Abstract
Proliferin (PLF) is an angiogenic placental hormone. We now report that PLF gene expression can also occur in a progressive fibrosarcoma mouse tumor cell model. PLF mRNA and protein are detectable at very low levels in cell lines derived from the mild noninvasive stage of tumor development. Expression is greatly augmented in cell lines from the aggressively invasive stage of development, a stage at which the tumor becomes highly angiogenic, and PLF expression remains high in cell lines from the end stage of fibrosarcoma. Activator protein 1 factors present at high levels in the more invasive stages of the tumor may in part allow for increased PLF expression, as cells from the mild stage in which c-jun and junB are stably expressed secrete levels of PLF comparable to that of the advanced stages. Secreted PLF protein is functionally important in tumor cell angiogenic activity, as demonstrated by the reduction of angiogenic activity in fibrosarcoma cell culture medium by immunodepletion of PLF. These results suggest that an extraembryonic genetic program, which has evolved to support fetal growth, may be reactivated in certain tumors and contribute to tumor growth.
In eutherian mammals, the placenta has evolved to support fetal development not only as the physical link to maternal sources of nutrition and for waste management, but also as a source of circulating factors that regulate maternal physiology. Shortly after implantation, the needs of the growing fetus exceed the limited nourishment supplied by diffusing metabolites. In response, cells of trophoblastic origin acquire an invasive phenotype and adopt many endothelial cell attributes. As the placenta grows and differentiates, distinct trophoblast cell types arise and express a variety of hormones that act on maternal targets. In rodents, a large family of proteins closely related to prolactin is often uniquely expressed by the placenta (1). These hormones have diverse actions on maternal targets, including the prolactin-like leuteotropic effect of the placental lactogens (2), anti-cytolytic action on natural killer cells of prolactin-like protein A (3), and megakaryocytopoietic effects of prolactin-like protein E (4).
The prolactin-related hormone proliferin [PLF, also known as mitogen-regulated protein (MRP); ref. 5] was originally identified as a growth factor-inducible gene in immortalized mouse fibroblasts (6, 7), but in vivo is produced primarily by the trophoblast giant cells (8). Expression of PLF in the placenta is developmentally regulated, with hormone production peaking at mid-gestation (8, 9). PLF proteins with nearly identical amino acid sequences are encoded by four or more distinct genes, and all of these genes are expressed in the placenta (9–11). PLF is angiogenic (12) and seems to be important for the migration of uterine vascular endothelial cells toward the giant cells (13), the outer layer of the extraembryonic compartment at the implantation site. MRP/PLF has also been shown to stimulate DNA synthesis in uterine cells (14). Secreted PLF protein accumulates to high levels in both the maternal circulation and in the fetal compartment (8), and PLF binding to developing structures in the fetus suggests roles on both endothelial cells and on other cell types (15).
PLF expression has been found in vivo outside of the placenta, notably in skin (16, 17), consistent with the possibility that PLF may participate in angiogenesis more broadly than during pregnancy. The finding that PLF is expressed in certain immortalized cells suggests that reactivation of PLF expression may occur in tumor cells and contribute to tumor growth and metastasis, processes which depend on the ability of a tumor to secrete net angiogenic activity (18). Certain tumor types might be considered strong candidates for expressing and using PLF as an angiogenic factor. Inducible PLF gene transcription in response to serum growth factors involves activator protein 1 (AP-1; refs. 19 and 20) and is inhibited by glucocorticoids (19). Moreover, the effect of glucocorticoids through a specific PLF gene regulatory element can be converted to a positive response depending on the composition of AP-1 factors present, with jun dimers acting positively and jun-fos heterodimers acting negatively; this element was therefore designated as a “composite glucocorticoid response element” (21). Thus, tumors in which AP-1 activity increases as a result of elevated jun levels, and in which glucocorticoid responsiveness also increases, would be particularly attractive as candidates for the participation of PLF in tumor growth.
