Abstract
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited heart disease, associated with a high risk of sudden cardiac death. ARVC has been termed a ‘disease of the desmosome’ based on the fact that in many cases, it is caused by mutations in genes encoding desmosomal proteins at the specialised intercellular junctions between cardiomyocytes, the intercalated discs. Desmosomes maintain the structural integrity of the ventricular myocardium and are also implicated in signal transduction pathways. Mutated desmosomal proteins are thought to cause detachment of cardiac myocytes by the loss of cellular adhesions and also affect signalling pathways, leading to cell death and substitution by fibrofatty adipocytic tissue. However, mutations in desmosomal proteins are not the sole cause for ARVC as mutations in non-desmosomal genes were also implicated in its pathogenesis. This review will consider the pathology, genetic basis and mechanisms of pathogenesis for ARVC.
Keywords: Desmosomes, Arrhythmogenic right ventricular cardiomyopathy, Desmoglein, Desmocollin, Desmoplakin, Plakoglobin, Plakophilin, Sudden cardiac death
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a rare (1:2000–1:5000) inherited cardiac condition (Pilichou et al. 2016). Sudden cardiac death can often be the first presentation of the disease in previously asymptomatic individuals, making this condition life-threatening and difficult to diagnose (Thiene et al. 1998). It affects young people and athletes, as strenuous exercise can exacerbate the incidence of arrhythmias and cause sudden cardiac death. Of note, 20% of non-exercise-related sudden cardiac deaths in young people in the Europe and USA were attributed to ARVC (Shen et al. 1995).
Diagnosis is based on ‘Task Force Criteria’, a scoring system that takes into account structural and electrical abnormalities, family history of the disease and genetic findings and is divided into major and minor criteria (Marcus et al. 2010). This results in the classification of ‘definite’, ‘borderline’ or ‘possible’ diagnosis of ARVC. By taking the diagnosis of a first degree relative into consideration, the criteria are more stringent for cases of familial ARVC.
The histological hallmark of ARVC is the substitution of cardiac myocytes by fibrofatty tissue (Sen-Chowdhry et al. 2005). ARVC is commonly an autosomal dominant inherited disease with variable clinical manifestations (Corrado et al. 2017). Mutations in desmosomal genes, specifically desmoplakin, plakophilin-2 and desmoglein-2 were found, in addition to non-desmosomal genes encoding for transforming growth factor (TGF) β3, human ryanodine receptor (RyR) 2 and the transmembrane protein (TMEM) 43 (Herren et al. 2009).
Treatment aims both at delaying the progression of heart failure and the prevention of arrhythmic events. This is achieved by general lifestyle changes, pharmacological interventions such as beta-blockers or anti-arrhythmic drugs, by surgical means including catheter ablation and implantable cardioverter defibrillator (ICD) or ultimately by heart transplantation (Corrado et al. 2017).
Pathology
ARVC was initially categorised as a dysplasia based on histological findings (hence also referred to as ARVD), but closer analysis led to the characterisation of the disease as being a genetically predisposed cardiomyopathy (Basso et al. 1996; Nava et al. 1988). Defining pathological characteristics of ARVC include cardiac myocyte depletion and substitution with fibrofatty tissue (Sen-Chowdhry et al. 2005). Fibrous scar tissue replacement of cardiac myocytes occurs initially in the epicardium of the heart but over time infiltrates transmurally into the endocardium, resulting in thinning of the right ventricular walls. In ARVC, the three areas of the heart affected most include the anterior infundibulum, the apex of the right ventricle and the inferior wall of the right ventricle. These areas, which are collectively coined the ‘triangle of dysplasia’, appear especially susceptible to stress and stretch and had the most fibrofatty replacement of myocardial tissue and the thinnest walls of the right ventricle (Gerull et al. 2004; Marcus et al. 1982). Despite the fact that it is called ‘arrythmogenic right ventricular cardiomyopathy’, the disease is not limited solely to the right ventricle, as fibrofatty replacement of the left ventricular myocardium may also occur (Beffagna et al. 2005). Although it is less common, myocyte degeneration may also be seen predominantly in the left ventricle and the interventricular septum (Beffagna et al. 2005). Hence the term ‘arrhythmogenic cardiomyopathy’ (AC) has been proposed (Basso et al. 2018).
Genetics of ARVC
Mutations in genes encoding for desmosomal proteins
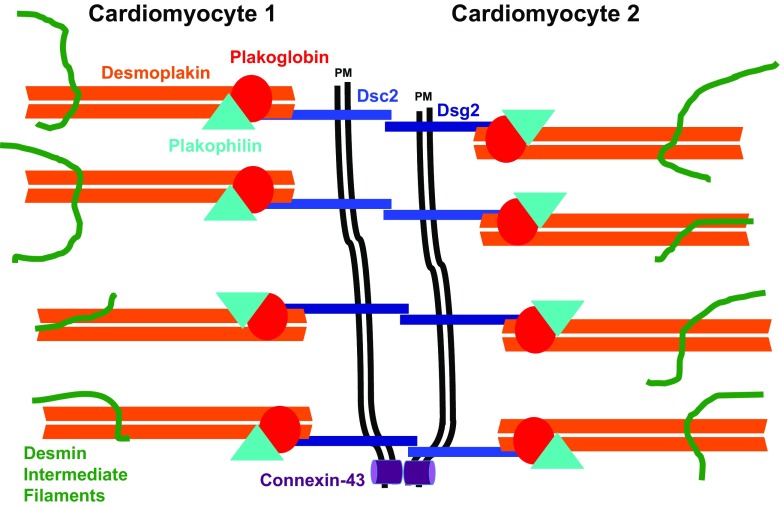
Desmosomes are integral cellular adhesion structures found at junctions between adjacent cardiomyocytes called intercalated discs (Vermij et al. 2017). A simplified scheme of a desmosome is depicted in Fig. 1. Desmosomal cadherins—desmoglein (Dsg)-2 and desmocollin (Dsc)-2—are transmembrane proteins that link neighbouring cardiomyocytes via homophilic or heterophilic interactions. Their cytoplasmic domains interact with the armadillo proteins, plakoglobin and plakophilin. These two proteins link to desmoplakin, which binds strongly to intracellular intermediate filaments, which are composed of desmin in cardiomyocytes (reviewed in Kottke et al. 2006). These cellular adhesions are essential for the heart to be able to withstand mechanical stresses caused by the contractile cycle. Additionally, desmosomes are implicated in intercellular signalling cascades (reviewed in Broussard et al. 2015) and perturbation of these signalling pathways could contribute to the development of ARVC. More recently the exclusive molecular composition of desmosomes versus adherens junctions, which are actin-anchoring contacts at the intercalated disc, was challenged and the concept of the Area composita was proposed, which suggests a more dynamic distribution of proteins between these two types of cell-cell contact (Franke et al. 2006).
