Abstract
Oligodendroglial tumors are chemosensitive with a favorable prognosis compared with other histological subtypes. The genetic hallmark of co-deletion of 1p and 19q determines both treatment response and prognosis. While this test now forms part of routine histopathology diagnosis in many laboratories, alternative noninvasive imaging biomarkers of tumor genotype remain an attractive proposition. This review will focus on imaging biomarkers of molecular genetics in oligodendroglial tumors.
KEYWORDS : genotype, MRI, oligoastrocytoma, oligodendroglioma, SPECT
Practice points.
The literature is limited by small studies of mixed histological grade or subtype, with associations not always reaching highly statistically significant levels. Furthermore, the lack of independent validations sets and unclear specificity and sensitivity limits the clinical usefulness of imaging techniques in the distinction of molecular subtypes of oligodendroglial tumors.
Associations between 1p/19q status and imaging (seen in some but not necessarily all studies) include:
On conventional MRI 1p/19q co-deletion is associated with:
Peripheral tumor location.
Bi-hemispherical growth pattern.
Ill-defined margins on T1 images.
Heterogeneous signal intensity on T1 and T2 images.
Paramagnetic susceptibility effect and calcification.
On multimodal MRI 1p/19q co-deletion is associated with:
Higher relative cerebral blood volume.
Lower apparent diffusion coefficient.
Although genotype differences can be identified via imaging modalities, associations between imaging and 1p/19q status are not robust enough for a diagnostic test; molecular genetics remains the gold standard.
MRS identification of the presence of the metabolite 2-hydroxyglutarate (2HG) has been demonstrated to correlate well with IDH1 mutation status, showing promise for future noninvasive evaluation of IDH1 mutation status as a diagnostic and prognostic biomarker.
Since the seminal observations by Cairncross in the 1990s that oligodendroglial tumors are sensitive to chemotherapy, the genetics and biology of gliomas have been extensively investigated [1]. Molecular classification of oligodendroglial tumors according to genotype is a useful adjunct to histopathology diagnosis, and 1p/19q co-deletion is considered the genetic hallmark of chemosensitivity associated with a favorable prognosis [2]. Other genetic alterations such as IDH1 and IDH2 mutations and MGMT promoter methylation are also important biomarkers with diagnostic and prognostic significance [3].
The histological features used to classify gliomas are reflected macroscopically in their imaging characteristics. In recent decades, advances in neuroimaging have led to the noninvasive characterization of cerebral tumors, providing improved diagnosis and prognostication. Research studies have moved from simple image analysis to extraction of semiquantiative and quantitative biomarker data from multimodal MRI sequences related to the physiological and metabolic characteristics of gliomas. The clinical utility of these imaging biomarkers is being actively investigated.
These various imaging modalities have been used as a noninvasive tool not only to distinguish tumor from normal brain but also to predict histological type and grade and thereby prognosis. In addition, many studies have investigated whether imaging might also provide a means to distinguish between genetic subtypes of glioma. In this article, we will examine the imaging characteristics of oligodendroglial tumors in relation to their genetic subtypes with emphasis on 1p/19q and IDH status. Specifically, we aim to address the question: “Can neuroimaging noninvasively distinguish the genetic subtypes of oligodendroglial tumors?”
Conventional imaging
Conventional anatomical neuroimaging comprises computed tomography (CT) and MRI. T1-weighted (± gadolinium), T2-weighted and fluid attenuated inversion recovery (FLAIR) MRI are used routinely for noninvasive diagnosis of brain tumors and provide excellent soft tissue contrast but do not provide information on angiogenesis, metabolism or cellularity, which are important parameters in tumor identification and prognostication. However, some research studies have shown interesting correlations between the features commonly seen in conventional MRI and genotype.
The evidence relating MRI features to genotype in oligodendroglial tumors is frequently based on comparisons made in small clinical series, often resulting in conflicting data. A preferential distribution of 1p/19q co-deleted tumors in frontal, parietal and occipital lobes, or nontemporal locations has been reported, but other studies have failed to find significant differences between tumor location and 1p/19q status [4]. Similarly, preferential localization of tumors with intact 1p/19q to insular regions is also subject to controversy [5,6]. 1p/19q co-deletion has been associated with bilateral growth pattern [7], lower cross-sectional area in T2-weighted MRI [8] and in untreated tumors with slower growth rate measured on serial MRI scans [9].