One tumor model satisfying these criteria is a progressive fibrosarcoma that develops in transgenic mice harboring the bovine papillomavirus type 1 genome (22). After about 12 months of age, these mice acquire dermal fibrosarcomas. Analysis of tissue biopsies allows for the segregation of dermal dysplasia into distinct stages of tumor progression: mild fibromatosis, a superficial proliferative stage; aggressive fibromatosis, a less differentiated stage engaged in blood vessel recruitment and violation of the basal membrane; and fibrosarcoma, a well vascularized, anaplastic, and invasive stage. Analysis of cell lines derived from these tumor stages has revealed that increased expression of junB and c-jun, but not junD and fos, occurs in aggressive fibromatosis and fibrosarcoma and contributes to the progression of the tumor (23); junB and c-jun also are the primary jun family mediators of serum-dependent PLF expression in mouse fibroblasts (20). Glucocorticoid receptor activity also increases with tumor stage (24). We therefore asked whether PLF is expressed in cell lines derived from a late stage, but not from an early stage, of tumor progression. If PLF is expressed in later stages, then the contribution of PLF to tumor angiogenesis can also be evaluated.
Materials and Methods
Cell Culture.
The mild fibromatosis, aggressive fibromatosis, and fibrosarcoma cell lines have been described, as has the mild fibromatosis cell line stably transfected with c-jun and junB expression constructs (23). All cell lines were propagated in DMEM supplemented with 10% FBS, 2 mM glutamine, and a combination of antibiotic-antimycotic (all from Life Technologies, Rockville, MD) in an atmosphere containing 5% CO2.
Human dermal microvascular endothelial cells (HMVECs) were cultured from foreskins as described (25), by panning on the anti-endothelial cell mAb EN4. Cells were grown in MCDB131 medium (Sigma) supplemented with endothelial growth supplements (EGM bullet kit, BioWhittaker). Migrations were performed as described (26). Briefly, cells were plated in serum-free medium in a modified Boyden chamber (Neuro Probe, Gaithersburg, MD) on the bottom side of the microporous gelatinized polycarbonate membrane and allowed to migrate up the gradient of the test substance. Membranes were fixed and stained with Diff-Quick (Fisher), and the number of cells that had migrated to the top side of the membrane in 10 high-powered fields was subsequently counted. Each condition was tested in quadruplicate.
RNA Analysis.
Total RNA was prepared from the various cell lines with Tri-Reagent (Sigma) according to the manufacturer's instructions. A coupled reverse transcription (RT)-PCR was used to detect PLF mRNA with oligonucleotide primers specific to PLF. Included in the reactions was an [α-32P]deoxyribonucleotide to generate radiolabeled products. The extent of amplification was varied to enable comparisons among reaction product amounts with increasing cycle number. To distinguish among the various PLF mRNAs, RT-PCR products were treated with diagnostic restriction endonucleases based on the approach described for the PLF/MRP mRNAs by Nilsen-Hamilton and colleagues (16).
For filter hybridizations, 20 μg of total RNA per lane was separated on 1% formaldehyde-agarose gels, transferred to nylon membranes (Schleicher & Schuell), and exposed to UV light to cross-link the RNA to the membranes. 32P-labeled riboprobes were synthesized by means of random priming and purified by using MicroSpin S-300 h columns (Amersham Pharmacia Biotech). Hybridizations were carried out for 12–16 h at 68°C in 50% formamide/5× SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7)/4× Denhardt's solution (0.02% polyvinylpyrrolidone/0.02% Ficoll/0.02% BSA)/0.1% SDS/0.1% sodium pyrophosphate. After hybridization, membranes were washed twice in 2× SSC, twice in 0.5× SSC/0.1% SDS at 65°C, and twice in 0.1× SSC/0.1% SDS at 65°C.
Protein Preparation and Analysis.
Cell cultures were washed several times with PBS, and medium was replaced with serum-free medium for a 24-h period. Conditioned medium was concentrated before further analysis by using Ultrafree-15/30 kilodalton cut-off centrifugal filters (Millipore). For immunoblot analysis, equal amounts of secreted protein, as determined with the Bio-Rad protein assay solution (a dye-binding spectrophotometric assay), were fractionated by gel electrophoresis and probed with mAb 209 against PLF followed by sheep-anti-mouse Ab coupled to horseradish peroxidase (Amersham Pharmacia Biotech). The anti-PLF mAb was purified by using HiTrap Protein G columns (Amersham Pharmacia Biotech) from culture medium of a hybridoma cell line generated by S.-J. Lee and D. Nathans (The Johns Hopkins University School of Medicine, Baltimore).
