Abstract
Intercellular bridges are conserved structures that allow neighboring cells to exchange cytoplasmic material; defects in intercellular bridges can lead to infertility in many organisms. Here, we use the Drosophila egg chamber to study the mechanisms that regulate intercellular bridges. Within the developing egg chamber, the germ cells (15 nurse cells and 1 oocyte) are connected to each other through intercellular bridges called ring canals, which expand over the course of oogenesis to support the transfer of materials from the nurse cells to the oocyte. The ring canals are enriched in actin and actin binding proteins, and many proteins have been identified that localize to the germline ring canals and control their expansion and stability. Here, we demonstrate a novel role for the Ste20 family kinase, Misshapen (Msn), in regulation of the size of the germline ring canals. Msn localizes to ring canals throughout most of oogenesis, and depletion of Msn led to the formation of larger ring canals. Over-expression of Msn decreased ring canal diameter, and expression of a membrane tethered form of Msn caused ring canal detachment and nurse cell fusion. Altering the levels or localization of Msn also led to changes in the actin cytoskeleton and altered the localization of E-cadherin, which suggests that Msn could be indirectly limiting ring canal size by altering the structure or dynamics of the actin cytoskeleton and/or adherens junctions.
Keywords: oogenesis, ring canal, egg chamber, Misshapen (Msn), Drosophila
Graphical abstract

Introduction
Intercellular bridges are important, evolutionarily conserved structures that allow neighboring cells to maintain a cytoplasmic connection to each other. Intercellular bridges can facilitate the transfer of proteins, mRNAs, and even organelles to allow cells to coordinate behaviors or to support development. In most sexually reproducing organisms, sperm and eggs are physically connected to each other or to supporting cells at some point during their development, and defects in intercellular bridges can lead to sterility (Robinson and Cooley, 1996). Therefore, understanding the molecular mechanisms that control the formation, stability, and expansion of these structures is essential.
The developing Drosophila egg chamber is an excellent model system to study the formation and growth of intercellular bridges. The egg chamber is a multicellular structure composed of a central cluster of 16 germ cells (15 nurse cells that support the development of a single oocyte) that is surrounded by a layer of somatic epithelial cells, the follicle cells (Spradling, 1993). The germ cell cluster is formed in region 1 of the germarium (Fig. 1A) from a single stem cell daughter that undergoes four rounds of mitosis followed by incomplete cytokinesis. At the end of each mitosis, the contractile ring does not fully close, maintaining a cytoplasmic connection between the daughter cells. The arrested contractile ring undergoes a maturation process to form an intercellular bridge, or ring canal. The outer rim of the ring canal is formed by the thickening of the plasma membrane in the region of the furrow (Mahowald, 1971), and actin and other proteins such as Hts-RC and Kelch are recruited to form the inner rim (Fig. 1A; Robinson et al., 1994). There is an additional population of actin filaments peripheral to the outer rim which are associated with microvilli structures that help to anchor the ring canals to the plasma membrane (Loyer et al., 2015; Tilney et al., 1996). Hts-RC is an adducin-like protein that is recruited to ring canals in region 2a of the germarium; it is required to localize actin and to form the inner rim (Robinson et al., 1994; Yue and Spradling, 1992). Kelch, which is recruited in region 3 of the germarium (Robinson et al., 1994), has been implicated in actin binding and turnover as well as in the regulation of ubiquitination in the germline (Hudson and Cooley, 2010; Hudson et al., 2015; Kelso et al., 2002; Robinson et al., 1994; Tilney et al., 1996; Xue and Cooley, 1993). Cheerio, or filamin, is an actin cross-linking protein, and it is found in both the inner and outer rims, likely providing a link between the plasma membrane and the actin-rich inner rim (Li et al., 1999; Robinson et al., 1997; Sokol and Cooley, 1999).
Figure 1. Misshapen localizes to the germline ring canals and nurse cell membranes throughout oogenesis.
(A) Schematic of the germarium and inner and outer rim structure of the ring canals. (B–D) Single plane confocal images of egg chambers expressing a MsnYFP protein trap. Samples were stained with an anti-GFP antibody, phalloidin, and DAPI. Arrowheads point to MsnYFP on nurse cell membranes. (E) Maximum intensity projection of confocal images of egg chambers stained with an anti-GFP antibody, phalloidin, and DAPI. (F) Single plane confocal images of MsnYFP-expressing egg chambers stained with an Hts-RC antibody and DAPI. Boxes indicate magnified regions.
During egg chamber development, the germline ring canals undergo a 20-fold increase in diameter. The expansion is essential to accommodate transfer of materials throughout oogenesis, but especially during the process of nurse cell dumping, which occurs during stage 11 of oogenesis. During this process, the nurse cells squeeze their entire cytoplasmic contents to the oocyte, which leads to a doubling of the oocyte volume in ~30 minutes. After dumping, the nurse cells undergo apoptosis (Spradling, 1993). Conditions that affect ring canal growth or stability cause defects in this dumping process, leading to the formation of a smaller egg.
Ring canal expansion requires the dynamic regulation of actin and actin-binding proteins. Electron microscopy has revealed that ring canal expansion is associated with an increase in the number of actin filaments per ring canal, from ~80 filaments at stage 2 to over 700 filaments at stage 6 (Tilney et al., 1996). Although the number of actin filaments remains fairly constant from stage 6 to stage 10 (Tilney et al., 1996), these filaments are dynamic, at least during stage 10a (Kelso et al., 2002). Dynamic behavior of the ring canal actin filaments likely depends on proper regulation of actin binding proteins, such as Kelch (Kelso et al., 2002), as well as nucleation of new actin filaments. The Arp2/3 complex is a candidate nucleator of actin at the ring canals, as mutations in Arp2/3 complex members, ArpC1 or Arp3, lead to the formation of smaller or collapsed ring canals and defects in nurse cell dumping (Hudson and Cooley, 2002).
In addition to the structural components of the ring canal, two kinases have been identified that are required for ring canal expansion and stability. The Src family kinase, Src64, promotes actin turnover, likely by phosphorylating Kelch (Kelso et al., 2002). Mutations in Src64 lead to smaller ring canals, which can become detached from the plasma membrane (Dodson et al., 1998; Guarnieri et al., 1998; O’Reilly et al., 2006; Roulier et al., 1998). Src64 also phosphorylates the Tec family kinase, Btk29, to promote its ring canal localization (Dodson et al., 1998; Guarnieri et al., 1998; Lu et al., 2004; Roulier et al., 1998). Recent work has demonstrated that Btk29 phosphorylates β-catenin (Hamada-Kawaguchi et al., 2015), and this modification contributes to ring canal growth.
Here, we identify a novel role for the Ste20 family kinase, Misshapen, in the regulation of ring canal size. Misshapen localizes to the ring canals and nurse cell membranes throughout oogenesis, and reducing the levels of Misshapen in the germline significantly increases ring canal diameter. In contrast, over-expression of Msn decreases ring canal diameter. To our knowledge, this is the first protein whose levels are able to modulate the size of the germline ring canals without altering the rate of expansion. Depletion of Msn disrupts actin organization and can lead to the ectopic accumulation of E-cadherin. These data suggest that Msn could be limiting ring canal size through effects on actin or adherens junction stability. Further, expression of a membrane-tethered form of Msn leads to ring canal detachment, nurse cell fusion, and defects in border cell migration. Therefore, alterations in Msn levels or localization could have additional effects in the germline distinct from its role at the ring canals.
Materials and Methods
Fly Genetics and maintenance
Full genotypes and incubation conditions are listed in Table S1. The following stocks were obtained from the Bloomington Drosophila Stock Center: the maternal triple driver abbreviated as MTD-GAL4 (otu-Gal4; nos-Gal4; nos-Gal4; #31777), matαTub-GAL4 (#7062 on chromosome 2 or #7063 on chromosome 3), and UAS-msn-RNAi (#42518). The Misshapen-YFP (#115454) and Cheerio-YFP (#115123) protein trap lines were obtained from the Drosophila Genomics and Genetic Resources (DGGR; Kyoto Institute of Technology, Kyoto, Japan (Lowe et al., 2014; Lye et al., 2014; Rees et al., 2011)). The UAS-Myr-HA-MsnWT line was from L. Xu (Kaneko et al., 2011).
Generation of UAS lines
Sequences corresponding to HA-MsnWT, HA-MsnKR, or Myr-HA-MsnKR were amplified from pMK33 containing the Myr-HA-MsnWT coding sequence (gift from L. Xu) and inserted into pENTR3c Dual using In-Fusion HD Cloning (Takara Bio USA). For the kinase inactive lines, there was a lysine to arginine change corresponding to amino acid 61 of the unmodified Msn protein (Kaneko et al., 2011; Li et al., 2014; Li et al., 2015). Gateway LR Clonase II Enzyme Mix (ThermoFisher Scientific) reactions were used to recombine the coding sequences into the UASp-pPW vector (Drosophila Gateway, Carnegie Institution for Science). Sequence-verified, purified plasmids were injected into w1118 flies and balanced using either CyO or TM3,Sb (BestGene, Inc).
Quantification of RNA levels
Ovaries from well-fed female flies were dissected in Schneider s media (Genesee Scientific). Total RNA was extracted using the RNeasy Plus Mini kit (Qiagen). RNA was reverse transcribed into cDNA and used for qPCR using the QuantiNova SYBR Green RT-PCR kit (Qiagen). The following primer pairs were used: Misshapen- 5′-TCCCTTGGACAGCAGCGATT-3′ and 5′ – AGTTCCATCGTTCCTAGCC – 3′. The primers for the control, GAPDH – 5′ – AAATCGCGGAGCCAAGTAGT - 3′ and 5′ – CACGATTTTCGCTATGGCCG – 3′ were from (Giuliani et al., 2014). The data in Fig. S1A represents the average of 4 independent experiments. Each reaction was done in triplicate for each experiment, and the Msn expression levels were calculated using the 2−ΔCq method as described in (Livak and Schmittgen, 2001). ΔCq was the difference in the Cq values for the GAPDH and the Msn reactions.