Fig. 1.
Schematic drawing of a desmosome: Intercellular connections between two cardiomyocytes (only partially shown) are mediated by the transmembrane proteins, the desmosomal cadherins desmocollin 2 (Dsc2) and desmoglein 2 (Dsg2). The link to the cytoskeleton, the intermediate filaments composed of desmin, is mediated by a complex consisting of desmoplakin, plakoglobin and plakophilin-2. Dsc2 also interacts with the gap junction protein connexin-43. PM stands for plasma membrane
ARVC is most commonly inherited autosomal dominantly with incomplete penetrance and variable phenotypic expressivity, although it can also be recessively inherited (reviewed in Herren et al. 2009). There are eight autosomal dominant forms of the disease (Beffagna et al. 2005; Rampazzo et al. 1994), and 50% of these cases are the result of mutations in the five genes JUP, DSP, PKP2, DSG2 and DSC2 encoding the desmosomal proteins plakoglobin, desmoplakin, plakophilin 2, desmoglein 2 and desmocollin 2, respectively (Marcus et al. 2010). Together with mutations in genes coding for non-desmosomal proteins, 15 disease genes were identified so far (Table 1).
Table 1.
Reported disease genes for ARVC
| Gene | Encoded protein | Subcellular localisation | Chromosomal locus | Reference |
|---|---|---|---|---|
| JUP | Plakoglobin | Desmosome | 17q21.2 | McKoy et al. (2000) |
| DSP | Desmoplakin | Desmosome | 6p24.3 | Rampazzo et al. (2002) |
| PKP2 | Plakophilin-2 | Desmosome | 12p11.21 | Gerull et al. (2004) |
| DSG2 | Desmoglein-2 | Desmosome | 18q12.1 | Pilichou et al. (2006) |
| DSC2 | Desmocollin-2 | Desmosome | 18q12.1 | Syrris et al. (2006) |
| CDH2 | N-Cadherin | Area composita | 18q12.1 | Mayosi et al. (2017) |
| LMNA | Lamin A/C | Nuclear envelope | 1q22 | Quarta et al. (2012) |
| DES | Desmin | Intermediate filament | 2q35 | van Tintelen et al. (2009) |
| CTNNA3 | Alpha-T-catenin | Area composita | 10q21.3 | van Hengel et al. (2013) |
| PLN | Phospholamban | Sarcoplasmic reticulum | 6q22.31 | van der Zwaag et al. (2012) |
| RYR2 | Ryanodine receptor 2 | Sarcoplasmic reticulum | 1q43 | Tiso et al. (2001) |
| TGFB3 | Transforming growth factor β3 | Growth factor | 14q24.3 | Beffagna et al. (2005) |
| TTN | Titin | Sarcomere | 2q31.2 | Taylor et al. (2011) |
| SCN5A | Sodium voltage-gated channel alpha subunit 5 | Sodium channel Intercalated disk | 3p22.2 | Te Riele et al. (2017) |
| TMEM43 | Transmembrane protein 43 | Nuclear envelope | 3p25.1 | Merner et al. (2008) |
Naxos disease and Carvajal syndrome
Naxos disease and Carvajal syndrome are both known as cardiocutaneous disorders as, in addition to cardiomyocyte dysfunction, they are accompanied by skin disorders (Protonotarios and Tsatsopoulou 2004). The first mutation in a desmosomal protein that was unequivocally linked with the ARVC-implicated autosomal recessive Naxos disease was a homozygous frameshift-causing deletion mutation at chromosomal locus 17q21 for the JUP gene, encoding plakoglobin (McKoy et al. 2000). Homozygous mutations in the DSP gene encoding desmoplakin at locus 6p24 cause the recessively inherited Carvajal syndrome, which is also characterised by woolly hair, keratoderma and ARVC with a prominent left ventricular manifestation (Norgett et al. 2000; Rampazzo et al. 2002).
Plakophilin-2 (PKP2)
Mutations in the PKP2 gene encoding plakophilin-2, the main cardiac plakophilin, at locus 12p11 are a common cause of ARVC. It was shown that mutations in the PKP2 gene were present in 27% of 120 probands of Western European descent (Gerull et al. 2004), and all were ultimately diagnosed with ARVC. Furthermore, it appears that the nucleotide residue 235 is a mutational hotspot as six cases of nonsense mutations occurred at this position (Mertens et al. 1996). An additional study further validated the correlation between PKP2 mutations and the prevalence of ARVC as 43% of a cohort of 58 ARVC cases contained PKP2 mutations (Dalal et al. 2006).
Desmoglein-2 (DSG2)
Mutations in the DSG2 gene encoding desmoglein-2 at locus 18q12 were reported in ARVC patients (Pilichou et al. 2006). Desmoglein-2 is synthesised as a pro-protein, which is post-translationally cleaved by the protease furin, at an R-X-K-R motif, into its fully functional form. However, mutations in residues R45Q and R48H appear to interfere with this cleavage, thus preventing the formation of the active form of desmoglein-2 (Awad et al. 2006). Dieding and colleagues used atomic force microscopy-based single molecule force spectroscopy and cell-cell adhesion assays to study the kinetic properties of wild-type desmoglein-2 and compared it to two ARVC-associated mutants (p.D105E and p.V343I; Dieding et al. 2017). While D105E, which is closely located to a calcium ion coordination site, seemed to lead to stronger binding and an increased lifetime, potentially affecting the dynamics of desmosomal turnover, no significant differences were found in these molecular assays for the V343I mutant, indicating it may be a genetic modifier (Dieding et al. 2017).
Desmoplakin (DSP)
As discussed above, recessive mutations in the gene that encodes desmoplakin at locus 6p24 give rise to Carvajal disease (Norgett et al. 2000). Autosomal dominant mutations in the DSP gene can give rise to ARVC without cutaneous involvement (Rampazzo et al. 2002). It was suggested that mutations resulting in the truncation of the desmoplakin protein due to a premature incorporation of a stop codon result in a more severe phenotypic expression of the disorder (Lopez-Ayala et al. 2014). Moreover, desmoplakin mutations appear to lead to a higher frequency of left ventricular dysfunctional forms of the disease (Castelletti et al. 2017). The mutant form of desmoplakin has a high tendency to dimerise; hence, aggregates are likely to be found in desmosomal protein complexes (Sen-Chowdhry et al. 2005).
Plakoglobin (JUP)
Autosomal dominant mutations in JUP coding for plakoglobin are rare contributors to ARVC and apart from Naxos disease, so far, only few cases were reported, e.g. JUP p. S39_K40insS (Asimaki et al. 2007), which leads to the insertion of a serine into the N-terminus of plakoglobin. In this case, only the heart is affected and there are no signs of a skin or hair phenotype.
