Abstract
Anaplastic oligodendrogliomas (AOs) are rare brain tumors responsive to chemotherapy with procarbazine, lomustine (CCNU) and vincristine (PCV), especially when harboring 1p19q codeletion. However, with the emergence of temozolomide as an easier to administer and less toxic alternative regimen, PCV fell out of favor. Now, long-term results of two Phase III studies conceived in the 1990s, Radiation Therapy Oncology Group (RTOG) 9402 and European Organisation for Research and Treatment of Cancer (EORTC) 26951, resurrected debate about the potential role of PCV. No adequately powered prospective trial has compared chemotherapy alone with PCV versus temozolomide for newly diagnosed 1p19q codeleted AOs. Available data suggest responses may be both more frequent and more durable with PCV, and survival may be longer. Which regimen is ‘better’, therefore, depends on the importance of different metrics (i.e., toxicity, complexity, efficacy), and await definitive results from the important ongoing and recently redesigned CODEL international Phase III trial.
KEYWORDS : 1p19q, anaplastic, CCNU, chemotherapy, glioma, lomustine, malignant glioma, mixed glioma, oligoastrocytoma, oligodendroglioma, PCV, procarbazine, radiotherapy, temozolomide, vincristine
Practice points.
Anaplastic oligodendroglial tumors (oligodendrogliomas and oligoastrocytomas) are rare primary brain tumors. However, they are more responsive to treatment with radiotherapy and chemotherapy than other brain tumor subtypes.
Codeletion of chromosomes 1p and 19q is both a favorable prognostic factor regardless of treatment, and predicts benefit from DNA alkylator chemotherapy.
Radiotherapy and chemotherapy with the combination of procarbazine, lomustine (CCNU) and vincristine (PCV) leads to longer survival than radiotherapy alone for patients with 1p19q codeleted tumors.
No preplanned, powered, randomized study has been conducted comparing PCV versus temozolomide alone for newly diagnosed 1p19q codeleted anaplastic oligodendroglial tumors.
It remains unclear which regimen is ‘better’, PCV or temozolomide, for newly diagnosed 1p19q codeleted anaplastic oligodendroglial tumors.
Definitive results await completion of the important and recently redesigned CODEL international Phase III clinical trial. However, that is unlikely to occur for several years if not a decade or more.
Available data demonstrate with fair clarity that temozolomide is less toxic, easier to prescribe and less complicated for the patient.
Responses may be more frequent and more durable, and survival may be longer with PCV than with temozolomide.
Nonetheless, it may be that some patients (and practitioners) would want to use temozolomide even if efficacy is inferior, depending on the magnitude of survival and response rate differences.
New discoveries into the molecular biology of gliomas will hopefully lead to newer and ‘better’ therapies.
Anaplastic oligodendrogliomas (AOs) are rare primary brain tumors [1]. In 1988 it was first reported that recurrent, AOs that progressed despite radiotherapy respond well to alkylator chemotherapy [2]. At that time, the most commonly prescribed regimen of chemotherapy was PCV, the combination of procarbazine, lomustine (CCNU) and vincristine [3]. However, since then, temozolomide (TMZ) emerged as an alternative to PCV. Following publication of favorable results in recurrent anaplastic astrocytic gliomas in the late 1990s [4], TMZ became part of the standard of care for newly diagnosed glioblastoma in 2005 [5]. In the ensuing years, TMZ replaced PCV as the chemotherapy regimen of choice for all gliomas, including newly diagnosed AOs [6].
However, surprisingly favorable long-term results of two Phase III studies, Radiation Therapy Oncology Group (RTOG) trial 9402 and European Organisation for Research and Treatment of Cancer (EORTC) trial 26951, first conceived in the early 1990s but not published in mature form until 2013 [7,8], have now resurrected debate about the potential role of PCV in treatment of newly diagnosed AOs.
Which is ‘better’?
The evaluation depends on how ‘better’ is defined. Merriam-Webster's online dictionary defines ‘better’ first as ‘higher in quality’ [9], which does not clarify the issue. Certainly if one regimen were less toxic, simpler, induced responses more frequently, and improved survival versus the other, then it would be easy to define one as ‘better’. The analysis, unfortunately, is more complex.
Toxicity
There is general agreement that PCV is more toxic than TMZ. For example, among 24 patients who received an intensified PCV regimen in a Phase II study published in 1994 by the National Cancer Institute of Canada, toxicities were both frequent and harsh. These included the loss of more then 5% of body weight in approximately one-half of patients, debilitating fatigue in approximately a third and neuropathy in the majority, including paralytic ileus in 8% [10].