Ovary Dissection and Staining Procedures
Ovaries from well-fed females were dissected in Schneider’s media (Genesee Scientific). Tissue was fixed for 15 minutes using 4% EM grade formaldehyde (Polysciences) diluted in PBS or PBS +0.1% Triton X-100 (PBT). Ovaries were washed in PBS + 0.1–0.3% Triton X-100 and stained with DAPI (1:500, 1 mg/mL stock, D3571 ThermoFisher Scientific), phalloidin (1:500, TRITC or FITC conjugated, ECM Biosciences), and one of the antibodies diluted in PBS + 0.3% Triton X-100 + 5 mg/mL BSA. The following antibodies were used in this study: Hts-RC (1:20, htsRC, DSHB), GFP (1:10, 12A6, DSHB), Kelch (1:10, kel1B, DSHB), anti-HA (1:200, Cat. #71-5500, ThermoFisher Scientific), DE-Cadherin (1:20, DCAD2, DSHB), β-catenin pTyr142 (1:200, CP1081, ECM Biosciences), FasIII (1:20, 7G10, DSHB), and phospho-Tyrosine clone 27B10 (1:300, APY03, Cytoskeleton). Secondary antibodies conjugated to Alexa Fluor 488/FITC or Alexa Fluor 555/TRITC (donkey anti-rabbit and donkey anti-mouse from ThermoFisher Scientific, goat anti-rat from Jackson ImmunoResearch) were used at a 1:200 dilution in PBS + 0.3% Triton X-100 + 5 mg/mL BSA. Stained ovaries were mounted using SlowFade Antifade (ThermoFisher Scientific).
Mature Egg Measurements and Embryonic Viability
Mature eggs were collected on apple juice plates with yeast paste. Mature eggs were imaged with a 10× objective on a Zeiss standard microscope equipped with a ProgRes MF camera (Jenoptik) controlled by ProgRes Capture Pro software (Jenoptik). The length and width of the eggs were measured using the line tool in Fiji/ImageJ, and the volume was calculated using the equation: Volume = (1/6)π(width)2(length). To assess embryonic viability, embryos were collected on apple juice plates with yeast paste at 29°C and then transferred to fresh apple juice plates. Embryos were incubated for 50 hours at 25°C, and the number of larvae that had hatched was determined.
Imaging and Analysis
Most egg chambers were imaged with a Leica DM5500B compound fluorescence microscope with a Leica DFC7000T camera with motorized z-stage and controlled by Leica Application Suite X (LASX) Software. Images were obtained with either a 20× dry, 40× dry (0.65 NA Fl Plan), or 63× oil (1.3 NA PlanApo) objective without binning. Some images (Fig. 2D,E, 5B) were deconvolved using the 3D Blind Deconvolution module in LASX using 5 total iterations. Images of the MsnYFP (Fig. 1) and E-Cadherin staining (Fig. 5A,C) were collected with a laser-scanning confocal microscope (either Zeiss LSM800 or Leica SPE) using a 40× oil objective (1.3 NA PlanApo). For quantification of ring canal diameters, z-stacks were taken through the egg chamber. The Hts-RC stain was used to identify the plane where each ring canal was in focus, and the maximum outer diameter of the Hts-RC stain for each ring canal was measured using the line tool in Fiji/Image J. All ring canals connecting nurse cells with a clear lumen were measured for each egg chamber, and egg chambers were staged based on their size, DAPI-staining of DNA, and the described follicle cell behaviors that accompany each developmental stage (Jia et al., 2016; Spradling, 1993). Collapsed ring canals were excluded from outer diameter measurements.
Figure 2. Depletion of Misshapen increases ring canal diameter and causes ring canal collapse.
Depletion of Misshapen beginning at stage 2 of oogenesis was performed using matαTub-GAL4. (A) Images of stage 10B control and msn-RNAi egg chambers. (B) Scatter plot showing the outer diameter of individual ring canals connecting nurse cells in control and msn-RNAi egg chambers at each stage. Bars indicate the average outer diameter at each stage. n = 33–101 ring canals/stage for each condition. Asterisks indicate significant difference compared to control (p<0.05, 2-tailed t-test). (C) Average number of ring canals per egg chamber that have a clear lumen or were collapsed. n=6–12 egg chambers/stage. Error bars are SEM. Images of stage 10B control and msn-RNAi egg chambers stained with antibodies against (D) Kelch or (E) phospho-tyrosine. Arrows in (D,E) point to collapsed ring canals. Images in E are maximum intensity projections. Boxes in A, D, and E indicate magnified regions shown in panels to the right. Graphs in A,D,E show the average fluorescence intensity of Hts-RC (n=25 for control, n=19 for msn-RNAi), Kelch (n=20 for each condition), or pTyr (n=12 for control, n=18 for msn-RNAi) staining in ring canals of stage 10 control and msn-RNAi egg chambers. Error bars are SEM. Average full width at half maximum +/− SEM is shown for each stain in each condition. Asterisks indicate significant difference compared to control (p<0.05, 2-tailed t-test).
Figure 5. Altering Msn levels leads to changes in E-cadherin localization and defects in border cell migration.
Images of stage 9 control and msn-RNAi egg chambers stained with antibodies against (A) E-cadherin or (B) phosphorylated β-catenin (pY142). Crosses were performed with either (A) MTD-GAL4 or (B) matαTub-GAL4. (C) Fluorescence images of stage 9 egg chambers expressing Myr-HA-MsnWT (Gal80ts;nanos-GAL4) and stained with the E-cadherin antibody. Asterisk marks the border cell cluster in (A) and (C), and boxes indicate regions of magnification. (D) Images of stage 10 control and Myr-HA-MsnWT expressing egg chambers (matαTub-GAL4) stained with a FasIII antibody to label the polar cells, which are found in the center of the border cell cluster, and an Hts-RC antibody to label the ring canals. Arrows indicate the position of the border cell cluster. The number of ring canals and degree of border cell migration is indicated for the examples shown. (E) Scatter plot showing the number of ring canals and the percent border cell migration in individual Myr-HA-MsnWT expressing stage 10 egg chambers (n=24). All control egg chambers contained 15 ring canals, and the border cells had migrated 100% (n=30).
To quantify the fluorescence intensity of phalloidin (Fig. S2B), Hts-RC (Fig. 2A), Kelch (Fig. 2D), Cheerio-YFP (Fig. S2D), and pTyr (Fig. 2E), the BAR Multichannel Plot Profile tool (Fiji) was used to determine the fluorescence intensity along a line drawn perpendicular to the curve of horizontally-oriented ring canals from stage 10 egg chambers. Lines were always drawn in the cortical to lumenal direction, and the cortical background fluorescence was subtracted for each scan. The scans were aligned at their peaks, and the average fluorescence intensity +/− SEM was plotted for each condition (Fig. S2A). The graph of phalloidin intensity (Fig. S2B) was generated by combining the data from experiments shown in Fig. 2A,D,E, and S2C,D. The full width at half maximum was determined using the Origin Graphing Software (version 6.1; OriginLab Corp); a non-linear curve fit and Gaussian distribution were performed.
To assess border cell migration, measurements from the anterior end of the egg chamber to the anterior edge of the oocyte and from the anterior end of the egg chamber to the posterior edge of the border cell cluster were made using the line tool in Fiji/Image J. These measurements were then used to calculate the percent border cell migration.
Western Blotting
Ovaries from 10 well-fed females were dissected in Schneider’s media (Genesee Scientific) and then ground in homogenization buffer (83mM Tris pH 6.8, 2.7% SDS, 189mM β-mercaptoethanol, and 1X concentration of Proteoguard (Clontech)). Samples were boiled for 10 minutes, spun at 14,000 rpm for 5 minutes, and the supernatant was mixed with 4× Laemmli sample buffer and loaded onto a 4–15% gradient gel (BioRad). The following antibodies were used: Rabbit anti-HA (1:250, Cat. #71-5500, ThermoFisher Scientific), mouse anti-tubulin (1:200, E7; DSHB), anti-mouse DyLight 650 (1:1000, cat #84545 ThermoFisher Scientific) and anti-Rabbit IgG HRP (1:5000, GE Healthcare). To visualize the HA-tagged proteins, Clarity Western ECL substrate was used (BioRad). Immunoblots were imaged using a FluorChemQ (Protein Simple).
Statistical Analysis
A two-tailed student-s t-test was performed with assumed equal variance. Significance was determined at p-values less than 0.05.
Further information about the resources used in this study can be found in the Key Resources Table (KRT).