Desmocollin-2 (DSC2)
Autosomal dominant mutations in DSC2 at 18q12.1 have been reported to be causative for ARVC (Beffagna et al. 2007; Gehmlich et al. 2011b). Both missense and truncating mutations were described. For some of the missense mutations, trafficking defects, i.e. the failure of the protein to locate to the desmosomes, were suggested as the pathogenic mechanism. In contrast, a common, very C-terminal truncation variant DSC2 p.A897KfsX4 affects only the DSC2a isoform and is now thought to be clinically silent (De Bortoli et al. 2010).
Since multiple desmosomal protein mutations have been found to be linked with causing ARVC, it could be proposed that these all lead to the deregulation of one final common pathway via the disruption of cellular adhesions between cardiac myocytes (Vatta et al. 2007), resulting in a loss of plakoglobin from desmosomes (Asimaki et al. 2009). The latter was also suggested as a diagnostic feature for the disease. However, more recent evidence showed that the disappearance of the plakoglobin signal from the intercalated disc in an ARVC mouse model and samples from human patients is observed just with one antibody but not with others specific for plakoglobin, suggesting that epitope masking (potentially through post-translational modifications) may have contributed to this interpretation and that plakoglobin is retained to a certain extent also in disease (Kant et al. 2016). With more and more mutations being structurally mapped (for a review see Al-Jassar et al. 2013), it has become clear that a one-fits-all explanation may be too optimistic, since proven disease causing missense mutations may have several consequences, such as affecting stability, protein-protein interactions, trafficking as well as signalling roles of a desmosomal protein.
Mutations in genes encoding for non-desmosomal proteins
In addition to desmosomal mutations, also mutations in genes encoding non-desmosomal proteins were reported as causes of ARVC. These include—among others—TGF-β3, cardiac RyR2 and TMEM43 (Herren et al. 2009).
Transforming growth factor-β3 (TGFB3)
The transforming growth factor-β3 (TGF-β3) gene encodes a cytokine (Beffagna et al. 2005) that plays a role in regulating cellular adhesions and was found to be involved in the classic autosomal dominant form of the disease, ARVD1 (Sen-Chowdhry et al. 2005; Sporn and Roberts 1992). Direct sequencing of genomic DNA highlighted two mutations, which were localised to regulatory 3′ untranslated region (UTR) and 5′ UTR regions of the TGF-β3 gene that may have a role in the disorder. Studies suggest that UTR mutations, which induce TGF-β3 overexpression, may also be associated with the distinctive fibrosis observed of the cardiac myocardium in ARVC (Beffagna et al. 2005). More recently, another link between TGFbeta signalling and desmosomes was established, when it was discovered that reduced expression of PKP2 in cultured cardiomyocytes activated TGFbeta1 and p38-mitogen-activated protein kinase signalling with the final readout of increased expression of profibrotic genes (Dubash et al. 2016).
Ryanodine receptor 2 (RYR2)
Another non-desmosomal gene that was linked to ARVC is ryanodine receptor 2 (RYR2; Tiso et al. 2001), mapping to chromosomal locus 1q42-q43 and encoding a large calcium ion channel responsible for eliciting excitation-contraction coupling in the heart (Lehnart et al. 2008). Four heterozygous missense mutations were identified in the gene, classified as arrhythmogenic right ventricular dysplasia 2 (ARVD2). Although structural abnormalities in ARVD2 are very limited, the disease is thought to be allelic with familial catecholaminergic polymorphic ventricular tachycardia as there are many phenotypic overlaps (Sen-Chowdhry et al. 2005). Comparing the two similar phenotypic ryanodine receptor-induced disorders allows for a better understanding of the disease-causing mechanism of ARVC. Calcium-induced calcium release from the sarcoplasmic reticulum into the cytosol is induced by the cardiac ryanodine receptor 2. Therefore, RyR2 mutations may result in the leakage of calcium ions from the sarcoplasmic reticulum due to defective channels (Laitinen et al. 2001). This consequently contributes to dysfunctional cardiac excitability in response to sympathetic stimulation, giving rise to arrhythmias induced by strenuous exercise, as is observed in ARVC (Lehnart et al. 2008).
Transmembrane protein 43 (TMEM43)
Missense mutations in the TMEM43 gene which encodes the transmembrane protein 43 (also called Luma) were described to cause the ARVD5 form of this cardiomyopathy. A potentially deleterious missense mutation in TMEM43 (S358L) was observed in all ancestral haplotypes across 15 families with ARVD5 (also termed ‘Newfoundland’ mutation). It should be noted that the TMEM43 gene contains a response element for peroxisome proliferator-activated receptor-γ, PPARγ (Merner et al. 2008). PPARγ is an adipocyte growth factor and may be associated with the differentiation of fibroadipocyte progenitor cells into myocardial adipocytes—thus offering an explanation for the phenomenon of fibrofatty infiltration of the cardiac myocardium (Corrado et al. 2017). However, two recent mouse models, a knock-out and a knock-in of the S358L missense mutation, display no cardiac abnormalities (Stroud et al. 2018). Moreover, the recent work establishes TMEM43 as a nuclear envelope protein (Stroud et al. 2018).
Sodium voltage-gated channel alpha subunit, 5 (SCN5A)
Mutations in the gene SCN5A, which encodes the sodium voltage-gated channel alpha subunit, 5 (Nav1.5), found in myocardial cells were also hypothesised as being significant in causing ARVC (Te Riele et al. 2017). A similar cardiomyopathy, Brugada Syndrome, arises due to impairment of the same sodium channel and shares many phenotypic features with ARVC, in particular the high frequency of ventricular tachycardia and sudden cardiac death (Stokoe et al. 2007).
There are multiple reports of variants identified in further genes in patients with ARVC (see Table 1). The affected proteins localise to various cellular compartments and pathogenic mechanisms are largely unknown. A further confounding factor is the phenotypic overlap of ARVC with other inherited cardiac diseases, for example, predominantly left ventricular forms of ARVC share a similarity with dilated cardiomyopathy.
Pathogenic processes
The two main pathogenic mechanisms that are observed at the histological level are apoptosis and inflammation. Apoptosis leads to programmed cardiomyocyte death and contributes to the observed progressive atrophy of the myocardium (Thiene et al. 2000). Features of apoptotic cells include chromatin condensation, nuclear fragmentation and the presence of protease CPP-32 (also called caspase-3; Herren et al. 2009). In a study using TUNEL assay to identify apoptotic cardiomyocytes, 7 out of 20 cases of ARVC displayed characteristics of apoptosis (Thiene et al. 1998). Immunohistological investigation of the expression of CPP-32 in right ventricles of normal subjects and ARVC patients revealed high levels of CPP-32 in cardiomyocytes of ARVC patients, whereas unaffected individuals had cardiomyocytes with undetectably low levels of the protease (Mallat et al. 1996). Apoptosis was also documented in a transgenic mouse model with a mutation in the desmosomal protein desmoglein-2 (Pilichou et al. 2009).