More recent studies also demonstrated that PCV is a toxic regimen. For example, in prospective Phase III studies, up to 38% of patients were required to discontinue PCV (either standard [11,12] or intensified [13] dosing) because of toxicity [11–13] versus up to 8% for TMZ [5,11], and up to 9% refused to continue [12,13] versus 4% for TMZ (Table 1) [5]. Moreover, severe toxicities were more frequent with PCV [11–13], including fatalities [13]. Finally, dose reductions were necessary more frequently and dose interruptions were both more frequent and longer with PCV than TMZ [11].
Table 1. . Toxicities from PCV and temozolomide causing treatment cessations in Phase III studies of newly diagnosed anaplastic gliomas or glioblastoma.
| Result | Procarbazine, iomustine and vincristine | Temozolomide |
|---|---|---|
| Discontinued treatment | ||
| Toxicity (%) | 9–38 [11–13] | 0–8 [5,11] |
| Refusal (%) | 5–9 [12,13] | 4 [5] |
| Toxicity | ||
| Grade 3–4 (%) | 20–56 [11–13] | 4–14 [5,11] |
| Grade 5 (death) | 2 (of 148) [13] | 0 [5,11] |
| Treatment modifications | ||
| Dose reductions | 16 [11] | 6 [11] |
| Interruptions | ||
| Frequency (%) | 18 [11] | 6 [11] |
| Duration (median, days) | 14 [11] | 10 [11] |
Complexity
In addition to the more frequent and more severe toxicities associated with PCV in comparison to TMZ, PCV is also more difficult to administer. The regimen is complex – three agents administered over a period of 6 [10,13] to 8 [2,3,12] weeks depending on the intensity. For example, a typical regimen of PCV consists of CCNU at 110 mg/m2 on day 1 followed by procarbazine at 60 mg/m2/day on days 8–21, all oral and at home. Patients with brain tumors often have cognitive impairments, which can contribute to treatment noncompliance. Complicating matters further, procarbazine is available only in flat doses of 50 mg per tablet, forcing the total dose to be rounded off and then divided over the 14 days of administration. By the time day 29 is reached, myelosuppresion may preclude intravenous vincristine (administered at 1.3 mg/m2 with a 2 mg cap on days 8 and 29). In brief, this is a more difficult regimen for the patient to take, and also a complicated one to prescribe. When intensified with higher doses and shorter cycle length, the complexities and risks become more substantial.
By contrast, TMZ is relatively simple. At a typical dose of 150–200 mg/m2 for days 1–5 of 28 [5], the flat dose is typically 250–400 mg. This is easily calculated from available capsule sizes. While a calendar is typically important in prescribing PCV, it is often unnecessary in prescribing TMZ.
These differences in toxicity and treatment logistics led to abandonment of PCV in the field until recently. For example, analysis of treatment patterns over time from a large (1013) retrospective series of patients with anaplastic oligodendroglial tumors revealed that since 2005, 3% of patients prescribed chemotherapy received PCV versus 97% who received TMZ [6]. This almost certainly resulted from the perception that the regimens were equi-efficacious, or at least that any superior of PCV in efficacy did not justify the increased toxicity and/or complexity.
Efficacy
Going back in time, RTOG 9402 and EORTC 26951 were conceived in the early 1990s when PCV was the standard, and arguably the only, chemotherapy regimen available. They were both designed to determine whether the addition of chemotherapy with PCV to radiotherapy improved survival relative to radiotherapy alone for newly diagnosed AO or anaplastic oligoastrocytoma (AOA) (Figure 1). They were simultaneously reported in 2006 as demonstrating no survival benefit from the addition of PCV to radiotherapy [12,13]. This reinforced the view, already prominent, that PCV was a toxic regimen that was no more effective than TMZ, despite an absence of any comparative data.