KEY RESOURCES TABLE.
| Reagent or resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
|
| ||
| Hts-RC | Developmental Studies Hybridoma Bank | htsRC |
| GFP | Developmental Studies Hybridoma Bank | 12A6 |
| Kelch | Developmental Studies Hybridoma Bank | kel1B |
| HA | ThermoFisher Scientific | 71-5500 |
| DE-Cadherin | Developmental Studies Hybridoma Bank | DCAD2 |
| β-catenin pTyr142 | ECM Biosciences | CP1081 |
| FasIII | Developmental Studies Hybridoma Bank | 7G10 |
| pTyr clone 27B10 | Cytoskeleton | APY03 |
| Anti-tubulin | Developmental Studies Hybridoma Bank | E7 |
|
| ||
| Critical Commercial Assays | ||
|
| ||
| RNeasy Plus Mini kit | Qiagen | Cat No. 74134 |
| QuantiNova SYBR Green RT-PCR kit | Qiagen | Cat No. 208152 |
| Clarity Western ECL substrate | BioRad | Cat No. 1705060S |
|
| ||
| Experimental Models: Organisms/Strains | ||
|
| ||
| MTD-GAL4 P{w[+mC]=otu-GAL4::VP16.R}1, w[*]; P{w[+mC]=GAL4-nos.NGT}40; P{w[+mC]=GAL4::VP16-nos.UTR}CG6325[MVD1] |
Bloomington Drosophila Stock Center | 31777 |
|
matαTub-GAL4 w[*]; P{w[+mC]=matalpha4-GAL-VP16}V2H |
Bloomington Drosophila Stock Center | 7062 |
|
matαTub-GAL4 w[*]; P{w[+mC]=matalpha4-GAL-VP16}V37 |
Bloomington Drosophila Stock Center | 7063 |
|
UAS-msn-RNAi y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.HMJ02084}attP40 |
Bloomington Drosophila Stock Center | 42518 |
| Misshapen-YFP w[1118]; PBac{802.P.SVS-2}msn[CPTI003908] |
KYOTO Stock Center (DGGR) | 115454 |
| Cheerio-YFP w[1118]; PBac{602.P.SVS-1}cher[CPTI000847] |
KYOTO Stock Center (DGGR) | 115123 |
| w1118;;UAS-Myr-HA-MsnWT | L. Xu | |
| w1118;;UAS-HA-MsnWT | This paper | |
| w1118;;UAS-HA-MsnKR | This paper | |
| w1118;UAS-Myr-HA-MsnKR | This paper | |
|
| ||
| Oligonucleotides | ||
|
| ||
| Misshapen qRT-PCR | ||
| 5′-TCCCTTGGACAGCAGCGATT-3′ | ||
| 5′ – AGTTCCATCGTTCCTAGCC – 3′ | ||
| GAPDH qRT-PCR | (Giuliani et al., 2014) | |
| 5′ – AAATCGCGGAGCCAAGTAGT - 3′ | ||
| 5′ – CACGATTTTCGCTATGGCCG – 3′ | ||
|
| ||
| Recombinant DNA | ||
|
| ||
| pMK33 with Myr-HA-MsnWT | L. Xu | |
| pENTR3c Dual with HA-MsnWT | This paper | |
| pENTR3c Dual with HA-MsnKR | This paper | |
| pENTR3c Dual with Myr-HA-MsnKR | This paper | |
| UASp-pPW with HA-MsnWT | This paper | |
| UASp-pPW with HA-MsnKR | This paper | |
| UASp-pPW with Myr-HA-MsnKR | This paper | |
|
| ||
| Software and Algorithms | ||
|
| ||
| Fiji/ImageJ | https://fiji.sc/ | |
| Origin | OriginLab Corp | Version 6.1 |
| LASX | Leica | Version 3.3.3.16958 |
|
| ||
| Other | ||
Results
Misshapen localizes to germline ring canals
While studying the role for Misshapen in the rotational migration of the follicle cells (Lewellyn et al., 2013), we noticed that the endogenously tagged MsnYFP localizes to germline ring canals in addition to the somatic follicle cells. MsnYFP is first seen at ring canals in the germarium. In region 1 of the germarium, we found a few examples of MsnYFP localized to ring-like structures (Fig. 1B, upper); however, MsnYFP was more consistently localized at ring canals in region 2a, where actin, Hts-RC, and Cheerio are recruited (Fig. 1B, lower; Robinson et al., 1994). In the early germ cell clusters, MsnYFP is not observed at every ring canal, but when it is visible, it colocalizes with Hts-RC and actin (Fig. 1B,F). In mid-oogenesis, MsnYFP is more consistently found at ring canals; it localizes to both ring canals connecting nurse cells to each other as well as ring canals connecting nurse cells to the oocyte (Fig. 1C). In addition to its co-localization with actin in the inner rim, MsnYFP is enriched just outside of the actin ring (Fig. 1C,D,E); this suggests that there could be a population of Msn that localizes to the outer rim, microvilli structures, or nearby cortex. MsnYFP also localizes to nurse cell membranes, which is most evident in the center of the germ cell cluster where multiple nurse cells contact each other (Fig. 1C,D; arrowhead). The enrichment of MsnYFP on germline ring canals and nurse cell membranes is maintained throughout oogenesis (Fig. 1D), and this localization suggests that it may play a role in the formation, expansion, or stability of these structures.
Misshapen regulates the size and stability of the germline ring canals
Based on the localization of MsnYFP to germline ring canals, we tested whether Msn is required for ring canal structure or function. To avoid defects in formation of the germline cyst, matαTub-GAL4 was used to express the UAS-msn-RNAi transgene beginning at stage 2 of oogenesis (Hudson and Cooley, 2014). This RNAi line has been previously verified to reduce msn mRNA levels by 95% in embryos (Sopko et al., 2014), and qRT-PCR from whole ovaries demonstrated that there was an average of ~50% reduction in msn mRNA in this condition (Fig. S1A, n=4). This level of depletion is consistent with the expression of UAS-msn-RNAi specifically in the germline; the remaining msn mRNA would be found either in the somatic follicle cells, which should be unaffected in this condition, or in the germ cells of younger egg chambers that would not yet be expressing GAL4. Depletion of Msn in MsnYFP-expressing egg chambers further confirmed the specificity of this UAS-RNAi line (Fig. S1B).
Depletion of Msn led to a significant change in the size of the germline ring canals. Using the Hts-RC stain, the outer diameter of ring canals connecting nurse cells was measured at each stage; depletion of Msn led to a significant increase in the diameter of the ring canals from stage 5 through stage 10b (Fig. 2A,B, p<0.05). Despite this increase in diameter, the ring canals expanded with a rate that was similar to controls (from stage 5–10a, there was a 2.70 and 2.75 fold increase for control and msn-RNAi respectively). These data suggest that Msn is required to limit ring canal size but does not affect the ability of the ring canals to expand.
At younger stages, the msn-RNAi egg chambers always contained 15 ring canals, each with a clear lumen; however, as they continued to develop, we found evidence of instability. Collapsed ring canals were observed beginning around stage 9 and increased in frequency through stage 10b (Fig. 2A,C). These collapsed ring canals did not have a clear lumen, and therefore would be unable to support the transfer of cytoplasmic materials. Imaging of late stage egg chambers revealed that there were defects in the cytoplasmic transfer of materials from the nurse cells to the oocyte that occurs during nurse cell dumping; the nurse cells in msn-RNAi egg chambers often contained cytoplasmic material well after stage 11 (Fig. S1C). This ultimately led to the formation of significantly smaller mature eggs; the average volume of control eggs was 9.99 +/− 0.09 × 10−3 mm3, whereas the average volume of the msn-RNAi egg was 9.72 +/− 0.10 × 10−3 mm3 (Fig. S1D; p<0.05). There was also a significant impact on embryonic viability; none of the msn-RNAi embryos hatched compared to 87.5% of control embryos (Fig. S1E; n=48 for controls and n=60 for msn-RNAi). This suggests that Msn is required to maintain ring canal stability and to insure proper nurse cell dumping.
To further characterize the ring canals in msn-RNAi egg chambers, we used linescan analysis to assess the levels and distribution of ring canal markers (Fig. S2A). Although actin was present within the ring canals throughout oogenesis in the msn-RNAi egg chambers, the fluorescence intensity was reduced compared to control egg chambers (Fig. S2B), and the actin was often disorganized around large or abnormally shaped ring canals (Fig. 2A). The inner rim ring canal proteins, Hts-RC and Kelch, were both recruited to ring canals in egg chambers depleted of Msn; however, the fluorescence intensity was reduced, and there was a significant increase in the full width at half maximum for the scans (Fig. 2A,D; p<0.05). Cheerio is a protein that localizes to both the inner and outer rims of the ring canals (Sokol and Cooley, 1999); in msn-RNAi egg chambers, an endogenously tagged Cheerio-YFP still localized to both expanded and collapsed ring canals (Fig. S2C,D). Phospho-tyrosine is one of the first markers that localizes to the ring canals in region 1 of the germarium (Robinson et al., 1994), and this epitope depends on the presence of the Src-family kinase, Src64, and the Tec family kinase, Btk29 (Dodson et al., 1998; Guarnieri et al., 1998; Kelso et al., 2002; Lu et al., 2004). In the msn-RNAi egg chambers, pTyr localized to nurse cell membranes and to most intact and collapsed ring canals; however, the fluorescence intensity varied, even between ring canals in the same egg chamber (Fig. 2E). On the smaller or collapsed ring canals, there was robust pTyr staining, but on some of the larger ring canals, there was a significant reduction in pTyr signal (Fig. 2E). Therefore, although Msn is dispensable for the recruitment of actin and other ring canal proteins, it may promote their continued maintenance or normal turnover throughout oogenesis.
To determine whether Msn is required to regulate ring canal size prior to stage 2, the maternal triple driver (otu-GAL4; nanos-GAL4; nanos-GAL4; referred to as MTD-GAL4) was used to deplete Msn throughout oogenesis. In egg chambers depleted of Msn using the MTD-GAL4, ring canals were significantly larger throughout oogenesis from stages 2a/2b in the germarium through stage 10b (Fig. S2E,F). Ring canal collapse was also observed in these egg chambers, demonstrating that Misshapen is required to limit ring canal size and promote stability throughout oogenesis.
Overexpression of Misshapen reduces ring canal diameter
Because reducing Msn levels led to an increase in the diameter of the germline ring canals, we tested the effect of over-expressing an HA-tagged wild-type (HA-MsnWT) or kinase-inactive form of Msn (HA-MsnKR). The kinase inactive form contains a lysine to an arginine change at position 61, which is important for ATP binding and has been previously used to compromise Msn kinase activity (Kaneko et al., 2011; Li et al., 2014; Li et al., 2015). Expression of HA-MsnWT and HA-MsnKR was verified using antibody staining against the HA tag both in tissue (Fig. 3A,B) and through Western blotting of whole ovary lysate (Fig. 3C). HA-MsnWT is primarily cytosolic and sometimes enriched in puncta; however, it is not strongly localized at ring canals or nurse cell membranes (Fig. 3A,B). In addition to its cytosolic localization, HA-MsnKR localizes to nurse cell membranes and to some ring canals (Fig. 3A,B).