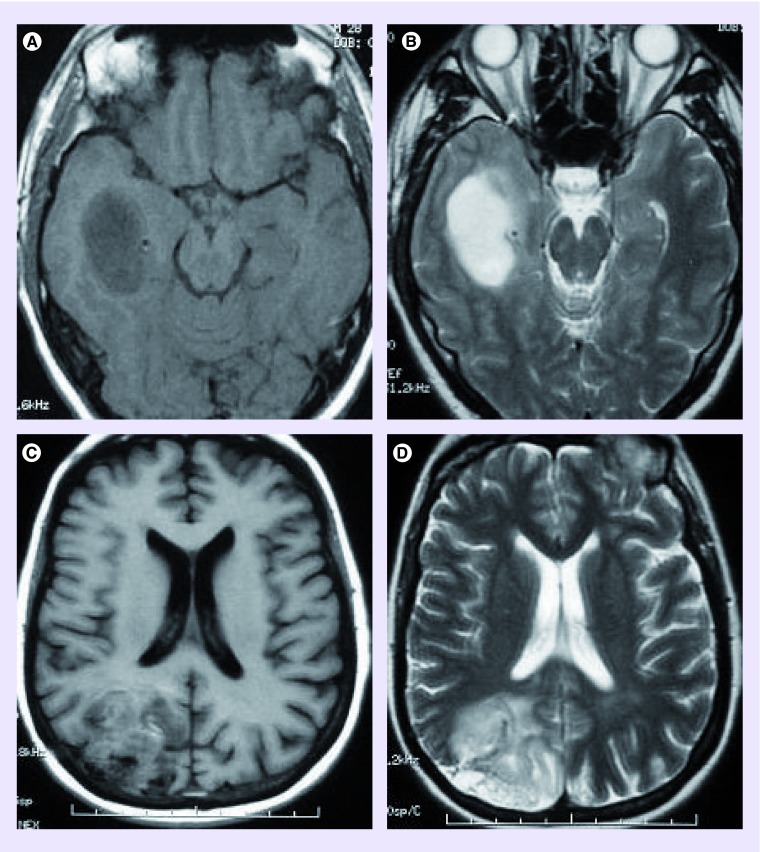
Oligodendroglial tumors with co-deleted 1p/19q are more likely to have heterogeneous signal intensities on T1- and T2-weighted MRI [10–12]. However, in other studies signal intensity did not differ with 1p/19q status [13], and signal heterogeneity was associated with intact 1p/19q in oligoastrocytomas [14]. Oligodendroglial tumors with co-deleted 1p/19q are more likely to have indistinct borders on T1- or T2-weighted images (Figure 1) [10,12]. Infiltrative growth was more common in tumors with intact 1p/19q in a study investigating histological growth patterns in relation to co-registered clinical imaging [11]. Transition in cellularity at the margin was found to be similar, irrespective of genotype; however, tumors with a sharp, smooth border and homogeneous signal intensity were more likely to have intact 1p/19q [11]. 1p/19q co-deletion could be predicted by measuring MRI image texture with a sensitivity of 93% and a specificity of 96% [15]. 1p/19q status did not influence contrast enhancement or cortical involvement [11,12,16]. In keeping with the known calcification of oligodendrogliomas, some but not all studies have reported associations between paramagnetic susceptibility effect or calcification and 1p/19q co-deletion [10–13]. The biological basis of these differences in imaging characteristics on conventional MRI is yet to be fully elucidated, although it has been hypothesized that differences in signal intensity and tumor borders may be due to increased ‘invasiveness’ in tumors with 1p/19q co-deletion [10].
Figure 1. . Conventional MRI characteristics of 1p/19q status (a,c - MRI T1 axial images; b,d - MRI T2 axial images).
(A–B) Sharp smooth T1 and T2 border in a grade II oligodendroglioma with intact 1p/19q; (C–D) Indistinct, irregular border in a grade III oligodendroglioma with 1p/19q co-deletion.
Adapted with permission from [11] © Oxford University Press.
Other genetic alterations common in oligodendroglial tumors have been investigated in relation to MRI. Genetic analysis confirmed tumor aggressiveness by detection of chromosome 10 losses in oligoastrocytomas displaying MRI features of high-grade glioma [14]. Mutations in the IDH1 gene are present in 60–80% of glioma grades II and III and secondary glioblastomas. IDH2 mutations are less common and seen in approximately 2–5% of these tumors. Patients with IDH mutated gliomas have a better 5-year survival than those with wild-type IDH, and IDH mutations are significantly associated with 1p/19q co-deleted genotype and p53 immunopositivity [17]. Equally the identification of tumors with wild-type IDH is prognostically important. In conventional MRI, tumors lacking IDH mutations more frequently involved the insula, displayed an infiltrative pattern and had larger T2 diameters [17].
Although MGMT methylation status in glioblastomas may be distinguished by texture analysis [18] and ill-defined margins are seen more frequently in MGMT methylated high-grade gliomas [19], associations between MGMT methylation and imaging features have not been reported in oligodendroglial tumors (Table 1).