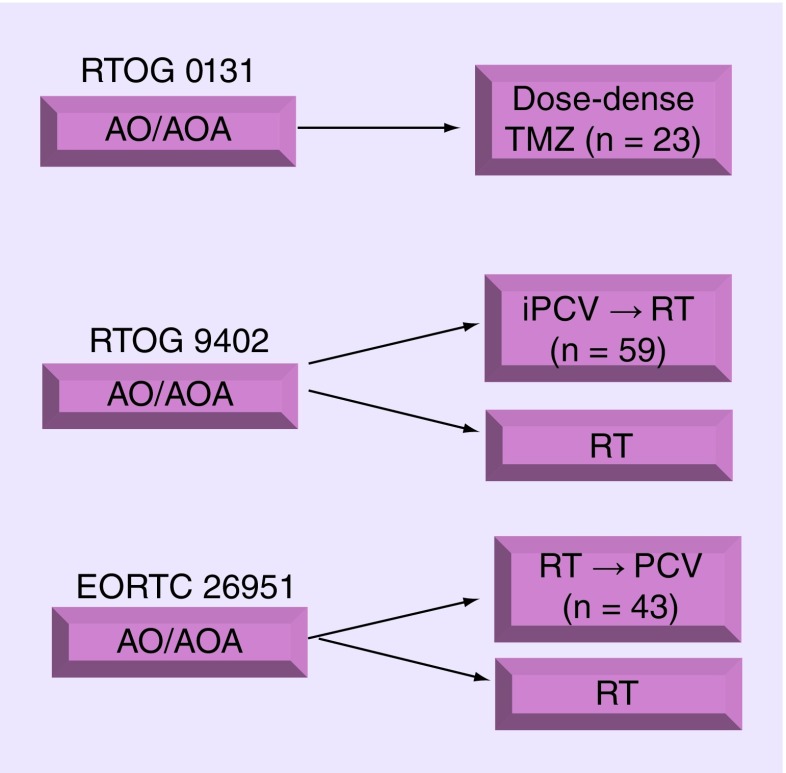
Figure 1. . Schema for clinical trials RTOG 0131 (single-arm Phase II study), RTOG 9402, and EORTC 26951.
The number (n) of patients with 1p19q co-deleted tumors treated with chemotherapy (TMZ or PCV) is indicated.
AO: Anaplastic oligodendrolglioma; AOA: Anaplastic oligoastrocytoma; iPCV: Intensive PCV; RT: Radiotherapy; TMZ: Temozolomide.
Shortly thereafter, NOA-04, a Phase III trial by the Neuro-Oncology Working Group of the German Cancer Society, also supported that conclusion [11]. NO-04 randomized patients with newly diagnosed anaplastic gliomas (including AOs, AOAs and anaplastic astrocytomas that are generally considered more chemotherapy resistant) to receive either RT followed by chemotherapy or vice versa, and the chemotherapy was further randomized to PCV or TMZ. On first glance, the results suggested TMZ was not only less toxic than PCV but was also not associated with an inferior progression-free or overall survival [11]. However, when evaluating the results in more detail, it was underpowered for such a conclusion. For example, there were only 33 patients with 1p19q codeleted anaplastic gliomas treated with primary chemotherapy for whom the comparison is most clear [14,15]. In addition, the results overall were immature with only 43% reaching the primary end point of time to progression after radiotherapy + chemotherapy [15]. More time is required to allow the results to mature, as in studies of other grade II and III gliomas that require long follow-up periods [16].
RTOG 9402 and EORTC 26951 were also, in retrospect, reported prematurely in 2006. [12,13] In 2013, long-term results were published (median follow-up of over 11 years). In addition, since their launch in 1994, it became clear that most histologically typical oligodendrogliomas were observed to harbor chromosome 1p and 19q codeletion [17], and codeletion appeared to predict response in small prospective or retrospective series [18,19]. The favorable prognostic value of 1p19q deletion was confirmed by the initial report of RTOG 9402 and EORTC 26951 [12,13]. When mature results were analyzed, both surprisingly demonstrated that codeletetion also predicted benefit from PCV [7,8]. Most impressively, survival was doubled among patients with AOs harboring deletion of chromosomes 1p and 19q treated with PCV and radiotherapy in comparison to radiotherapy alone [7,8]. In RTOG 9402, median survival among 1p19q codeleted cases treated with (intensive) PCV and radiotherapy was 14.7 years versus 7.3 years among those treated with radiotherapy alone (n = 59 vs 67; hazard ratio (HR): 0.59; 95% CI: 0.37–0.95; p = 0.03) [7]. In EORTC 26951, median survival was not reached versus 9.3 years (n = 43 vs 37, hazard ratio (HR): 0.56–1.03; 95% CI: 0.31–1.03; p = 0.0594) [8].