Inflammation is another mechanism thought to contribute to the pathological features of ARVC. Inflammation may arise following myocardial degeneration as histology has revealed that patchy inflammatory infiltrates of T-lymphocytes were present in 50% of ARVC patients studied (Campuzano et al. 2012). It was speculated that the fibrofatty replacement of the myocardium occurs as a healing process in response to the inflammatory disease such as chronic myocarditis (Thiene et al. 1991) and that inflammation is a trigger of ARVC in genetically predisposed individuals (Thiene et al. 2000). In support of this, a study involving BALB/c mice subjected to Coxsackie virus led to selective right ventricular myocarditis and wall thinning, which is consistent with the predominant right ventricular involvement observed in the pathology of ARVC (Matsumori and Kawai 1980). However, Coxsackie virus infection can also upregulate the expression of miR-21 which leads to reduced desmin protein levels, the cytoskeletal filament to which desmosomes attach (see Fig. 1), which might again lead to a destabilisation of desmosomes (Ye et al. 2014).
Both apoptosis and inflammation may only be secondary mechanisms that are ultimately be due to a compromised resistance of the cardiac tissue to mechanical stress due to impaired function of desmosomes. Experiments on cultured cells provided evidence for mutated plakophilin-2 directly affecting the assembly and stability of desmosomes (Hall et al. 2009). This may lead to cell detachment and induce necrotic and apoptotic events.
From a histological point of view, it has been a mystery for a long time, why a disease that is caused by mutated proteins that make up a subcellular structure such as the desmosome that is found throughout the heart is predominantly observed in the right ventricle, which is usually exposed to less mechanical stress. Recent research has highlighted that the underlying cause for this might be due to differential embryonic origin of parts of the heart, since it was shown that cardiac progenitor cells from the epicardium can be stimulated to transdifferentiate into adipocytes upon nuclear translocation of plakoglobin (Garcia-Gras et al. 2006; Lombardi and Marian 2010). Since these cardiac stem cells are predominantly derived from the secondary heart field, which contributes more to the right ventricle than the left ventricle (Kelly et al. 2014), this suggests that the higher adopigenic potential may be due to a differential embryonic origin.
Pathological events at the cellular level
Plakoglobin and Wnt signalling pathways
The desmosomal armadillo protein plakoglobin may play a role in the pathogenesis of ARVC. Under normal conditions, plakoglobin is exclusively found at the cell-cell contacts (intercalated discs). However, plakoglobin is also known as γ-catenin and is related to β-catenin, a well-known factor in the classical Wnt signalling pathway (Zhurinsky et al. 2000). In a cardiac cell line, the HL-1 cells, suppression of desmoplakin expression leads to a nuclear translocation of plakoglobin and suppression of the Wnt/β-catenin signalling pathway by binding to the Tcf/Lef1 transcription factors (Garcia-Gras et al. 2006). A similar nuclear concentration of plakoglobin was also demonstrated for whole tissue extracts from hearts from desmoplakin-deficient mice (Garcia-Gras et al. 2006). However, since only 15% of the nuclei in heart tissue are from cardiomyocytes (Soonpaa et al. 1996), it cannot be excluded that the reported effect on Wnt signalling at the tissue level occurs mainly in non-cardiomyocytes. Nuclear plakoglobin was also detected in neonatal cardiomyocytes that expressed a truncated version of the protein (2057del2) but was not really obvious in the nuclei of induced pluripotent stem cell (iPSC)-derived cardiomyocytes from ARVC patients (Asimaki et al. 2014), despite the well-known immature status of this kind of cells. Glycogen synthase kinase (GSK) 3β, which has a suppressive role in the canonical Wnt signalling pathway, shows differential subcellular distribution in mouse models for ARVC (Chelko et al. 2016). In control mice, GSK3β was localised to the cytoplasm; however, in mice with an excision of exon 4 and 5 from the desmoglein 2 gene, GSK3β was found abnormally redistributed to the intercalated disc (Chelko et al. 2016). There is also potential crosstalk between β-catenin and the Hippo signalling pathway in ARVC: Following molecular remodelling of the intercalated disc, it was discovered that levels of protein kinase C α, a signalling molecule which localises to the intercalated disc upon interactions with desmosomal plakophilin-2, decreased (Chopra et al. 2011). As a result, neurofibromin-2 (or Merlin) becomes activated and in turn phosphorylates and inactivates the effector of the Hippo pathway, Yes-associated protein, YAP (Zhou and Hanemann 2012). YAP is then free to bind to β-catenin and γ-catenin (plakoglobin) and therefore drives adipogenesis in myocardial tissue. The Hippo pathway has a number of functions but notably plays a role in the regulation of cell differentiation and maintenance of tissue homeostasis (Yu and Guan 2013). Significantly, activation of the Hippo pathway has a negative feedback effect on the canonical Wnt pathway, thus reinforcing the hypothesis that the two pathways might be implicated in the pathogenesis of ARVC (Chen et al. 2014).
Trafficking
Since potentially fatal arrhythmias are one of the main characteristics of ARVC, it is important to determine which histological or cellular changes lead to this detrimental phenotype. On one hand, it may be caused by cardiomyocyte death and fibro-fatty replacement interfering with coordinated conduction; on the other hand, it has become clear that also more subtle alterations in ion channel expression levels and localisation are observed at the cellular level. A lack of interaction of a Dsc2a mutant with connexin-43, which leads to its altered phosphorylation and decreased presence (Gehmlich et al. 2011a) and dysregulation of Nav1.5 sodium channels in Pkp2 knockdown mice (Cerrone et al. 2012) were described. Immunohistochemical analysis of heart tissue from ARVC patients also showed decreased signals for plakoglobin, connexin-43, and Nav1.5, suggesting that this remodelling may be a general phenomenon, contributing to a vulnerability to arrhythmias (Noorman et al. 2013). Trafficking of desmosomal proteins is not very well understood, but for desmoplakin, an involvement of microtubules was demonstrated as it was shown that disease mutations in desmoplakin interfere with its binding to EB1, a microtubule binding protein (Huang et al. 2014). In addition to its interaction with Desmocollin-2, connexin-43 was also shown to bind to ankyrin-G and plakophilin (Sato et al. 2011). Since plakophilin is required for the transcription of RyR, ankyrin-B, and other proteins involved in the control of calcium cycling (Cav1.2, triadin, calsequestrin2) (Cerrone et al. 2017), signalling crosstalk from the intercalated disc to the nucleus obviously contributes to the development of an ARVC phenotype. Interestingly, in a recent screen using a zebrafish model of ARVC, a drug, SB216763, was identified (Asimaki et al. 2014), which changed the subcellular localisation of SAP97 (also known as DLG-1), a protein known to be involved in the trafficking of Nav1.5. SB216763 can act as an inhibitor of GSK3β and seemed to increase expression levels of SAP97 as well as preserve its plasma membrane location. In ARVC model systems (neonatal rat cardiomyocytes expressing mutant plakoglobin and iPSC-derived cardiomoycytes from human ARVC patients) treated with this drug, this was accompanied by connexin-43 and Nav1.5 being retained at cell-cell contacts (Asimaki et al. 2014). This suggests that it may be possible to interfere with subcellular trafficking issues that appear to be a hallmark of ARVC and thereby lead to a functional improvement.