To remove PLF protein from fibrosarcoma-conditioned medium, two different methods gave similar results. In one, medium was passed repeatedly over an immunoaffinity column prepared by using anti-PLF mAb 209 coupled to Affi-Gel 10 according to manufacturer's instructions (Bio-Rad). Alternatively, concentrated medium was incubated with mAb 209 or with a control Ab against PLF-related protein, monoclonal 28.1.1 (27). After an overnight incubation at 4°C, protein G Sepharose beads preequilibrated with PBS were added to the samples to remove Ab and Ab-antigen conjugates.
Neovascularization Assay.
Slow-release Hydron pellets containing test substances were placed in corneal micropockets surgically created in the eyes of anesthetized 6-week-old Fisher rats (National Cancer Institute). After 7 days, corneal neovascularization was assessed by using slit-lamp microscopy (16-fold magnification). All animal experiments were approved by the Northwestern University Animal Care and Use Committee.
Results
PLF Expression During Fibrosarcoma Development.
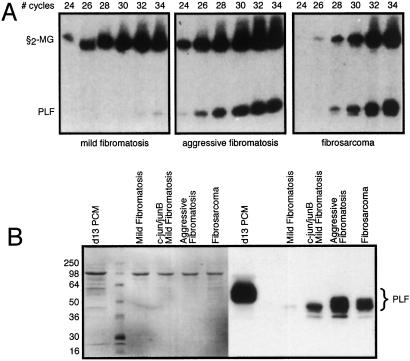
To determine whether PLF is expressed during fibrosarcoma development, RNA was isolated from mild fibromatosis, aggressive fibromatosis, and fibrosarcoma-derived cell lines and analyzed by RT-PCR. Several independently derived cell lines were examined, and although some variation in expression levels was noted, the level of PLF expression correlates strongly with the degree of cell transformation and tumorigenicity. PLF mRNA is barely detectable in the mild fibromatosis stage but is greatly elevated in both aggressive fibromatosis and fibrosarcoma (Fig. 1A); the amount of β2-microglobulin mRNA served as an internal RT-PCR control in this experiment. Consistent with these results, PLF protein is secreted at much greater levels by aggressive fibromatosis and fibrosarcoma cells than by the mild fibromatosis cells (Fig. 1B). Equal loading of each lane was verified by staining with Ponceau-S (Sigma; Fig. 1B Left). PLF is a glycoprotein, and based on electrophoretic mobility, the forms of PLF secreted by the tumor cells appear to be less extensively glycosylated than PLF protein secreted from cultures of placental tissue (PCM in Fig. 1B).
Figure 1.
Tumor stage-specific expression of PLF. (A) RT-PCR with primers specific for PLF and β2-macroglobulin mRNAs was carried out with total RNA from mild fibromatosis (Left), aggressive fibromatosis (Center), and fibrosarcoma (Right) cells. Products were analyzed after 24–34 PCR cycles. The amount of PLF mRNA detected at the fibrosarcoma stage is ≈20-fold greater than the level at the mild fibromatosis stage. (B) Serum-free media were collected from cultured mild fibromatosis, aggressive fibromatosis, and fibrosarcoma cells, and from mild fibromatosis cells stably expressing the AP-1 factors c-jun and junB. Proteins in these samples were fractionated by gel electrophoresis and PLF then detected by immunoblotting (Right). Medium from cultured placental tissue collected on day 13 of gestation served as a positive control, and Ponceau-S staining (Left) demonstrates equal protein loading.