Neither RTOG 9402 nor EORTC 26951 compared PCV against TMZ. They also did not treat patients with chemotherapy alone. However, the surprising final results, and the magnitude of the survival benefit observed, led to a renewed interest in PCV. It also forced a redesign of the international CODEL trial, initially entitled ‘Phase III Intergroup Study of Radiotherapy Versus Temozolomide Alone Versus Radiotherapy With Concomitant and Adjuvant Temozolomide for Patients With 1p/19q Codeleted Anaplastic Glioma’, led the North Central Cancer Treatment Group (now the ALLIANCE). CODEL now includes an arm containing PCV and radiotherapy in light of the results of RTOG and EORTC.
To date, no level 1 evidence exists comparing PCV against TMZ for newly diagnosed 1p19q codeleted AOs. RTOG 0131 was a single arm Phase II trial in which patients with AOs or anaplastic oligoastrocytomas were treated with an intensified regimen of TMZ and those with less than a complete response then received radiotherapy (Figure 1). Results were favorable (Table 2), with 0% of patients with codeleted tumors suffering progression during chemotherapy versus 10–15% progressing during pre-radiotherapy intensive-PCV in RTOG 9402 [13,20,21,13] (Gregory J Cairncross, personal communication). Perhaps most intriguing, long-term follow-up demonstrated that the 6 years overall survival rate was 82% in RTOG 0131 [21], which compared favorably against RTOG 9402 (67%; p = 0.07) [21] and 69% in EORTC 26951 (Martin van den Bent, personal communication). However, RTOG 0131 was a single-arm study, it was not designed as a comparator against PCV, and such analysis neither preplanned nor was it nor appropriately powered, enrolling only 40 eligible patients among whom 23 harbored 1p19q codeleted tumors. Nonetheless, these results of this small study generate the hypothesis that TMZ may not be inferior to PCV, and CODEL will test that hypothesis formally (with radiotherapy).
Table 2. . Efficacy results from trials RTOG 0131, RTOG 9402 and EORTC 26951.
| 1p19q codeleted population | RTOG 9402/EORTC 26951 procarbazine, iomustine and vincristine (+ radiotherapy) | RTOG 0131 temozolomide (+ radiotherapy) |
|---|---|---|
| 3-year progression-free survival rate (%) | 70–80 [12,13,20] | 77 [20,21] |
| Progressive disease during chemotherapy (%) | 10–15 [13] | 0 [21] |
| 6-year overall survival rate (%) | 67–69 [7,8,12,13,21] | 82 [21] |
Other studies have reported response rates to PCV that appear higher than for TMZ (Table 3) among chemotherapy-naive patients treated for progressive disease [10,18,22–25] and newly diagnosed patients treated with chemotherapy alone [20,26–28]. However, these studies used different response criteria, included heterogeneous histologies, and were not consistently reported according to predictive or prognostic biomarkers such as 1p19q deletion. Restricting the analysis to studies reporting by 1p19q deletion status, response rates are also higher for PCV (Table 4). While intriguing, any such comparisons must be interpreted with substantial caution given the small cohort sizes (n = 7–22, Table 4), and mixed reporting methods.
Table 3. . Radiographic response rates to procarbazine, lomustine and vincristine or temozolomide.
Table 4. . Radiographic response rates to procarbazine, lomustine and vincristine or temozolomide for 1p19q codeleted anaplastic oligodendroglial tumors.
Nonetheless, it is of interest that responses to PCV appear not only more frequent but also more durable. For example, several studies (not exclusively AO) demonstrated that responses to PCV continued after the regimen was stopped [31–33]. Peyre et al. reported that tumor size did not reach nadir until an average of 2.7 years after PCV was discontinued [31–33]. By contrast, tumor growth appeared to resume immediately upon cessation of TMZ [34].
Perhaps the most important efficacy measure is survival. An international multicenter retrospective study also demonstrated that progression-free survival was more than twice is long following PCV (any iteration) than TMZ alone in 1p19q codeleted tumors (7.2 vs 3.2 years, n = 21 vs 68; p = 0.0186) [14]. Survival was also longer (10.5 vs 7.6 years) although immaturity of results (median of 7 years among surviving patients treated with PCV, 3.6 years for TMZ) contributed to a lack of statistical significance (p = 0.16).
Therefore, all of the available data is imperfect, and cross-comparisons are fraught with difficulty because of differences in response criteria, histopathologic diagnostic criteria, and other factors over time. Nonetheless, available data suggest PCV efficacy may, in fact, be ‘better’ than that of TMZ.
Thus, beauty is in the eye of the beholder when weighing the importance of toxicity, complexity and efficacy, and taking into account the levels of evidence for the available comparative data of PCV versus TMZ. However, that these regimens remain in common use despite decades of research, is not beautiful by any measure; rather it gives the field a black eye [35]. New discoveries in the molecular biology of brain tumors will hopefully lead to ‘better’ therapies.