Figure 3. Over-expression of Misshapen decreases ring canal diameter.
(A,B) Fluorescence images of stage 6 and stage 10 HA-MsnWT or HA-MsnKR expressing egg chambers stained with an HA antibody. Boxes indicate regions of magnification shown to the right. The brightness/contrast settings were altered from the larger panels to better visualize ring canal localization. (C) Western blot of whole ovary lysate from control, HA-MsnWT, or HA-MsnKR expressing flies. (D) Fluorescence images of stage 10 control, HA-MsnWT, or HA-MsnKR expressing egg chambers. Boxes indicate regions of magnification shown to the right. (E) Scatter plot showing the outer diameter of individual ring canals connecting nurse cells in control, HA-MsnWT, or HA-MsnKR expressing egg chambers. Bars indicate the average outer diameter at each stage. n = 104–165 ring canals/stage for each condition. Asterisks indicate significant difference compared to control (p<0.05, 2-tailed t-test). All crosses were set up with the maternal triple driver, MTD-GAL4.
Despite the diffuse localization of the HA-MsnWT in the germline, over-expression led to a significant change in ring canal size, without affecting the ability of the ring canals to expand. Expression of HA-MsnWT throughout oogenesis using the maternal triple driver led to a significant decrease in the outer diameter of the ring canals in stages 5, 6, 8, 9, 10a, and 10b (Fig. 3D,E). Although we did not observe evidence of ring canal collapse, we did find examples of extremely small ring canals in egg chambers overexpressing HA-MsnWT. Despite their smaller size, the ring canals expanded at a similar rate as in controls (from stage 5–10a, there was 3.11 and 3.07 fold increase in the outer diameter for control and HA-MsnWT expressing egg chambers). In contrast, over-expression of HA-MsnKR led to a significant increase in ring canal diameter at stages 7, 9, and 10b (Fig. 3E), which is consistent with a mild dominant negative effect of this form of Msn. Overall, this shows that increasing Msn levels reduces ring canal size, again without affecting the ability of the ring canals to expand.
Expression of an activated form of Misshapen leads to ring canal instability and nurse cell fusion
Previous work in multiple tissue types has demonstrated that tethering Msn to the membrane using myristoylation is consistent with constitutive activation (Kaneko et al., 2011; Lewellyn et al., 2013; Mishra et al., 2015). Therefore, we tested whether expression of an activated form of Msn would have an even stronger effect on ring canal size. The nanos-GAL4 driver controlled by a temperature sensitive Gal80 repressor (Gal80ts; nanos-GAL4) was used to express Myr-HA-MsnWT in the germline. Crosses were raised at 18°C, and adult female flies were shifted to 29°C for 48 hours prior to dissection to activate expression of the UAS transgene. The nanos-GAL4 line shows two peaks of GAL4 expression; an early peak in the germline stem cells within the germarium, and then a later peak in mid-oogenesis (Hudson and Cooley, 2014). Antibody staining revealed that Myr-HA-MsnWT was expressed at low levels during early oogenesis, but it began to accumulate at ring canals and nurse cell membranes during the middle stages (Fig. 4A,B), demonstrating that the myristoylation tag enhanced the localization of Msn to these structures.
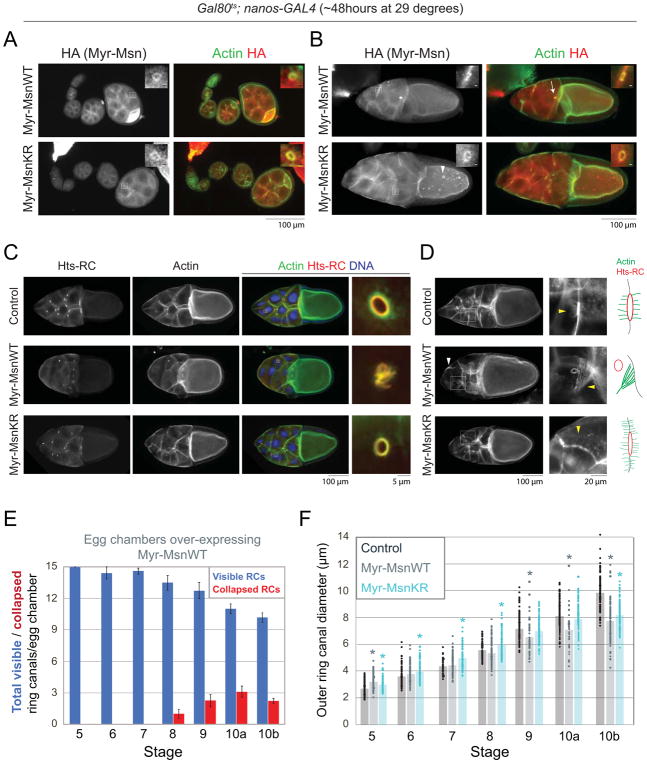
Figure 4. Expression of a membrane-tethered form of Misshapen leads to defects in ring canal stability and nurse cell fusion.
(A,B) Fluorescence images of egg chambers expressing Myr-HA-MsnWT or Myr-HA-MsnKR stained with an HA antibody. In addition to the ring canals and nurse cell membranes, Myr-HA-MsnWT and Myr-HA-MsnKR localize to punctae in the nurse cells and oocyte (arrowhead). Arrow in (B) points to a collapsed ring canal in a stage 10 egg chamber expressing Myr-HA-MsnWT. Boxes indicate regions of magnification. For magnified regions, scale bars are 2μm. (C,D) Fluorescence images of stage 10 control, Myr-HA-MsnWT, or Myr-HA-MsnKR expressing egg chambers. Boxes indicate regions of magnification in panels on the right. White arrowhead in (D) points to large actin bundle. Yellow arrowheads point to actin structures shown in the cartoons to the right. (E) Average number of visible and collapsed ring canals in egg chambers expressing Myr-HA-MsnWT. n=8–17 egg chambers/stage. Error bars are SEM. (F) Scatter plot showing the outer diameter of individual ring canals connecting nurse cells in control, Myr-HA-MsnWT, and Myr-HA-MsnKR expressing egg chambers. Bars indicate the average outer diameter at each stage. n = 44–159 ring canals/stage for each condition. Crosses were set up with the Gal80ts;nanos-GAL4 driver. Asterisks indicate significant difference compared to control (p<0.05, 2-tailed t-test).
Expression of Myr-HA-MsnWT dramatically disrupted the stability of the nurse cells. Beginning in mid-oogenesis, nurse cells begin to fuse with each other, resulting in more than one nucleus per cell. Whereas all of the stage 5 egg chambers contained the expected 15 ring canals, we began to observe an increasing number of missing and collapsed ring canals beginning in stage 6 (Fig. 4B,C,E); this suggests that the multinucleation is caused by nurse cell fusion and not by defects in incomplete cytokinesis. In later stages, up to 90% of the egg chambers contained multinucleate nurse cells. In these cases, the ring canals were often abnormally clustered together, floating in the cytoplasm (Fig. 4D). This phenotype is consistent with a defect in ring canal anchoring, which has been reported in a number of mutants that affect E-cadherin and membrane trafficking (Bogard et al., 2007; Coutelis and Ephrussi, 2007; Dodson et al., 1998; Januschke et al., 2007; Loyer et al., 2015; Murthy et al., 2005; Murthy and Schwarz, 2004; O’Reilly et al., 2006; Peifer et al., 1993; Tan et al., 2014; Vaccari et al., 2009; White et al., 1998). In addition to nurse cell fusion, expression of Myr-HA-MsnWT also caused a significant amount of ring canal collapse (Fig. 4C,E). For the ring canals that did have a clear lumen, the average outer diameter was significantly smaller than in control egg chambers in stages 9, 10a and 10b (Figure 4F, p<0.05). Because Myr-HA-MsnWT localizes to nurse cell membranes and to the remnants of the collapsed ring canals (Fig. 4A,B), this suggests that constitutive activation of Msn destabilizes both of these structures.
We next tested whether the kinase activity was required to produce the nurse cell fusion phenotype observed in egg chambers expressing the membrane-tethered form of Msn. Although Myr-HA-MsnKR localized to ring canals and to nurse cell membranes (Fig. 4A,B), its expression did not cause nurse cell fusion; all egg chambers analyzed contained 15 ring canals. The average outer diameter of the ring canals was significantly larger than controls at stages 5, 6, 7, and 8 (Fig. 4F, p<0.05), again suggesting that the kinase-inactive form of Msn can cause a mild dominant negative effect.
Expression of the membrane-tethered forms of Msn also led to significant changes in the actin cytoskeleton. In stage 10 control egg chambers, there is typically a cloud of actin peripheral to the Hts-RC stain (Fig 1A,4C), which is associated with microvilli structures necessary for ring canal attachment (Loyer et al., 2015; Tilney et al., 1996). When Myr-HA-MsnKR was expressed in the germline, there was still actin visible at the ring canals, but the cloud of actin surrounding the Hts-RC stain was less robust (Fig. 4C). Further, in late stage 10B egg chambers, there were changes to the actin structures that assemble in anticipation of nurse cell dumping (Cooley et al., 1992; Guild et al., 1997; Huelsmann et al., 2013). In control egg chambers, actin cables extend from the ring canals as well as from nurse cell membranes. In egg chambers expressing Myr-HA-MsnWT, there was little actin surrounding many of the ring canals, including the floating ring canals. Actin cables were able to form; however, they were sometimes thicker and less organized compared to controls (Fig. 4D, arrowhead). In egg chambers expressing Myr-HA-MsnKR, the actin cables appeared thinner and were not enriched at ring canals, which produced a “fuzzy membrane” effect (Fig. 4D). These data suggest that tethering Msn to the membrane not only affects ring canal anchoring and membrane integrity, but it can also impact actin structure.
