.1089/aid.2012.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nglazi MD, Kranzer K, Holele P, Kaplan R, Mark D, Jaspan H, Bekker L-G. Treatment outcomes in HIV-infected adolescents attending a community-based antiretroviral therapy clinic in South Africa. BMC Infectious Diseases. 2012;12:21. doi: 10.1186/1471-2334-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bygrave H, Mtangirwa J, Ncube K, Ford N, Kranzer K, Munyaradzi D. Antiretroviral therapy outcomes among adolescents and youth in rural zimbabwe. PloS One. 2012;7(12):e52856. doi: 10.1371/journal.pone.0052856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shroufi A, Gunguwo H, Dixon M, Nyathi M, Ndebele W, Saint-Sauveur JF, Ferrand RA. HIV-infected adolescents in southern africa can achieve good treatment outcomes: Results from a retrospective cohort study. AIDS (London, England) 2013;27(12):1971–1978. doi: 10.1097/QAD.0b013e32836149ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochieng-Ooko V, Ochieng D, Sidle JE, Holdsworth M, Wools-Kaloustian K, Siika AM, Braitstein P. Influence of gender on loss to follow-up in a large HIV treatment programme in western kenya. Bulletin of the World Health Organization. 2010;88(9):681–688. doi: 10.2471/BLT.09.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez-Uria G, Naik PK, Pakam R, Midde M. Factors associated with attrition, mortality, and loss to follow up after antiretroviral therapy initiation: Data from an HIV cohort study in india. Global Health Action. 2013;6:21682. doi: 10.3402/gha.v6i0.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berheto TM, Haile DB, Mohammed S. Predictors of loss to follow-up in patients living with HIV/AIDS after initiation of antiretroviral therapy. North American Journal of Medical Sciences. 2014;6(9):453–459. doi: 10.4103/1947-2714.141636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kranzer K, Bradley J, Musaazi J, Nyathi M, Gunguwo H, Ndebele W, Ferrand RA. Loss to follow-up among children and adolescents growing up with HIV infection: Age really matters. Journal of the International AIDS Society. 2017;20(1):1–7. doi: 10.7448/IAS.20.1.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz IT, Ryu AE, Onuegbu AG, Psaros C, Weiser SD, Bangsberg DR, Tsai AC. Impact of HIV-related stigma on treatment adherence: Systematic review and meta-synthesis. Journal of the International AIDS Society. 2013;16(3 Suppl 2):18640. doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu L, Munir K, Kanabkaew C, Le Coeur S. Factors influencing antiretroviral treatment suboptimal adherence among perinatally HIV-infected adolescents in thailand. PloS One. 2017;12(2):e0172392. doi: 10.1371/journal.pone.0172392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benson JE, Johnson MK. Adolescent family context and adult identity formation. Journal of Family Issues. 2009;30(9):1265–1286. doi: 10.1177/0192513X09332967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon JA, Wickrama KA. Linking family economic pressure and supportive parenting to adolescent health behaviors: Two developmental pathways leading to health promoting and health risk behaviors. Journal of Youth and Adolescence. 2014;43(7):1176–1190. doi: 10.1007/s10964-013-0060-0. [DOI] [PubMed] [Google Scholar]
- 25.Cluver LD, Hodes RJ, Sherr L, Orkin FM, Meinck F, Lim Ah Ken P, Vicari M. Social protection: Potential for improving HIV outcomes among adolescents. Journal of the International AIDS Society. 2015;18(Suppl 6):20260. doi: 10.7448/IAS.18.7.20260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cluver LD, Toska E, Orkin FM, Meinck F, Hodes R, Yakubovich AR, Sherr L. Achieving equity in HIV-treatment outcomes: Can social protection improve adolescent ART-adherence in south africa? AIDS Care. 2016;28(Suppl 2):73–82. doi: 10.1080/09540121.2016.1179008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UNICEF India. [Retrieved August 28, 2017];Adolescence Education Programme: teachers to be awarded for imparting quality HIV/AIDS awareness. (n.d.) from http://unicef.in/PressReleases/268/Adolescence-Education-Programme-teachers-to-be-awarded-for-imparting-quality-HIVAIDS-awarene.
- 28.Suryavanshi N, Raval G, Kanade S, Nimkar S, Nadgiri V, Sahu P, Shankar A. Challenges to disclosure of HIV status to perinatally infected children: A study of caregiver perspectives in Pune, India. Journal of Pediatric Infectious Diseases. 2014;9(2):71–84. doi: 10.3233/JPI-140418. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.