Table 1. . Conventional MRI features associated with 1p/19q co-deletion.
| Study | n | Histology | MRI features | Ref. |
|---|---|---|---|---|
| Brown R et al. | 55 | Low-grade OD and mixed OA | Quantitative S-transform texture on T2 images | [15] |
| Chawla S et al. | 40 | WHO grade II and III OD | Paramagnetic susceptibility effect | [13] |
| Jenkinson MD et al. | 86 | WHO grade II and III OD and OA | Calcification heterogeneous intensity signal, indistinct/irregular borders | [11] |
| Kim JW et al. | 56 | WHO grade III OD and OA | Frontal lobe location Indistinct tumor borders, heterogeneous intensity signal | [12] |
| Megyesi JF et al. | 40 | WHO grade II and III OD | Calcification heterogeneous intensity signal Indistinct border, paramagnetic susceptibility effect | [10] |
| Zlatescu M et al. | 64 | WHO grade III OD | Frontal, parietal and occipital location, bilateral growth pattern | [7] |
AS: Astrocytomas; OA: Oligoastrocytomas; OD: Oligodendroglioma.
Multimodal MRI
Advanced MRI sequences allow quantitative and semi-quantitative measurement of physiological processes including cerebral blood volume and flow, water movement and the chemical composition of tissue. These MRI modalities which include proton MR spectroscopy (MRS), perfusion-weighted imaging (PWI) and diffusion-weighted imaging (DWI) are becoming well accepted as additional methods for tumor characterization (Table 2).
Table 2. . Imaging biomarkers and their biological correlates.
| Imaging modality | Imaging biomarker | Biological correlate |
|---|---|---|
| MRS | Cho | Membrane turnover, cell proliferation |
| NAA | Neuronal integrity | |
| Lipid | Intracellular lipid droplets and cell necrosis | |
| Lactate | Anaerobic respiration, cell hypoxia | |
| 2HG | IDH1 mutation | |
| PWI | rCBV | Blood volume |
| rCBF | Blood flow | |
| Ktrans | Vascular permeability | |
| DWI | ADC | Reduced water mobility reflecting cellular density and physical properties of the extracellular matrix |
| Metabolic Imaging | 18F-FDG and 201Tl uptake, radiolabeled amino acid uptake | Cell proliferation and metabolism, amino acid transport through the cell membrane |
ADC: Apparent diffusion coefficient; DWI: Diffusion-weighted imaging; MRS: MR spectroscopy; PWI: Perfusion-weighted imaging.
• Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) provides an assessment of the metabolic signature and chemical markers of neoplastic activity. The mitotic index of oligodendrogliomas is a reflection of their proliferation rate, which increases progressively from low to high grade. In high-grade gliomas (HGGs), increased cell division is associated with an elevated choline (Cho) signal, reflecting cell membrane turnover. Increased cellularity in HGGs results in fewer neurons within tumor tissues and a decreased N-acetyl aspartate (NAA) signal. The high growth rates necessitate increased metabolism, which becomes anaerobic as growth outstrips oxygen supply, and hypoxia and necrosis result, associated with elevated lipid and lactate signals [4].
In oligodendroglial tumors, MRS may be used to distinguish tumor grade but not 1p/19q status [4]. In a study of 48 patients, oligodendroglial tumors with 1p/19q co-deletion could not be distinguished from those with intact 1p/19q using routine clinical MRS [20].
The mutations in the enzyme IDH1 impair the normal ability of IDH1 to convert isocitrate to α-ketoglutarate (αKG) and result in excess production and accumulation of the metabolite 2-hydroxyglutarate (2HG). Recently, the production of 2HG has been detected noninvasively, presurgery, in a series of glioma patients with IDH1 mutations, but not in glioma patients with wild-type IDH1 or in healthy volunteers using optimized in vivo spectral editing and two-dimensional correlation MRS methods [21]. These methods offer an advanced compared with traditional one-dimensional spectroscopy, as the similarity of 2HG to other metabolites, such as glutamate and glutamine, can provide false-positive data owing to spectral overlap [21]. Similar results in the detection of 2HG have been demonstrated using 3 Tesla MRI [22,23].
As well as in clinical studies in vivo, 2HG has been detected in ex vivo tumor samples using proton high-resolution magic angle spinning (1H HR-MAS) nuclear magnetic resonance (NMR) spectroscopy resulting in 86% agreement with the IDH1 mutation status of the tumor samples [24]. In a similar 1HR-MAS NMR study, 2HG detection identified gliomas with mutated IDH1/2 genes with a 96% sensitivity and 98% accuracy [25].
• Perfusion-weighted imaging
Perfusion-weighted imaging (PWI) can be acquired by two techniques:
Dynamic susceptibility contrast MRI (DSC-MRI): a first pass technique that uses rapid measurement of T2- or T2*-weighted signal change after injection of a bolus of paramagnetic compound (i.e., gadolinium-based contrast material);
Dynamic contrast-enhanced MRI (DCE-MRI): based on the T1-weighted signal change produced after an interval of 5–10 min following injection of gadolinium-based contrast material.
These techniques can provide parameters such as:
Relative cerebral blood volume (rCBV) – the fraction occupied by blood vessels within a cerebral tumor relative to contralateral normal brain; this parameter is termed relative tumor blood volume in some studies (rTBV);
Relative cerebral blood flow (rCBF) – the flow of blood through cerebral tissue over time which reflects variations in angiogenesis and vascular density;
Contrast transfer coefficient (Ktrans) – measure of vascular permeability.