Outlook and further perspectives
ARVC is an inherited cardiac disease with a wide phenotypic spectrum. Diagnosis can be challenging, especially hindered by the incomplete penetrance. Therefore, the first manifestation of the disease can be life-threatening arrhythmias, which may result in sudden cardiac death. The majority of familial cases show autosomal dominant inheritance. Even though the majority of mutations are found in desmosomal genes, also mutations in non-desmosomal proteins were reported to cause ARVC. Next generating sequencing and its wider use in clinical practice will pave the way for a “molecular diagnosis” of ARVC; however, the evaluation of identified variants can be challenging, since presumed disease-causing missense mutations may also be present in the control population (Andreasen et al. 2013). It is also becoming more and more apparent that compound and digenic heterozygosity of desmosomal gene mutations contribute to the disease (Xu et al. 2010). Recently, it was suggested that the ARVD/C database might be a useful tool for practitioners (Lazzarini et al. 2015), listing 1400 variants in 12 ARVC-related genes, both desmosomal and non-desmosomal ones (as of 2014). However, also in this database the majority of variants are classified as of ‘unknown significance’, highlighting the dilemma of assigning causality to variants identified in individuals. The analysis of patient material and in particular the thorough study of animal and cellular disease models and in vitro functional studies has provided insights into the disease mechanisms of ARVC. However, a deeper understanding of aberrant signalling in the presence of desmosomal impairment is required to develop novel therapies to combat myocardial remodelling and conduction defects.
Compliance with ethical standards
Anita Kiran Vimalanathan declares that she has no conflict of interest. Elisabeth Ehler declares that she has no conflict of interest. Katja Gehmlich declares that she has no conflict of interest.
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on ‘Heart Failure Due to Non-Myofibrillar Defects’ edited by Elisabeth Ehler and Katja Gehmlich.
References
- Al-Jassar C, Bikker H, Overduin M, Chidgey M. Mechanistic basis of desmosome-targeted diseases. J Mol Biol. 2013;425:4006–4022. doi: 10.1016/j.jmb.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen C, Nielsen JB, Refsgaard L, Holst AG, Christensen AH, Andreasen L, Sajadieh A, Haunso S, Svendsen JH, Olesen MS. New population-based exome data are questioning the pathogenicity of previously cardiomyopathy-associated genetic variants. Eur J Hum Genet. 2013;21:918–928. doi: 10.1038/ejhg.2012.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimaki A, Syrris P, Wichter T, Matthias P, Saffitz JE, McKenna WJ. A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2007;81:964–973. doi: 10.1086/521633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimaki A, Tandri H, Huang H, Halushka MK, Gautam S, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, McKenna WJ, Calkins H, Saffitz JE. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–1084. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]
- Asimaki A, Kapoor S, Plovie E, Karin Arndt A, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J, Wu JC, MacRae CA, Kleber AG, Saffitz JE. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med. 2014;6:240ra74. doi: 10.1126/scitranslmed.3008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad MM, Dalal D, Cho E, Amat-Alarcon N, James C, Tichnell C, Tucker A, Russell SD, Bluemke DA, Dietz HC, Calkins H, Judge DP. DSG2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Hum Genet. 2006;79:136–142. doi: 10.1086/504393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation. 1996;94:983–991. doi: 10.1161/01.CIR.94.5.983. [DOI] [PubMed] [Google Scholar]
- Basso C, Pilichou K, Bauce B, Corrado D, Thiene G. Diagnostic criteria, genetics, and molecular basis of arrhythmogenic cardiomyopathy. Heart Fail Clin. 2018;14:201–213. doi: 10.1016/j.hfc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Beffagna G, Occhi G, Nava A, Vitiello L, Ditadi A, Basso C, Bauce B, Carraro G, Thiene G, Towbin JA, Danieli GA, Rampazzo A. Regulatory mutations in transforming growth factor-beta3 gene cause arrhythmogenic right ventricular cardiomyopathy type 1. Cardiovasc Res. 2005;65:366–373. doi: 10.1016/j.cardiores.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Beffagna G, De Bortoli M, Nava A, Salamon M, Lorenzon A, Zaccolo M, Mancuso L, Sigalotti L, Bauce B, Occhi G, Basso C, Lanfranchi G, Towbin JA, Thiene G, Danieli GA, Rampazzo A. Missense mutations in desmocollin-2 N-terminus, associated with arrhythmogenic right ventricular cardiomyopathy, affect intracellular localization of desmocollin-2 in vitro. BMC Med Genet. 2007;8:65. doi: 10.1186/1471-2350-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JA, Getsios S, Green KJ. Desmosome regulation and signaling in disease. Cell Tissue Res. 2015;360:501–512. doi: 10.1007/s00441-015-2136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campuzano O, Alcalde M, Iglesias A, Barahona-Dussault C, Sarquella-Brugada G, Benito B, Arzamendi D, Flores J, Leung TK, Talajic M, Oliva A, Brugada R. Arrhythmogenic right ventricular cardiomyopathy: severe structural alterations are associated with inflammation. J Clin Pathol. 2012;65:1077–1083. doi: 10.1136/jclinpath-2012-201022. [DOI] [PubMed] [Google Scholar]