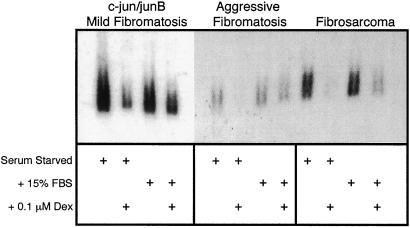
Because c-jun and junB expression is elevated in aggressive fibromatosis and fibrosarcoma compared with mild fibromatosis (23), and because these components of AP-1 stimulate PLF gene expression (20), the ability of c-jun and junB to convert mild fibromatosis cells to strong PLF-expressing cells was examined. As predicted, stable transfection of c-jun and junB expression constructs into mild fibromatosis cells activates PLF synthesis (Fig. 1B). Not only does constitutive expression of c-jun and junB cause an increase in PLF production, but PLF mRNA levels remain high when cells are maintained in 15% serum or are placed in conditions of serum starvation (Fig. 2). In contrast, PLF expression in BALB/c 3T3 fibroblasts is tightly regulated by serum (7). Aggressive fibromatosis and fibrosarcoma cells also display serum-independent PLF synthesis (Fig. 2), consistent with elevated AP-1 activity in these tumor cells. Glucocorticoid treatment blocks serum-inducible PLF gene expression in BALB/c 3T3 fibroblasts (19). Similarly, addition of dexamethasone to c-jun/junB-expressing mild fibromatosis cells, to aggressive fibromatosis cells, and to fibrosarcoma cells reduces PLF expression both in the presence and absence of serum (Fig. 2). In the presence of serum, however, the inhibitory effect of dexamethasone on PLF mRNA levels is less severe.
Figure 2.
Serum and glucocorticoid regulation of PLF expression in tumor cells. Mild fibromatosis cells expressing c-jun and junB, aggressive fibromatosis cells, and fibrosarcoma cells were serum-starved for 48 h in the absence or presence of 0.1 μM dexamethasone or starved and then stimulated with 15% FBS for 24 h, again with or without dexamethasone. Total RNA from these cells was fractionated by formaldehyde gel electrophoresis, transferred to a filter, and hybridized to detect PLF mRNA.
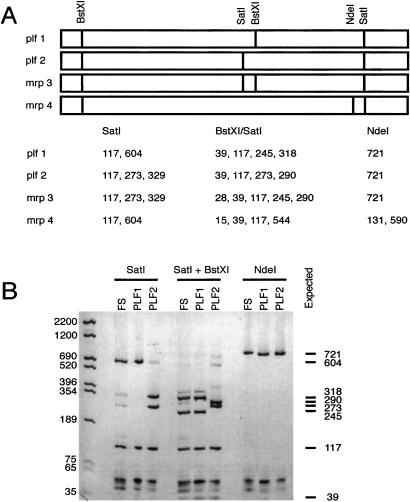
Four distinct PLF genes have been characterized (10, 11). Sequence identity of the mRNA products from these genes is greater than 90%, thus RT-PCR followed by diagnostic restriction digestion was used to ascertain the relative abundance of each specific mRNA. BstXI, NdeI, and SatI restriction endonuclease sites provide informative patterns of PLF cDNA to identify which PLF genes are expressed (Fig. 3A). Based on the NdeI digestion, MRP4 mRNA is not expressed at detectable levels in fibrosarcoma cells (Fig. 3B). The BstXI/SatI digestion pattern indicates that PLF2 mRNA is also absent (no detectable 273-bp fragment), and that MRP3 mRNA is present at low levels (note the low intensity of the 290-bp band). The predominant form of PLF mRNA in the fibrosarcoma cells is PLF1.
Figure 3.
PLF1 expression in fibrosarcoma cells. (A) Schematic diagrams of the four known PLF mRNA sequences are shown with the locations of cleavage sites for the BstXI, SatI, and NdeI restriction endonucleases. Fragment sizes in base pairs that would result from cleavage with these enzymes are listed below. (B) PLF1, PLF2, and fibrosarcoma cDNAs were amplified by PCR, using primers corresponding to nucleotide sequences found in all PLF transcripts. Reaction products were digested with the indicated restriction endonucleases, resolved by PAGE, and detected by ethidium bromide staining. Size markers are labeled on the left; all expected fragment sizes are listed on the right.
PLF Activity in Fibrosarcoma Angiogenesis.