The vascular architecture of oligodendrogliomas is a key feature in their histopathology diagnosis and allows imaging associations with grade through perfusion-weighted imaging parameters [26]. The vessels within lower grade tumor tissue may be more numerous than in normal brain and may adopt a ‘chicken-wire’ pattern, but vessel structure is unaltered and the blood–brain barrier remains intact. Malignant transformation and biological aggressiveness in oligodendrogliomas are associated with neovascularization in response to hypoxia, and degradation and remodeling of extracellular matrix macromolecules, leading to loss of blood–brain barrier integrity which is reflected in perfusion-weighted MRI.
In addition to distinction of grade, associations between histopathology subtype or genotype and PWI parameters have been observed in oligodendroglial tumors. In several studies, oligodendroglial tumors with 1p/19q co-deletion were more likely to have high rCBV and rCBF than those with intact 1p/19q, however rCBV did not predict chemosensitivity [27–32]. These findings are based mainly in studies of low grade or mixed grade (WHO grades II and III) oligodendroglial tumors. Despite promising differences in rCBV in low-grade oligodendroglial tumors with or without 1p/19q co-deletion, significant differences in rCBV between the two genotypes have not been observed in high-grade oligodendroglial tumors, rendering rCBV-based tumor grading potentially inaccurate [28]. In contrast when using perfusion CT, CBV and neovascular permeability did not differ between high- and low-grade tumors or between those with and without 1p/19q co-deletion [33].
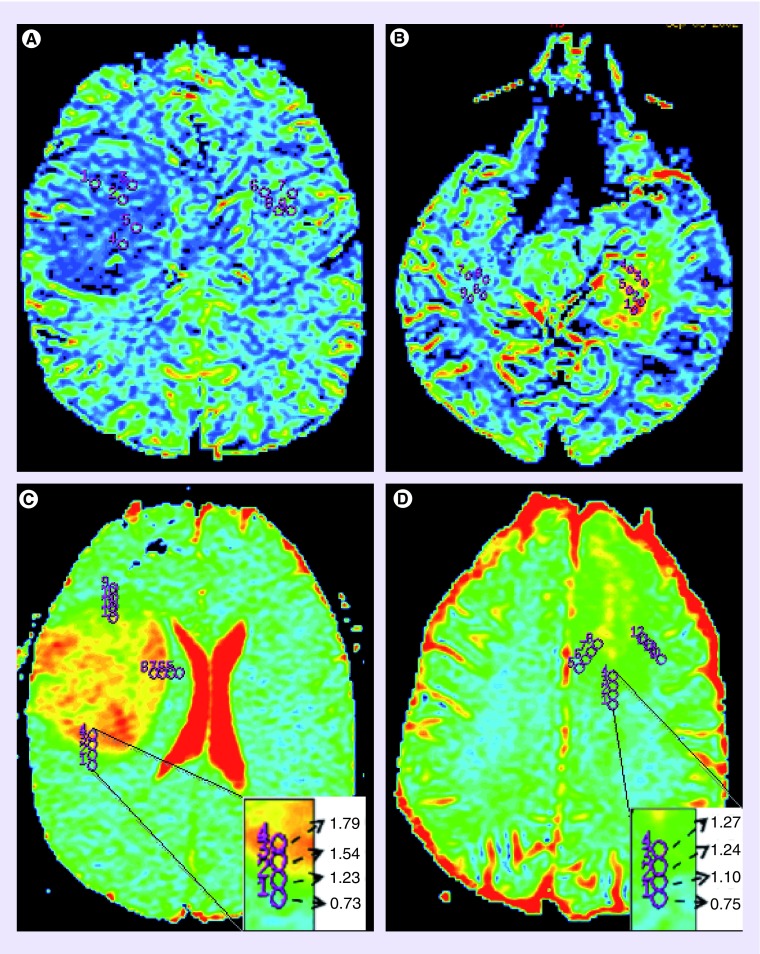
Elevated rCBV in oligodendroglial tumors with 1p/19q co-deletion suggests upregulation of angiogenesis in these tumors. In a study relating rCBV to expression of angiogenesis and growth factors, Kapoor et al. showed significantly higher rTBV in low-grade oligodendrogliomas with 1p/19q co-deletion in association with increased expression of angiogenesis markers VEGF, CD31 and CD105 [31]. In addition, significant associations of rTBV with 1p/19q co-deletion and expression of EGFR and VEGF were found following multivariate linear regression analysis [31,34]. These data suggest high rCBV in 1p/19q co-deleted tumors may reflect differences in biology according to genotype and not histological grade (Figure 2).
Figure 2. . Cereberal blood volume and apparent diffusion coefficient maps demonstrating characteristics of 1p/19q status.
(A & C) are grade II oligoastrocytoma with intact 1p/19q, (B & D) are grade II oligoastrocytoma with 1p/19q co-deletion. As can be seen by the color differences the tumor in (A) has low relative CBV whereas the 1p/19q co-deleted tumor in (B) has high relative CBV. Again as can be seen by the color differences and changes in ADC values (C) has the largest ADC transition coefficient, signifying a sharp change in free water diffusion, whereas the 1p/19q co-deleted tumor in (D) has a smaller ADC transition coefficient, signifying a gradual change in free water diffusion.