Altering the levels of Misshapen Affects E-cadherin localization
Because egg chambers expressing Myr-HA-MsnWT resembled mutants that affect adherens junctions, and regulation of E-cadherin/Shotgun and β-catenin/Armadillo is necessary for proper ring canal anchoring and growth (Hamada-Kawaguchi et al., 2015; Loyer et al., 2015; Oda et al., 1997; Peifer et al., 1993), we tested whether altering the levels or activity of Msn affected the localization of adherens junction proteins. As was previously reported, E-cadherin normally localizes to nurse cell membranes and is slightly enriched around ring canals (Fig. 5A; Loyer et al., 2015). When Msn was depleted from the germline, the levels of E-cadherin at the nurse cell membranes and within the border cell cluster were comparable to controls; however, there was a strong accumulation of E-cadherin surrounding some of the ring canals in later stage egg chambers (Fig. 5A). This enrichment was not observed at every ring canal in the egg chambers depleted of Msn, but it was seen at collapsed or irregularly-shaped ring canals in 90% of stage 9 and 10 egg chambers and was never observed in control egg chambers (n=10 and n=11 egg chambers respectively). This suggests that part of the msn-RNAi phenotype could be caused by localized defects in E-cadherin regulation at the ring canals.
Recent work has demonstrated that Btk29-mediated phosphorylation of β-catenin on Tyr150 is associated with ring canal growth (Hamada-Kawaguchi et al., 2015). An antibody raised against the Tyr142-phosphorylated form of the mammalian β-catenin is able to recognize this modification (Hamada-Kawaguchi et al., 2014); this antibody was used to stain control and msn-RNAi egg chambers. In control egg chambers, pTyr142 staining was enriched at nurse cell membranes and ring canals. In msn-RNAi egg chambers, pTyr142 staining was still seen at nurse cell membranes and surrounding both intact and collapsed ring canals; however, there were reduced levels of pTyr142 staining at some ring canals (Fig. 5B; ~50% of stage 9 and 10 egg chambers showed reduced levels of pTyr142 on at least 1 ring canal (n=7)). This suggests that there is not a global defect in phosphorylation of β-catenin in the msn-RNAi egg chambers, but there could be local effects on its proper regulation that contribute to the changes in ring canal size and stability.
Because we observed that E-cadherin was enriched surrounding some ring canals following depletion of Msn, we examined E-cadherin staining in egg chambers expressing the membrane-tethered form of Msn. Although there was variability in the levels of E-cadherin between different samples, we observed some egg chambers with a clear decrease in the levels of E-cadherin at the nurse cell membranes compared to the levels in the somatic follicle cells and border cells (Fig 5C). This reduction in staining is consistent with a role for Msn in negatively regulating E-cadherin.
Further evidence that Myr-HA-MsnWT expression reduces E-cadherin levels on the surface of nurse cells came from assessment of border cell migration in this genetic background. During stage 9 of oogenesis, the border cells delaminate from the follicular epithelium and undergo a collective migration from the anterior of the egg chamber to the oocyte at the posterior. Cadherin-cadherin based adhesion between the border cell cluster and the nurse cells is necessary for the migration (Cai et al., 2014; Fulga and Rorth, 2002; Niewiadomska et al., 1999; Oda et al., 1997; Peifer et al., 1993). Live imaging of border cell migration has demonstrated that altering E-cadherin levels in the germline did not affect migration ability, per se, but it did limit the directional persistence of the migration (Cai et al., 2014). Consistent with the reduction in E-cadherin levels observed by immunofluorescence, egg chambers expressing Myr-HA-MsnWT showed a significant defect in border cell migration. In the control egg chambers, the border cell cluster was always able to reach the anterior end of the oocyte by stage 10 (n=30). In contrast, half of the egg chambers expressing Myr-HA-MsnWT had not completed border cell migration by the end of stage 10 (12/24); half of those border cell clusters (6/12) exhibited off target migration and were located in peripheral regions of the egg chamber (Fig. 5D,E). Nurse cell fusion could contribute to the defect in border cell migration; however, we did not see a strict correlation between the number of ring canals present (used to estimate the degree of nurse cell fusion) and the extent of border cell migration in the Myr-HA-MsnWT expressing egg chambers (Fig. 5E). Although we cannot rule out an effect on the speed or persistence of migration, depletion of Msn or expression of the wild type or kinase inactive form of Msn did not significantly affect completion of border cell migration (24/24 msn-RNAi, 25/26 HA-MsnWT expressing, and 23/24 HA-MsnKR expressing stage 10 egg chambers had completed border cell migration compared to 63/64 control egg chambers). Therefore, these results suggest that when Msn is tethered to the membrane, it is able to negatively regulate the levels of E-cadherin at this location.
Discussion
Here, we have shown a novel role for the Ste20 family kinase, Msn, in modulating the size and stability of the germline ring canals. Msn localizes to ring canals and to nurse cell membranes throughout most of oogenesis (Fig. 1), and altering the levels or localization of Msn in the germline leads to changes in ring canal diameter (Fig. 2,3), changes in actin organization (Fig. 2,4), and altered distribution of E-cadherin (Fig. 5). In addition to its role at the ring canals, tethering Msn to the membrane can disrupt nurse cell stability and collective migration of the border cells (Fig. 4,5).
Msn regulates ring canal size and stability
To our knowledge this is the first protein that has been identified whose levels appear to both positively and negatively control ring canal diameter. Depletion of Msn resulted in ring canals that were significantly larger than controls at all stages analyzed (Fig. 2B, S2E,F), whereas over-expression of HA-MsnWT led to smaller ring canals (Fig. 3D,E). A number of mutations have been identified that disrupt ring canal expansion; however, msn-RNAi is one of only a few conditions that increases ring canal diameter (Hamada-Kawaguchi et al., 2015; Morawe et al., 2011) without abolishing the localization of Hts-RC, Kelch, Cheerio, or actin (Fig. 2A,D, S2B–D). Because we have not been able to recover homozygous msn/msn germline clones, we cannot rule out the possibility that Msn is involved in incomplete cytokinesis and ring canal formation in the germline. However, our data support the model that Msn is required to regulate ring canal size and stability, but it is dispensable for ring canal formation and the initial recruitment of ring canal proteins. This suggests that there are special mechanisms in place that limit the size of the ring canals.
Because their role is to facilitate the transfer of material from the nurse cells to the oocyte, it is interesting to consider why limiting ring canal size would be essential during oogenesis. Our data suggest that if ring canals get too large, this can lead to collapse prior to nurse cell dumping. In the msn-RNAi egg chambers, we observed a significant number of collapsed structures, which were labeled with actin, Hts-RC, Kelch, pTyr, and Cheerio (Fig. 2A,D,E, S2C). Because these collapsed structures were not observed during earlier stages of oogenesis (Fig. 2C), it suggests that they are derived from ring canals that have become unstable, potentially via unregulated expansion. Therefore, limiting ring canal size may be an essential mechanism required to maintain long-term stability of these intercellular bridges.
Combining our work with that of others suggests that there could be opposing kinase-dependent pathways that determine the optimal size of the ring canals. As with depletion of Msn, mutation of the Src family kinase, Src64, or the Tec family kinase, Btk29 does not block the recruitment of actin, Kelch, or Hts-RC, but in these mutants, the ring canals are significantly smaller than controls (Dodson et al., 1998; Guarnieri et al., 1998; Lu et al., 2004; Roulier et al., 1998). Despite their smaller size, between stage 5 and stage 10a, the outer diameter of ring canals in Src64 or Btk29 mutant egg chambers still doubles in size; during the same time period, ring canal diameter in wild type egg chambers tripled in size (Guarnieri et al., 1998), which suggests that although the ring canals expand, the normal recruitment and/or turnover of ring canal components is slightly compromised when the level of Src64 or Btk29 is reduced. Interestingly, neither depletion of Msn nor over-expression of HA-MsnWT affected the rate of ring canal expansion from stage 5–10a compared to controls (Fig. 2B,3E; 2.70 and 2.75 fold for control and msn-RNAi; 3.11 and 3.07 fold for control and HA-MsnWT). Although over-expression of Src64 did not significantly affect ring canal size (O’Reilly et al., 2006), mutation of the parcas gene, which is a negative regulator of Btk29, enhances the localization of Btk29 to the ring canal and increases ring canal diameter (Hamada-Kawaguchi et al., 2015). This suggests that Msn, Btk29 and/or Src64 could play opposing roles in controlling ring canal size, and this size control mechanism could function at least partially independently of the regulation of ring canal expansion.