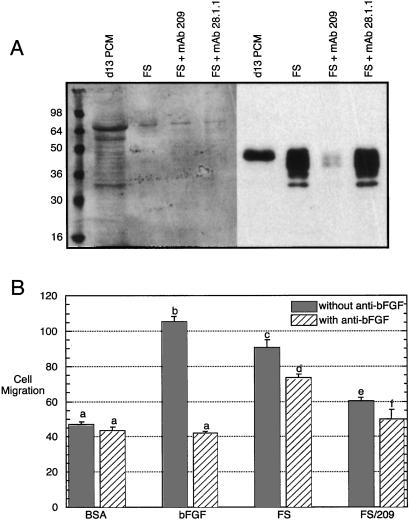
During progression to the fibrosarcoma stage, an increase in angiogenic activity is observed that correlates with the increased secretion of basic fibroblast growth factor (bFGF) and the consequent mitogenic stimulation of endothelial cells (28). Angiogenesis may result simply, or primarily, in response to bFGF binding to receptors on endothelial cells, but it is also possible that a combination of angiogenic factors from fibrosarcoma cells is functionally important. Conditioned medium from fibrosarcoma cell cultures was collected and clarified, and low molecular weight compounds were removed by filtration. PLF protein was selectively removed from tumor cell-conditioned medium by immunodepletion. Effective removal of this protein is obtained with mAb 209; neither protein G Sepharose beads by themselves, nor these beads coupled to a mAb which recognizes PLF-related protein, removes PLF from the medium (Fig. 4A).
Figure 4.
Cell culture angiogenic activity secreted by fibrosarcoma cells . (A) Medium collected from cultured fibrosarcoma cells (FS) was untreated or was incubated with mAb 209 (specific for PLF) or 28.1.1 (nonspecific control). Ab-antigen complexes were removed by using protein G beads. After these treatments, the medium was analyzed by gel electrophoresis and immunoblotting for the removal of PLF protein. Medium from cultured mouse placentas isolated on day 13 of gestation was used as a positive control for PLF detection; Ponceau-S staining shows equal protein loading (Left). (B) Basal endothelial cell migration was measured in a Boyden chamber in the presence of 0.1% BSA, and maximally induced migration was determined after addition of 10 ng/ml of bFGF. Fibrosarcoma culture medium, either intact or after removal of PLF, was also tested. Each sample was also assayed after addition of a neutralizing Ab against bFGF. Results were analyzed by a one-way ANOVA followed by a Tukey's post hoc test. n = 4, and the results were reproduced with an independently generated set of samples. a vs. b, c, or d: P < 0.001; c vs. d: P < 0.01; c vs. e: P < 0.001; d vs. f: P < 0.001.
Conditioned medium from fibrosarcoma cell cultures, either before or after removal of PLF protein, was examined for its ability to stimulate endothelial cell migration (Fig. 4B). Compared with the basal migration rate in the presence of BSA, complete fibrosarcoma-conditioned medium stimulates endothelial cell migration ≈2-fold. Addition of a neutralizing Ab against bFGF, which completely blocks the stimulatory effects of purified bFGF, reduces angiogenic activity (P < 0.01 for the comparison of fibrosarcoma-conditioned medium with and without anti-bFGF), but significant angiogenic activity remains (P < 0.001 for the comparison of BSA-treated cells to fibrosarcoma-conditioned medium with anti-bFGF). In contrast, the migration of endothelial cells in the presence of fibrosarcoma-conditioned medium from which PLF had been removed, with or without the bFGF neutralizing Ab, is not significantly higher than the migration of BSA-treated cells.
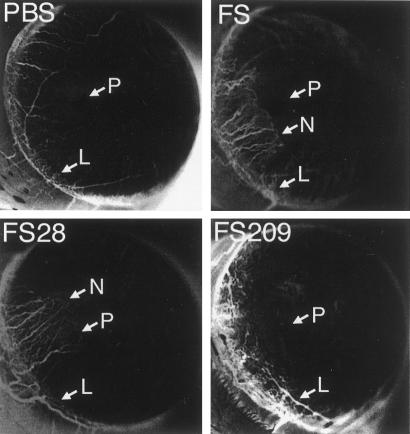
The effect of removing PLF from the fibrosarcoma culture-conditioned medium was also assayed in vivo. Pellets were prepared with buffer, fibrosarcoma culture-conditioned medium, this medium from which PLF had been removed, or this medium after immunodepletion with a mAb that does not recognize PLF. Samples were inserted into rat corneas, and neovascularization was scored as the growth of blood vessels from the limbus toward the pellet. Fibrosarcoma culture-conditioned medium, or that medium treated with the irrelevant mAb, induces blood vessel growth in the cornea; in contrast, medium after removal of PLF or buffer alone has no detectable angiogenic activity (Fig. 5).