• Diffusion-weighted imaging
DWI uses strong magnetic field gradients applied in multiple directions to probe the structure of biologic tissues at a microscopic level by measuring the Brownian motion of water molecules. In the brain, water movement is not truly random and is restricted by the presence of cell membranes, nerve fibers and extracellular macromolecules. Since diffusion is a microscopic process that is beyond the spatial resolution of MRI, true water diffusion cannot be measured. Instead, using a statistical model, isotropic mean diffusivity or apparent diffusion coefficient (ADC) is derived from DWI and reflects the ease of movement of water molecules within tissue microstructures. Low ADC values result from more solid tissues with high cell density, while high ADC values arise from tissue with low cellularity. Fractional anisotropy (FA), which is a measure of directional diffusivity can also be measured using this method [26,35].
In DWI of grade II gliomas, lower median ADC [26] or ADC histogram [36] correlated well with oligodendrogliomas as opposed to astrocytomas or oligoastrocytomas. Similarly, oligodendrogliomas were distinguished from astrocytomas with 92% sensitivity and 91% specificity using a threshold median normalized ADC (generated by dividing diffusion image maps by the median ADC within the normal appearing white matter) [35]. In other studies, ADC values assessed by placing regions of interest on regions of lowest ADC (minimum ADC), were significantly higher in grade II compared with grade III tumors but no difference in minimum ADC was demonstrated between tumors with 1p/19q co-deletion and those with intact 1p/19q [28]. In another study, minimum ADC did not distinguish genotype, but tumors with intact 1p/19q had higher maximum ADC (regions of interest placed on areas of highest ADC). These findings were reflected in the ADC histograms where tumors with 1p/19q co-deletion tended to have a narrow ADC histogram peak skewed toward low ADC, whereas those with intact 1p/19q had broader histogram peaks skewed toward higher ADC values. Tumors with intact 1p/19q also had greater ADC transition coefficient (ATC: the rate of change in ADC across the tumor margin) compared with those with 1p/19q co-deletion, although this was not reflected in their cellularity. While the higher ATC may be reflected in a more diffuse T2 MRI appearance, diffusion measurements gave greater discrimination between genotypes. [37,38].
Metabolic imaging
Metabolic imaging with radiolabeled tracers such as [18F]fluorodeoxyglucose (18F-FDG), 201Thallium (201Tl) or radiolabeled amino acids has been used to assess glioma biology. FDG, an analog of glucose, is transported into the cell by facilitated diffusion, where it is subsequently phosphorylated by hexokinase to fluoroglucose-6-phosphate, but unlike glucose, this is not a substrate for further metabolism and becomes trapped intracellularly. Malignant cells are characterized by high rates of glucose consumption, reflecting increased energy demand related to proliferation, increased expression of glucose transporters or deregulation of hexokinase activity, and show elevated uptake of 18F-FDG. 201Tl has a low uptake in normal cerebral tissue because of restricted passive diffusion across the blood–brain barrier. Its uptake in gliomas is thought to depend on disruption of the blood–brain barrier and activity of the Na/K ATPase pump, indicating cell viability and to a lesser extent blood flow and consequently increases in association with the malignancy grade in gliomas [39]. Radiolabeled amino acids uptake involves a sodium-dependent cell membrane transport system and is influenced by the proliferation rate and environmental factors such as intracellular pH, hormones, growth factors and amino acid availability.
A study based on 59 oligodendroglial tumors using 18F-FDG and 201Tl single-photon emission CT (SPECT) demonstrated a clear association between metabolism and genotype as oligodendroglial tumors with 1p/19q co-deletion were more likely to show increased 201Tl uptake and, to a lesser extent, increased FDG uptake [39]. Similar findings have been reported using FDG uptake by PET imaging [40].
In grade II gliomas 11C-methionine uptake ratios (tumor/normal tissue [T/N] ratio) detected using PET were higher in oligodendrogliomas than in diffuse astrocytomas independent of their proliferative activity [41]. In the 24 oligodendroglial tumors in this study, the uptake ratio was significantly higher in tumors with intact 1p/19q than in those with the co-deletion whether the analysis included both grade II and III tumors or if grade II cases were analyzed separately [41]. Incidentally, Ki67 labeling index did not significantly correlate with T/N ratio in oligodendrogliomas [41]. In contrast, other studies have shown that within grade II tumors, the mean T/N ratio of tumors with 1p/19q co-deletion was significantly higher than that of tumors with intact 1p/19q, while no significant differences were observed in the mean T/N ratio in grade III tumors with or without 1p/19q co-deletion [42].