- Castelletti S, Vischer AS, Syrris P, Crotti L, Spazzolini C, Ghidoni A, Parati G, Jenkins S, Kotta MC, McKenna WJ, Schwartz PJ, Pantazis A. Desmoplakin missense and non-missense mutations in arrhythmogenic right ventricular cardiomyopathy: genotype-phenotype correlation. Int J Cardiol. 2017;249:268–273. doi: 10.1016/j.ijcard.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Cerrone M, Noorman M, Lin X, Chkourko H, Liang FX, van der Nagel R, Hund T, Birchmeier W, Mohler P, van Veen TA, van Rijen HV, Delmar M. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc Res. 2012;95:460–468. doi: 10.1093/cvr/cvs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrone M, Montnach J, Lin X, Zhao YT, Zhang M, Agullo-Pascual E, Leo-Macias A, Alvarado FJ, Dolgalev I, Karathanos TV, Malkani K, Van Opbergen CJM, van Bavel JJA, Yang HQ, Vasquez C, Tester D, Fowler S, Liang F, Rothenberg E, Heguy A, Morley GE, Coetzee WA, Trayanova NA, Ackerman MJ, van Veen TAB, Valdivia HH, Delmar M. Plakophilin-2 is required for transcription of genes that control calcium cycling and cardiac rhythm. Nat Commun. 2017;8:106. doi: 10.1038/s41467-017-00127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelko SP, Asimaki A, Andersen P, Bedja D, Amat-Alarcon N, DeMazumder D, Jasti R, MacRae CA, Leber R, Kleber AG, Saffitz JE, Judge DP (2016) Central role for GSK3beta in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight 1 [DOI] [PMC free article] [PubMed]
- Chen SN, Gurha P, Lombardi R, Ruggiero A, Willerson JT, Marian AJ. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ Res. 2014;114:454–468. doi: 10.1161/CIRCRESAHA.114.302810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra A, Tabdanov E, Patel H, Janmey PA, Kresh JY. Cardiac myocyte remodeling mediated by N-cadherin-dependent mechanosensing. Am J Physiol Heart Circ Physiol. 2011;300:H1252–H1266. doi: 10.1152/ajpheart.00515.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrado D, Basso C, Judge DP. Arrhythmogenic cardiomyopathy. Circ Res. 2017;121:784–802. doi: 10.1161/CIRCRESAHA.117.309345. [DOI] [PubMed] [Google Scholar]
- Dalal D, Molin LH, Piccini J, Tichnell C, James C, Bomma C, Prakasa K, Towbin JA, Marcus FI, Spevak PJ, Bluemke DA, Abraham T, Russell SD, Calkins H, Judge DP. Clinical features of arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in plakophilin-2. Circulation. 2006;113:1641–1649. doi: 10.1161/CIRCULATIONAHA.105.568642. [DOI] [PubMed] [Google Scholar]
- De Bortoli M, Beffagna G, Bauce B, Lorenzon A, Smaniotto G, Rigato I, Calore M, Li Mura IE, Basso C, Thiene G, Lanfranchi G, Danieli GA, Nava A, Rampazzo A. The p.A897KfsX4 frameshift variation in desmocollin-2 is not a causative mutation in arrhythmogenic right ventricular cardiomyopathy. Eur J Hum Genet. 2010;18:776–782. doi: 10.1038/ejhg.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieding M, Debus JD, Kerkhoff R, Gaertner-Rommel A, Walhorn V, Milting H, Anselmetti D. Arrhythmogenic cardiomyopathy related DSG2 mutations affect desmosomal cadherin binding kinetics. Sci Rep. 2017;7:13791. doi: 10.1038/s41598-017-13737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubash AD, Kam CY, Aguado BA, Patel DM, Delmar M, Shea LD, Green KJ. Plakophilin-2 loss promotes TGF-beta1/p38 MAPK-dependent fibrotic gene expression in cardiomyocytes. J Cell Biol. 2016;212:425–438. doi: 10.1083/jcb.201507018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW, Borrmann CM, Grund C, Pieperhoff S. The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur J Cell Biol. 2006;85:69–82. doi: 10.1016/j.ejcb.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–2021. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehmlich K, Lambiase PD, Asimaki A, Ciaccio EJ, Ehler E, Syrris P, Saffitz JE, McKenna WJ. A novel desmocollin-2 mutation reveals insights into the molecular link between desmosomes and gap junctions. Heart Rhythm. 2011;8:711–718. doi: 10.1016/j.hrthm.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehmlich K, Syrris P, Peskett E, Evans A, Ehler E, Asimaki A, Anastasakis A, Tsatsopoulou A, Vouliotis AI, Stefanadis C, Saffitz JE, Protonotarios N, McKenna WJ. Mechanistic insights into arrhythmogenic right ventricular cardiomyopathy caused by desmocollin-2 mutations. Cardiovasc Res. 2011;90:77–87. doi: 10.1093/cvr/cvq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, Lerman BB, Markowitz SM, Ellinor PT, MacRae CA, Peters S, Grossmann KS, Drenckhahn J, Michely B, Sasse-Klaassen S, Birchmeier W, Dietz R, Breithardt G, Schulze-Bahr E, Thierfelder L. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- Hall C, Li S, Li H, Creason V, Wahl JK., 3rd Arrhythmogenic right ventricular cardiomyopathy plakophilin-2 mutations disrupt desmosome assembly and stability. Cell Commun Adhes. 2009;16:15–27. doi: 10.1080/15419060903009329. [DOI] [PubMed] [Google Scholar]
- Herren T, Gerber PA, Duru F. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: a not so rare “disease of the desmosome” with multiple clinical presentations. Clin Res Cardiol. 2009;98:141–158. doi: 10.1007/s00392-009-0751-4. [DOI] [PubMed] [Google Scholar]
- Huang NN, Becker S, Boularan C, Kamenyeva O, Vural A, Hwang IY, Shi CS, Kehrl JH. Canonical and noncanonical g-protein signaling helps coordinate actin dynamics to promote macrophage phagocytosis of zymosan. Mol Cell Biol. 2014;34:4186–4199. doi: 10.1128/MCB.00325-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant S, Krusche CA, Gaertner A, Milting H, Leube RE. Loss of plakoglobin immunoreactivity in intercalated discs in arrhythmogenic right ventricular cardiomyopathy: protein mislocalization versus epitope masking. Cardiovasc Res. 2016;109:260–271. doi: 10.1093/cvr/cvv270. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Buckingham ME, Moorman AF (2014) Heart fields and cardiac morphogenesis. Cold Spring Harb Perspect Med 4 [DOI] [PMC free article] [PubMed]
- Kottke MD, Delva E, Kowalczyk AP. The desmosome: cell science lessons from human diseases. J Cell Sci. 2006;119:797–806. doi: 10.1242/jcs.02888. [DOI] [PubMed] [Google Scholar]
- Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, Donarum EA, Marino M, Tiso N, Viitasalo M, Toivonen L, Stephan DA, Kontula K. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.CIR.103.4.485. [DOI] [PubMed] [Google Scholar]