Figure 5.
In vivo angiogenic activity secreted by fibrosarcoma cells. Hydron pellets were prepared containing buffer (PBS), fibrosarcoma culture-conditioned medium (FS), tumor cell medium treated with anti-PLF-related protein mAb 28.1.1, which does not recognize PLF (FS28), or tumor cell medium from which PLF had been removed by immunodepletion with mAb 209 (FS209). The pellets were placed in rat corneas, and neovascularization was monitored after 7 days. Vigorous ingrowth of the new vessels from the limbus (L) in the direction of the pellet (P) was scored as neovascularization (N). Inverted grayscale images are shown to enhance visualization of neovascularization. Positive responses were detected in 0/4 corneas with PBS, 4/4 with FS, 3/4 with FS28, and 0/4 for FS209.
Discussion
The placenta has many properties similar to a tumor: both grow rapidly and generate a blood supply by secreting angiogenic factors, both are invasive, and both escape immune surveillance (29). Reactivation of genes that have evolved to promote fetal growth by redirecting the maternal vasculature therefore seems to be a logical mechanism which would be selected for during tumor growth. The finding that the PLF gene is expressed in later-stage cell lines from a progressive fibrosarcoma is consistent with this prediction. Indeed, PLF may be just one example of potential gene reactivation in a broader genetic program that normally supports extraembryonic invasiveness and angiogenesis, but which can also be adopted by tumors.
That junB is important for PLF gene transcription in mouse fibroblasts (20) and in placental trophoblasts (30) suggests that one means by which elevated or oncogenic AP-1 components stimulate not only cell transformation but also tumor growth is to promote the production of angiogenic factors. This expectation is consistent with the finding that constitutively high c-jun and junB levels convert mild fibromatosis cells to strong PLF secretors. However, not all tumors that arise from high levels of oncogenic AP-1 factors express PLF. In v-jun transgenic mice, angiogenic tumors arise at sites of wounding (31), and PLF gene expression in wild-type mice has recently been found to be induced at wound sites (17). Nevertheless, we were unable to detect significant PLF expression in these v-jun tumors (J. Groskopf and D.I.H.L., unpublished observations). Thus, other factors may need to combine with AP-1 to induce PLF gene reactivation, or inhibitory factors need to be reduced to detect AP-1-dependent PLF expression.
Based on the effect of stably transfecting the mild fibromatosis cells with c-jun and junB expression constructs, the elevated and serum-independent expression of PLF in the aggressive fibromatosis and fibrosarcoma cells is presumably the result of the increased level of AP-1 activity. Despite the higher contribution of jun proteins to the AP-1 complexes, and the noted positive cooperation of jun dimers with glucocorticoid receptor to stimulate transcription from a PLF gene-regulatory element (21), glucocorticoids continue to inhibit PLF gene expression. The ability of the PLF “composite glucocorticoid response element” to respond positively to glucocorticoids in cooperation with jun dimers, but negatively with jun-fos heterodimers, has been demonstrated in constructs with the isolated element, not for the intact PLF gene-regulatory region (21). The current data suggest that the more complex architecture of the native promoter may not mimic the regulatory effects seen with this one isolated element, at least in this cell background.
The primary PLF gene expressed in fibrosarcoma cells is that encoding the PLF1 mRNA. Reactivation of PLF gene expression outside the placenta has previously been observed, but in these cases different PLF genes were found to be expressed. The PLF/MRP mRNA-designated Mrp4 was detected in tail and ear skin (16), and Mrp3 mRNA has recently been identified in skin wound sites and in hair follicles (17). Although all four of the identified PLF/MRP genes are expressed in the placenta and therefore are likely to share similar or identical placental enhancer elements, the group of PLF/MRP genes must also possess distinct regulatory features that give rise to gene-specific patterns of expression outside of the placenta. That the gene encoding PLF1 is expressed in fibrosarcoma-derived cell lines is consistent with the high level of serum-inducible and AP-1-dependent expression of this mRNA in immortalized mouse fibroblasts.