Combined modalities
Distinction between oligodendroglial genotypes via imaging may be improved by combining multimodal advanced MRI techniques. Studies involving DCS-MRI-guided 1H-MRS demonstrated ability to distinguish 1p/19q co-deleted oligodendrogliomas from those with intact alleles with a sensitivity of 82.6% and a specificity of 64.7%, if rCBVmax values and metabolite ratios were incorporated [13]. Although combined modalities gave higher diagnostic accuracy than single parameters, further advances are necessary for these techniques to be used clinically to distinguish oligodendroglial genotypes.
In a study of 50 adult patients with grade II and III oligodendrogliomas and oligoastrocytomas DWI, PWI and MR spectroscopy showed no significant difference between tumors with and without 1p/19q co-deletion. However following multivariate analysis using random forest methods [43], separation between tumor grades and genotypes with conventional MRI alone showed 31 and 48% misclassification rates, respectively. Application of multivariate analysis to multimodal MR imaging data helped to determine tumor grade and 1p/19q genotype more accurately with misclassification rates of 17 and 40%, respectively [28] (Table 3), but further discrimination would be required for clinical utility.
Table 3. . Multimodal MRI biomarkers associated with 1p/19q co-deletion.
| Study | n | Histology | MRI features | Ref. |
|---|---|---|---|---|
| Chawla S et al. | 40 | WHO grade II and III OD | rCBVmax and metabolite ratios | [13] |
| Emblem K et al. | 52 | WHO grade II and III OD and OA | High rCBV | [32] |
| Fellah S et al. | 50 | WHO grade II and III OD and OA | Higher rCBV and rCBF within grade II oligodendroglial subgroup | [28] |
| Jenkinson MD et al. | 37 | WHO grade II and III OD and OA | Higher rCBV (>1.59) | [27] |
| Jenkinson MD et al. | 17 | WHO grade II and III OD and OA | Lower maximum and histogram ADC Lower ATC | [38] |
| Khayal IS et al. | 30 | WHO grade II AS, OD and OA | Lower median normalized ADC (<1.8) | [35] |
| Walker C et al. | 59 | WHO grade II and III OD and OA | Increased 201Tl uptake | [39] |
ADC: Apparent diffusion coefficient; ATC: ADC transition coefficient; AS: Astrocytomas; OA: Oligoastrocytomas; OD: Oligodendroglioma; rCBF: Relative cerebral blood flow; rCBV: Relative cerebral blood volume.
Conclusion & future perspective
The current data are limited by small studies with varying tumor types that limit the applicability in routine clinical practice. With the pending introduction of the latest version of the WHO Classification of Tumors of the Central Nervous System (expected 2016/2017) molecular subtype is expected to be incorporated into the classification system. Since this is not routinely available in all laboratories, a reliable noninvasive imaging biomarker remains desirable. Increasingly the use of large imaging dataset (so called ‘big data’) will be needed to answer this important research question and enable its translation into routine clinical practice.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J. Natl Cancer Inst. 1998;90(19):1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 2.Fuller CE, Perry A. Molecular diagnostics in central nervous system tumors. Adv. Anat. Pathol. 2005;12(4):180–194. doi: 10.1097/01.pap.0000175117.47918.f7. [DOI] [PubMed] [Google Scholar]
- 3.Bourne TD, Schiff D. Update on molecular findings, management and outcome in low-grade gliomas. Nat. Rev. Neurol. 2010;6(12):695–701. doi: 10.1038/nrneurol.2010.159. [DOI] [PubMed] [Google Scholar]
- 4.Walker C, Baborie A, Crooks D, Wilkins S, Jenkinson MD. Biology, genetics and imaging of glial cell tumours. Br. J. Radiol. 2011;84:S90–S106. doi: 10.1259/bjr/23430927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gozé C, Rigau V, Gibert L, Maudelonde T, Duffau H. Lack of complete 1p19q deletion in a consecutive series of 12 WHO grade II gliomas involving the insula: a marker of worse prognosis? J. Neurooncol. 2009;91(1):1–5. doi: 10.1007/s11060-008-9680-8. [DOI] [PubMed] [Google Scholar]
- 6.Wu A, Aldape K, Lang F. High rate of deletion of chromosomes 1p and 19q in insular oligodendroglial tumors. J. Neurooncol. 2010;99(1):57–64. doi: 10.1007/s11060-009-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zlatescu MC, TehraniYazdi A, Sasaki H, et al. Tumor location and growth pattern correlate with genetic signature in oligodendroglial neoplasms. Cancer Res. 2001;61(18):6713–6715. [PubMed] [Google Scholar]