Misshapen may regulate ring canal size and stability through an effect on actin, contractility, and/or adherens junction turnover
Misshapen could control size and stability by altering the organization or turnover rate of actin in the ring canals. Actin is an important component of the ring canals that is initially recruited in the germarium around stage 2a (Fig. 1A; Robinson et al., 1994; Theurkauf et al., 1993). Over the course of oogenesis, there is an almost 10-fold increase in the number of actin filaments in the ring canals as well as a change in filament organization from a more homogeneous structure to a net of bundles; this reorganization depends on the actin-binding protein, Kelch (Tilney et al., 1996), and it may require sliding of actin filaments to allow ring canals to increase in diameter. In addition to the actin within the ring canals, there are also actin filaments associated with microvilli on the nurse cell membranes, especially surrounding the ring canals; these microvilli are organized by E-cadherin and are necessary for ring canal anchoring (Fig. 1A; Loyer et al., 2015; Tilney et al., 1996). Therefore, there are multiple populations of actin filaments that must be controlled and coordinated to regulate ring canal size and anchoring. The Arp2/3 complex is an essential actin nucleator in the germline. Egg chambers with germline mutations in Arp2/3 complex subunits contained smaller ring canals with reduced actin and showed ring canal detachment (Hudson and Cooley, 2002). In msn-RNAi egg chambers, there was a reduction in the fluorescence intensity of the phalloidin stain (Fig. S2B), the actin surrounding the enlarged ring canals was often disorganized (Fig. 2A), expression of Myr-HA-MsnWT led to ring canal detachment (Fig. 4C,D), and expression of Myr-HA-MsnKR largely eliminated the actin peripheral to the Hts-RC stain (Fig. 4C), which likely corresponds to the microvilli structures. Based on these phenotypes, Msn could regulate: 1) the rate of actin nucleation or turnover, 2) actin bundling or sliding, or 3) organization of the microvilli structures to control ring canal size and stability. A homolog of Msn, NIK, phosphorylates the Arp2 subunit to activate the Arp2/3 complex (LeClaire et al., 2015), and during development of the fly eye, Msn functions upstream of the actin-binding protein, Bifocal, to regulate actin organization and promote R cell targeting (Ruan et al., 2002). Although it was shown that actin turns over in the ring canals during stage 10 (Kelso et al., 2002), additional studies will be required to further characterize filament dynamics and to determine the contribution of actin nucleators, actin binding proteins, and other regulators, such as Msn, to these dynamics.
Actomyosin-based contraction is another mechanism that can regulate the size of intercellular bridges. Mutations in members of a myosin light chain phosphatase, Drosophila myosin phosphatase targeting protein (DYMPT), or the serine/threonine phosphatase, Flapwing, lead to over-constriction of the ring canal (Ong et al., 2010; Yamamoto et al., 2013). In C. elegans, another Ste20 family kinase, GCK-1, inhibits the localization of the actin-binding protein, anillin, and non-muscle myosin II, to intercellular bridges in the hermaphrodite gonad. Depletion of GCK-1 or an interacting protein, CCM-3, decreased the perimeter of these intercellular bridges (Rehain-Bell et al., 2017), which suggests that regulation of actomyosin-based contractility is a conserved mechanism to regulate the size of intercellular bridges. In the msn-RNAi egg chambers, we observed both expanded and collapsed ring canals; it is possible that both of these phenotypes could be explained by imbalances in contractile forces in the germline. Higher extrinsic forces could lead to ring canal expansion and potential rupture, and then elasticity or contractility could cause collapse. Future work could test whether reducing Msn levels alters the balance of actomyosin based contractility required to control ring canal size, or whether changes in actin structure indirectly affect contractility in the msn-RNAi egg chambers.
Misshapen could also regulate ring canal size through an effect on E-cadherin recruitment or adherens junction turnover. In the germline, E-cadherin and β-catenin localize to nurse cell membranes, and E-cadherin is weakly enriched around ring canals (Fig. 5A; Loyer et al., 2015; Peifer et al., 1993). Btk29-mediated phosphorylation of β-catenin is required to promote ring canal growth (Hamada-Kawaguchi et al., 2015), likely by increasing adherens junction turnover. Depletion of Msn led to the accumulation of E-cadherin near ring canals (Fig. 5A), which suggests that Msn could negatively regulate E-cadherin levels in the germline. Misshapen and its homologs have been shown to play a similar role in E-cadherin regulation in other systems and contexts (Cobreros-Reguera et al., 2010; Feng et al., 2016; Mishra et al., 2015). In the germline, expression of a dominant negative Rab11 disrupted the normal enrichment of E-cadherin at ring canals (Loyer et al., 2015) and led to ring canal detachment from the plasma membrane (Bogard et al., 2007; Loyer et al., 2015), suggesting that Rab11 could be another key regulator of E-cadherin levels in this context. Because reducing Msn levels partially rescued a Rab11 mutant phenotype in the eye (Tiwari and Roy, 2009), it will be important to test whether Msn and Rab11 function in the same or different pathways to regulate E-cadherin levels and ring canal size in the germline.
Msn could directly regulate actin and adherens junctions to control ring canal size, or it could indirectly control these aspects of egg chamber development through one or more of the other kinases. We observed a decrease in pTyr staining at some ring canals in msn-RNAi egg chambers (Fig. 2E). Because this epitope depends on Src64 and Btk29 (Dodson et al., 1998; Guarnieri et al., 1998; Lu et al., 2004; Roulier et al., 1998), this suggests that decreasing Msn levels could partially reduce the localization or activity of one of these kinases, which could explain some of the observed phenotypes. Actin turnover in the ring canal depends on phosphorylation of Kelch by Src64 (Kelso et al., 2002); therefore, if Msn alters Src64 activity, this could indirectly affect actin turnover and ring canal size. We also found some ring canals in msn-RNAi egg chambers with reduced β-catenin phosphorylation (Fig. 5B), which would be consistent with a decrease in Btk29 activity (Hamada-Kawaguchi et al., 2015) and a local effect on adherens junction turnover. The other Drosophila Src family kinase, Src42A, has been shown to interact with and phosphorylate β-catenin. This β-catenin phosphorylation promotes both adherens junction turnover and activates expression of E-cadherin, allowing for adherens junction remodeling during tissue morphogenesis (Shindo et al., 2008; Takahashi et al., 2005). Src, which can be activated by mechanical stretching (Han et al., 2004; Wang et al., 2005), could act as a sensor to regulate adherens junction remodeling either directly through phosphorylation of β-catenin or indirectly through activation of Btk29 (Guarnieri et al., 1998; Lu et al., 2004; Roulier et al., 1998), to control ring canal size. In the context of dorsal closure, Src42A recruitment to the leading edge cells was dependent on E-cadherin (Takahashi et al., 2005). Therefore, there could be a more complex feedback mechanism to control Src recruitment to and activation at the ring canals, and additional work will be required to determine how Msn fits into this network of regulatory kinases.
Misshapen may also have ring canal-independent roles in the germline
The Msn protein localizes to both ring canals and nurse cell membranes, and over-expression experiments suggest that it could have ring canal-independent roles in the germline. Mutations in E-cadherin, β-catenin, or proteins involved in membrane trafficking (the clathrin adaptor, AP-1, Rab6, Rab11, the exocyst subunits, Sec5/6, the ESCRT complex, or the phospholipid kinase, PI4KIIIα) generate many of the same phenotypes observed in egg chambers expressing Myr-Msn-HA-WT, including changes in actin structures, ring canal detachment, and nurse cell fusion (Fig. 4; Bogard et al., 2007; Coutelis and Ephrussi, 2007; Langevin et al., 2005; Loyer et al., 2015; Murthy et al., 2005; Murthy and Schwarz, 2004; Oda et al., 1997; Peifer et al., 1993; Tan et al., 2014; Vaccari et al., 2009). Further, in egg chambers expressing the membrane-tethered Msn, we observed a defect in border cell migration (Fig. 5D,E), which is consistent with reduced levels of E-cadherin on nurse cell membranes (Cai et al., 2014; Fulga and Rorth, 2002; Niewiadomska et al., 1999; Oda et al., 1997). Although based on an over-expression phenotype, it suggests that Msn could play a more global role in the regulation of adherens junction turnover. Additional experiments will be required to determine the molecular mechanisms that control the spatial and temporal activation of Msn in the germline.
Supplementary Material
(A) Average level of misshapen mRNA remaining in whole ovary extract assessed by qRT-PCR (n=4). (B) Fluorescence images of MsnYFP-expressing control or msn-RNAi egg chambers stained with DAPI. Arrowheads point to MsnYFP localized to nurse cell membranes in the control egg chambers, which is not seen in the msn-RNAi egg chambers. Asterisks mark the somatic border cell clusters; expression of MsnYFP in the border cells should not be affected by germline depletion of Msn. (C) Images of stage 13/14 control and msn-RNAi egg chambers. Egg chambers depleted of Msn often showed defects in the cytoplasmic transfer (nurse cell dumping) that occurs during stage 11. Arrows point to the remaining cytoplasmic contents in the nurse cells of msn-RNAi egg chambers. (D) Scatter plot showing the volumes of individual mature eggs. Average volume +/− SEM for control and msn-RNAi eggs is indicated (n=57 and 58 for control and msn-RNAi). Asterisks indicate significant difference compared to control (p<0.05, 2-tailed t-test). (E) Hatching rate of embryos that developed from control egg chambers and egg chambers depleted of Msn from the germline.
(A) Schematic of the linescan analysis. (B) Average fluorescence intensity of phalloidin stain in stage 10 ring canals in control (n=66) and msn-RNAi (n=69) egg chambers from experiments shown in Fig. 2A,D,E, and S2C. Average full width at half max +/− SEM is indicated for each. (C) CherYFP localization in control and msn-RNAi egg chambers. Box indicates region of magnification. Arrow points to a collapsed ring canal. (D) Average fluorescence intensity of CherYFP in ring canals from control (n=9) and msn-RNAi (n=12) stage 10 egg chambers. Average full width at half max +/− SEM is indicated for each. (A-D) Crosses were performed using matαTub-GAL4. The maternal triple driver (MTD-GAL4) was also used to deplete the Msn protein throughout oogenesis. Average outer diameter of ring canals connecting nurse cells in control and msn-RNAi egg chambers in (E) early and (F) mid-late oogenesis. n= 42–230 or 11–132 ring canals/stage/condition for E and F respectively. All error bars are SEM. Asterisks indicate significant difference compared to control (p<0.05, 2-tailed t-test).
Highlights.