The protein product of the PLF1 mRNA was the form of PLF that was originally shown to be angiogenic (12), and thus secretion of this protein by fibrosarcomas would be expected to contribute to tumor angiogenesis. Indeed, the elimination of PLF from the soluble mixture of factors released by fibrosarcoma cells reduces endothelial cell migration to background levels and eliminates corneal neovascularization in these cell culture and in vivo assays. These results demonstrate that PLF is a significant component of the angiogenic phenotype. Inhibition of bFGF activity also has a significant effect on the ability of the products secreted by fibrosarcoma cells to induce endothelial cell migration; additional low molecular weight compounds, which were excluded in this analysis, may also participate in fibrosarcoma-induced vessel growth. Endothelial cell migration is only one step in angiogenesis, and bFGF has been shown to be the major endothelial cell mitogen secreted by these fibrosarcoma cells (28). Thus, the independent actions of bFGF on endothelial cell proliferation and PLF on endothelial cell migration may be expected to cooperate in stimulating a maximal angiogenic response.
Acknowledgments
We thank Diane Christiansen for expert technical assistance and Doug Hanahan for reagents and advice. This work was supported by National Institutes of Health Grant HD24518 (to D.I.H.L.), by American Cancer Society Grant RSG-01-099-01-CSM (to O.V.), and by the Robert H. Lurie Comprehensive Cancer Center of Northwestern University (National Institutes of Health Grant P30 CA60553). D.J.T. has received support from the Medical Scientist and Endocrinology Training Programs at Northwestern University.
Abbreviations
- AP-1
activator protein 1
- bFGF
basic fibroblast growth factor
- MRP
mitogen-regulated protein
- PLF
proliferin
- RT
reverse transcription
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Soares M J, Muller H, Orwig K E, Peters T J, Dai G. Biol Reprod. 1998;58:273–284. doi: 10.1095/biolreprod58.2.273. [DOI] [PubMed] [Google Scholar]
- 2.Galosy S S, Talamantes F. Endocrinology. 1995;136:3993–4003. doi: 10.1210/endo.136.9.7649108. [DOI] [PubMed] [Google Scholar]
- 3.Müller H, Liu B, Croy B A, Head J R, Hunt J S, Dai G, Soares M J. Endocrinology. 1999;140:2711–2720. doi: 10.1210/endo.140.6.6828. [DOI] [PubMed] [Google Scholar]
- 4.Lin J, Linzer D I H. J Biol Chem. 1999;274:21485–21489. doi: 10.1074/jbc.274.30.21485. [DOI] [PubMed] [Google Scholar]
- 5.Nilsen-Hamilton M, Hamilton R T, Alvarez-Azaustre E. Gene. 1987;51:163–170. doi: 10.1016/0378-1119(87)90304-0. [DOI] [PubMed] [Google Scholar]
- 6.Nilsen-Hamilton M, Shapiro J M, Massoglia S L, Hamilton R T. Cell. 1980;20:19–28. doi: 10.1016/0092-8674(80)90230-5. [DOI] [PubMed] [Google Scholar]
- 7.Linzer D I H, Nathans D. Proc Natl Acad Sci USA. 1984;81:4255–4259. doi: 10.1073/pnas.81.14.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S-J, Talamantes F, Wilder E, Linzer D I H, Nathans D. Endocrinology. 1988;122:1761–1768. doi: 10.1210/endo-122-5-1761. [DOI] [PubMed] [Google Scholar]