- 8.Sherman JH, Prevedello DM, Shah L, et al. MR imaging characteristics of oligodendroglial tumors with assessment of 1p/19q deletion status. Acta Neurochir. 2010;152(11):1827–1834. doi: 10.1007/s00701-010-0743-1. [DOI] [PubMed] [Google Scholar]
- 9.Ricard D, Kaloshi G, Amiel-Benouaich A, et al. Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann. Neurol. 2007;61(5):484–490. doi: 10.1002/ana.21125. [DOI] [PubMed] [Google Scholar]
- 10.Megyesi JF, Kachur E, Lee DH, et al. Imaging correlates of molecular signatures in oligodendrogliomas. Clin. Cancer Res. 2004;10(13):4303–4306. doi: 10.1158/1078-0432.CCR-04-0209. [DOI] [PubMed] [Google Scholar]
- 11.Jenkinson MD, du Plessis DG, Smith TS, Joyce KA, Warnke PC, Walker C. Histological growth patterns and genotype in oligodendroglial tumours: correlation with MRI features. Brain. 2006;129:1884–1891. doi: 10.1093/brain/awl108. [DOI] [PubMed] [Google Scholar]
- 12.Kim JW, Park C-K, Park S-H, et al. Relationship between radiological characteristics and combined 1p and 19q deletion in World Health Organization grade III oligodendroglial tumours. J. Neurol. Neurosurg. Psychiatry. 2011;82(2):224–227. doi: 10.1136/jnnp.2009.178806. [DOI] [PubMed] [Google Scholar]
- 13.Chawla S, Krejza J, Vossough A, et al. Differentiation between oligodendroglioma genotypes using dynamic susceptibility contrast perfusion-weighted imaging and proton MR spectroscopy. AJNR Am. J. Neuroradiol. 2013;34(8):1542–1549. doi: 10.3174/ajnr.A3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eoli M, Bissola L, Bruzzone MG, et al. Reclassification of oligoastrocytomas by loss of heterozygosity studies. Int. J. Cancer. 2006;119(1):84–90. doi: 10.1002/ijc.21759. [DOI] [PubMed] [Google Scholar]
- 15.Brown R, Zlatescu M, Sijben A, et al. The use of magnetic resonance imaging to noninvasively detect genetic signatures in oligodendroglioma. Clin. Cancer Res. 2008;14(8):2357–2362. doi: 10.1158/1078-0432.CCR-07-1964. [DOI] [PubMed] [Google Scholar]
- 16.Sankar T, Moore NZ, Johnson J, et al. Magnetic resonance imaging volumetric assessment of the extent of contrast enhancement and resection in oligodendroglial tumors. J. Neurosurg. 2012;116(6):1172–1181. doi: 10.3171/2012.2.JNS102032. [DOI] [PubMed] [Google Scholar]
- 17.Metellus P, Coulibaly B, Colin C, et al. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010;120(6):719–729. doi: 10.1007/s00401-010-0777-8. [DOI] [PubMed] [Google Scholar]
- 18.Drabycz S, Roldán G, De Robles P, et al. An analysis of image texture, tumor location, and MGMT promoter methylation in glioblastoma using magnetic resonance imaging. NeuroImage. 2010;49(2):1398–1405. doi: 10.1016/j.neuroimage.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 19.Moon W-J, Choi JW, Roh HG, Lim SD, Koh Y-C. Imaging parameters of high grade gliomas in relation to the MGMT promoter methylation status: the CT, diffusion tensor imaging, and perfusion MR imaging. Neuroradiology. 2012;54(6):555–563. doi: 10.1007/s00234-011-0947-y. [DOI] [PubMed] [Google Scholar]
- 20.Jenkinson MD, Smith TS, Joyce K, et al. MRS of oligodendroglial tumors: correlation with histopathology and genetic subtypes. Neurology. 2005;64(12):2085–2089. doi: 10.1212/01.WNL.0000165998.73779.D9. [DOI] [PubMed] [Google Scholar]
- 21.Andronesi OC, Kim GS, Gerstner E, et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci. Transl. Med. 2012;4(116):116ra4. doi: 10.1126/scitranslmed.3002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi C, Ganji SK, DeBerardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 2012;18(4):624–629. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pope WB, Prins RM, Albert Thomas M, et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J. Neurooncol. 2012;107(1):197–205. doi: 10.1007/s11060-011-0737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elkhaled A, Jalbert LE, Phillips JJ, et al. Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas. Sci. Transl. Med. 2012;4(116):116ra5. doi: 10.1126/scitranslmed.3002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalinina J, Carroll A, Wang L, et al. Detection of “oncometabolite” 2-hydroxyglutarate by magnetic resonance analysis as a biomarker of IDH1/2 mutations in glioma. J. Mol. Med. (Berl.). 2012;90(10):1161–1171. doi: 10.1007/s00109-012-0888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bian W, Khayal IS, Lupo JM, et al. Multiparametric characterization of Grade 2 glioma subtypes using magnetic resonance spectroscopic, perfusion, and diffusion imaging. Transl. Oncol. 2009;2(4):271–280. doi: 10.1593/tlo.09178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkinson MD, Smith TS, Joyce KA, et al. Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumours. Neuroradiology. 2006;48(10):703–713. doi: 10.1007/s00234-006-0122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fellah S, Caudal D, De Paula AM, et al. Multimodal MR imaging (diffusion, perfusion and spectroscopy): is it possible to distinguish oligodendroglial tumor grade and 1p/19q codeletion in the pretherapeutic diagnosis? AJNR Am. J. Neuroradiol. 2012;4(7):1326–1333. doi: 10.3174/ajnr.A3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Law M, Brodsky JE, Babb J, et al. High cerebral blood volume in human gliomas predicts deletion of chromosome 1p: preliminary results of molecular studies in gliomas with elevated perfusion. J. Magn. Reson. Imaging. 2007;25(6):1113–1119. doi: 10.1002/jmri.20920. [DOI] [PubMed] [Google Scholar]
- 30.Whitmore RG, Krejza J, Kapoor GS, et al. Prediction of oligodendroglial tumor subtype and grade using perfusion weighted magnetic resonance imaging. J. Neurosurg. 2007;107(3):600–609. doi: 10.3171/JNS-07/09/0600. [DOI] [PubMed] [Google Scholar]
- 31.Kapoor GS, Gocke TA, Chawla S, et al. Magnetic resonance perfusion-weighted imaging defines angiogenic subtypes of oligodendroglioma according to 1p19q and EGFR status. J. Neurooncol. 2009;92(3):373–386. doi: 10.1007/s11060-009-9880-x. [DOI] [PubMed] [Google Scholar]
- 32.Emblem KE, Scheie D, Due-Tonnessen P, et al. Histogram analysis of MR imaging-derived cerebral blood volume maps: combined glioma grading and identification of low-grade oligodendroglial subtypes. AJNR Am. J. Neuroradiol. 2008;29(9):1664–1670. doi: 10.3174/ajnr.A1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narang J, Jain R, Scarpace L, et al. Tumor vascular leakiness and blood volume estimates in oligodendrogliomas using perfusion CT: an analysis of perfusion parameters helping further characterize genetic subtypes as well as differentiate from astroglial tumors. J. Neurooncol. 2011;102(2):287–293. doi: 10.1007/s11060-010-0317-3. [DOI] [PubMed] [Google Scholar]
- 34.Bruzzone MG, Eoli M, Cuccarini V, Grisoli M, Valletta L, Finocchiaro G. Genetic signature of adult gliomas and correlation with MRI features. Expert Rev. Mol. Diagn. 2009;9(7):709–720. doi: 10.1586/erm.09.44. [DOI] [PubMed] [Google Scholar]
- 35.Khayal IS, Vandenberg SR, Smith KJ, et al. MRI apparent diffusion coefficient reflects histopathologic subtype, axonal disruption, and tumor fraction in diffuse-type grade II gliomas. Neuro Oncol. 2011;13(11):1192–1201. doi: 10.1093/neuonc/nor122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tozer DJ, Jäger HR, Danchaivijitr N, et al. Apparent diffusion coefficient histograms may predict low-grade glioma subtype. NMR Biomed. 2007;20(1):49–57. doi: 10.1002/nbm.1091. [DOI] [PubMed] [Google Scholar]
- 37.Jenkinson MD, du Plessis DG, Smith TS, Brodbelt AR, Joyce KA, Walker C. Cellularity and apparent diffusion coefficient in oligodendroglial tumours characterized by genotype. J. Neurooncol. 2010;96(3):385–392. doi: 10.1007/s11060-009-9970-9. [DOI] [PubMed] [Google Scholar]
- 38.Jenkinson MD, Smith TS, Brodbelt AR, et al. Apparent diffusion coefficients in oligodendroglial tumors characterized by genotype. J. Magn. Reson. Imaging. 2007;26:1405–1412. doi: 10.1002/jmri.21062. [DOI] [PubMed] [Google Scholar]
- 39.Walker C, Du Plessis DG, Fildes D, et al. Correlation of molecular genetics with molecular and morphological imaging in gliomas with an oligodendroglial component. Clin. Cancer Res. 2004;10(21):7182–7191. doi: 10.1158/1078-0432.CCR-04-0681. [DOI] [PubMed] [Google Scholar]
- 40.Stockhammer F, Thomale U-W, Plotkin M, Hartmann C, Von Deimling A. Association between fluorine-18-labeled fluorodeoxyglucose uptake and 1p and 19q loss of heterozygosity in World Health Organization Grade II gliomas. J. Neurosurg. 2007;106(4):633–637. doi: 10.3171/jns.2007.106.4.633. [DOI] [PubMed] [Google Scholar]
- 41.Shinozaki N, Uchino Y, Yoshikawa K, et al. Discrimination between low-grade oligodendrogliomas and diffuse astrocytoma with the aid of 11C-methionine positron emission tomography. J. Neurosurg. 2011;114(6):1640–1647. doi: 10.3171/2010.11.JNS10553. [DOI] [PubMed] [Google Scholar]
- 42.Saito T, Maruyama T, Muragaki Y, et al. 11C-methionine uptake correlates with combined 1p and 19q loss of heterozygosity in oligodendroglial tumors. AJNR Am. J. Neuroradiol. 2013;34(1):85–91. doi: 10.3174/ajnr.A3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breiman L. Random forests. Machine Learning. 2001;45(1):5–32. [Google Scholar]