The Ste20 kinase, Misshapen (Msn), localizes to germline ring canals and nurse cell membranes in the Drosophila egg chamber
Depletion of Msn increases ring canal diameter and causes ring canal collapse, whereas over-expression of Msn reduces ring canal diameter
Expression of a membrane-tethered form of Misshapen causes ring canal detachment and nurse cell fusion
Changing the levels or localization of Misshapen alters E-cadherin distribution and border cell migration
Acknowledgments
We are grateful to Sally Horne-Badovinac and Maureen Cetera for insightful comments on this manuscript. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number 1R15HD084243-01A1 (L.L.). Multiple antibodies used in this study were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bogard N, Lan L, Xu J, Cohen RS. Rab11 maintains connections between germline stem cells and niche cells in the Drosophila ovary. Development. 2007;134:3413–3418. doi: 10.1242/dev.008466. [DOI] [PubMed] [Google Scholar]
- Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, Sawyer JK, Danuser G, Montell DJ. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell. 2014;157:1146–1159. doi: 10.1016/j.cell.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobreros-Reguera L, Fernandez-Minan A, Fernandez-Espartero CH, Lopez-Schier H, Gonzalez-Reyes A, Martin-Bermudo MD. The Ste20 kinase misshapen is essential for the invasive behaviour of ovarian epithelial cells in Drosophila. EMBO Rep. 2010;11:943–949. doi: 10.1038/embor.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley L, Verheyen E, Ayers K. chickadee encodes a profilin required for intercellular cytoplasm transport during Drosophila oogenesis. Cell. 1992;69:173–184. doi: 10.1016/0092-8674(92)90128-y. [DOI] [PubMed] [Google Scholar]
- Coutelis JB, Ephrussi A. Rab6 mediates membrane organization and determinant localization during Drosophila oogenesis. Development. 2007;134:1419–1430. doi: 10.1242/dev.02821. [DOI] [PubMed] [Google Scholar]
- Dodson GS, Guarnieri DJ, Simon MA. Src64 is required for ovarian ring canal morphogenesis during Drosophila oogenesis. Development. 1998;125:2883–2892. doi: 10.1242/dev.125.15.2883. [DOI] [PubMed] [Google Scholar]
- Feng XJ, Pan Q, Wang SM, Pan YC, Wang Q, Zhang HH, Zhu MH, Zhang SH. MAP4K4 promotes epithelial-mesenchymal transition and metastasis in hepatocellular carcinoma. Tumour Biol. 2016;37:11457–11467. doi: 10.1007/s13277-016-5022-1. [DOI] [PubMed] [Google Scholar]
- Fulga TA, Rorth P. Invasive cell migration is initiated by guided growth of long cellular extensions. Nat Cell Biol. 2002;4:715–719. doi: 10.1038/ncb848. [DOI] [PubMed] [Google Scholar]
- Giuliani G, Giuliani F, Volk T, Rabouille C. The Drosophila RNA-binding protein HOW controls the stability of dgrasp mRNA in the follicular epithelium. Nucleic Acids Res. 2014;42:1970–1986. doi: 10.1093/nar/gkt1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri DJ, Dodson GS, Simon MA. SRC64 regulates the localization of a Tec-family kinase required for Drosophila ring canal growth. Molecular cell. 1998;1:831–840. doi: 10.1016/s1097-2765(00)80082-9. [DOI] [PubMed] [Google Scholar]
- Guild GM, Connelly PS, Shaw MK, Tilney LG. Actin filament cables in Drosophila nurse cells are composed of modules that slide passively past one another during dumping. The Journal of cell biology. 1997;138:783–797. doi: 10.1083/jcb.138.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada-Kawaguchi N, Nishida Y, Yamamoto D. Btk29A-mediated tyrosine phosphorylation of armadillo/beta-catenin promotes ring canal growth in Drosophila oogenesis. PLOS ONE. 2015;10:e0121484. doi: 10.1371/journal.pone.0121484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada-Kawaguchi N, Nore BF, Kuwada Y, Smith CI, Yamamoto D. Btk29A promotes Wnt4 signaling in the niche to terminate germ cell proliferation in Drosophila. Science. 2014;343:294–297. doi: 10.1126/science.1244512. [DOI] [PubMed] [Google Scholar]
- Han B, Bai XH, Lodyga M, Xu J, Yang BB, Keshavjee S, Post M, Liu M. Conversion of mechanical force into biochemical signaling. J Biol Chem. 2004;279:54793–54801. doi: 10.1074/jbc.M406880200. [DOI] [PubMed] [Google Scholar]
- Hudson AM, Cooley L. A subset of dynamic actin rearrangements in Drosophila requires the Arp2/3 complex. The Journal of cell biology. 2002;156:677–687. doi: 10.1083/jcb.200109065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AM, Cooley L. Drosophila Kelch functions with Cullin-3 to organize the ring canal actin cytoskeleton. The Journal of cell biology. 2010;188:29–37. doi: 10.1083/jcb.200909017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AM, Cooley L. Methods for studying oogenesis. Methods. 2014;68:207–217. doi: 10.1016/j.ymeth.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AM, Mannix KM, Cooley L. Actin Cytoskeletal Organization in Drosophila Germline Ring Canals Depends on Kelch Function in a Cullin-RING E3 Ligase. Genetics. 2015;201:1117–1131. doi: 10.1534/genetics.115.181289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsmann S, Ylanne J, Brown NH. Filopodia-like actin cables position nuclei in association with perinuclear actin in Drosophila nurse cells. Developmental cell. 2013;26:604–615. doi: 10.1016/j.devcel.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januschke J, Nicolas E, Compagnon J, Formstecher E, Goud B, Guichet A. Rab6 and the secretory pathway affect oocyte polarity in Drosophila. Development. 2007;134:3419–3425. doi: 10.1242/dev.008078. [DOI] [PubMed] [Google Scholar]
- Jia D, Xu Q, Xie Q, Mio W, Deng WM. Automatic stage identification of Drosophila egg chamber based on DAPI images. Sci Rep. 2016;6:18850. doi: 10.1038/srep18850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Chen X, Lu P, Yao X, Wright TG, Rajurkar M, Kariya K, Mao J, Ip YT, Xu L. Smad inhibition by the Ste20 kinase Misshapen. Proc Natl Acad Sci U S A. 2011;108:11127–11132. doi: 10.1073/pnas.1104128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso RJ, Hudson AM, Cooley L. Drosophila Kelch regulates actin organization via Src64-dependent tyrosine phosphorylation. The Journal of cell biology. 2002;156:703–713. doi: 10.1083/jcb.200110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, Camonis J, Bellaiche Y. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Developmental cell. 2005;9:365–376. doi: 10.1016/j.devcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- LeClaire LL, Rana M, Baumgartner M, Barber DL. The Nck-interacting kinase NIK increases Arp2/3 complex activity by phosphorylating the Arp2 subunit. The Journal of cell biology. 2015;208:161–170. doi: 10.1083/jcb.201404095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewellyn L, Cetera M, Horne-Badovinac S. Misshapen decreases integrin levels to promote epithelial motility and planar polarity in Drosophila. The Journal of cell biology. 2013;200:721–729. doi: 10.1083/jcb.201209129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MG, Serr M, Edwards K, Ludmann S, Yamamoto D, Tilney LG, Field CM, Hays TS. Filamin is required for ring canal assembly and actin organization during Drosophila oogenesis. The Journal of cell biology. 1999;146:1061–1074. doi: 10.1083/jcb.146.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Li S, Mana-Capelli S, Roth Flach RJ, Danai LV, Amcheslavsky A, Nie Y, Kaneko S, Yao X, Chen X, Cotton JL, Mao J, McCollum D, Jiang J, Czech MP, Xu L, Ip YT. The conserved misshapen-warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Developmental cell. 2014;31:291–304. doi: 10.1016/j.devcel.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cho YS, Yue T, Ip YT, Jiang J. Overlapping functions of the MAP4K family kinases Hppy and Msn in Hippo signaling. Cell Discov. 2015;1:15038. doi: 10.1038/celldisc.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lowe N, Rees JS, Roote J, Ryder E, Armean IM, Johnson G, Drummond E, Spriggs H, Drummond J, Magbanua JP, Naylor H, Sanson B, Bastock R, Huelsmann S, Trovisco V, Landgraf M, Knowles-Barley S, Armstrong JD, White-Cooper H, Hansen C, Phillips RG, Lilley KS, Russell S, St Johnston D Consortium UKDPTS. Analysis of the expression patterns, subcellular localisations and interaction partners of Drosophila proteins using a pigP protein trap library. Development. 2014;141:3994–4005. doi: 10.1242/dev.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyer N, Kolotuev I, Pinot M, Le Borgne R. Drosophila E-cadherin is required for the maintenance of ring canals anchoring to mechanically withstand tissue growth. Proc Natl Acad Sci U S A. 2015;112:12717–12722. doi: 10.1073/pnas.1504455112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu N, Guarnieri DJ, Simon MA. Localization of Tec29 to ring canals is mediated by Src64 and PtdIns(3,4,5)P3-dependent mechanisms. The EMBO journal. 2004;23:1089–1100. doi: 10.1038/sj.emboj.7600127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye CM, Naylor HW, Sanson B. Subcellular localisations of the CPTI collection of YFP-tagged proteins in Drosophila embryos. Development. 2014;141:4006–4017. doi: 10.1242/dev.111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald AP. The formation of ring canals by cell furrows in Drosophila. Zeitschrift fur Zellforschung und mikroskopische Anatomie. 1971;118:162–167. doi: 10.1007/BF00341561. [DOI] [PubMed] [Google Scholar]