- 9.Linzer D I H, Lee S-J, Ogren L, Talamantes F, Nathans D. Proc Natl Acad Sci USA. 1985;82:4356–4359. doi: 10.1073/pnas.82.13.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilder E L, Linzer D I H. Mol Cell Biol. 1986;6:3283–3286. doi: 10.1128/mcb.6.9.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang Y, Lepont P, Fassett J T, Ford S P, Mubaidin A, Hamilton R T, Nilsen-Hamilton M. Endocrinology. 1999;140:5239–5249. doi: 10.1210/endo.140.11.7142. [DOI] [PubMed] [Google Scholar]
- 12.Jackson D, Volpert O, Bouck N, Linzer D I H. Science. 1994;266:1581–1584. doi: 10.1126/science.7527157. [DOI] [PubMed] [Google Scholar]
- 13.Ma G T, Roth M E, Groskopf J C, Tsai F-Y, Orkin S H, Grosveld F, Engel J D, Linzer D I H. Development (Cambridge, UK) 1997;124:907–914. doi: 10.1242/dev.124.4.907. [DOI] [PubMed] [Google Scholar]
- 14.Nelson J T, Rosenzweig N, Nilsen-Hamilton M. Endocrinology. 1995;136:283–298. doi: 10.1210/endo.136.1.7828542. [DOI] [PubMed] [Google Scholar]
- 15.Jackson D, Linzer D I H. Endocrinology. 1997;138:149–155. doi: 10.1210/endo.138.1.4828. [DOI] [PubMed] [Google Scholar]
- 16.Fassett J T, Hamilton R T, Nilsen-Hamilton M. Endocrinology. 2000;141:1863–1871. doi: 10.1210/endo.141.5.7479. [DOI] [PubMed] [Google Scholar]
- 17.Fassett J T, Nilsen-Hamilton M. Endocrinology. 2001;142:2129–2137. doi: 10.1210/endo.142.5.8132. [DOI] [PubMed] [Google Scholar]
- 18.Folkman J. Semin Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- 19.Mordacq J C, Linzer D I H. Genes Dev. 1989;3:760–769. doi: 10.1101/gad.3.6.760. [DOI] [PubMed] [Google Scholar]
- 20.Groskopf J C, Linzer D I H. Mol Cell Biol. 1994;14:6013–6020. doi: 10.1128/mcb.14.9.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond M I, Miner J N, Yoshinaga S K, Yamamoto K R. Science. 1990;249:1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- 22.Lacey M, Alpert S, Hanahan D. Nature (London) 1986;322:609–612. doi: 10.1038/322609a0. [DOI] [PubMed] [Google Scholar]
- 23.Bossy-Wetzel E, Bravo R, Hanahan D. Genes Dev. 1992;6:2340–2351. doi: 10.1101/gad.6.12a.2340. [DOI] [PubMed] [Google Scholar]
- 24.Vivanco M D, Johnson R, Galante P E, Hanahan D, Yamamoto K R. EMBO J. 1995;14:2217–2228. doi: 10.1002/j.1460-2075.1995.tb07216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta K, Ramakrishnan S, Browne P V, Solovey A, Hebbel R P. Exp Cell Res. 1997;230:244–251. doi: 10.1006/excr.1996.3421. [DOI] [PubMed] [Google Scholar]
- 26.Good D J, Polverini P J, Rastinejad F, Le Beau M M, Lemons R S, Frazier W A, Bouck N P. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin J, Toft D J, Bengtson N W, Linzer D I H. Recent Prog Horm Res. 2000;55:37–52. doi: 10.1016/s0009-2509(99)00304-8. [DOI] [PubMed] [Google Scholar]
- 28.Kandel J, Bossy-Wetzel E, Radvanyi F, Klagsbrun M, Folkman J, Hanahan D. Cell. 1991;66:1095–1104. doi: 10.1016/0092-8674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- 29.Strickland S, Richards W G. Cell. 1992;71:355–357. doi: 10.1016/0092-8674(92)90503-5. [DOI] [PubMed] [Google Scholar]
- 30.Schorpp-Kistner M, Wang Z Q, Angel P, Wagner E F. EMBO J. 1999;18:934–948. doi: 10.1093/emboj/18.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuh A C, Keating S J, Monteclaro F S, Vogt P K, Breitman M L. Nature (London) 1990;346:756–760. doi: 10.1038/346756a0. [DOI] [PubMed] [Google Scholar]