- Lazzarini E, Jongbloed JD, Pilichou K, Thiene G, Basso C, Bikker H, Charbon B, Swertz M, van Tintelen JP, van der Zwaag PA. The ARVD/C genetic variants database: 2014 update. Hum Mutat. 2015;36:403–410. doi: 10.1002/humu.22765. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, Reiken S, Wronska A, Drew LJ, Ward CW, Lederer WJ, Kass RS, Morley G, Marks AR. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–2245. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi R, Marian AJ. Arrhythmogenic right ventricular cardiomyopathy is a disease of cardiac stem cells. Curr Opin Cardiol. 2010;25:222–228. doi: 10.1097/HCO.0b013e3283376daf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ayala JM, Gomez-Milanes I, Sanchez Munoz JJ, Ruiz-Espejo F, Ortiz M, Gonzalez-Carrillo J, Lopez-Cuenca D, Oliva-Sandoval MJ, Monserrat L, Valdes M, Gimeno JR. Desmoplakin truncations and arrhythmogenic left ventricular cardiomyopathy: characterizing a phenotype. Europace. 2014;16:1838–1846. doi: 10.1093/europace/euu128. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Tedgui A, Fontaliran F, Frank R, Durigon M, Fontaine G. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N Engl J Med. 1996;335:1190–1196. doi: 10.1056/NEJM199610173351604. [DOI] [PubMed] [Google Scholar]
- Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–398. doi: 10.1161/01.CIR.65.2.384. [DOI] [PubMed] [Google Scholar]
- Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumori A, Kawai C. Coxsackie virus B3 perimyocarditis in BALB/c mice: experimental model of chronic perimyocarditis in the right ventricle. J Pathol. 1980;131:97–106. doi: 10.1002/path.1711310202. [DOI] [PubMed] [Google Scholar]
- Mayosi BM, Fish M, Shaboodien G, Mastantuono E, Kraus S, Wieland T, Kotta MC, Chin A, Laing N, Ntusi NB, Chong M, Horsfall C, Pimstone SN, Gentilini D, Parati G, Strom TM, Meitinger T, Pare G, Schwartz PJ, Crotti L (2017) Identification of cadherin 2 (CDH2) mutations in arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Genet 10 [DOI] [PubMed]
- McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–2124. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- Merner ND, Hodgkinson KA, Haywood AF, Connors S, French VM, Drenckhahn JD, Kupprion C, Ramadanova K, Thierfelder L, McKenna W, Gallagher B, Morris-Larkin L, Bassett AS, Parfrey PS, Young TL. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am J Hum Genet. 2008;82:809–821. doi: 10.1016/j.ajhg.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens C, Kuhn C, Franke WW. Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol. 1996;135:1009–1025. doi: 10.1083/jcb.135.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava A, Thiene G, Canciani B, Scognamiglio R, Daliento L, Buja G, Martini B, Stritoni P, Fasoli G. Familial occurrence of right ventricular dysplasia: a study involving nine families. J Am Coll Cardiol. 1988;12:1222–1228. doi: 10.1016/0735-1097(88)92603-4. [DOI] [PubMed] [Google Scholar]
- Noorman M, Hakim S, Kessler E, Groeneweg JA, Cox MG, Asimaki A, van Rijen HV, van Stuijvenberg L, Chkourko H, van der Heyden MA, Vos MA, de Jonge N, van der Smagt JJ, Dooijes D, Vink A, de Weger RA, Varro A, de Bakker JM, Saffitz JE, Hund TJ, Mohler PJ, Delmar M, Hauer RN, van Veen TA. Remodeling of the cardiac sodium channel, connexin43, and plakoglobin at the intercalated disk in patients with arrhythmogenic cardiomyopathy. Heart Rhythm. 2013;10:412–419. doi: 10.1016/j.hrthm.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, Purkis PE, Whittock N, Leigh IM, Stevens HP, Kelsell DP. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet. 2000;9:2761–2766. doi: 10.1093/hmg/9.18.2761. [DOI] [PubMed] [Google Scholar]
- Pilichou K, Nava A, Basso C, Beffagna G, Bauce B, Lorenzon A, Frigo G, Vettori A, Valente M, Towbin J, Thiene G, Danieli GA, Rampazzo A. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:1171–1179. doi: 10.1161/CIRCULATIONAHA.105.583674. [DOI] [PubMed] [Google Scholar]
- Pilichou K, Remme CA, Basso C, Campian ME, Rizzo S, Barnett P, Scicluna BP, Bauce B, van den Hoff MJ, de Bakker JM, Tan HL, Valente M, Nava A, Wilde AA, Moorman AF, Thiene G, Bezzina CR. Myocyte necrosis underlies progressive myocardial dystrophy in mouse dsg2-related arrhythmogenic right ventricular cardiomyopathy. J Exp Med. 2009;206:1787–1802. doi: 10.1084/jem.20090641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilichou K, Thiene G, Bauce B, Rigato I, Lazzarini E, Migliore F, Perazzolo Marra M, Rizzo S, Zorzi A, Daliento L, Corrado D, Basso C. Arrhythmogenic cardiomyopathy. Orphanet J Rare Dis. 2016;11:33. doi: 10.1186/s13023-016-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protonotarios N, Tsatsopoulou A. Naxos disease and Carvajal syndrome: cardiocutaneous disorders that highlight the pathogenesis and broaden the spectrum of arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Pathol. 2004;13:185–194. doi: 10.1016/j.carpath.2004.03.609. [DOI] [PubMed] [Google Scholar]
- Quarta G, Syrris P, Ashworth M, Jenkins S, Zuborne Alapi K, Morgan J, Muir A, Pantazis A, McKenna WJ, Elliott PM. Mutations in the Lamin a/C gene mimic arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2012;33:1128–1136. doi: 10.1093/eurheartj/ehr451. [DOI] [PubMed] [Google Scholar]
- Rampazzo A, Nava A, Danieli GA, Buja G, Daliento L, Fasoli G, Scognamiglio R, Corrado D, Thiene G. The gene for arrhythmogenic right ventricular cardiomyopathy maps to chromosome 14q23-q24. Hum Mol Genet. 1994;3:959–962. doi: 10.1093/hmg/3.6.959. [DOI] [PubMed] [Google Scholar]
- Rampazzo A, Nava A, Malacrida S, Beffagna G, Bauce B, Rossi V, Zimbello R, Simionati B, Basso C, Thiene G, Towbin JA, Danieli GA. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2002;71:1200–1206. doi: 10.1086/344208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato PY, Coombs W, Lin X, Nekrasova O, Green KJ, Isom LL, Taffet SM, Delmar M. Interactions between ankyrin-G, plakophilin-2, and connexin43 at the cardiac intercalated disc. Circ Res. 2011;109:193–201. doi: 10.1161/CIRCRESAHA.111.247023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen-Chowdhry S, Syrris P, McKenna WJ. Genetics of right ventricular cardiomyopathy. J Cardiovasc Electrophysiol. 2005;16:927–935. doi: 10.1111/j.1540-8167.2005.40842.x. [DOI] [PubMed] [Google Scholar]
- Shen WK, Edwards WD, Hammill SC, Bailey KR, Ballard DJ, Gersh BJ. Sudden unexpected nontraumatic death in 54 young adults: a 30-year population-based study. Am J Cardiol. 1995;76:148–152. doi: 10.1016/S0002-9149(99)80047-2. [DOI] [PubMed] [Google Scholar]
- Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Phys. 1996;271:H2183–H2189. doi: 10.1152/ajpheart.1996.271.5.H2183. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Roberts AB. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992;119:1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokoe KS, Thomas G, Goddard CA, Colledge WH, Grace AA, Huang CL. Effects of flecainide and quinidine on arrhythmogenic properties of Scn5a+/Delta murine hearts modelling long QT syndrome 3. J Physiol. 2007;578:69–84. doi: 10.1113/jphysiol.2006.117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud MJ, Fang X, Zhang J, Guimaraes-Camboa N, Veevers J, Dalton ND, Gu Y, Bradford WH, Peterson KL, Evans SM, Gerace L, Chen J. Luma is not essential for murine cardiac development and function. Cardiovasc Res. 2018;114:378–388. doi: 10.1093/cvr/cvx205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrris P, Ward D, Evans A, Asimaki A, Gandjbakhch E, Sen-Chowdhry S, McKenna WJ. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am J Hum Genet. 2006;79:978–984. doi: 10.1086/509122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M, Graw S, Sinagra G, Barnes C, Slavov D, Brun F, Pinamonti B, Salcedo EE, Sauer W, Pyxaras S, Anderson B, Simon B, Bogomolovas J, Labeit S, Granzier H, Mestroni L. Genetic variation in titin in arrhythmogenic right ventricular cardiomyopathy-overlap syndromes. Circulation. 2011;124:876–885. doi: 10.1161/CIRCULATIONAHA.110.005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Riele AS, Agullo-Pascual E, James CA, Leo-Macias A, Cerrone M, Zhang M, Lin X, Lin B, Sobreira NL, Amat-Alarcon N, Marsman RF, Murray B, Tichnell C, van der Heijden JF, Dooijes D, van Veen TA, Tandri H, Fowler SJ, Hauer RN, Tomaselli G, van den Berg MP, Taylor MR, Brun F, Sinagra G, Wilde AA, Mestroni L, Bezzina CR, Calkins H, Peter van Tintelen J, Bu L, Delmar M, Judge DP. Multilevel analyses of SCN5A mutations in arrhythmogenic right ventricular dysplasia/cardiomyopathy suggest non-canonical mechanisms for disease pathogenesis. Cardiovasc Res. 2017;113:102–111. doi: 10.1093/cvr/cvw234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiene G, Corrado D, Nava A, Rossi L, Poletti A, Boffa GM, Daliento L, Pennelli N. Right ventricular cardiomyopathy: is there evidence of an inflammatory aetiology? Eur Heart J. 1991;12(Suppl D):22–25. doi: 10.1093/eurheartj/12.suppl_D.22. [DOI] [PubMed] [Google Scholar]
- Thiene G, Basso C, Angelini A, Calabrese F, Valente M. Morbid anatomy and pathobiology of arrhythmogenic right ventricular cardiomyopathy. Herzschrittmacherther Elektrophysiol. 1998;9:147–154. doi: 10.1007/s003990050024. [DOI] [Google Scholar]
- Thiene G, Basso C, Calabrese F, Angelini A, Valente M. Pathology and pathogenesis of arrhythmogenic right ventricular cardiomyopathy. Herz. 2000;25:210–215. doi: 10.1007/s000590050008. [DOI] [PubMed] [Google Scholar]
- Tiso N, Stephan DA, Nava A, Bagattin A, Devaney JM, Stanchi F, Larderet G, Brahmbhatt B, Brown K, Bauce B, Muriago M, Basso C, Thiene G, Danieli GA, Rampazzo A. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2) Hum Mol Genet. 2001;10:189–194. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- van der Zwaag PA, van Rijsingen IA, Asimaki A, Jongbloed JD, van Veldhuisen DJ, Wiesfeld AC, Cox MG, van Lochem LT, de Boer RA, Hofstra RM, Christiaans I, van Spaendonck-Zwarts KY, Lekanne dit Deprez RH, Judge DP, Calkins H, Suurmeijer AJ, Hauer RN, Saffitz JE, Wilde AA, van den Berg MP, van Tintelen JP. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail. 2012;14:1199–1207. doi: 10.1093/eurjhf/hfs119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hengel J, Calore M, Bauce B, Dazzo E, Mazzotti E, De Bortoli M, Lorenzon A, Li Mura IE, Beffagna G, Rigato I, Vleeschouwers M, Tyberghein K, Hulpiau P, van Hamme E, Zaglia T, Corrado D, Basso C, Thiene G, Daliento L, Nava A, van Roy F, Rampazzo A. Mutations in the area composita protein alphaT-catenin are associated with arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2013;34:201–210. doi: 10.1093/eurheartj/ehs373. [DOI] [PubMed] [Google Scholar]
- van Tintelen JP, Van Gelder IC, Asimaki A, Suurmeijer AJ, Wiesfeld AC, Jongbloed JD, van den Wijngaard A, Kuks JB, van Spaendonck-Zwarts KY, Notermans N, Boven L, van den Heuvel F, Veenstra-Knol HE, Saffitz JE, Hofstra RM, van den Berg MP. Severe cardiac phenotype with right ventricular predominance in a large cohort of patients with a single missense mutation in the DES gene. Heart Rhythm. 2009;6:1574–1583. doi: 10.1016/j.hrthm.2009.07.041. [DOI] [PubMed] [Google Scholar]
- Vatta M, Marcus F, Towbin JA. Arrhythmogenic right ventricular cardiomyopathy: a 'final common pathway' that defines clinical phenotype. Eur Heart J. 2007;28:529–530. doi: 10.1093/eurheartj/ehl530. [DOI] [PubMed] [Google Scholar]
- Vermij SH, Abriel H, van Veen TA. Refining the molecular organization of the cardiac intercalated disc. Cardiovasc Res. 2017;113:259–275. doi: 10.1093/cvr/cvw259. [DOI] [PubMed] [Google Scholar]
- Xu T, Yang Z, Vatta M, Rampazzo A, Beffagna G, Pilichou K, Scherer SE, Saffitz J, Kravitz J, Zareba W, Danieli GA, Lorenzon A, Nava A, Bauce B, Thiene G, Basso C, Calkins H, Gear K, Marcus F, Towbin JA, Multidisciplinary Study of Right Ventricular Dysplasia I Compound and digenic heterozygosity contributes to arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2010;55:587–597. doi: 10.1016/j.jacc.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Zhang HM, Qiu Y, Hanson PJ, Hemida MG, Wei W, Hoodless PA, Chu F, Yang D. Coxsackievirus-induced miR-21 disrupts cardiomyocyte interactions via the downregulation of intercalated disk components. PLoS Pathog. 2014;10:e1004070. doi: 10.1371/journal.ppat.1004070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Guan KL. The hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Hanemann CO. Merlin, a multi-suppressor from cell membrane to the nucleus. FEBS Lett. 2012;586:1403–1408. doi: 10.1016/j.febslet.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Zhurinsky J, Shtutman M, Ben-Ze'ev A. Differential mechanisms of LEF/TCF family-dependent transcriptional activation by beta-catenin and plakoglobin. Mol Cell Biol. 2000;20:4238–4252. doi: 10.1128/MCB.20.12.4238-4252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]