- Mishra AK, Sachan N, Mutsuddi M, Mukherjee A. Kinase active Misshapen regulates Notch signaling in Drosophila melanogaster. Experimental cell research. 2015;339:51–60. doi: 10.1016/j.yexcr.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Morawe T, Honemann-Capito M, von Stein W, Wodarz A. Loss of the extraproteasomal ubiquitin receptor Rings lost impairs ring canal growth in Drosophila oogenesis. The Journal of cell biology. 2011;193:71–80. doi: 10.1083/jcb.201009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M, Ranjan R, Denef N, Higashi ME, Schupbach T, Schwarz TL. Sec6 mutations and the Drosophila exocyst complex. Journal of cell science. 2005;118:1139–1150. doi: 10.1242/jcs.01644. [DOI] [PubMed] [Google Scholar]
- Murthy M, Schwarz TL. The exocyst component Sec5 is required for membrane traffic and polarity in the Drosophila ovary. Development. 2004;131:377–388. doi: 10.1242/dev.00931. [DOI] [PubMed] [Google Scholar]
- Niewiadomska P, Godt D, Tepass U. DE-Cadherin is required for intercellular motility during Drosophila oogenesis. The Journal of cell biology. 1999;144:533–547. doi: 10.1083/jcb.144.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly AM, Ballew AC, Miyazawa B, Stocker H, Hafen E, Simon MA. Csk differentially regulates Src64 during distinct morphological events in Drosophila germ cells. Development. 2006;133:2627–2638. doi: 10.1242/dev.02423. [DOI] [PubMed] [Google Scholar]
- Oda H, Uemura T, Takeichi M. Phenotypic analysis of null mutants for DE-cadherin and Armadillo in Drosophila ovaries reveals distinct aspects of their functions in cell adhesion and cytoskeletal organization. Genes Cells. 1997;2:29–40. doi: 10.1046/j.1365-2443.1997.d01-284.x. [DOI] [PubMed] [Google Scholar]
- Ong S, Foote C, Tan C. Mutations of DMYPT cause over constriction of contractile rings and ring canals during Drosophila germline cyst formation. Developmental biology. 2010;346:161–169. doi: 10.1016/j.ydbio.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Peifer M, Orsulic S, Sweeton D, Wieschaus E. A role for the Drosophila segment polarity gene armadillo in cell adhesion and cytoskeletal integrity during oogenesis. Development. 1993;118:1191–1207. doi: 10.1242/dev.118.4.1191. [DOI] [PubMed] [Google Scholar]
- Rees JS, Lowe N, Armean IM, Roote J, Johnson G, Drummond E, Spriggs H, Ryder E, Russell S, St Johnston D, Lilley KS. In vivo analysis of proteomes and interactomes using Parallel Affinity Capture (iPAC) coupled to mass spectrometry. Mol Cell Proteomics. 2011;10:M110002386. doi: 10.1074/mcp.M110.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehain-Bell K, Love A, Werner ME, MacLeod I, Yates JR, 3rd, Maddox AS. A Sterile 20 Family Kinase and Its Co-factor CCM-3 Regulate Contractile Ring Proteins on Germline Intercellular Bridges. Current biology : CB. 2017;27:860–867. doi: 10.1016/j.cub.2017.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DN, Cant K, Cooley L. Morphogenesis of Drosophila ovarian ring canals. Development. 1994;120:2015–2025. doi: 10.1242/dev.120.7.2015. [DOI] [PubMed] [Google Scholar]
- Robinson DN, Cooley L. Stable intercellular bridges in development: the cytoskeleton lining the tunnel. Trends in cell biology. 1996;6:474–479. doi: 10.1016/0962-8924(96)84945-2. [DOI] [PubMed] [Google Scholar]
- Robinson DN, Smith-Leiker TA, Sokol NS, Hudson AM, Cooley L. Formation of the Drosophila ovarian ring canal inner rim depends on cheerio. Genetics. 1997;145:1063–1072. doi: 10.1093/genetics/145.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulier EM, Panzer S, Beckendorf SK. The Tec29 tyrosine kinase is required during Drosophila embryogenesis and interacts with Src64 in ring canal development. Molecular cell. 1998;1:819–829. doi: 10.1016/s1097-2765(00)80081-7. [DOI] [PubMed] [Google Scholar]
- Ruan W, Long H, Vuong DH, Rao Y. Bifocal is a downstream target of the Ste20-like serine/threonine kinase misshapen in regulating photoreceptor growth cone targeting in Drosophila. Neuron. 2002;36:831–842. doi: 10.1016/s0896-6273(02)01027-9. [DOI] [PubMed] [Google Scholar]
- Shindo M, Wada H, Kaido M, Tateno M, Aigaki T, Tsuda L, Hayashi S. Dual function of Src in the maintenance of adherens junctions during tracheal epithelial morphogenesis. Development. 2008;135:1355–1364. doi: 10.1242/dev.015982. [DOI] [PubMed] [Google Scholar]
- Sokol NS, Cooley L. Drosophila filamin encoded by the cheerio locus is a component of ovarian ring canals. Current biology : CB. 1999;9:1221–1230. doi: 10.1016/s0960-9822(99)80502-8. [DOI] [PubMed] [Google Scholar]
- Sopko R, Foos M, Vinayagam A, Zhai B, Binari R, Hu Y, Randklev S, Perkins LA, Gygi SP, Perrimon N. Combining genetic perturbations and proteomics to examine kinase-phosphatase networks in Drosophila embryos. Developmental cell. 2014;31:114–127. doi: 10.1016/j.devcel.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC. Developmental genetics of oogenesis. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; 1993. pp. 1–70. [Google Scholar]
- Takahashi M, Takahashi F, Ui-Tei K, Kojima T, Saigo K. Requirements of genetic interactions between Src42A, armadillo and shotgun, a gene encoding E-cadherin, for normal development in Drosophila. Development. 2005;132:2547–2559. doi: 10.1242/dev.01850. [DOI] [PubMed] [Google Scholar]
- Tan J, Oh K, Burgess J, Hipfner DR, Brill JA. PI4KIIIalpha is required for cortical integrity and cell polarity during Drosophila oogenesis. Journal of cell science. 2014;127:954–966. doi: 10.1242/jcs.129031. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Alberts BM, Jan YN, Jongens TA. A central role for microtubules in the differentiation of Drosophila oocytes. Development. 1993;118:1169–1180. doi: 10.1242/dev.118.4.1169. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, Guild GM. Formation of actin filament bundles in the ring canals of developing Drosophila follicles. The Journal of cell biology. 1996;133:61–74. doi: 10.1083/jcb.133.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari AK, Roy JK. Mutation in Rab11 results in abnormal organization of ommatidial cells and activation of JNK signaling in the Drosophila eye. Eur J Cell Biol. 2009;88:445–460. doi: 10.1016/j.ejcb.2009.02.188. [DOI] [PubMed] [Google Scholar]
- Vaccari T, Rusten TE, Menut L, Nezis IP, Brech A, Stenmark H, Bilder D. Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. Journal of cell science. 2009;122:2413–2423. doi: 10.1242/jcs.046391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- White P, Aberle H, Vincent JP. Signaling and adhesion activities of mammalian beta-catenin and plakoglobin in Drosophila. The Journal of cell biology. 1998;140:183–195. doi: 10.1083/jcb.140.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Cooley L. kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell. 1993;72:681–693. doi: 10.1016/0092-8674(93)90397-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Bayat V, Bellen HJ, Tan C. Protein phosphatase 1β limits ring canal constriction during Drosophila germline cyst formation. PloS one. 2013;8:e70502. doi: 10.1371/journal.pone.0070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Spradling AC. hu-li tai shao, a gene required for ring canal formation during Drosophila oogenesis, encodes a homolog of adducin. Genes & development. 1992;6:2443–2454. doi: 10.1101/gad.6.12b.2443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Average level of misshapen mRNA remaining in whole ovary extract assessed by qRT-PCR (n=4). (B) Fluorescence images of MsnYFP-expressing control or msn-RNAi egg chambers stained with DAPI. Arrowheads point to MsnYFP localized to nurse cell membranes in the control egg chambers, which is not seen in the msn-RNAi egg chambers. Asterisks mark the somatic border cell clusters; expression of MsnYFP in the border cells should not be affected by germline depletion of Msn. (C) Images of stage 13/14 control and msn-RNAi egg chambers. Egg chambers depleted of Msn often showed defects in the cytoplasmic transfer (nurse cell dumping) that occurs during stage 11. Arrows point to the remaining cytoplasmic contents in the nurse cells of msn-RNAi egg chambers. (D) Scatter plot showing the volumes of individual mature eggs. Average volume +/− SEM for control and msn-RNAi eggs is indicated (n=57 and 58 for control and msn-RNAi). Asterisks indicate significant difference compared to control (p<0.05, 2-tailed t-test). (E) Hatching rate of embryos that developed from control egg chambers and egg chambers depleted of Msn from the germline.
(A) Schematic of the linescan analysis. (B) Average fluorescence intensity of phalloidin stain in stage 10 ring canals in control (n=66) and msn-RNAi (n=69) egg chambers from experiments shown in Fig. 2A,D,E, and S2C. Average full width at half max +/− SEM is indicated for each. (C) CherYFP localization in control and msn-RNAi egg chambers. Box indicates region of magnification. Arrow points to a collapsed ring canal. (D) Average fluorescence intensity of CherYFP in ring canals from control (n=9) and msn-RNAi (n=12) stage 10 egg chambers. Average full width at half max +/− SEM is indicated for each. (A-D) Crosses were performed using matαTub-GAL4. The maternal triple driver (MTD-GAL4) was also used to deplete the Msn protein throughout oogenesis. Average outer diameter of ring canals connecting nurse cells in control and msn-RNAi egg chambers in (E) early and (F) mid-late oogenesis. n= 42–230 or 11–132 ring canals/stage/condition for E and F respectively. All error bars are SEM. Asterisks indicate significant difference compared to control (p<0.05, 2-tailed t-test).