Footnotes
Financial & competing interests disclosure
In the last 12 months, A Lassman has potentially relevant financial relationships with Abbvie, Genentech, Regeneron, Novartis, Heron Therapeutics and Foundation Medicine. The content of this chapter was presented as part of a symposium entitled ‘Anaplastic glioma: new challenges in the era of molecularly based treatment’ at the Society for Neuro-Oncology (SNO) annual meeting supported by the SNO Foundation, November 2012, Washington, DC, USA. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavanee WK. WHO Classification of Tumours of the Central Nervous System (4th Edition) International Agency for Research on Cancer; Lyon, France: 2007. [Google Scholar]
- 2.Cairncross JG, Macdonald DR. Successful chemotherapy for recurrent malignant oligodendroglioma. Ann. Neurol. 1988;23(4):360–364. doi: 10.1002/ana.410230408. [DOI] [PubMed] [Google Scholar]
- 3.Levin VA, Edwards MS, Wright DC, et al. Modified procarbazine, CCNU, and vincristine (PCV 3) combination chemotherapy in the treatment of malignant brain tumors. Cancer Treat. Rep. 1980;64(2–3):237–244. [PubMed] [Google Scholar]
- 4.Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter Phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group [Erratum in J. Clin. Oncol. 1999 17(11), 3693] J. Clin. Oncol. 1999;17(9):2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Panageas KS, Iwamoto FM, Cloughesy TF, et al. Initial treatment patterns over time for anaplastic oligodendroglial tumors. Neuro Oncol. 2012;14(6):761–767. doi: 10.1093/neuonc/nos065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J. Clin. Oncol. 2013;31(3):337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013;31(3):344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 9.Merriam-Webster definition: ‘better’. http://www.merriam-webster.com/dictionary/better?show=0&t=1379534932
- 10.Cairncross G, Macdonald D, Ludwin S, et al. Chemotherapy for anaplastic oligodendroglioma. National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 1994;12(10):2013–2021. doi: 10.1200/JCO.1994.12.10.2013. [DOI] [PubMed] [Google Scholar]
- 11.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized Phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J. Clin. Oncol. 2009;27(35):5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 12.van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer Phase III trial. J. Clin. Oncol. 2006;24(18):2715–2722. doi: 10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 13.Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J. Clin. Oncol. 2006;24(18):2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 14.Lassman AB, Iwamoto FM, Cloughesy TF, et al. International retrospective study of over 1000 adults with anaplastic oligodendroglial tumors. Neuro Oncol. 2011;13(6):649–659. doi: 10.1093/neuonc/nor040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deangelis LM. Anaplastic glioma: how to prognosticate outcome and choose a treatment strategy [corrected] J. Clin. Oncol. 2009;27(35):5861–5862. doi: 10.1200/JCO.2009.24.5985. [DOI] [PubMed] [Google Scholar]
- 16.van den Bent MJ, Jaeckle K, Baumert B, Wick W. RTOG 9802: good wines need aging. J. Clin. Oncol. 2013;31(5):653–654. doi: 10.1200/JCO.2012.46.6896. [DOI] [PubMed] [Google Scholar]
- 17.Reifenberger J, Reifenberger G, Liu L, James CD, Wechsler W, Collins VP. Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am. J. Pathol. 1994;145(5):1175–1190. [PMC free article] [PubMed] [Google Scholar]
- 18.Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J. Natl Cancer Inst. 1998;90(19):1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 19.Ino Y, Betensky RA, Zlatescu MC, et al. Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin. Cancer Res. 2001;7(4):839–845. [PubMed] [Google Scholar]
- 20.Vogelbaum MA, Berkey B, Peereboom D, et al. Phase II trial of preirradiation and concurrent temozolomide in patients with newly diagnosed anaplastic oligodendrogliomas and mixed anaplastic oligoastrocytomas: RTOG BR0131. Neuro Oncol. 2009;11(2):167–175. doi: 10.1215/15228517-2008-073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogelbaum MA, Wang M, Peereboom DM, et al. RTOG 0131: Phase II trial of pre-irradiation and concurrent temozolomide in patients with newly diagnosed anaplastic oligodendrogliomas and mixed anaplastic oligoastrocytomas – updated survival and progression free survival analysis [Abstract NO-56] Neuro Oncol. 2012;14(Suppl. 6):vi75–vi76. [Google Scholar]
- 22.van den Bent MJ, Kros JM, Heimans JJ, et al. Response rate and prognostic factors of recurrent oligodendroglioma treated with procarbazine, CCNU, and vincristine chemotherapy. Dutch Neuro-Oncology Group. Neurology. 1998;51(4):1140–1145. doi: 10.1212/wnl.51.4.1140. [DOI] [PubMed] [Google Scholar]
- 23.Paleologos NA, Macdonald DR, Vick NA, Cairncross JG. Neoadjuvant procarbazine, CCNU, and vincristine for anaplastic and aggressive oligodendroglioma. Neurology. 1999;53(5):1141–1143. doi: 10.1212/wnl.53.5.1141. [DOI] [PubMed] [Google Scholar]
- 24.Glass J, Hochberg FH, Gruber ML, Louis DN, Smith D, Rattner B. The treatment of oligodendrogliomas and mixed oligodendroglioma-astrocytomas with PCV chemotherapy. J. Neurosurg. 1992;76(5):741–745. doi: 10.3171/jns.1992.76.5.0741. [DOI] [PubMed] [Google Scholar]
- 25.van den Bent MJ, Looijenga LH, Langenberg K, et al. Chromosomal anomalies in oligodendroglial tumors are correlated with clinical features. Cancer. 2003;97(5):1276–1284. doi: 10.1002/cncr.11187. [DOI] [PubMed] [Google Scholar]
- 26.van den Bent MJ, Taphoorn MJ, Brandes AA, et al. Phase II study of first-line chemotherapy with temozolomide in recurrent oligodendroglial tumors: the European Organization for Research and Treatment of Cancer Brain Tumor Group Study 26971. J. Clin. Oncol. 2003;21(13):2525–2528. doi: 10.1200/JCO.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Taliansky-Aronov A, Bokstein F, Lavon I, Siegal T. Temozolomide treatment for newly diagnosed anaplastic oligodendrogliomas: a clinical efficacy trial. J. Neurooncol. 2006;79(2):153–157. doi: 10.1007/s11060-005-9020-1. [DOI] [PubMed] [Google Scholar]
- 28.Peereboom D, Brewer C, Schiff D, et al. Dose-intense temozolomide in patients with newly diagnosed pure and mixed anaplastic oligodendroglioma: Phase II multicenter study [abstract TA-40] Neuro Oncol. 2006;8(4):448. doi: 10.1007/s11060-014-1684-y. [DOI] [PubMed] [Google Scholar]
- 29.Kouwenhoven MC, Kros JM, French PJ, et al. 1p/19q loss within oligodendroglioma is predictive for response to first line temozolomide but not to salvage treatment. Eur. J. Cancer. 2006;42(15):2499–2503. doi: 10.1016/j.ejca.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Brandes AA, Tosoni A, Cavallo G, et al. Correlations between O6-methylguanine DNA methyltransferase promoter methylation status, 1p and 19q deletions, and response to temozolomide in anaplastic and recurrent oligodendroglioma: a prospective GICNO study. J. Clin. Oncol. 2006;24(29):4746–4753. doi: 10.1200/JCO.2006.06.3891. [DOI] [PubMed] [Google Scholar]
- 31.Peyre M, Cartalat-Carel S, Meyronet D, et al. Prolonged response without prolonged chemotherapy: a lesson from PCV chemotherapy in low-grade gliomas. Neuro Oncol. 2010;12(10):1078–1082. doi: 10.1093/neuonc/noq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason WP, Krol GS, Deangelis LM. Low-grade oligodendroglioma responds to chemotherapy. Neurology. 1996;46(1):203–207. doi: 10.1212/wnl.46.1.203. [DOI] [PubMed] [Google Scholar]
- 33.Stege EM, Kros JM, De Bruin HG, et al. Successful treatment of low-grade oligodendroglial tumors with a chemotherapy regimen of procarbazine, lomustine, and vincristine. Cancer. 2005;103(4):802–809. doi: 10.1002/cncr.20828. [DOI] [PubMed] [Google Scholar]
- 34.Ricard D, Kaloshi G, Amiel-Benouaich A, et al. Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann. Neurol. 2007;61(5):484–490. doi: 10.1002/ana.21125. [DOI] [PubMed] [Google Scholar]
- 35.Jim Henson. Quote by Miss Piggy. http://www.goodreads.com/quotes/22-beauty-is-in-the-eye-of-the-beholder-and-it